Abstract
Background
Surgical site infection (SSI) is one of the leading complications in health care. Negative pressure wound therapy (NPWT) is meanwhile widely prophylactically used for preventing SSIs. For evaluating the results of the implantation of this technique, we used the Simon single-arm study design and examine whether NPWT has a prophylactic effect on reducing SSIs in a cohort of general surgery patients.
Methods
This single-arm, two-stage study includes 81 elective general surgery patients and corresponds to the Simon's design. The sample size calculation was based on a reduction in the superficial SSI rate from 12 to 4% (power 80%, significance level 5%) using a NPWT system. In compliance with Simon's two-Stage design, the study required the recruitment of 34 patients in stage I and 47 patients in stage II. The two-stage design method would be discarded in case of a wound infection in 3 or more patients in stage I or 6 or more patients in stage II. Using the NPWT system in the operating room, a negative pressure wound dressing was applied post-operatively and removed after 7 days. According to the criteria of the Centres for Disease Control and Prevention (CDC), post-operative wound documentation followed on day 7 and 30.
Results
In stage I, no SSI was apparent. In stage II, 3 patients had SSIs (CDC grade I).
Conclusion
A prophylactic NPWT can significantly reduce the wound infection rate in elective general surgery.
Keywords: Negative pressure wound therapy, Surgical site infection, Simon design
Introduction
Surgical site infection (SSI) is one of the leading complications following surgical interventions. In Europe and the USA, SSI represents the 2nd most common infection in the health care system [1]. The report “Surveillance of surgical site infections in Europe 2013–2014” documents the highest cumulative incidence rate in colorectal surgery at 9.5% [2]. Many studies document a range of 9–33% [3, 4] SSI incidence rate in patients undergoing colorectal surgery. The necessity for delayed treatment of SSI prolongs the average length of hospitalization in Europe by 6.5 days and increases treatment costs around 3 times. The financial loss in the European Union amounts to 1.47–19.1 billion €. Furthermore, cost analyses indicate that the economic burden could be significantly higher [5]. Cost analyses for SSI therapy in the USA range between 3.2 and 8.6 billion US$ [6].
In a literature review, negative pressure wound therapy shows the potential to reduce the rate of wound infection [7, 8]. At the same time, the review emphasizes the necessity for further, well designed studies to fill considerable evidence gaps and to determine the effect of specific interventions on SSI incidences [9]. Moreover, the Global Guidelines for the Prevention of SSI of the WHO 2016 state: “Overall low quality evidence shows that prophylactic negative pressure wound therapy has a benefit in reducing the risk of SSI in patients with a primarily closed surgical incision following high-risk wounds when compared to conventional post-operative wound dressings (conditional recommendation, low quality of evidence)” [10].
A number of randomized controlled trial (RCT) protocols have been published (PONIY and DRESSING) for which no results have yet been disclosed [11, 12]. Evrard et al. [13] criticize the concept of “RCT or nothing” and propose the Simon's design as an alternative to gain knowledge in surgery. The two-stage design has the advantage of generating high-quality data within a short period of time [13] and therefore avoids the excessive amount of organization, duration, and financing necessary for a surgical full-scale RCT.
Based on this approach and against the background that meanwhile prophylactic NPWT is already widely accepted, the present study aims to evaluate this technique in elective general surgery patients on reducing the SSI rate during the implementation in our clinic using the two-stage single-arm Simon study design.
Material and Methods
Study Design
This two-stage, single-arm Simon's design phase 2 study, carried out in the Asklepios Clinic Langen (Germany) is according to Evrard et al. [13] a completely underutilized tool in surgery that could raise the level of scientific reporting. The threshold for efficacy and nonefficacy is defined and with statistical power (risks a and b), the cohort size is calculated (usually, between 30 and 60 patients). The use of interim stopping rules allows reduction of the required number of patients.
The report is prepared in accordance with the SQUIRE guidelines (Standards for Quality Improvement Reporting Excellence; http://www.squire-statement.org/). The study is registered on DRKS (www.drks.de, DRKS-ID: DRKS00015531) and was approved by the Ethics Committee of the Hessen State Medical Association (FF165/2016).
Setting
The Asklepios Clinic Langen (Germany) is an acute and standard care hospital with 400 beds and is an academic teaching hospital of the Goethe University Frankfurt/Main, Germany. The Department of General, Visceral, and Thoracic Surgery has a capacity of 60 beds and performs over 2, 000 surgical interventions per year with focus on oncological, colorectal, minimally invasive, and hernia surgery. Wound management has a high priority in the daily surgical routine with 2 in-house employed and certified wound managers. As a regional centre, the department is integrated into the surgical study network CHIR-Net of the German Society for Surgery (www.chir-net.de).
Statistics
In accordance with the PROUD study and data from our hospital's patient population, the basic rate for SSI in elective, general surgery patients is 12% [14]. With the NPWT system, the basic rate is to be reduced to 4%. According to the Simon's design (power 80%, significance level 5%), in 2 stages, a total of 81 patients has to be recruited:
In stage I, a total of 34 patients were recruited. The method is discarded if no infection occurs in 31 or fewer patients or if 3 or more patients develop an infection. Otherwise, stage II begins.
Stage II (stage I and stage II include a total of 81 patients): The method is discarded if in 75 or fewer patients, no infection occurs or if 6 or more patients develop an infection.
With these assumptions, the null hypothesis (SSI rate is less than 12%) can be rejected at a significance level of 5% with a statistical power of 80% (with an actual SSI rate of 4%). If the SSI rate cannot be reduced and is still at 12%, there is a 79.2% probability that it will be recognized after stage I.
Patient Population and Criteria
All adult patients with elective laparotomies capable of giving consent were included. Patients in emergency criteria and minor patients were excluded. The primary endpoint of the study is the post-operative SSI defined according to the SSI classification of the Centres for Disease Control and Prevention/USA (CDC): grade 1 (superficial), grade 2 (deep), and grade 3 (organs and body cavities) [15].
Study Initiation, Organization, and Data
Medical and nursing staff were informed about the study and introduced to the study through further training. An e-learning module (ROSSINI) is used to assess and standardize SSI [16]. The study director, study nurse, and medical and nursing staff of the department were responsible for the implementation and quality control of the study processes. Supervised by the wound manager, all wound controls and wound documentation were recorded in written and pictorial form. The study nurse developed all study documentation. In monthly jour fixe meetings, the study was monitored by the participating staff.
In addition to basic data (age, gender, weight, body mass index, comorbidities, smoking status, diagnosis, surgical procedure, duration of surgery, perioperative antibiotic prophylaxis, disinfection of the operation area, degree of contamination, and wound length) and the NNIS Risc Index Score of the CDC USA [17], the Clavien-Dindo classification of complications [18] was collected for all patients.
Intervention
According to WHO recommendations [19] and NICE instructions [20], perioperatively the modified SSI Care Bundle Management [21] was performed for all patients with elective laparotomies who have been informed and included in the study. Any incision wound of the abdominal wall that communicates with the abdominal cavity and is 5 cm or more in length is defined as a laparatomy. In laparoscopic colon surgery, only the incision wound to retrieve the specimen is considered and measured as a laparotomy and not the sum of all port incisions. Post-operatively, based on the CDC/USA [15], the surgeon determines the degree of wound contamination and documents the wound length. After the abdomen has been closed, under sterile conditions, the negative pressure wound therapy system (PICO7®, Fa. Smith & Nephew, Hamburg, Germany) is applied in the operating room.
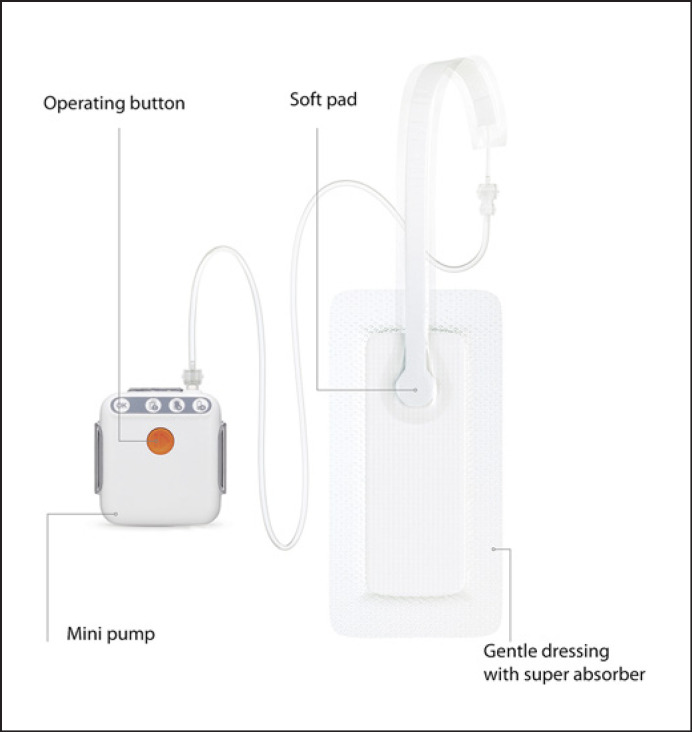
The used NPWT-system is a battery-operated, portable, and active pump system for negative pressure wound therapy. Special features of the used system are: small (7.2 × 6.4 × 2.1 cm in size), light (mini pump 84 g, including batteries), without exudate canister, single-use product, film with high water vapour permeability, and quiet operation, can be used for up to 7 days, continuous negative pressure of −80 mm Hg, operation using 1-button technology (Fig. 1).
Fig. 1.
Negative pressure wound system (PICO7®).
To assure a stable tightness of the negative pressure wound therapy system, special attention was paid to the adequate distance from the drainage and/or stoma points. With a film, the activated mini pump was fixed ventrally to the exposed abdominal wall, usually in the upper/middle abdomen. In this way, the patient could be guaranteed an undisturbed mobility.
The bandage is removed after 7 days and the wound recorded in a digital database. At the same time, the clinic's medical staff or the certified wound manager assess the wound and, if necessary, classify the SSI according to CDC criteria (superficial, deep, organs, and body cavities). According to the same scheme, the wound is assessed again on the day of discharge. If the patient is already discharged after 30 days following surgery (majority of the patients), a patient interview and questionnaire based on the Protocol for the Surveillance of SSI of Surveillance Services/Public Health England (online suppl. material; for all online suppl. material, see www.karger.com/doi/10.1159/000520464) via telephone and postal is conducted. If there are any abnormalities, the patient is called in for a clinical checkup.
Results
Over a period of 8 months from December 2019 to July 2020, stage I and stage II of the study were performed including a total of 81 patients (Table 1). Forty two patients were female and 39 were male. The mean age of the study population was 68.3 years (31–92 years). With an average value of 28.6, the range of the calculated body mass index was between 19.1 and 46.2. In accordance to the American Society of Anaesthesiologist Risk Score (ASA-Score), 1 patient (1.2%) was allocated to ASA I, 54 patients (66.7%) to ASA II, 25 patients (30.9%) to ASA III, and 1 patient (1.2%) to ASA IV. Sixteen patients (19.8%) were active smokers. Type II diabetes mellitus was previously known in 18 patients (22.2%). In dependence of surgical procedures, we performed 21 hernioplasties (25.9%), 17 right hemicolectomies (21.0%), 14 sigmoidectomies (17.3%), 6 rectum resections (7.4%), 5 rectosigmoidectomies (6.2%), 5 left hemicolectomies (6.2%), 5 ileostomy reversals (6.2%), 2 Hartmann reversal procedures (2.4%), as well as 1 (1.2%) liver resection, 1 resection of transverse colon, 1 subtotal colectomy, 1 gastrectomy, 1 reversal of loop colostomy, and 1 open cholecystectomy. In the study collective, in 42 cases (51.9%) the diagnosis was benign and in 39 cases (48.1%) the diagnosis was malignant. Forty-two (51.9%) open surgeries and 39 (48.1%) laparoscopic surgeries were performed. According to the Surgical Wound Classification of the CDC [22, 23] we documented the contamination class I (clean) in 20 interventions (24.7%), the contamination class II (clean-contaminated) in 8 interventions (9.9%), and the contamination class III (contaminated) in 53 interventions (65.4%). The average length of all wounds was 12.8 cm. The average post-operative length of stay was 8.4 days (2–28 days). Based on the Clavien-Dindo classification of complications, the patients were classified into 3 groups. Eight patients (9.9%) were classified in group I (grade 2) and II (grade 3a), and 1 patient (1.2%) was classified in group III (grade 4). In 64 patients (79.0%), the post-operative course showed no complications (Table 2).
Table 1.
Detailed study overview considering factors of risk stratification
| Pat | Sex | Age | BMI | ASA | Smoking | Diabetes | Diagnosis | Surgery | Wound length | Contamination class | Clavien-Dindo | SSI according CDC day 7 | SSI according CDC day 30 | Post-op days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 79 | 23.1 | No | No | Carcinoma of ascending colon, liver metastases | Laparoscopy-assisted right hemicolectomy | 9 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 2 | F | 57 | 23.6 | Yes | No | Existing ileostomy | Reversal of ileostomy | 7 cm | II | None | None | None | 5 | |
|
| ||||||||||||||
| 3 | M | 67 | 26.3 | III | Yes | No | Recurrence of epigastric incisional hernia | Open IPOM | 8 cm | None | None | None | 3 | |
|
| ||||||||||||||
| 4 | M | 44 | 26.1 | Yes | No | Sigmoid diverticulitis | Laparoscopy-assisted sigmoidectomy | 8 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 5 | M | 52 | 22.2 | No | No | Sigmoid diverticulitis | Laparoscopy-assisted sigmoidectomy | 7 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 6 | F | 70 | 33.3 | No | Yes Typ II |
Epigastric incisional hernia | Open hernioplasty (sublay) | 18 cm | None | None | None | 11 | ||
|
| ||||||||||||||
| 7 | F | 80 | 28.3 | No | No | Carcinoma of ascending colon | Laparoscopy-assisted right hemicolectomy | 7 cm | III | None | None | None | 12 | |
|
| ||||||||||||||
| 8 | F | 66 | 26.7 | No | No | Insufficiency of pelvic floor | Laparoscopy-assisted, deep anterior rectal resection with rectopexy | 7 cm | III | None | None | None | 9 | |
|
| ||||||||||||||
| 9 | M | 39 | 23.6 | No | No | Double colon carcinoma–left and right flexure | Laparoscopy-assisted subtotal colektomie | 8 cm | III | Grade II | None | None | 14 | |
|
| ||||||||||||||
| 10 | F | 67 | 33.0 | Yes | No | Hernia of abdominal wall left upper abdomen | Open IPOM | 8 cm | None | None | None | 6 | ||
|
| ||||||||||||||
| 11 | M | 44 | 30.8 | No | No | Sigmoid diverticulitis | Laparoscopy-assisted sigmoidectomy | 9 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 12 | F | 70 | 37.8 | III | No | Yes Typ II |
Sigmoid diverticulitis with stenosis | Laparoscopiy-assisted sigmoidectomy | 10 cm | III | None | None | None | 9 |
|
| ||||||||||||||
| 13 | M | 88 | 31.4 | III | No | Yes Typ II |
Carcinoma of colon transversum | Laparoscopy-assisted resection of transverse colon | 13 cm | III | None | None | None | 9 |
|
| ||||||||||||||
| 14 | F | 83 | 21.8 | III | No | No | Sigmoid colon carcinoma | Open sigmoid resection | 28 cm | III | None | None | None | 19 |
|
| ||||||||||||||
| 15 | F | 82 | 24.2 | No | Yes Typ II |
Carcinoma of ascending colon | Laparoscopy-assisted right hemicolectomy | 8 cm | III | Grade IIIa |
None | None | 20 | |
|
| ||||||||||||||
| 16 | F | 73 | 26.7 | No | No | Existing ileostomy | Reversal of ileostomy | 6 cm | II | None | None | None | 7 | |
|
| ||||||||||||||
| 17 | F | 82 | 25.4 | III | No | Yes Typ II |
Rectal carcinoma | Laparoscopy-assisted deep rectal resection and ileostomy | 8 cm | III | None | None | None | 13 |
|
| ||||||||||||||
| 18 | M | 82 | 28.9 | III | No | Yes Typ II |
Epigastric incisional hernia | Open IPOM | 34 cm | None | None | None | 9 | |
|
| ||||||||||||||
| 19 | M | 55 | 28.0 | No | No | Carcinoma of right colonic flexure | Laparoscopy-assisted right hemicolectomy | 12 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 20 | M | 87 | 26.8 | IV | Yes | Yes Typ II |
HGIEN of ascending colon | Open right hemicolectomy | 20 cm | III | Grade IIIa |
None | None | 11 |
|
| ||||||||||||||
| 21 | F | 82 | 25.4 | III | No | Yes Typ II |
Existing ileostomy | Reversal of ileostomy | 7 cm | II | None | None | None | 7 |
|
| ||||||||||||||
| 22 | F | 76 | 33.9 | III | No | No | Sigmoid colon carcinoma | Laparoscopy-assisted rectosigmoid resection | 8 cm | III | None | None | None | 13 |
|
| ||||||||||||||
| 23 | M | 71 | 27.5 | Yes | Yes Typ II |
Carcinoma of rectosigmoid | Laparoscopy-assisted rectosigmoid resection | 8 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 24 | M | 67 | 20.5 | III | No | No | Existing ileostomy | Reversal of ileostomy | 11 cm | II | None | None | None | 6 |
|
| ||||||||||||||
| 25 | M | 55 | 23.5 | Yes | No | Epigastric incisional hernia | Open hernioplasty (sublay) | 12 cm | None | None | None | 4 | ||
|
| ||||||||||||||
| 26 | F | 72 | 25.4 | No | No | Carcinoma of ascending colon | Laparoscopy-assisted right hemicolectomy | 8 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 27 | F | 59 | 32.9 | Yes | No | Recurrence sigmoid diverticulitis | Laparoscopy-assisted left hemicolectomy | 12 cm | III | Grade IIIa |
None | None | 8 | |
|
| ||||||||||||||
| 28 | F | 83 | 32.5 | No | No | Carcinoma of right colonic flexure | Laparoscopy-assisted right hemicolectomy | 7 cm | III | None | None | None | 14 | |
|
| ||||||||||||||
| 29 | M | 79 | 22.9 | II | Yes | No | Existing hartmann procedure | Hartmann reversal procedure | 14 cm | III | None | None | None | 7 |
|
| ||||||||||||||
| 30 | M | 67 | 31.8 | No | No | Hepatic metastatic carcinoma of pancreatic head | Explorative laparotomy PE's liver metastases, cholecystektomy |
26 cm | II | None | None | None | 11 | |
|
| ||||||||||||||
| 31 | M | 87 | 29.1 | III | No | No | Large, tubulovillous adenoma of the right colonic flexure | Laparoscopy-assisted right hemicolectomy | 8 cm | III | None | None | None | 7 |
|
| ||||||||||||||
| 32 | F | 60 | 35.3 | II | No | No | Supraumbilical incisional and umbilical hernia | Open IPOM | 8 cm | I | None | None | None | 7 |
|
| ||||||||||||||
| 33 | F | 77 | 29.3 | III | No | No | Carcinoma of coecum | Open right hemicolectomy | 8 cm | III | None | None | None | 9 |
|
| ||||||||||||||
| 34 | F | 84 | 20.0 | III | No | No | Sigmoid diverticulitis with stenosis | Laparoscopy-assisted sigmoidectomy and left adnexectomy | 8 cm | III | Grade IIIa |
None | None | 13 |
|
| ||||||||||||||
| 35 | M | 76 | 28.3 | II | No | No | Epigastric and periumbilical incisional hernia | Open hernioplasty (sublay) | 24 cm | I | None | None | None | 6 |
|
| ||||||||||||||
| 36 | F | 77 | 28.7 | III | No | No | Carcinoma of coecum | Laparoscopy-assisted right hemicolectomy | 8 cm | III | None | None | None | 12 |
|
| ||||||||||||||
| 37 | M | 49 | 26.9 | II | Yes | No | Rectal carcinoma | Laparoscopy-assisted deep anterior rectal resection | 8 cm | III | None | None | None | 6 |
|
| ||||||||||||||
| 38 | F | 59 | 30.1 | II | No | No | Epigastric and periumbilical incisional hernia | Open hernioplasty (sublay) | 18 cm | I | Grade II | None | None | 11 |
|
| ||||||||||||||
| 39 | F | 68 | 35.0 | No | Yes Typ II |
Carcinoma of descending colon | Open left hemicolectomy | 30 cm | III | Grade IIIa |
Yes Grade 1 + histology Pyoderma gangraeno sum |
None | 20 | |
|
| ||||||||||||||
| 40 | F | 80 | 22.4 | II | No | No | Existing hartmann procedure | Hartmann reversal procedure | 20 cm | III | None | None | None | 8 |
|
| ||||||||||||||
| 41 | M | 65 | 30.4 | III | No | No | Recurrence of sigmoid diverticulitis | Laparoscopy-assisted sigmoidectomy | 8 cm | III | None | None | None | 10 |
|
| ||||||||||||||
| 42 | F | 86 | 23.8 | III | No | No | Carcinoma of ascending colon | Open right hemicolectomy | 24 cm | III | Grade IVa | None | None | 28 |
|
| ||||||||||||||
| 43 | F | 76 | 21.8 | II | No | No | Infraumbilical incisional hernia | Open hernioplasty (sublay) | 12 cm | I | None | None | None | 3 |
|
| ||||||||||||||
| 44 | M | 67 | 37.6 | II | No | No | Carcinoma of ascending colon | Laparoscopy-assisted right hemicolectomy | 7 cm | III | None | None | None | 7 |
|
| ||||||||||||||
| 45 | F | 63 | 40.6 | II | No | No | Epigastric and umbilical incisional hernia | Open hernioplasty (sublay) | 12 cm | I | None | None | None | 6 |
|
| ||||||||||||||
| 46 | M | 57 | 24.9 | No | No | Rectal carcinoma | Laparoscopy-assisted deep anterior rectal resection and ileostomy | 8 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 47 | M | 64 | 33.4 | II | No | No | Sigmoid colon carcinoma | Laparoscopy-assisted sigmoidectomy | 10 cm | III | None | None | None | 7 |
|
| ||||||||||||||
| 48 | M | 58 | 32.1 | II | No | No | Carcinoma of right colonic flexure | Laparoscopy-assisted right hemicolectomy | 6 cm | III | None | None | None | 6 |
|
| ||||||||||||||
| 49 | F | 57 | 29.6 | III | No | No | Epigastric and periumbilical incisional hernia | Laparoscopy-assisted hernioplasty (MILOS/sublay) | 10 cm | I | None | None | None | 5 |
|
| ||||||||||||||
| 50 | F | 56 | 33.8 | III | No | No | Epigastric incisional hernia | Open hernioplasty (sublay) | 11 cm | I | None | None | None | 7 |
|
| ||||||||||||||
| 51 | M | 54 | 27.2 | II | No | No | Epigastric incisional hernia | Open hernioplasty (sublay) | 7 cm | I | None | None | None | 5 |
|
| ||||||||||||||
| 52 | F | 72 | 22.2 | II | No | Yes Typ II |
Rectal carcinoma | Laparoscopy-assisted deep anterior rectal resection and ileostomy | 7 cm | III | None | None | None | 8 |
|
| ||||||||||||||
| 53 | M | 68 | 22.0 | III | No | No | Supraumbilical/umbilical incisional hernia | Open hernioplasty (sublay) | 19 cm | I | Grade II | None | None | 8 |
|
| ||||||||||||||
| 54 | M | 61 | 22.0 | II | No | No | Carcinoma of descending colon | Laparoscopy-assisted left hemicolectomy | 7 cm | III | None | None | None | 7 |
|
| ||||||||||||||
| 55 | M | 64 | 28.0 | II | No | No | Sigmoid colon carcinoma | Laparoscopy-assisted sigmoidectomy | 11 cm | III | Grade IIIa | None | None | 7 |
|
| ||||||||||||||
| 56 | F | 71 | 34.4 | III | Yes | Yes Typ II |
Sigmoid diverticulitis with sigmovesical fistula | Open sigmoidectomy and fistula removal | 30 cm | III | Grade II | Yes Grade 1 |
None | 8 |
|
| ||||||||||||||
| 57 | M | 31 | 46.2 | Yes | No | Large umbilical hernia | Open hernioplasty (sublay) | 7 cm | None | None | None | 2 | ||
|
| ||||||||||||||
| 58 | F | 87 | 22.3 | No | Yes Typ II |
Double carcinoma => Carcinoma of ascending colon + rectal carcinoma |
Open right hemicolectomy and deep anterior rectal resection | 30 cm | III | Grade II | None | None | 13 | |
|
| ||||||||||||||
| 59 | M | 75 | 23.8 | No | No | Epigastric incisional hernia | Open hernioplasty (sublay) | 8 cm | None | None | None | 5 | ||
|
| ||||||||||||||
| 60 | M | 60 | 33.0 | No | No | Carcinoma of colon transversum close to left flexure | Laparoscopy-assisted left hemicolectomy | 12 cm | III | Grade II | None | None | 8 | |
|
| ||||||||||||||
| 61 | M | 57 | 48.3 | No | No | Large umbilical hernia | Open IPOM | 7 cm | None | None | None | 2 | ||
|
| ||||||||||||||
| 62 | M | 86 | 28.4 | III | No | No | Sigmoid colon carcinoma | Laparoscopy-assisted rectosigmoid resektion | 7 cm | III | None | None | None | 8 |
|
| ||||||||||||||
| 63 | M | 77 | 31.9 | Yes | Yes Typ II |
Supraumbilical/umbilical hernia | Open hernioplasty (sublay) | 7 cm | None | None | None | 3 | ||
|
| ||||||||||||||
| 64 | F | 67 | 29.5 | No | No | Sigmoid diverticulitis | Laparoscopy-assisted sigmoidectomy | 10 cm | III | Grade II | None | None | 6 | |
|
| ||||||||||||||
| 65 | F | 43 | 28.1 | No | No | Sigmoid diverticulitis | Laparoscopy-assisted sigmoidectomy | 7 cm | III | None | None | None | 6 | |
|
| ||||||||||||||
| 66 | F | 86 | 24.4 | III | Yes | No | Hepatic metastatic carcinoma of rectosigmoid | Open rectosigmoid resection and resection of liver segment V | 35 cm | III | None | None | None | 9 |
|
| ||||||||||||||
| 67 | F | 58 | 33.9 | No | No | Carcinoma of coecum | Laparoscopy-assisted right hemicolectomy | 8 cm | III | None | None | None | 5 | |
|
| ||||||||||||||
| 68 | M | 68 | 34.8 | No | No | Incisional hernia | Open hernioplasty (sublay) and omphalectomy | 7 cm | II | None | None | None | 4 | |
|
| ||||||||||||||
| 69 | F | 80 | 30.5 | No | No | Sigmoid colon carcinoma | Laparoscopy-assisted sigmoidectomy with left adnexectomy | 10 cm | III | None | None | None | 6 | |
|
| ||||||||||||||
| 70 | M | 55 | 28.0 | No | No | Recurrence of incisional hernia in middle abdomen + rectus diastasis | Open hernioplasty (sublay) and reconstruction diastasis recti | 25 cm | None | None | None | 5 | ||
|
| ||||||||||||||
| 71 | F | 78 | 37.0 | III | No | Yes Typ II |
Carcinoma of ascending colon | Laparoscopy-assisted right hemicolectomy | 10 cm | III | None | None | None | 9 |
|
| ||||||||||||||
| 72 | F | 64 | 19.1 | III | Yes | No | Double carcinoma => coecum und C. transversum close to left flexure | Open right and left hemicolectomy | 25 cm | III | None | None | None | 11 |
|
| ||||||||||||||
| 73 | M | 72 | 28.7 | No | No | Epigastric incisional hernia | Open hernioplasty (sublay) | 12 cm | None | None | None | 6 | ||
|
| ||||||||||||||
| 74 | F | 80 | 27.7 | No | No | Existing ileostomy | Reversal of ileostomy | 8 cm | III | None | None | None | 5 | |
|
| ||||||||||||||
| 75 | M | 92 | 21.3 | III | No | No | Double carcinoma => coecum und ascending colon | Open right hemicolectomy + construction of artificial anus | 20 cm | III | None | None | None | 8 |
|
| ||||||||||||||
| 76 | F | 66 | 35.4 | No | Yes Typ II |
Carcinoma of descending colon | Open left hemicolectomy | 20 cm | III | None | None | None | 7 | |
|
| ||||||||||||||
| 77 | F | 47 | 24.5 | No | Yes Typ II |
Existing artificial anus | Reversal of artificial anus | 11 cm | III | Grade II | None | None | 9 | |
|
| ||||||||||||||
| 78 | M | 78 | 25.3 | No | Yes Typ II |
Carcinoma of coecum with infiltration of r ectosigmoid | Open right hemicolectomy and rectosigmoid resection | 27 cm | III | Grade IIIa |
None | None | 17 | |
|
| ||||||||||||||
| 79 | M | 46 | 26.2 | Yes | No | Adenocarcinoma of gastric antrum | Laparoscopy-assisted gastrectomy | 10 cm | II | Grade IIIa |
None | Yes Grade 1 |
12 | |
|
| ||||||||||||||
| 80 | F | 68 | 30.4 | No | No | Hepatic metastatic sigmoid colon carcinoma | Laparoscopy-assisted sigmoidectomy | 9 cm | III | None | None | None | 8 | |
|
| ||||||||||||||
| 81 | F | 78 | 27.3 | III | No | No | Solitary liver metastasis seg. VII | Open resection of liver metastasis | 25 cm | II | None | None | None | 4 |
Clavien-Dindo, Clavien-Dindo Complication Grade (grade 1–5). SSI nach CDC, surgical site infection-classification of Centres for Disease Control and Prevention/USA (grade 1 − superficial/grade 2 − deep); ASA, American Society of Anaesthesiologist Risk; BMI, body mass index.
Table 2.
Summary table (stage I and II)
| N (Range/%) | |
|---|---|
| Patients, n | 81 |
| Sex | |
| Male | 39 |
| Female | 42 |
| Mean age, years | 68.3 (31–92) |
| BMI, Ø | 28.6 (19.1–4.2) |
| ASA grade, n (%) | |
| I | 1 (1.2) |
| II | 54 (66.7) |
| III | 25 (30.9) |
| IV | 1 (1.2) |
| Smoker status, n (%) | |
| Smoker | 16 (19.8) |
| Non-smoker | 65 (80.2) |
| Type II diabetes, n (%) | |
| Yes | 18 (22.2) |
| No | 63 (77.8) |
| Diagnosis, n (%) | |
| Benign | 42 (51.9) |
| Malignant | 39 (48.1) |
| Surgical technique, n (%) | |
| Laparoscopic | 39 (48.1) |
| Open | 42 (51.9) |
| Surgical procedure, n (%) | |
| Hernioplasty | 21 (25.9) |
| Right hemicolectomy | 17 (21.0) |
| Sigmoidectomy | 14 (17.3) |
| Rectum resection | 6 (7.4) |
| Rectosigmoidectomy | 5 (6.2) |
| Left hemicolectomy | 5 (6.2) |
| Ileostomy reversal | 5 (6.2) |
| Hartmann reversal procedure | 2 (2.4) |
| Liver resection | 1 (1.2) |
| Resection of transverse colon | 1 (1.2) |
| Subtotal colectomy | 1 (1.2) |
| Gastrectomy | 1 (1.2) |
| Reversal of a loop colostomy | 1 (1.2) |
| Open cholecystectomy | 1 (1.2) |
| Contamination class, n (%) | |
| Class I (clean) | 20 (24.7) |
| Class II (clean-contaminated) | 8 (9.9) |
| Class III (contaminated) | 53 (65.4) |
| Wound length, Ø cm | 12.8 |
| Post-op lenght of stay (Ø days) | 8.4 (2–28 d) |
| Clavien-Dindo classification, n (%) | |
| Grade 2/Group I | 8 (9.9) |
| Grade 3a/Group II | 8 (9.9) |
| Grade 4/Group III | 1 (1.2) |
| SSI according CDC criteria | |
| Study stage I + II | 3 CDC grade 1 (superficial) |
| Study stage I | 0 |
| Study stage II | 3 CDC grade 1 (superficial) |
BMI, body mass index, ASA, American Society of Anaesthesiologists, CDC, Centre for Disease Control and Prevention/USA; SSIs, surgical site infection.
After 34 patients were recruited, stage I of the study was terminated and the available data were analysed. SSIs could not be documented in any of these patients (Table 1). In accordance with the study design, the data collected indicated that the method used could reduce the SSI rate with a high statistical probability (79.2%). With no SSIs in stage I, the negative pressure dressing used on the laparotomy wound was retained and the study continued with stage II.
In the course of stage II, we recruited another 47 patients and the study could be completed with a total of 81 elective patients. The follow-up was double secured by postal and telephone enquiries and completed with a 100% follow-up rate. In 2 cases, a clinical re-evaluation of the wound was necessary in our clinic. In the above cases, an SSI has been excluded. According to the SSI classification of the CDC, a superficial wound infection was found in 3 patients (grade 1) in stage II (Table 3). The necessary controls and the care of infected wounds took place in the wound outpatient department of our clinic.
Table 3.
Patients with SSI − study stage II
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Sex | Female | Female | Male |
| Age | 68 | 71 | 46 |
| BMI | 35.0 | 34.4 | 26.2 |
| ASA | II | III | II |
| Smoking | No | 15 py | 25 py |
| Diabetes mellitus | Yes/Type II | Yes/Type II | No |
| Diagnosis | Carcinoma of colon descendants | Sigmoid diverticulitis with sigmoid-vesicle fistula | Adenocarcinoma of the gastric antrum |
| Surgery | Open surgical left hemicolectomy | Open surgical sigmoidectomy with fistula surgery | Laparoscopy-assisted gastrectomy |
| Contamination class | III | III | II |
| Wound length | 30 cm | 30 cm | 10 cm |
| Clavien-Dindo | Grade IIIa | Grade II | Grade IIIa |
| SSI according CDC | Yes − grade 1 | Yes − grade 1 | None |
| 7th post-operative day | Histo: Pyoderma gangraenosum | ||
| SSI according CDC | None | None | Yes − grade 1 |
| 30th post-operative day | |||
| Post-operative length of stay | 20 | 8 | 12 |
Clavien-Dindo, Clavien-Dindo Complication Grade (grade 1–5). SSI according CDC, surgical site infection classification of the Centres for Disease Control and Prevention/USA. (grade 1 − superficial/grade 2 deep).
Out of 81 patients, a final data analysis revealed a superficial wound infection (CDC grade 1) in 3 cases. The specific number of 6 or more SSI per 81 patients specified at the beginning was not reached or exceeded. This shows that the negative pressure wound therapy system effectively reduces the rate of SSI in laparotomy wounds with a high statistical probability. Furthermore, the determined null hypothesis (SSI rate less than 12%) could be rejected with a statistical power of 80%.
Discussion
Summary
The results of our study analysis clearly support a positive prophylactic effect of negative pressure wound therapy reducing SSI in elective laparotomies. This study based on the Simon's two-stage design demonstrates that a negative pressure wound therapy system has the potential to reduce SSI from assumed 12–4% (statistical power 80%, significance level 5%). Moreover, the specific strength of our study stems from the fact that the data reflect a real-world scenario of general surgery patients.
Interpretation
Based on evidence, the project examines a certain intervention and its effectiveness on SSI after laparotomies and is therefore of particular importance [7, 8, 24]. Various surgical disciplines are concerned with the effectiveness of negative pressure wound therapy on wound healing after surgery. The current data document heterogeneous methods using negative pressure wound therapy with divergent variables and controversial results [9]. A literature research by the WHO identified 19 publications that report on 21 studies, including 6 RCTs and 14 observation studies testing the effect of negative pressure wound therapy on SSI. A systematic review by the WHO including the abovementioned studies show “a benefit of negative pressure wound therapy in reducing surgical site infections in patients with primarily closed surgical wounds compared with conventional post-operative dressings” [24].
Depending on the type of procedure, this effect is observed in abdominal and cardiac surgery, not significantly in orthopaedics and traumatology. Prophylactic negative pressure wound therapy on primarily closed, high-risk surgical wounds of adults is recommended to prevent SSI. However, this recommendation is insubstantial due to low quality evidence. The current clinical evidence of the used NPWT system reflects a divergence in results with regard to the SSI reduction [25, 26, 27, 28].
The effectiveness of negative pressure wound therapy on SSI reduction is multifactorial and can vary depending on the type of surgical procedure, the contamination class of the surgical wound, the type, duration, and pressure level of the negative pressure wound therapy, the duration of the operation, patient comorbidities, and perioperative care bundle giving an explanation for controversial study results [21, 29]. Shen et al. [30] demonstrated a non-significant effect of negative pressure wound therapy on SSI reduction in a RCT, where 265 patients with gastrointestinal, pancreatic, or peritoneal malignancies were treated with laparotomy. A more recent RCT (NEPTUNE) by Murphy et al. [31] also could not demonstrate a prophylactic effect of negative pressure wound therapy on reducing the rate of SSI considering 300 patients undergoing elective open colorectal surgery. Then again, in an RCT by O´Leary et al. [8] including a smaller group of 50 patients undergoing elective or emergency laparotomy and in an RCT by Javed et al. [32], including 123 patients undergoing pancreaticoduodenectomies show a significant SSI reduction through negative pressure wound therapy.
There are few studies that consider the effectiveness of negative pressure wound therapy in laparotomies [33]. In particular, the data on colorectal surgical interventions are inadequate and incomplete. Our study including a mixed study collective with a high percentage of colorectal surgical patients with wound contamination classes II and III (n = 57, 70.4%) and the data it generated documents a significantly positive effect on negative pressure wound therapy to reduce SSI.
With our project, we support the efforts of various committees for the comprehensive implementation and standardization of specific and innovative prophylactic measures to reduce SSI [10]. In our experience, there were significant advantages in everyday clinical practice using the method: simple application, simplification of work processes, better adaptation of the wound margins, support of the early mobilization of the patient, and ensured patient comfort. A locally adjusted, critical cost-benefit assessment, as well as an assessment that contains a rational estimation of all effect vectors, possibly assuming opportunity costs, is essential.
In addition, we saw the following advantages of the single-arm, Simon's two-stage design study: organizational and temporal simplicity, statistically valid and plausible number of cases. In addition, this study method usually enables an uncomplicated patient recruitment. As the study fell in the beginning of the pandemic, this effect was not observed. Moreover, the data obtained are of high mathematical and biostatistical quality. The early possibility to recognize the effectiveness of the negative pressure wound therapy in stage I is another advantage of the Simon's two-stage design. Finally, early realization if a method was ineffective and therefore allowing an early termination of the study is a potential organizational and economic benefit of the Simon's design [34].
Limitations
The single-arm Simon's two-stage design only allows the analysation of one primary endpoint. In addition, the study cannot quantify the various data. As a result, we were only able to benefit to a limited extent from secondary information gained through the interaction analysis of the documented risk variables [35]. Certainly, the single-arm Simon's two-stage design study has inherent limitations and is not comparable to an RCT. However, it offers a competitive alternative to time-consuming, organizationally difficult and often expensive complex studies [13]. Following the analytical evaluation of the study elements, the ecological concern (single-use product) and the procurement costs of the used NPWT-system must be examined critically.
Conclusion
Our implementation study statistically underpins the efficacy of negative pressure wound therapy in abdominal wound healing and can be recommended as an efficient method to reduce superficial SSI. There are advantages, especially, regarding the practicality of wound management and the patient comfort using a portable system.
Statement of Ethics
Written informed consent was obtained from all individual participants included in the study. The study was approved by the Ethics Committee of the Hessen State Medical Association (Landesärtzekammer Hessen No. FF165/2016). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Conflict of Interest Statement
V. Piroski, E. Müller, E. Herrmann, E. Hanisch, and A. Buia have no conflict of interest or financial ties to disclose.
Funding Sources
There were no funding sources.
Author Contributions
V. Piroski did the data acquisition and interpretation, literature research, and wrote and revised the manuscript; E. Müller did the literature research and revised the manuscript; E. Herrmann did the statistical analyses, data interpretation, wrote the statistical part, and revised the manuscript; E. Hanisch did the literature research and wrote and revised the manuscript; and A. Buia did the literature research, wrote, and revised the manuscript.
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary files. Further enquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
We thank Mr. Thomas Pinkney, Senior Lecturer, and Consultant Colorectal Surgeon, Academic Department of Surgery, University of Birmingham; Clinical Director, Birmingham Surgical Trials Consortium, NIHR Clinical Research Speciality Lead, General Surgery − CRN West Midlands for his kindness that we could use his e-learning module (ROSSINI) in the process of evaluation and standardizing SSI.
References
- 1.World Health Organization . Global guidelines for the prevention of surgical site infection. 2nd ed. World Health Organization; 2018. [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control . Annual epidemiological report for 2014 Surgical site infections. ECDC; 2016. [Google Scholar]
- 3.Belda FJ, Aguilera L, García de la Asunción J, Alberti J, Vicente R, Ferrándiz L, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294((16)):2035–42. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 4.Krieger BR, Davis DM, Sanchez JE, Mateka JJL, Nfonsam VN, Frattini JC, et al. The use of silver nylon in preventing surgical site infections following colon and rectal surgery. Dis Colon Rectum. 2011;54((8)):1014–9. doi: 10.1097/DCR.0b013e31821c495d. [DOI] [PubMed] [Google Scholar]
- 5.Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, et al. Surgical site infection: a European perspective of incidence and economic burden. Int Wound J. 2004;1((4)):247–73. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Cdc; 2009. Mar 13, [Google Scholar]
- 7.Pellino G, Sciaudone G, Selvaggi F, Canonico S. Prophylactic negative pressure wound therapy in colorectal surgery. Effects on surgical site events: current status and call to action. Updates Surg. 2015;67((3)):235–45. doi: 10.1007/s13304-015-0298-z. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary DP, Peirce C, Anglim B, Burton M, Concannon E, Carter M, et al. Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations. Ann Surg. 2017;265((6)):1082–6. doi: 10.1097/SLA.0000000000002098. [DOI] [PubMed] [Google Scholar]
- 9.Scalise A, Calamita R, Tartaglione C, Pierangeli M, Bolletta E, Gioacchini M, et al. Improving wound healing and preventing surgical site complications of closed surgical incisions: a possible role of incisional negative pressure wound therapy. A systematic review of the literature. Int Wound J. 2016;13((6)):1260–81. doi: 10.1111/iwj.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leaper DJ, Edmiston CE. World Health Organization: global guidelines for the prevention of surgical site infection. J Hosp Infect. 2017;95((2)):135–6. doi: 10.1016/j.jhin.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Mihaljevic AL, Schirren R, Müller TC, Kehl V, Friess H, Kleeff J. Postoperative negative-pressure incision therapy following open colorectal surgery (Poniy): study protocol for a randomized controlled trial. Trials. 2015;16((1)):471. doi: 10.1186/s13063-015-0995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillespie BM, Webster J, Ellwood D, Stapleton H, Whitty JA, Thalib L, et al. ADding negative pRESSure to improve healING (the DRESSING trial): a RCT protocol. BMJ Open. 2016;6((2)):e010287. doi: 10.1136/bmjopen-2015-010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evrard S, Audisio R, Poston G, Caballero C, Kataoka K, Fontein D, et al. From a comic opera to surcare an open letter to whom clinical research in surgery is a concern. Ann Surg. 2016;264((6)):911–2. doi: 10.1097/SLA.0000000000001700. [DOI] [PubMed] [Google Scholar]
- 14.Diener MK, Knebel P, Kieser M, Schüler P, Schiergens TS, Atanassov V, et al. Effectiveness of triclosan-coated PDS plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: the randomised controlled PROUD trial. Lancet. 2014;384((9938)):142–52. doi: 10.1016/S0140-6736(14)60238-5. [DOI] [PubMed] [Google Scholar]
- 15.The Centers for Disease Control Draft guideline for the prevention of surgical site infection, 1998: CDC. Notice. Fed Regist. 1998;63((116)):33168–92. [PubMed] [Google Scholar]
- 16.Pinkney T, Bartlett D, Gheorghe A, Dowswell G, Morton D, Calvert M. Use of an online e-learning module to standardise the assessment and reporting of a subjective endpoint in a multicentre rct. Trials. 2013;14:S1. [Google Scholar]
- 17.Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. Am J Med. 1991 Sep 16;91((3B)):152S–7S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240((2)):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaper D, Edmiston C. WHO: Global guidelines for the prevention of surgical site infection. J Hosp Infect. 2017;95((2)):135–6. doi: 10.1016/j.jhin.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 20.NICE Guidelines update team . Surgical site infections: prevention and treatment CG74. NICE; 2018. [Google Scholar]
- 21.Weiser MR, Gonen M, Usiak S, Pottinger T, Samedy P, Patel D, et al. Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. Br J Surg. 2018 Nov;105((12)):1680–7. doi: 10.1002/bjs.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garner JS. CDC guideline for prevention of surgical wound infections, 1985. Supersedes guideline for prevention of surgical wound infections published in 1982. (Originally published in November 1985). Revised. Infect Control. 1986;7((3)):193–200. doi: 10.1017/s0195941700064080. [DOI] [PubMed] [Google Scholar]
- 23.Simmons BP. Guideline for prevention of surgical wound infections. Am J Infect Control. 1983 Aug;11((4)):133–43. doi: 10.1016/0196-6553(83)90030-5. [DOI] [PubMed] [Google Scholar]
- 24.Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. The Lancet Inf Dis. 2016;16((12)):e276–87. doi: 10.1016/S1473-3099(16)30398-X. [DOI] [PubMed] [Google Scholar]
- 25.Sahebally SM, McKevitt K, Stephens I, Fitzpatrick F, Deasy J, Burke JP, et al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg. 2018;153((11)):e183467. doi: 10.1001/jamasurg.2018.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuper TM, Murphy PB, Kaur B, Ott MC. Prophylactic negative pressure wound therapy for closed laparotomy incisions: a meta-analysis of randomized controlled trials. Ann Surg. 2020;271((1)):67–74. doi: 10.1097/SLA.0000000000003435. [DOI] [PubMed] [Google Scholar]
- 27.Shiroky J, Lillie E, Muaddi H, Sevigny M, Choi WJ, Karanicolas PJ. The impact of negative pressure wound therapy for closed surgical incisions on surgical site infection: a systematic review and meta-analysis. Surgery. 2020;167((6)):1001–9. doi: 10.1016/j.surg.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence PICO negative pressure wound dressings for closed surgical incisions. Med Technol Guid. 2019;NTG43:1–16. [Google Scholar]
- 29.Tufts LS, Jarnagin ED, Flynn JR, Gonen M, Guillem JG, Paty PB, et al. A perioperative multidisciplinary care bundle reduces surgical site infections in patients undergoing synchronous colorectal and liver resection. HPB. 2018;21((2)):181–6. doi: 10.1016/j.hpb.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen P, Blackham AU, Lewis S, Clark CJ, Howerton R, Mogal HD, et al. Phase II randomized trial of negative-pressure wound therapy to decrease surgical site infection in patients undergoing laparotomy for gastrointestinal, pancreatic, and peritoneal surface malignancies. J Am Col Surg. 2017 Apr;224((4)):726–37. doi: 10.1016/j.jamcollsurg.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy PB, Knowles S, Chadi SA, Vogt K, Brackstone M, Koughnett JAV, et al. Negative pressure wound therapy use to decrease surgical nosocomial events in colorectal resections (neptune): a randomized controlled trial. Ann Surg. 2019;270((1)):38–42. doi: 10.1097/SLA.0000000000003111. [DOI] [PubMed] [Google Scholar]
- 32.Javed AA, Teinor J, Wright M, Ding D, Burkhart RA, Hundt J, et al. Negative pressure wound therapy for surgical-site infections: a randomized trial. Ann Surg. 2019;269((6)):1034–40. doi: 10.1097/SLA.0000000000003056. [DOI] [PubMed] [Google Scholar]
- 33.Seidel D, Diedrich S, Herrle F, Thielemann H, Marusch F, Schirren R, et al. Negative pressure wound therapy vs conventional wound treatment in subcutaneous abdominal wound healing impairment: the sawhi randomized clinical trial. JAMA Surg. 2020;155((6)):469–78. doi: 10.1001/jamasurg.2020.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mander AP, Thompson SG. Two-stage designs optimal under the alternative hypothesis for phase II cancer clinical trials. Contemp Clin Trials. 2010;31((6)):572–8. doi: 10.1016/j.cct.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunz CU, Kieser M. Optimal two-stage designs for single-arm phase II oncology trials with two binary endpoints. Methods Inf Med. 2011;50((4)):372–7. doi: 10.3414/ME10-01-0037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary files. Further enquiries can be directed to the corresponding author.