Abstract
Background
The incidence of Grade C postoperative pancreatic fistula ranges from 2 to 11% depending on the type of pancreatic resection. This complication may frequently require early relaparotomy and the surgical approach remains technically challenging and is still associated with a high mortality. Infectious complications and post-operative hemorrhage are the two most common causes of reoperation.
Summary
The best management of grade C pancreatic fistulas remains controversial and ranges from conservative approaches up to completion pancreatectomy. The choice of the technique depends on the patient's conditions, intraoperative findings, and surgeon's discretion. A pancreas-preserving strategy appears to be attractive, including from simple to more complex procedures such as debridement and drainage, and external wirsungostomy. Completion pancreatectomy should be reserved for selected cases, including stable patients with severe infection complication or hemorrhage after pancreatic fistula who do not respond to pancreas-preserving procedures.
Key Messages
This review describes the current options for management of grade C pancreatic fistula after pancreatoduodenectomy with regard to indication, choice of procedure and outcomes of the different approaches.
Keywords: Pancreatic fistula, Pancreatoduodenectomy, Grade C fistula, Postoperative pancreatic fistula, Whipple, Surgical management
Introduction
Pancreatoduodenectomy associated with chemotherapy is the only treatment option to achieve long-term survival for patients with pancreatic ductal adenocarcinoma of the head of the pancreas; furthermore, pancreatoduodenectomy is a standard treatment for other benign pathologies in the pancreatic head [1, 2, 3]. Pancreatoduodenectomy is a complex procedure associated with a mortality of around 5% in high-volume centers and complications such as bleeding, delayed gastric emptying, intra-abdominal abscess, and postoperative pancreatic fistula. Complications related to pancreatic fistula are associated with prolonged hospital stay, elevated costs, and even postoperative mortality. Furthermore, the best approach to manage patients with pancreatic fistula after pancreatoduodenectomy is still under debate [4, 5, 6, 7].
In contrast to mortality, postoperative morbidity after pancreatoduodenectomy is still high and pancreatic fistula is by far the most important complication. The incidence of pancreatic fistula according to the definition of the International Study Group for Pancreatic Surgery (ISGPS) ranges from 3% to 45% and grade C pancreatic fistula represents between 2% and 11%. Various preoperative risk factors have been identified, among which soft texture of the pancreas and duct size less than 3 mm are two of the most important ones. Modifications of pancreato-enteric anastomosis, use of stents, and omission of octreotide have been investigated to decrease the rate of this dreaded complication, yet high-level evidence for mitigation remains scarce [1, 2, 3, 4]. Regarding management, however, surgery is only considered after failure of all reasonable non-operative procedures, including especially, interventional radiology [4, 5, 6, 7].
Pancreatic fistula can frequently cause other major complications, such as intra-abdominal abscess, hemorrhage, wound infection, and bile leak, due to a highly erosive nature of the fluid. Relaparotomy is indicated for pancreatic fistula associated with uncontrolled hemorrhage, peritonitis, and intra-abdominal abscess, complications of which can be fatal in these patients [5, 7, 8, 9].
Some preventive procedures to minimize the risk of pancreatic fistula such as primary total pancreatectomy, second-stage pancreatojejunostomy, and prophylactic occlusion of the pancreatic duct without anastomosis have been proposed, but not been generally accepted as also after such measures, complications have been observed. Patients with postoperative pancreatic fistula represent a heterogenous group; some can be managed merely with a conservative approach or radiological intervention for variable periods, whereas others need early surgical treatment to reduce associated complications [7, 8, 9].
If grade C pancreatic fistula after pancreatoduodenectomy requires early relaparotomy, surgical management still remains technically challenging and is associated with a high mortality. Postoperative hemorrhage and sepsis are the most serious complications related to leaking of pancreatic anastomosis after cephalic pancreatectomy and are the two most common causes for reoperation. Serious hemorrhage from pseudoaneurysms can further complicate pancreatic leakage, and after failure of radiologic endovascular procedures, emergency relaparotomy can be required for bleeding control. Sepsis is associated with failure of non-operative management, and the risk of dying is higher in cases of delayed surgical treatment [8, 9].
The current literature provides various surgical strategies for surgical management of grade C pancreatic fistula. Improvements in terms of management of postoperative pancreatic fistula have been achieved with the use of radiologic drainage of infected collections and arterial embolization or stenting in case of massive bleeding. Morbidity and mortality depend on the capacity to control the source of bleeding or sepsis. However, nearly half of these patients will require a second operation [10, 11, 12]. The aim of this review was to describe the current options for management of grade C pancreatic fistula after pancreatoduodenectomy and attempt to identify the ideal technique according to the clinical condition of the patient and intraoperative findings.
Definitions
The ISGPS defined pancreatic fistula as an abnormal communication between the pancreatic ductal “system” and another epithelial surface containing pancreas-derived, enzyme-rich fluid. For the diagnosis, any volume after postoperative day 3 with a value >3 times the upper limit of normal amylase is the necessary threshold. However, whenever an amylase activity is found without impact on the clinical outcome, no fistula should be reported as pancreatic fistula is only regarded when clinically relevant and then graded according to the ISGPS (Table 1) [7, 8, 9].
Table 1.
Pancreatic fistula according to ISGPF
| Pancreatic fistula (ISGPF) | ||
|---|---|---|
| BL | Grade B | Grade C |
|
| ||
| Amylase >3 times upper limit institutional normal serum amylase value | Persistent drainage >3 weeks Clinically relevant change in management of POPF Percutaneous or endoscopic drainage Angiographic procedures for bleeding Signs of infection without organ failure |
Reoperation Organ failure Death |
POPF, postoperative pancreatic fistula; BL, biochemical leak.
This comprises two grades related to severity, namely, grade B (intermediate) and grade C (severe), whereas a mild enzyme secretion without any clinical consequence is termed a biochemical leak only. Grade C pancreatic fistula is associated with mortality rates of up to 50%. Appropriate identification and treatment of patients with grade C pancreatic fistula who are at risk of rapid deterioration is vital to reduce postoperative mortality [10, 11, 12]. No clear criterion for revisional surgery in grade C pancreatic fistula has been described to guide the surgeon and the choice of the technique is relying on the patient's conditions, intraoperative findings, and surgeon's discretion [12, 13, 14, 15].
According to the ISGPF definition [7], grade C pancreatic fistula requires reoperation and is related to organ failure and death. Its incidence varies between 2% and 11% and is associated with 20–35% mortality rate. Reoperation remains an undeniable valuable option in cases where abdominal complications such as severe peritonitis, sepsis, or massive bleeding cannot be controlled by an interventional approach and the life-threatening nature of grade C pancreatic fistula becomes the indication for revisional surgery.
Some strategies have been described during reoperation for patients with grade C pancreatic fistula and are classified as completion pancreatectomy (CP) and procedures that preserve the pancreatic remnant. The decision to remove the pancreas remnant or to perform a pancreas-preserving procedure is a difficult task. The most common pancreas-preserving procedures are pancreas debridement with lavage and drains, primary suture, new pancreatic-enteric anastomosis, internal or external wirsungostomy, pancreatic duct occlusion, and salvage pancreatogastrostomy [8, 9]. These procedures are technically easier than CP and preserve pancreatic function. However, they often expose the patient to additional reoperations and associated complications because of the potential persistence of the pancreatic fistula [10, 11, 12, 13, 14, 15, 16]. Severe hemodynamic instability is an indication for performing a pancreas-preserving technique, particularly external wirsungostomy or debridement and drain placement. Low mortality rates in grade C pancreatic fistula can be expected in centers dedicated to pancreatic surgery and experienced in handling such complications. General and gastrointestinal surgeons must be aware of the available procedures when surgical management is necessary in these difficult situations [8, 9, 12, 13, 14, 15, 16].
Techniques
Indication for relaparotomy after pancreatoduodenectomy is associated with life-threatening complications such as uncontrolled late hemorrhage or peritoneal sepsis due to pancreatic fistula. Furthermore, it may be required after interventional bleeding control for hematoma evacuation and potentially focus control. During relaparotomy for grade C pancreatic fistula, the management of pancreatic stump includes different techniques and is classified as pancreas-preserving approaches or CP (Table 2) [12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. Intraoperative findings frequently dictate the choice of the procedure, which is often determined by clinical instability and duration as well as bleeding and collateral damage risk of the procedure[17, 18, 19, 21, 22, 23, 24] (Table 3).
Table 2.
Types of reinterventions for grade C pancreatic fistula
| Technique | Year | Author | Ref |
|---|---|---|---|
| 1 Primary suture (redo PJ anastomosis) | 2018 | Ma | [17] |
|
| |||
| 2 Debridement, lavage, drains | 2005 | De Castro | [12] |
| 2009 | Haddad | [16] | |
|
| |||
| 3 External wirsungostomy | 2012 | Denost | [20] |
| 2013 | Paye | [19] | |
| 2013 | Ribero | [18] | |
|
| |||
| 4 Internal wirsungostomy | 2010 | Xu | [21] |
| 2010 | Kent | [22] | |
|
| |||
| 5 Pancreatic duct occlusion | 2014 | Balzano | [15] |
|
| |||
| 6 Salvage pancreatogastrostomy | 2008 | Bachellier | [23] |
| 2012 | Govil | [24] | |
|
| |||
| 7 CP | 2006 | Tamijmarane | [31] |
Table 3.
Mortality after different treatment of grade C pancreatic fistula, %
| Author | Year | Drainage | Redo | Occlusion | PG | CP | Ext wir | Int wir | Ref |
|---|---|---|---|---|---|---|---|---|---|
| De Castro | 2005 | 25 | 0 | [12] | |||||
| Tamijmarane | 2006 | 56.5 | [31] | ||||||
| Muller | 2007 | 39.1 | [29] | ||||||
| Bachellier | 2008 | 0 | 50 | [23] | |||||
| Fuks | 2009 | 0 | 50 | [3] | |||||
| Haddad | 2009 | 11.1 | 40 | [16] | |||||
| Kent | 2010 | 0 | [22] | ||||||
| Xu | 2010 | 20 | 0 | [21] | |||||
| Denost | 2012 | 28.5 | [20] | ||||||
| Govil | 2012 | 67 | 0 | 50 | [24] | ||||
| Paye | 2013 | 0 | 50 | 8.3 | [19] | ||||
| Ribero | 2013 | 43.5 | 0 | [18] | |||||
| Balzano | 2014 | 30 | 29 | 21 | [15] | ||||
| Almond | 2015 | 52.6 | [37] | ||||||
| Nentwich | 2015 | 55 | [34] | ||||||
| Wiltberger | 2015 | 15.3 | [27] | ||||||
| Horvath | 2016 | 16.7 | [26] | ||||||
| Ma | 2018 | 26.7 | 33.3 | 25 | [17] | ||||
| Wronski | 2019 | 56.3 | 47.1 | 50 | [13] |
Debridement and Drainage
Debridement and drainage are the basis of operations for patients with grade C pancreatic fistula after failure of interventional procedures. The outcomes of debridement and drainage must be considered in these patients. Simple drainage of the abdominal cavity consists of debridement of necrotic tissue, hemostasis of the pancreatic parenchyma or other bleeding sources, lavage of the abdominal cavity, and use of drains (Fig. 1) [13, 25].
Fig. 1.
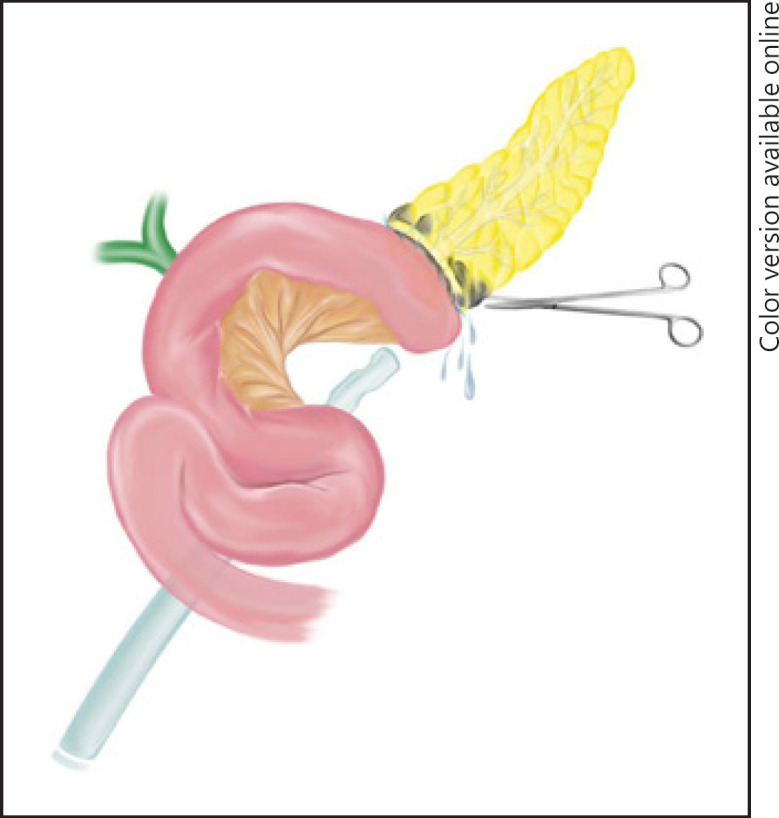
Debridement and drainage of grade C pancreatic fistula.
At the time of reoperation, surgical conditions in the abdominal cavity can be challenging, and debridement and drainage might remain the only option. In case of limited disruption of the pancreato-jejunal anastomosis, simple debridement and drainage is also indicated; oversewing of the anastomosis has little value and low success rates [13, 15, 25].
Hemodynamically unstable patients with metabolic acidosis and coagulopathies represent another indication for debridement and drainage. A very quick laparotomy is performed and rapid return of the patient to the ICU is mandatory. Simple drainage is frequently associated with high rates of reoperations − as known from surgery in necrotizing acute pancreatitis − when compared with CP or external wirsungostomy. However, the mortality is frequently low [13, 15, 25].
Main Pancreatic Duct Occlusion
In this case, the pancreatic remnant has no continuity and does not allow repeat reconstruction, and the small bowel is occluded with linear stapler. The pancreatic duct is identified and occluded with interrupted non-absorbable sutures, the pancreatic stump can be transfixed including the pancreatic duct, and a fine purse string could be made without cannulation of the duct (Fig. 2). To minimize secretion leaks from minor ducts, the pancreatic cut surface should be also occluded. More recently, some chemical substances have been proposed to occlude the main pancreatic duct such as cyanoacrylate (Dermabond® − Ethicon) or fibrin glue (Tissucol® − Baxter) [16, 25, 26].
Fig. 2.
a Main pancreatic duct occlusion. b Wirsungography. c Pancreatic duct occlusion with cyanoacrylate.
After occlusion of the pancreatic duct, the endocrine function may be preserved, and complete exocrine insufficiency may be minimized by enzymatic substitution. Complications related to the procedure are severe hemorrhage, postoperative pancreatitis, permanent exocrine insufficiency, and risk of late endocrine insufficiency due to pancreatic atrophy. This technique is reserved for selected patients with friable pancreas and small pancreatic duct. Duct occlusion includes the resection of the jejunal limb, conversion from an activated pancreatic fistula involving small bowel secretion to a pure pancreatic fistula avoiding the association and subsequent activation of pancreatic enzymes by the biliary and enteric secretion. In pancreatic duct occlusion, the theoretical advantages in preservation of the pancreas are associated with a high rate of second relaparotomy and risk of persistent pancreatic fistula. Therefore, the potential advantages of duct occlusion remain questionable when compared to debridement and drainage [16, 25, 26].
Additional relaparotomy is more common after pancreatic duct occlusion than after CP. Second reoperation may also cause blood loss. However, blood loss and blood transfusion have been described to be similar between CP and pancreatic duct occlusion. No difference in mortality was observed between CP and pancreatic duct occlusion (p = 0.610) [16, 25, 26].
External Wirsungostomy
This procedure is indicated for grade C pancreatic fistula and for patients in bad health condition when rapid laparotomy is necessary. The pancreatic-enteric anastomosis is dismantled, and the stump of the jejunum is closed with a stapler. An over suture is used to avoid bleeding. The remnant of the pancreatic duct is cannulated with a silicone tube with lateral holes at the internal end to maximize pancreatic juice drainage and fixed in the pancreatic duct. The free end of the catheter is passed through the abdominal wall and fixed to the skin, allowing drainage of fluids out of the abdomen (Fig. 3) [17, 27].
Fig. 3.
a External wirsungostomy. b Stent into the pancreatic duct. c External drainage.
Two drains are placed through the right and the left side in the abdominal cavity that are removed when the output is reduced to <50 mL/day. Feeding jejunostomy can be performed in some patients, distal to the gastric enteric anastomosis to provide enteral nutrition. Total parenteral nutrition is indicated in all patients and should start as soon as possible [17, 18, 19, 20, 21, 27]. Of the 21 patients who underwent wirsungostomy, 6 patients subsequently died of liver failure, refractory septic shock, or mesenteric ischemia (28.5%). The median length of hospital stay was 42 days (range: 34–60 days). Three patients (14.3%) developed diabetes mellitus during follow-up [20].
Ribero et al. [18] analyzed 42 patients with grade C pancreatic fistula who underwent surgical treatment including CP in 23 patients and external tube pancreatostomy in 9 patients. Indications for reoperation and operative time were similar in both procedures, while postoperative mortality was significantly higher after CP (43.5% vs. 0%, p = 0.03). Moreover, a second emergency reoperation was higher after CP (39.1% vs. 11.1%) [19, 20, 21].
External wirsungostomy was performed in 12 patients by Paye et al. [19], 2 patients died (17%), 10 patients developed postoperative complications, and reoperation was necessary in 3 patients. Salvage relaparotomy and repeat pancreatojejunostomy was attempted after a median delay of 130 days in 10 patients and was successful in 9 patients (90%). Long-term endocrine function was preserved in 66% of the patients [20].
In any case, a second procedure is necessary (re-pancreatojejunostomy) several months later, after stabilization and recovery of the patient (two-step procedure). External wirsungostomy is a safe and feasible alternative associated with excellent long-term results, preserving pancreatic endocrine and exocrine functions. However, further validation is necessary to confirm its feasibility and safety [17, 26, 28].
Modified external wirsungostomy is the preferred approach in patients with a viable pancreatic stump. Multiple reinterventions and deteriorating organ function are important factors associated with mortality after reoperation. A timely reintervention after the diagnosis of grade C pancreatic fistula may improve mortality by minimizing multiorgan failure. Still, external wirsungostomy may decrease the need for reintervention when compared with simple drainage of the pancreatic anastomosis [13].
Internal Wirsungostomy
In case of internal wirsungostomy (bridge anastomosis) with or without resection, the cut surface of the pancreas remnant is another possible procedure for grade C pancreatic fistula which has, however, been only anecdotally reported. A stent is introduced and fixed in the pancreatic duct and placed in the first jejunal loop by enterotomy and fixed by suture to avoid the risk of migration through bowel peristalsis (Fig. 4) [22, 23].
Fig. 4.

Internal wirsungostomy.
Kent et al. [22] performed this technique in 5 patients, all patients survived and were discharged from hospital after a median duration of 41 days. No pancreaticocutaneous fistula, stricture, or atrophy of the remnant was observed [23].
Seven patients underwent internal wirsungostomy by Xu et al. [21]. The blood loss and average in-hospital time were inferior when compared with CP (p < 0.05). After CP, the mortality was 20%, whereas no mortality was observed after internal wirsungostomy. Diabetes and diarrhea were found in all patients with CP compared to none of the patients with bridge anastomosis, however, slight hypertriglyceridemia controlled without insulin was observed in 1 patient [22]. Bridge anastomosis is a simple, easy, and safe procedure that can preserve the exocrine and endocrine function of the pancreas and decrease the mortality of reoperation.24
Pancreatogastrostomy
Salvage pancreatogastrostomy was analyzed in two studies after grade C pancreatic fistula following initial pancreatojejunostomy. Salvage pancreatogastrostomy was reported by Bachellier et al. [23]. They reported on 12 patients with PJ leaks after PD, 8 of whom were treated by CP, and 4 treated by pancreatogastrostomy. The decision to perform pancreatogastrostomy was made during laparotomy and based on (1) identification of viable pancreatic remnant, (2) major rupture of the pancreatojejunostomy, and (3) attempt to redo the previous pancreatojejunostomy which was considered hazardous [24, 28].
The technique consists of debridement of the necrotic portion of the pancreatic stump, to identify healthy tissue after taking down the pancreatojejunostomy. Sufficient mobilization of the pancreatic remnant off the splenic vein is necessary to allow telescoping into the gastric lumen. Two layers of absorbable sutures are used to secure the anastomosis in a small gastrotomy of approximately half of the diameter of the pancreas. The pancreatic stump is inserted through this gastrotomy into the stomach (Fig. 5). An anterior gastrotomy is performed to place additional sutures. Postoperative nasogastric decompression of the stomach is necessary to facilitate good drainage of pancreatic juice [24, 28]. Based on these reports, salvage pancreatogastrostomy is associated with lower rates or reinterventions as well as endocrine dysfunction compared to other procedures [24, 28].
Fig. 5.

Salvage pancreatogastrostomy.
In addition, no mortality was observed in these two studies which include 8 patients when compared with 50% mortality after CP. Salvage pancreatogastrostomy is technically easy because the location of the stomach and pancreas and the thickness and vascularity of the stomach wall, minimize the risk of infection complications. Yet, the procedure is restricted to patients with previous pancreatojejunostomy [28].
Completion Pancreatectomy
Once considered the standard approach as a salvage procedure for grade C pancreatic fistula, recently CP has been indicated in only selected patients due to its high rates of perioperative morbidity and mortality, as well as the inevitable development of endocrine and exocrine pancreatic insufficiency. Some authors have even suggested that CP should not be considered as treatment of grade C pancreatic fistula. However, CP seems to be the only option to achieve sterilization of the infection source and decrease the possibility of reoperation [29, 30, 31, 32, 33].
The operating field is commonly characterized by local sepsis, significant inflammation, and tissue necrosis. The pancreatic anastomosis should be evaluated by the extent of anastomotic dehiscence, the degree of surrounding inflammation, the presence of necrosis or abscess, the quality of the pancreatic parenchyma, the extent of the gap between the pancreas and the jejunal limb, and the quality of the jejunal serosa [30, 34, 35].
An intraoperative finding of necrotizing pancreatitis associated with fistula is an indication for CP. However, visceral adhesions in delayed reoperation and a difficult access to the pancreatic stump may reduce the possibility of CP. Some indications for CP are when the disruption exceeds 180° of the suture line, extensive necrosis of the pancreatic stump, and inability to find the main pancreatic duct [33, 34, 35, 36].
CP consists of the removal of the remnant pancreas with the distal segment of the jejunal reconstruction loop and usually splenectomy (Fig. 6). The bile duct anastomosis can generally be preserved unless the distance between the pancreato- and hepatojejunostomy is too short to safely cut and close the remaining jejunum. CP is an aggressive and difficult procedure because of poor intraoperative conditions. Splenectomy is associated to risk of ischemia of the gastric remnant. However, spleen-preserving emergency CP is technically sometimes even more difficult during relaparotomy. CP is associated with longer operative time when compared with simple drainage (p = 0.013) or external wirsungostomy (p = 0.044) [29, 34, 35, 36, 37].
Fig. 6.
Completion pancreatectomy.
Inevitably, CP results in a complete exocrine and endocrine pancreatic insufficiency, associated with a condition named “brittle” diabetes. This is potentially associated with complications of glucose regulation, even death due to acute hypoglycemia, long-term diabetes, and ketoacidosis. It therefore requires an excellent postoperative management of this type of diabetes. Following CP, hospital readmission rate has been observed in 56% of patients due to endocrine problems and impaired quality of life [29, 36, 37]. However, CP eliminates the problem of leakage and fluid collection. Recently total pancreatectomy associated with islet autotransplantation is an attractive option with lower incidence of postoperative diabetes. Still, this procedure is only available in some specialized centers due to complex requirements in terms of cell processing and regulatory limitations [30, 31, 32, 33, 34, 35, 37].
In conclusion, surgical treatment of pancreas anastomotic insufficiency remains a challenge after pancreatoduodenectomy. The literature provides various surgical therapeutic strategies. The choice of the technique depends on the patient's conditions, intraoperative findings, and surgeon's discretion. The pancreas-preserving strategy appears to be attractive, including simple to more complex procedures. Debridement and drainage are indicated in patient with uncomplicated dehiscence or in severe hemodynamic instability. Debridement appears to be simple, safe, and effective and associated with low mortality. External wirsungostomy is a safe and feasible technique and should be one of the techniques primarily considered. These techniques seem to be the preferred treatment option at present. CP should not be the first treatment option. The role of CP is declining in favor of more conservative strategies for patients with grade C pancreatic fistula. This technique is commonly indicated for selected stable patients with severe infection complication with extensive necrosis or hemorrhage after pancreatic fistula who do not respond to pancreas-preserving procedures. Severe hemodynamic instability is an indication for a pancreas-preserving technique, particularly external wirsungostomy or debridement and drain placement. A better understanding of endocrine and endocrine insufficiency is essential during the decision for CP. In some cases, the final decision depends on personal experience and preference of individual surgeon.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
Torres, O.J.M.: conception and design of study, acquisition of data, interpretation, drafting of manuscript, critical revision, and approval of final version. Moraes-Junior, J.M.A.: acquisition of data, interpretation, and approval of final version of manuscript. Fernandes, E.D.S.M.: conception and design of study, acquisition of data, critical revision, and approval of final version. Hackert, T.: acquisition of data, interpretation, critical revision, and approval of final version.
References
- 1.Torres OJM, Fernandes ESM, Vasques RR, Waechter FL, Amaral PCG, Rezende MB, et al. Pancreatoduodenectomy: Brazilian practice patterns. Arq Bras Cir Dig. 2017;30:190–6. doi: 10.1590/0102-6720201700030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres OJM, Costa RCNDC, Costa FFM, Neiva RF, Suleiman TS, Souza YLMS, et al. Modified Heidelberg technique for pancreatic anastomosis. Arq Bras Cir Dig. 2017;30:260–3. doi: 10.1590/0102-6720201700040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009;197((6)):702–9. doi: 10.1016/j.amjsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Miyagawa S, Makuuchi M, Kawasaki S, Ogiwara M. Second-stage pancreatojejunostomy following pancreatoduodenectomy in high-risk patients. Am J Surg. 1994;168:66–8. doi: 10.1016/s0002-9610(05)80073-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou YM, Zhou X, Wan T, Xu D, Si XY. An evidence-based approach to the surgical interventions for severe pancreatic fistula after pancreatoduodenectomy. Surgeon. 2018;16((2)):119–24. doi: 10.1016/j.surge.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Luu AM, Krasemann L, Fahlbusch T, Belyaev O, Janot-Matuschek M, Uhl W, et al. Facing the surgeon's nightmare: incidence and management of postoperative pancreatic fistulas grade C after pancreaticoduodenectomy based on the updated definition of the International Study Group of Pancreatic Surgery (ISGPS) J Hepatobiliary Pancreat Sci. 2020;27:171–81. doi: 10.1002/jhbp.713. [DOI] [PubMed] [Google Scholar]
- 7.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Smits FJ, van Santvoort HC, Besselink MG, Batenburg MCT, Slooff RAE, Boerma D, et al. Management of severe pancreatic fistula after pancreatoduodenectomy. JAMA Surg. 2017;152:540–8. doi: 10.1001/jamasurg.2016.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan MT, Vollmer CM, Jr, Asbun HJ, Ball CG, Bassi C, Beane JD, et al. The characterization and prediction of ISGPF grade C fistulas following pancreatoduodenectomy. J Gastrointest Surg. 2016;20:262–76. doi: 10.1007/s11605-015-2884-2. [DOI] [PubMed] [Google Scholar]
- 11.Hackert T, Werner J, Büchler MW. Postoperative pancreatic fistula. Surgeon. 2011;9:211–7. doi: 10.1016/j.surge.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 12.De Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92:1117–23. doi: 10.1002/bjs.5047. [DOI] [PubMed] [Google Scholar]
- 13.Wronski M, Cebulski W, Witkowski B, Guzel T, Karkocha D, Lech G, et al. Surgical management of the grade C pancreatic fistula after pancreatoduodenectomy. HPB. 2019;21:1166–74. doi: 10.1016/j.hpb.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Bouras AF, Marin H, Bouzid C, Pruvot FR, Zerbib P, Truant S. Pancreas-preserving management in reinterventions for severe pancreatic fistula after pancreatoduodenectomy: a systematic review. Langenbecks Arch Surg. 2016;401:141–9. doi: 10.1007/s00423-015-1357-0. [DOI] [PubMed] [Google Scholar]
- 15.Balzano G, Pecorelli N, Piemonti L, Ariotti R, Carvello M, Nano R, et al. Relaparotomy for a pancreatic fistula after a pancreaticoduodenectomy: a comparison of different surgical strategies. HPB. 2014;16:40–5. doi: 10.1111/hpb.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad LBP, Scatton O, Randone B, Andraus W, Massault PP, Dousset B, et al. Pancreatic fistula after pancreaticoduodenectomy: the conservative treatment of choice. HPB. 2009;11:203–9. doi: 10.1111/j.1477-2574.2009.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma T, Bai X, Chen W, Li G, Lao M, Liang T. Pancreas-preserving management of grade-C pancreatic fistula and a novel bridging technique for repeat pancreaticojejunostomy: an observational study. Int J Surg. 2018;52:243–7. doi: 10.1016/j.ijsu.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Ribero D, Amisano M, Zimmitti G, Giraldi F, Ferrero A, Capussotti L. External tube pancreatostomy reduces the risk of mortality associated with completion pancreatectomy for symptomatic fistulas complicating pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:332–8. doi: 10.1007/s11605-012-2100-6. [DOI] [PubMed] [Google Scholar]
- 19.Paye F, Lupinacci RM, Kraemer A, Lescot T, Chafaï N, Tiret E, et al. Surgical treatment of severe pancreatic fistula after pancreaticoduodenectomy by wirsungostomy and repeat pancreatico-jejunal anastomosis. Am J Surg. 2013;206:194–201. doi: 10.1016/j.amjsurg.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Denost Q, Pontallier A, Rault A, Ewald JA, Collet D, Masson B, et al. Wirsungostomy as a salvage procedure after pancreaticoduodenectomy. HPB. 2012;14:82–6. doi: 10.1111/j.1477-2574.2011.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Dai X, Bu X, Gao F, Zhang X. Pancreaticojejunal bridge anastomosis: a novel option for surgeon to preserve pancreatic body and tail in urgent reoperation for intra-abdominal massive hemorrhage after pancreaticoduodenectomy. World J Surg. 2010;34:2457–62. doi: 10.1007/s00268-010-0658-2. [DOI] [PubMed] [Google Scholar]
- 22.Kent TS, Callery MP, Vollmer CM., Jr The bridge stent technique for salvage of pancreaticojejunal anastomotic dehiscence. HPB. 2010;12:577–82. doi: 10.1111/j.1477-2574.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachellier P, Oussoultzoglou E, Rosso E, Scurtu R, Lucescu I, Oshita A, et al. Pancreatogastrostomy as a salvage procedure to treat severe postoperative pancreatic fistula after pancreatoduodenectomy. Arch Surg. 2008;143:966–70. doi: 10.1001/archsurg.143.10.966. discussion 971. [DOI] [PubMed] [Google Scholar]
- 24.Govil S. Salvage pancreaticogastrostomy for pancreatic fistulae after pancreaticoduodenectomy. Indian J Gastroenterol. 2012;31((5)):263–6. doi: 10.1007/s12664-012-0213-1. [DOI] [PubMed] [Google Scholar]
- 25.Conzo G, Gambardella C, Tartaglia E, Sciascia V, Mauriello C, Napolitano S, et al. Pancreatic fistula following pancreatoduodenectomy. Evaluation of different surgical approaches in the management of pancreatic stump. Literature review. Int J Surg. 2015;21(Suppl 1):S4–9. doi: 10.1016/j.ijsu.2015.04.088. [DOI] [PubMed] [Google Scholar]
- 26.Horvath P, Beckert S, Nadalin S, Königsrainer A, Königsrainer I. Pancreas-preserving surgical management of grade-C pancreatic fistulas after pancreatic head resection by external wirsungostomy. Langenbecks Arch Surg. 2016;401:457–62. doi: 10.1007/s00423-016-1423-2. [DOI] [PubMed] [Google Scholar]
- 27.Wiltberger G, Schmelzle M, Tautenhahn HM, Krenzien F, Atanasov G, Hau HM, et al. Alternative treatment of symptomatic pancreatic fistula. J Surg Res. 2015;196:82–9. doi: 10.1016/j.jss.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 28.Königsrainer I, Zieker D, Beckert S, Glatzle J, Schroeder TH, Heininger A, et al. A pancreas-preserving technique for the management of symptomatic pancreatic anastomotic insufficiency refractory to conservative treatment after pancreas head resection. Langenbecks Arch Surg. 2010;395:693–6. doi: 10.1007/s00423-009-0508-6. [DOI] [PubMed] [Google Scholar]
- 29.Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–74. doi: 10.1097/SLA.0b013e31815c2ca3. discussion 974–5. [DOI] [PubMed] [Google Scholar]
- 30.Gueroult S, Parc Y, Duron F, Paye F, Parc R. Completion pancreatectomy for postoperative peritonitis after pancreaticoduodenectomy: early and late outcome. Arch Surg. 2004;139:16–9. doi: 10.1001/archsurg.139.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Tamijmarane A, Ahmed I, Bhati CS, Mirza DF, Mayer AD, Buckels JA, et al. Role of completion pancreatectomy as a damage control option for post-pancreatic surgical complications. Dig Surg. 2006;23:229–34. doi: 10.1159/000095395. [DOI] [PubMed] [Google Scholar]
- 32.Smith CD, Sarr MG, vanHeerden JA. Completion pancreatectomy following pancreaticoduodenectomy: clinical experience. World J Surg. 1992;16:521–4. doi: 10.1007/BF02104459. [DOI] [PubMed] [Google Scholar]
- 33.Farley DR, Schwall G, Trede M. Completion pancreatectomy for surgical complications after pancreaticoduodenectomy. Br J Surg. 1996;83:176–9. [PubMed] [Google Scholar]
- 34.Nentwich MF, El Gammal AT, Lemcke T, Ghadban T, Bellon E, Melling N, et al. Salvage completion pancreatectomies as damage control for post-pancreatic Surgery complications: a single-center retrospective analysis. World J Surg. 2015;39:1550–6. doi: 10.1007/s00268-015-2969-9. [DOI] [PubMed] [Google Scholar]
- 35.Bressan AK, Wahba M, Dixon E, Ball CG. Completion pancreatectomy in the acute management of pancreatic fistula after pancreaticoduodenectomy: a systematic review and qualitative synthesis of the literature. HPB. 2018;20((1)):20–7. doi: 10.1016/j.hpb.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P, et al. Impact of total pancreatectomy: short- and long-term assessment. HPB. 2013;15:882–92. doi: 10.1111/hpb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, et al. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB. 2015;17:416–21. doi: 10.1111/hpb.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]





