Abstract
Introduction
The psychosocial background of subjects with severe obesity developed from childhood onset obesity (CO) and their outcomes after bariatric surgery have not been fully investigated.
Methods
305 subjects were enrolled in the J-SMART study, which examined the effects of laparoscopic sleeve gastrectomy (LSG) in Japan, and categorized into two groups: CO defined as onset up to 13 years of age (CO group) and post-puberty onset obesity defined as onset after 13 years of age (PPO group). The subjects were followed up for at least 2 years and up to 5 years after LSG. Changes in physical parameters and remission of obesity-related comorbidities were assessed at 2 years after LSG. Weight regain (WR) was also assessed by evaluating the nadir weight after LSG and maximum weight thereafter during follow-up period.
Results
The mean postoperative follow-up period was 3.0 ± 1.1 years. 40.0% of the subjects had CO and these subjects had higher BMI and HOMA-β and lower age, HbA1c, HDL cholesterol, and visceral/subcutaneous fat area ratio compared to those with PPO. The CO group was also characterized by having higher rates of mental retardation, developmental disorders, and obesity in either parent and lower rate of marriage compared to the PPO group. Two years after LSG, there were no differences in total weight loss and remission rates of diabetes, dyslipidemia, and sleep apnea syndrome between the two groups, although remission rate of hypertension was higher in the CO group. The CO group also had a higher rate of WR after LSG than the PPO group, with CO, BMI, mental disorder, and binge eating contributing to WR.
Conclusion
This study suggests that CO might be associated with genetic and psychosocial factors. CO and PPO probably differ in pathogenesis and may require different treatment strategies.
Keywords: Obesity, Childhood onset obesity, Weight regain, Bariatric surgery, Laparoscopic sleeve gastrectomy
Introduction
Childhood onset obesity (CO) develops into adult obesity, which in turn leads to the development of obesity-related comorbidities such as diabetes, hypertension, dyslipidemia, and obstructive sleep apnea syndrome (OSAS). Obese children were around five times more likely to be obese in adulthood than those who were not obese [1]. Recent studies have shown an acceleration of obesity in children and adolescents due to the increased time spent at home as a result of the COVID-19 pandemic, and this has become a serious problem [2, 3].
Although various factors may contribute to the development of CO, such as heredity, upbringing environment, and intellectual development [4], the contributing factors may be different between CO with onset before/during puberty and post-puberty onset obesity (PPO). Moreover, the effects of bariatric surgery may also be different between CO and PPO. The purpose of this study was to investigate the physical and psychosocial characteristics of CO among patients treated with bariatric surgery and compare the effects of bariatric surgery and the rate of postoperative weight regain (WR) between CO and PPO.
Methods
This study was a subgroup analysis of the Japanese Survey of Morbid and Treatment-Resistant Obesity (J-SMART) study [5], which retrospectively examined the effects of bariatric surgery on weight loss and remission of diabetes in a representative sample of centers performing bariatric surgery in Japan. The study design was retrospective and observational. The J-SMART study included 322 Japanese subjects who underwent laparoscopic sleeve gastrectomy (LSG) at 10 bariatric surgery facilities accredited by the Japanese Society for the Treatment of Obesity (JSTO) between January 2011 and December 2014, with a follow-up period of at least 2 years. The subjects were followed up for at least 2 years and up to 5 years after LSG. Changes in physical parameters and remission of obesity-related comorbidities were assessed at 2 years after LSG. WR was also assessed by evaluating the nadir weight after LSG and maximum weight thereafter.
In Japan, the percentage of overweight (POW) index is more commonly used for children than the age-percentile body mass index (BMI). The POW index is calculated based on measured weight and standard weight for height as follows: POW (%) = 100 × (measured weight − standard weight for height)/standard weight for height [6]. A POW ≥15% is considered to be mildly obese, ≥20% moderately obese, and ≥30% severely obese in children younger than 6 years of age. For children aged 6 years or older, a POW ≥20% is considered mildly obese, ≥30% moderately obese, and ≥50% severely obese [7, 8]. In the present study, the subjects were asked whether they had been diagnosed with obesity based on the above criteria during annual school health check and were classified as CO if they were obese up to the age of 13 years and PPO if they were obese after the age of 13 years.
The JSTO guidelines adopted the following as indications for bariatric surgery: primary obesity between the ages of 18 and 65 years, inadequate response to medical treatment, and one of the following conditions: (1) BMI of 35 kg/m2 or higher for the purpose of weight loss (weight loss surgery), (2) BMI of 32 or higher for the purpose of treating comorbidities (including diabetes, hypertension, dyslipidemia, liver dysfunction, and sleep apnea syndrome). In the present subgroup analysis, 305 subjects who underwent LSG based on these guidelines and whose obesity onset period was known were selected from the J-SMART database. From the subjects' records, the following data were extracted: sex, age, and physical and clinical parameters such as height, body weight, visceral fat area (VFA), subcutaneous fat area (SFA), blood pressure, glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), fasting serum connecting peptide immunoreactivity (CPR), and fasting immunoreactive insulin (IRI). The CPR index was calculated by the following formula: CPR index = 100 × fasting CPR (ng/mg)/FPG (mg/dL) [9, 10]. Homeostasis model assessment (HOMA)-beta cell function (HOMA-β) was calculated by the following formula: HOMA-β = (fasting IRI [μIU/mL] × 360)/(FPG [mg/dL] − 63) [11, 12]. HOMA-insulin resistance (HOMA-IR) was calculated as follows: HOMA-IR = (fasting IRI [μIU/mL] × FPG [mg/dL])/405 [11, 12]. Other extracted data were lipid level markers, serum creatinine, history of insulin administration, number of medications, and apnea-hypopnea index (AHI). Subjects with AHI ≥ 5 and sleep-related symptoms of OSAS were diagnosed as having OSAS. VFA and SFA were measured by computed tomography at the level of the umbilicus with the subject in supine position. Information on mental disorders, intelligence, economic status, and family background collected in the J-SMART was also used in the present analysis. Mental disorders, mental retardation, developmental disorders, and binge eating were diagnosed by skilled psychiatrists in doubtful cases, according to the criteria in Diagnostic and Statistical Manual of Mental Disorders 4th or 5th edition, or International Statistical Classification of Diseases and Related Health Problems 10th Revision. The prevalence of mental disorders was the sum of mental retardation, developmental disorders, binge eating, and/or other mental disorders. The Wechsler Adult Intelligence Scale 3rd edition (WAIS-III) was performed as the intelligence test in general practice. WAIS-III was administered and scored by a trained psychiatrist or psychologist. WAIS-III was used to estimate their postsurgical outcomes.
Complete remission and partial remission of diabetes were defined as HbA1c less than 6.0% and 6.5%, respectively, without using antidiabetic drugs [13]. Dyslipidemia remission was defined as total cholesterol level <220 mg/dL, triglyceride level <150 mg/dL, and high-density lipoprotein cholesterol level ≥40 mg/dL without drug treatment for dyslipidemia, based on the criteria of the Japanese Atherosclerosis Society guidelines [14]. Remission of hypertension was defined as systolic blood pressure less than 130 mmHg and diastolic blood pressure less than 80 mmHg in the absence of antihypertensive drugs, based on the normal range of the Japanese Society of Hypertension guidelines [15]. Postoperative WR was evaluated using three major indices in bariatric surgery as follows: (1) WR of more than 25% of lost weight from the nadir weight [16, 17], (2) WR of more than 10 kg from the nadir weight [18, 19, 20], and (3) regaining more than 5 BMI points from the BMI at nadir weight [17, 21].
All procedures and data collection were performed in accordance with the ethical standards of the institutional and Japanese research committees and the ethical standards of the 1975 Declaration of Helsinki. This study was approved by the Ethics Committee of Toho University Sakura Medical Center (Approval Number: S16026). Informed consent was obtained in the form of opt-out on the Website.
Statistical Analysis
Results were expressed as mean ± SD or median (interquartile range) or percentage. Normal distribution was tested using the Shapiro-Wilk test. For comparisons between the two groups, Student's t test was used to analyze parametric data and Mann-Whitney U test for nonparametric data. Fisher's exact test was used to detect significant differences between proportions and categorical variables. For analysis of the proportions of CO in various BMI categories, χ2 test for trend was used. Simple linear regression analysis was performed using Spearman's rank correlation. Multiple regression analysis was performed to identify factors that contribute to postoperative WR. These analyses were performed using SPSS software version 26 (IBM Corp, Armonk, NY, USA). A p value ≤0.05 was considered significant.
Results
Comparison of Baseline Physical Data between CO and PPO Groups
Of 305 subjects, 122 (40.0%) were classified in the CO group and 183 (60.0%) in the PPO group. Compared to the PPO group, the CO group had higher body weight, BMI, HOMA-β, and SFA and lower age, HbA1c, high-density lipoprotein cholesterol, VFA, VFA/SFA ratio, and number of antihypertensive drugs (Table 1). There were no differences between the two groups in sex ratio, FPG, total cholesterol, triglyceride, serum creatinine, uric acid, systolic blood pressure, diastolic blood pressure, HOMA-IR, percent diabetes, percent hypertension, percent dyslipidemia, OSAS, number of medications for diabetes and dyslipidemia, and percent insulin use.
Table 1.
Comparison of baseline physical data between CO and PPO groups
| CO (n = 122) | PPO (n = 183) | p value | |
|---|---|---|---|
| Age, years | 42.2±9.4 | 50.5±10.3 | 0.000* |
| Male, % | 45.9 | 42.1 | 0.509 |
| Body weight, kg | 125.3±31.4 | 114.2±26.7 | 0.002* |
| BMI, kg/m2 | 43.8 (38.6–50.6) | 40.3 (36.7–45.7) | 0.000* |
| FPG, mg/dL | 109.0 (98.0–128.8) | 115.5 (102.0–139.3) | 0.054 |
| HbA1c, % | 6.2 (5.5–7.3) | 6.7 (5.9–8.2) | 0.023* |
| TC, mg/dL | 195.4±45.5 | 204.0±38.9 | 0.212 |
| TG, mg/dL | 138.0 (98.0–205.0) | 160.0 (104.0–218.0) | 0.276 |
| HDL-C, mg/dL | 44.0 (37.5–50.5) | 46.0 (41.0–55.0) | 0.013* |
| Serum creatinine, mg/dL | 0.61 (0.55–0.75) | 0.69 (0.58–0.85) | 0.052 |
| Uric acid, mg/dL | 6.6±1.6 | 6.3±1.6 | 0.332 |
| SBP, mmHg | 140.2±20.0 | 139.5±19.9 | 0.870 |
| DBP, mmHg | 89.3±19.2 | 85.7±13.0 | 0.255 |
| CPR index | 2.9 (2.2–4.2) | 2.7 (2.0–3.6) | 0.068 |
| HOMA-IR | 4.2 (3.1–7.9) | 4.5 (3.0–7.7) | 0.782 |
| HOMA-β, % | 155.3 (66.5–263.3) | 93.9 (60.6–148.1) | 0.023* |
| VFA, cm2 | 163.2 (133.5–208.8) | 198.0 (165.0–244.7) | 0.000* |
| SFA, cm2 | 567.0 (434.4–658.9) | 483.3 (430.0–588.6) | 0.008* |
| VFA/SFA ratio | 0.37±0.20 | 0.48±0.23 | 0.000* |
| Rate of diabetes, % | 60.7 | 67.2 | 0.241 |
| Rate of hypertension, % | 73.7 | 81.8 | 0.098 |
| Rate of dyslipidemia, % | 98.4 | 95.1 | 0.132 |
| Antidiabetic drugs, n | 1 (0–2) | 1 (0–2) | 0.477 |
| Insulin use, % | 4.1 | 9.8 | 0.115 |
| Antihypertensive drugs, n | 0 (0–1) | 1 (0–2) | 0.004* |
| Lipid-lowering drugs, n | 0 (0–1) | 0 (0–1) | 0.439 |
| Rate of OSAS, % | 68.9 | 75.4 | 0.209 |
Data are presented as mean±SD or median (interquartile range) or percentage. CO, childhood onset obesity; PPO, post-puberty onset obesity; BMI, body mass index; FPG; fasting plasma glucose; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; CPR, connecting peptide immunoreactivity; HOMA-IR, homeostasis model assessment (HOMA)-insulin resistance; HOMA-β, HOMA for beta cell function; VFA, visceral fat area; SFA, subcutaneous fat area; OSAS, obstructive sleep apnea syndrome; SD, standard deviation.
p value ≤0.05.
Comparison of Baseline Psychological and Social Data between CO and PPO Groups
Psychological and social baseline data in the CO or PPO groups are shown in Table 2. Compared to the PPO group, the CO group had higher prevalence of mental retardation and developmental disorders and obesity of either parent. They were also less likely to be married. There were no differences between the two groups in the prevalence of mental disorder, full/verbal/performance intelligence quotient (IQ) evaluated by WAIS-III, economic independence, history of divorce, living with family, and binge eating.
Table 2.
Comparison of baseline psychological and social data between CO and PPO groups
| CO | PPO | p value | |
|---|---|---|---|
| Mental disorder, % | 18.9 | 21.3 | 0.841 |
| Mental retardation and developmental disorders, % | 4.4 | 0.6 | 0.027* |
| Full IQ (WAIS-III) | 93.8±20.8 | 98.8±14.0 | 0.385 |
| Verbal IQ (WAIS-III) | 97.2±20.3 | 98.3±12.7 | 0.834 |
| Performance IQ (WAIS-III) | 93.3±19.6 | 98.7±16.1 | 0.364 |
| Economic independence, % | 63.9 | 65.6 | 0.405 |
| Marriage, % | 36.9 | 63.4 | 0.000* |
| History of divorce, % | 9.0 | 11.5 | 0.726 |
| Living with family, % | 80.3 | 86.9 | 0.205 |
| Obesity of either parent, % | 61.5 | 28.3 | 0.001 * |
| Binge eating, % | 13.9 | 10.9 | 0.540 |
Data are presented as mean±SD or percentage. CO, childhood onset obesity; PPO, post-puberty onset obesity; IQ, intelligence quotient; WAIS-III, Wechsler Adult Intelligence Scale 3rd edition; SD, standard deviation.
p value ≤0.05.
Percentage of CO and PPO Subjects by BMI Category
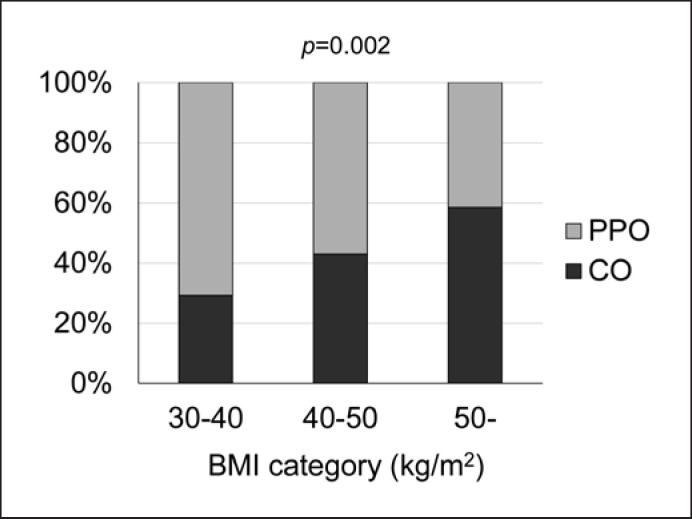
Figure 1 shows the percentage of CO and PPO subjects stratified by BMI category. The percentage of CO subjects increased and the percentage of PPO subjects decreased as BMI increased.
Fig. 1.
Percentage of CO and PPO subjects stratified by BMI category. The proportion of patients with CO increases as BMI increases. BMI, body mass index; CO, childhood onset obesity; PPO, post-puberty onset obesity.
Changes in Physical Parameters and Remission of Obesity-Related Comorbidities
The mean postoperative follow-up period was 3.0 ± 1.1 years. Changes in physical parameters and remission of obesity-related comorbidities were assessed at 2 years after LSG (Table 3). There were no differences between the two groups in body weight, BMI, percent total weight loss (%TWL), FPG, HbA1c, lipids levels, uric acid, blood pressure, VFA, SFA, and VFA/SFA ratio. The amount of change in serum creatinine was different between the CO and PPO groups. Remission rates of obesity-related comorbidities including complete and partial remission of diabetes, dyslipidemia, and OSAS were not significantly different between the two groups, but the remission rate of hypertension was higher in the CO group than in the PPO group. The amount of change in VFA was associated with complete or partial remission of diabetes and remission of dyslipidemia, and the amount of change in SFA was associated with remission of dyslipidemia and hypertension. Being CO or PPO did not contribute to changes in VFA or SFA (online suppl. Table S1; see www.karger.com/doi/10.1159/000524941 for all online suppl. material).
Table 3.
Comparison of changes in physical parameters and remission rates of obesity-related comorbidities 2 years after LSG
| CO | PPO | p value | |
|---|---|---|---|
| ΔBody weight, kg | −38.2±1.7 | −35.0±1.4 | 0.143 |
| ΔBMI, kg/m2 | −13.9±0.6 | −12.9±0.5 | 0.180 |
| %TWL, % | 30.1±11.7 | 29.7±10.6 | 0.772 |
| ΔFPG, mg/dL | −15.0 (−37.0 to [–5.0]) | −20.0 (−45.0 to [–8.0]) | 0.234 |
| ΔHbAlc, % | −0.7 (−1.7 to [–0.4]) | −1.0 (−2.0 to [–0.5]) | 0.163 |
| ΔTC, mg/dL | −0.6±6.5 | −8.3±4.2 | 0.298 |
| ΔTG, mg/dL | −57.0 (−110.8 to 1.0) | −61.0 (−106.0 to [–15.0]) | 0.372 |
| ΔHDL-C, mg/dL | 17.5±2.2 | 14.7±1.3 | 0.241 |
| ΔSerum creatinine, mg/dL | 0.40 (−0.43 to 0.90) | −0.20 (−0.70 to 0.50) | 0.014* |
| ΔUric acid, mg/dL | −0.9±0.2 | −0.8±0.1 | 0.693 |
| ΔSBP, mmHg | −3.2±2.6 | 1.6±1.6 | 0.115 |
| ΔDBP, mmHg | 0.3±1.9 | 0.8±1.2 | 0.819 |
| ΔVFA, cm2 | −84.7 (−123.9 to [–37.2]) | −132.7 (−186.8 to [–82.7]) | 0.063 |
| ΔSFA, cm2 | −145.1 (−254.9 to [–92.9]) | −108.7 (−274.5 to [–78.9]) | 0.520 |
| ΔVFA/SFA ratio | −0.07 (−0.17 to [–0.16]) | −0.10 (−0.24 to [–0.28]) | 0.324 |
| CR of diabetes, % | 80.0 | 65.6 | 0.061 |
| Complete or partial remission of diabetes, % | 83.6 | 77.1 | 0.337 |
| Remission of dyslipidemia, % | 57.7 | 65.6 | 0.196 |
| Remission of hypertension, % | 47.4 | 32.1 | 0.028* |
| Remission of OSAS, % | 58.4 | 66.2 | 0.236 |
Data are presented as mean±SD or median (interquartile range) or percentage. CO, childhood onset obesity; PPO, post-puberty onset obesity; BMI, body mass index; %TWL, percent total weight loss; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; VFA, visceral fat area; SFA, subcutaneous fat area; OSAS, obstructive sleep apnea syndrome; LSG, laparoscopic sleeve gastrectomy; SD, standard deviation; CR, complete remission.
p value ≤0.05.
Comparison of WR after LSG between CO and PPO Groups
Table 4 shows the differences in WR after LSG between the CO and PPO groups. There were no significant difference in the percentage of subjects with WR of more than 25% of lost weight from the nadir weight and WR of more than 10 kg from the nadir weight. However, the percentage of subjects who regained more than 5 BMI points from the BMI at nadir weight was significantly higher in the CO group.
Table 4.
Comparison of weight regain after LSG between CO and PPO groups
| CO | PPO | p value | |
|---|---|---|---|
| Regain of more than 25% of lost weight from the nadir weight, % | 32.1 | 25.7 | 0.305 |
| Regain of more than 10 kg from the nadir weight, % | 28.6 | 19.1 | 0.104 |
| Regain of more than 5 BMI points from the BMI at nadir weight, % | 17.9 | 5.1 | 0.002* |
Data are presented as percentage. CO, childhood onset obesity; PPO, post-puberty onset obesity; LSG, laparoscopic sleeve gastrectomy. * p value ≤0.05.
Relationship between WR of More than 5 BMI Points after LSG and Physical and Psychosocial Parameters
Based on the results of Table 4, the relationship between WR of more than 5 BMI points from the BMI at nadir weight after LSG and physical and psychosocial data was analyzed. In simple linear regression analysis, CO, age, BMI, mental disorder, and binge eating were associated with WR. In multiple regression analysis, CO, BMI, mental disorder, and binge eating were extracted as independent contributing factors for WR (Table 5). No significant correlation was found between WR of more than 5 BMI points from the BMI at nadir weight and changes in each parameter in all subjects (online suppl. Table S2).
Table 5.
Relationship between WR of more than 5 BMI points from the BMI at nadir weight (1) or not (0) and each parameter
| WR of more than 5 BMI points from the BMI at nadir weight (1) or not (0) |
||||
|---|---|---|---|---|
| univariate |
multivariate |
|||
| r | p value | β coefficient | p value | |
| CO (1) or PPO (0) | 0.206 | 0.002* | 0.132 | 0.05* |
| Age, years | −0.164 | 0.015* | −0.031 | 0.652 |
| Body weight, kg | 0.115 | 0.089 | ||
| BMI, kg/m2 | 0.19 | 0.005* | 0.226 | 0.001 * |
| FPG, mg/dL | −0.062 | 0.379 | ||
| HbA1c, % | −0.082 | 0.397 | ||
| TC, mg/dL | 0.031 | 0.75 | ||
| TG, mg/dL | 0.007 | 0.941 | ||
| HDL-C, mg/dL | −0.043 | 0.663 | ||
| Serum creatinine, mg/dL | 0.078 | 0.418 | ||
| Uric acid, mg/dL | 0.02 | 0.845 | ||
| SBP, mmHg | 0.101 | 0.163 | ||
| DBP, mmHg | −0.006 | 0.938 | ||
| OSAS (1) or not (0) | 0.062 | 0.37 | ||
| Mental disorder (1) or not (0) | 0.18 | 0.007* | 0.149 | 0.024* |
| Mental retardation and developmental disorders (1) or not (0) | 0.097 | 0.165 | ||
| Economic independence (1) or not (0) | −0.043 | 0.53 | ||
| Marriage (1) or not (0) | 0.037 | 0.589 | ||
| History of divorce (1) or not (0) | −0.012 | 0.863 | ||
| Living with family (1) or not (0) | −0.112 | 0.100 | ||
| Obesity of either parent (1) or not (0) | 0.011 | 0.930 | ||
| Binge eating (1) or not (0) | 0.173 | 0.011 * | 0.156 | 0.018* |
r, Spearman's rank correlation coefficient; WR, weight regain; CO, childhood onset obesity; PPO, post-puberty onset obesity; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; VFA, visceral fat area; SFA, subcutaneous fat area; OSAS, obstructive sleep apnea syndrome. The multivariate analysis model was r2 = 0.458, p = 0.021.
p value ≤0.05.
Discussion
This study analyzed the physical and psychosocial characteristics, the effects of bariatric surgery, and postoperative WR in CO patients who underwent bariatric surgery compared with PPO patients. In Japan, bariatric surgery has gradually become more widespread in recent years [22], but the number of patients undergoing surgery is still low compared to developed countries in the field of bariatric surgery. The J-SMART study is one of the largest databases of bariatric surgery in Japan, and the enrolled patients underwent LSG between 2011 and 2014. Although some time has passed since then, the characteristics of CO subjects needed to be clarified, and a sub-analysis of the J-SMART study was conducted in this study. The results suggest that background factors such as genetic predisposition (obesity of either parent), living environment, and mental problems were significantly associated with CO and that CO also contributed to postoperative WR.
Tables 1 and 2 presented the background characteristics of CO. A study of bariatric surgery candidates with obesity onset younger than 20 years showed that early onset of obesity was associated with higher total body fat mass [23], which is consistent with the finding of the CO subjects in our study. HbA1c was significantly lower in the CO group than in the PPO group, and the prevalence of diabetes and hypertension in the CO group tended to be lower, although not significantly different. It is well known that subcutaneous fat is predominant in childhood obesity [24, 25], and the amount of subcutaneous fat in young adults has been reported to be associated with BMI changes during both late childhood and adolescence [26]. Longitudinal studies suggest that excess fat and associated comorbidities persist into adulthood, with approximately 90% of children with severe obesity becoming adults having BMI ≥35 kg/m2 [27]. In the present study, the CO group was also obese with predominant accumulation of subcutaneous fat. Study has suggested that subcutaneous fat may have a protective effect against metabolic and cardiovascular diseases [28], which may support the lower HbA1c in the CO group than in the PPO group.
The remission rates of diabetes, dyslipidemia, and OSAS in this study were not different between the two groups, while the rate of hypertension remission was significantly higher in the CO group. In a sub-analysis of the SOS study, there was no difference in the remission of diabetes after bariatric surgery between subjects who were obese at age 20 and those who were not obese [29]. Since one of the purposes of this study was to identify the characteristics of CO, the subjects were classified by the cutoff age of 13 years, and there were no differences in remission rates of diabetes, dyslipidemia, and OSAS between the two groups. The reason for the significantly higher remission rate of hypertension in the CO group was unclear, but the PPO group was older and used a larger number of antihypertensive drugs than the CO group, which may suggest that the PPO subjects had longer duration of hypertension or more severe hypertension before LSG.
Another finding in this study was that CO, BMI, mental disorder, and binge eating were identified as contributing factors to WR after LSG. There are several major criteria for WR after bariatric surgery. In the present study, the prevalence of post-LSG increase in BMI of 5 or more was significantly higher in the CO group. Previous studies have reported a variety of factors associated with WR after bariatric surgery [30, 31, 32]. A systematic review reported the factors that contribute to postoperative WR [31], but there are few reports of the relationship between CO and WR. The reason for the association between CO and WR is not clear, but as there are reports about obesity and appetite abnormalities in children [33, 34], the presence of genetic abnormality influencing appetite regulation in the central nervous system in CO is plausible. On the other hand, for obese patients with CO and mental retardation or developmental disorders, genetic abnormalities such as Prader-Willi syndrome, Cohen syndrome, and Bardet-Biedl syndrome should be carefully excluded preoperatively [35]. In addition, in recent years, drugs such as setmelanotide may become a new treatment option for obesity caused by some specific genetic abnormalities [36, 37], and the possibility of genetic abnormalities should always be assumed in the practice of obesity.
BMI is also associated with WR [38], and WR is more likely to occur in patients with BMI over 50 [39]. A review found no association between preoperative BMI and WR [31], but careful interpretation is necessary because of the differences in surgical technique and ethnicity.
The finding of an association between WR and mental disorders and binge eating was also important; especially, depression and binge eating have been reported to be more common in candidates for bariatric surgery [40]. Because the current study focused on the association between CO and LSG, it did not focus on elucidating the factors that make it easier or harder for subjects to lose weight after LSG. On the other hand, the original J-SMART study showed weight trends for the entire cohort and further revealed that there was a higher prevalence of mental disorders in subjects with lower %TWL after LSG (%TWL <15%). Interestingly, subjects with higher %TWL (%TWL ≥45%) also had a higher prevalence of mental disorders [5]. In other reports, mental disorder and binge eating are also associated with WR after bariatric surgery [41, 42, 43]. In our study, there was no difference in the prevalence of mental disorder and binge eating between the CO and PPO groups. However, mental disorder and binge eating were extracted as factors that independently contributed to WR, confirming the importance of these factors for WR in Japanese subjects. IQ was not different between the CO and PPO groups and was not a contributing factor to WR. Although one report has shown that obese children have lower IQ than normal weight children and that lower IQ in childhood is associated with higher BMI in adulthood [44], there are few studies on the relationship between IQ or intellectual level and postoperative WR. On the other hand, severe mental retardation is often considered a contraindication for bariatric surgery [45]. The finding of no association between IQ and CO or WR in this study may be due to selection bias. It is necessary to examine whether bariatric surgery can be expected to improve the remission of obesity-related comorbidities in CO subjects with low IQ.
This study has several limitations. The retrospective observational study design and missing data in some subjects may have affected the results of the analysis. Furthermore, in dividing the subjects into CO and PPO groups, the original data were based on interviews with them, not on the existence of medical record data on their height and weight. It was also necessary to compare historical weight trends with Z -scores and percentiles based on pediatric growth curves for both the CO and PPO groups, but accurate historical weight data were not available and could not be analyzed. In the current study, most patients underwent LSG in their 40s or 50s; subjects in the CO group had been obese since childhood, but at what point they actually became obese enough to meet the criteria for surgery could not be determined without accurate historical weight records. In addition, since only Japanese subjects were included, the effects of racial differences could not be studied. It should also be noted that the analysis of WR was based on a maximum follow-up of 5 years and that the number of subjects included in this analysis has been decreasing over time.
Conclusion
In conclusion, this study suggests that severely obese patients with childhood onset tend to have more severe and subcutaneous fat-dominant obesity compared to those with post-puberty onset, and subcutaneous fat predominance may be associated with lower HbA1c in those with childhood onset. Compared to PPO, CO might be associated with genetic and psychosocial factors and shows a tendency to regain weight after bariatric surgery. These findings suggest CO and PPO may involve different pathogenesis, which may require different treatment strategies.
Statement of Ethics
This study was performed in accordance with the ethical standards of the institutional and Japanese research committees and the ethical standards of the 1975 Declaration of Helsinki. This study was approved by the Ethics Committee of Toho University Sakura Medical Center (Approval Number: S16026). Informed consent was obtained in the form of opt-out on the Website.
Conflict of Interest Statement
I.T. received lecture fee from Takeda Pharmaceutical Co., Ltd., and Novartis Pharma Ltd. and honorarium from Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Bayer Yakuhin, Ltd. T.N. received honorarium from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Medtronic, Inc., Johnson & Johnson, Inc., Olympus Corporation, Terumo Corporation, Sumitomo Bakelite Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck & Co., MC Medical, Inc., Daiichi Sankyo Co., Ltd., Bayer Yakuhin, Ltd., Nippon Boehringer lngelheim Co., Ltd., and Eli Lilly Japan K.K., research grant from Japan Society for the Promotion of Science, and Grant-in-Aid for Scientific Research (C) (#17K10575), Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Medtronic, Inc. Y.M. received honorarium from Otsuka Pharmaceutical Co., Ltd. and Covidien Japan Inc. M.T. received lecture fee from Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., and Ono Pharmaceutical Co., Ltd. K.Y. received grant from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd., Kao Corporation, Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., Teijin Pharma Limited, Shionogi Co., Ltd., Astellas Pharma Inc., Kowa Co., Ltd., and Bayer Yakuhin, Ltd., consulting fees from Kowa Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., Novo Nordisk Pharma Ltd., AstraZeneca K.K., Pfizer Japan Inc., and Bayer Yakuhin, Ltd., and honorarium from Kowa Co., Ltd., MSD K.K., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation, Amgen K.K., Takeda Pharmaceutical Co., Ltd., Sanofi K.K., Ono Pharmaceutical Co., LTD., AstraZeneca K.K., Daiichi Sankyo Co., Ltd, Novartis Pharma K.K., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., and Janssen Pharmaceutical K.K. K.K. received lecture fee from Ethicon and Covidien Japan Inc. Y.I. received honorarium from MSD K.K., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., and Mitsubishi Tanabe Pharma Corporation. Y.S. received research grant from Medtronic, Inc., Johnson & Johnson, Inc., Nikkiso Co., Ltd., Sunny Health Co., Ltd., and Daiwa Securities Health Foundation.
Funding Sources
J-SMART was supported by a grant for research on intractable diseases from the Ministry of Health, Labour and Welfare of Japan (H28-nanji-ippan-014).
Author Contributions
All authors made significant contributions to the study. A.S. and T.Y. designed the original concept. Y.W. wrote the initial draft of the manuscript. A.S. and I.T reviewed and edited the manuscript. All other authors contributed to data collection and interpretation and critically reviewed the manuscript. All the authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement
Data described in the article will be made available upon reasonable request from the corresponding author.
Supplementary Material
Supplementary data
Acknowledgements
The authors wish to thank Aki Hagiwara for the collection and assembly of data.
References
- 1.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016 Feb;17((2)):95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 2.Lange SJ, Kompaniyets L, Freedman DS, Kraus EM, Porter R, Blanck HM, et al. Longitudinal trends in body mass index before and during the COVID-19 pandemic among persons aged 2–19 years: United States, 2018–2020. MMWR Morb Mortal Wkly Rep. 2021 Sep 17;70((37)):1278–83. doi: 10.15585/mmwr.mm7037a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolford SJ, Sidell M, Li X, Else V, Young DR, Resnicow K, et al. Changes in body mass index among children and adolescents during the COVID-19 pandemic. JAMA. 2021 Oct 12;326((14)):1434–6. doi: 10.1001/jama.2021.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JD, Fu E, Kobayashi MA. Prevention and management of childhood obesity and its psychological and health comorbidities. Annu Rev Clin Psychol. 2020;16:351–78. doi: 10.1146/annurev-clinpsy-100219-060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saiki A, Yamaguchi T, Tanaka S, Sasaki A, Naitoh T, Seto Y, et al. Background characteristics and postoperative outcomes of insufficient weight loss after laparoscopic sleeve gastrectomy in Japanese patients. Ann Gastroenterol Surg. 2019;3((6)):638–47. doi: 10.1002/ags3.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asayama K, Ozeki T, Sugihara S, Ito K, Okada T, Tamai H, et al. Criteria for medical intervention in obese children: a new definition of “obesity disease” in Japanese children. Pediatr Int. 2003 Oct;45((5)):642–6. doi: 10.1046/j.1442-200x.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 7.Kubo T. Common approach to childhood obesity in Japan. J Pediatr Endocrinol Metab. 2014 Jul;27((7–8)):581–92. doi: 10.1515/jpem-2014-0047. [DOI] [PubMed] [Google Scholar]
- 8.Isojima T, Yokoya S. The value of anthropometric indices for childhood obesity in Japan. Ann Hum Biol. 2019 Jun;46((4)):293–7. doi: 10.1080/03014460.2019.1643404. [DOI] [PubMed] [Google Scholar]
- 9.Albareda M, Rigla M, Rodríguez-Espinosa J, Caballero A, Chico A, Cabezas R, et al. Influence of exogenous insulin on C-peptide levels in subjects with type 2 diabetes. Diabetes Res Clin Pract. 2005 Jun;68((3)):202–6. doi: 10.1016/j.diabres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, et al. Utility of indices using C-peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011 Aug 2;2((4)):297–303. doi: 10.1111/j.2040-1124.2010.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28((7)):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja V, Kadowaki T, Evans RW, Kadota A, Okamura T, El Khoudary SR, et al. Comparison of HOMA-IR, HOMA-β% and disposition index between US white men and Japanese men in Japan: the ERA JUMP study. Diabetologia. 2015 Feb;58((2)):265–71. doi: 10.1007/s00125-014-3414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015 May–Jun;11((3)):489–506. doi: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018 Sep 1;25((9)):846–984. doi: 10.5551/jat.GL2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019) Hypertens Res. 2019 Sep;42((9)):1235–481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu SY, Wong SK, Lam CC, Yung MY, Kong AP, Ng EK. Long-term results on weight loss and diabetes remission after laparoscopic sleeve gastrectomy for A morbidly obese Chinese population. Obes Surg. 2015 Oct;25((10)):1901–8. doi: 10.1007/s11695-015-1628-4. [DOI] [PubMed] [Google Scholar]
- 17.Lauti M, Lemanu D, Zeng ISL, Su'a B, Hill AG, MacCormick AD. Definition determines weight regain outcomes after sleeve gastrectomy. Surg Obes Relat Dis. 2017 Jul;13((7)):1123–9. doi: 10.1016/j.soard.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012 Dec;22((6)):479–86. doi: 10.1097/SLE.0b013e318262dc29. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah E, El Nakeeb A, Youssef T, Abdallah H, Ellatif MA, Lotfy A, et al. Impact of extent of antral resection on surgical outcomes of sleeve gastrectomy for morbid obesity (a prospective randomized study) Obes Surg. 2014 Oct;24((10)):1587–94. doi: 10.1007/s11695-014-1242-x. [DOI] [PubMed] [Google Scholar]
- 20.Casella G, Soricelli E, Giannotti D, Collalti M, Maselli R, Genco A, et al. Long-term results after laparoscopic sleeve gastrectomy in a large monocentric series. Surg Obes Relat Dis. 2016 May;12((4)):757–62. doi: 10.1016/j.soard.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013 Oct;258((4)):628–36. doi: 10.1097/SLA.0b013e3182a5034b. discussion 36–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshiro T, Kasama K, Nabekura T, Sato Y, Kitahara T, Matsunaga R, et al. Current status and issues associated with bariatric and metabolic surgeries in Japan. Obesity Surg. 2021;31((1)):343–9. doi: 10.1007/s11695-020-05056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrzosek M, Wiśniewska K, Sawicka A, Tałałaj M, Nowicka G. Early onset of obesity and adult onset of obesity as factors affecting patient characteristics prior to bariatric surgery. Obes Surg. 2018 Dec;28((12)):3902–9. doi: 10.1007/s11695-018-3381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benfield LL, Fox KR, Peters DM, Blake H, Rogers I, Grant C, et al. Magnetic resonance imaging of abdominal adiposity in a large cohort of British children. Int J Obes. 2008 Jan;32((1)):91–9. doi: 10.1038/sj.ijo.0803780. [DOI] [PubMed] [Google Scholar]
- 25.Suliga E. Visceral adipose tissue in children and adolescents: a review. Nutr Res Rev. 2009 Dec;22((2)):137–47. doi: 10.1017/S0954422409990096. [DOI] [PubMed] [Google Scholar]
- 26.Kindblom JM, Lorentzon M, Hellqvist A, Lönn L, Brandberg J, Nilsson S, et al. BMI changes during childhood and adolescence as predictors of amount of adult subcutaneous and visceral adipose tissue in men: the GOOD study. Diabetes. 2009 Apr;58((4)):867–74. doi: 10.2337/db08-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa heart study. J Pediatr. 2007 Jan;150((1)):12–e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009 Jun;32((6)):1068–75. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensson FM, Andersson-Assarsson JC, Svensson PA, Carlsson B, Peltonen M, Carlsson LMS. Effects of bariatric surgery in early- and adult-onset obesity in the prospective controlled Swedish obese subjects study. Diabetes Care. 2020;43((4)):860–6. doi: 10.2337/dc19-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaouk L, Hsu AT, Tanuseputro P, Jessri M. Modifiable factors associated with weight regain after bariatric surgery: a scoping review. F1000Res. 2019;8:615–5. doi: 10.12688/f1000research.18787.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Athanasiadis DI, Martin A, Kapsampelis P, Monfared S, Stefanidis D. Factors associated with weight regain post-bariatric surgery: a systematic review. Surg Endosc. 2021;35((8)):4069–84. doi: 10.1007/s00464-021-08329-w. [DOI] [PubMed] [Google Scholar]
- 32.El Ansari W, Elhag W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps: a scoping review. Obes Surg. 2021;31((4)):1755–66. doi: 10.1007/s11695-020-05160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutelle KN, Peterson CB, Crosby RD, Rydell SA, Zucker N, Harnack L. Overeating phenotypes in overweight and obese children. Appetite. 2014;76:95–100. doi: 10.1016/j.appet.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lansigan RK, Emond JA, Gilbert-Diamond D. Understanding eating in the absence of hunger among young children: a systematic review of existing studies. Appetite. 2015 Feb;85:36–47. doi: 10.1016/j.appet.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet. 2019 Jan;95((1)):23–40. doi: 10.1111/cge.13367. [DOI] [PubMed] [Google Scholar]
- 36.Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020 Dec;8((12)):960–70. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 37.Giannopoulou EZ, Zorn S, Schirmer M, Herrmann G, Heger S, Reinehr T, et al. Genetic obesity in children: overview of possible diagnoses with a focus on SH2B1 deletion. Horm Res Paediatr. 2021 Oct 22;:340–51. doi: 10.1159/000520402. [DOI] [PubMed] [Google Scholar]
- 38.Torrego-Ellacuría M, Barabash A, Larrad-Sainz A, Hernández-Nuñez GM, Matía-Martín P, Pérez-Ferre N, et al. Weight regain outcomes after bariatric surgery in the long-term follow-up: role of preoperative factors. Obes Surg. 2021 Sep;31((9)):3947–55. doi: 10.1007/s11695-021-05497-5. [DOI] [PubMed] [Google Scholar]
- 39.Baig SJ, Priya P, Mahawar KK, Shah S. Weight regain after bariatric surgery-a multicentre study of 9,617 patients from Indian bariatric surgery outcome reporting group. Obes Surg. 2019 May;29((5)):1583–92. doi: 10.1007/s11695-019-03734-6. [DOI] [PubMed] [Google Scholar]
- 40.Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016 Jan 12;315((2)):150–63. doi: 10.1001/jama.2015.18118. [DOI] [PubMed] [Google Scholar]
- 41.Kalarchian MA, King WC, Devlin MJ, Hinerman A, Marcus MD, Yanovski SZ, et al. Mental disorders and weight change in a prospective study of bariatric surgery patients: 7 years of follow-up. Surg Obes Relat Dis. 2019 May;15((5)):739–48. doi: 10.1016/j.soard.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauro M, Papelbaum M, Brasil MAA, Carneiro JRI, Coutinho ESF, Coutinho W, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obes Rev. 2019 Oct;20((10)):1413–25. doi: 10.1111/obr.12907. [DOI] [PubMed] [Google Scholar]
- 43.Freire CC, Zanella MT, Segal A, Arasaki CH, Matos MIR, Carneiro G. Associations between binge eating, depressive symptoms and anxiety and weight regain after Roux-en-Y gastric bypass surgery. Eat Weight Disord. 2021 Feb;26((1)):191–9. doi: 10.1007/s40519-019-00839-w. [DOI] [PubMed] [Google Scholar]
- 44.Yu ZB, Han SP, Cao XG, Guo XR. Intelligence in relation to obesity: a systematic review and meta-analysis. Obes Rev. 2010 Sep;11((9)):656–70. doi: 10.1111/j.1467-789X.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Bauchowitz AU, Gonder-Frederick LA, Olbrisch ME, Azarbad L, Ryee MY, Woodson M, et al. Psychosocial evaluation of bariatric surgery candidates: a survey of present practices. Psychosom Med. 2005 Sep–Oct;67((5)):825–32. doi: 10.1097/01.psy.0000174173.32271.01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Data described in the article will be made available upon reasonable request from the corresponding author.