Abstract
Background and Objectives
The effect of new-onset seizures in young stroke survivors on the subsequent development of dementia is poorly understood. This study aimed to assess the association between new onset of seizure and dementia in a population-based study of patients with stroke.
Methods
The IBM Watson Health MarketScan Commercial Claims and Encounters database for the years 2005–2014 served as the data source for this study. Using the International Classification of Diseases, Ninth Revision (ICD-9), we identified patients aged 18–60 years with ischemic strokes (ISs; 433.x1, 434.x1, and 436) and hemorrhagic strokes (HSs; 430, 431, 432.0, 432.1, and 432.9) between January 1, 2006, and December 31, 2009, which constituted our baseline study cohort. At baseline, all included participants were free of claims for dementia, brain tumors, toxin exposure, traumatic brain injury, and neuroinfectious diseases, identified using ICD-9 codes. They had at least 1-year continuous enrollment before the index stroke diagnosis and 5 years after, with no seizure claims within 1 year after the index date. The exposure of interest was seizures: a time-dependent variable. The study outcome of interest was dementia (ICD-9: 290.0, 290.10–13, 290.20–21, 290.3, 290.40–43, 291.2, 292.82, 294.10–11, 294.20–21, 294.8, 331.0, 331.11, 331.19, and 331.82), which occurred during the follow-up period from January 1, 2010, to December 31, 2014. A Cox proportional hazards regression model was applied to calculate the hazard ratio (HR) and 95% CI for the independent association of seizures with the occurrence of dementia.
Results
At the end of the baseline period, we identified 23,680 patients with stroke (IS: 20,642 and HS: 3,038). The cumulative incidence of seizure was 6.7%, 6.4%, and 8.3% for all strokes, IS, and HS, respectively. The cumulative incidence of dementia was 1.3%, 1.4%, and 0.9% for all strokes, IS, and HS, respectively. After multivariable adjustment, young patients with stroke who developed seizures had a greater risk of dementia compared with those without seizures (all strokes adjusted HR: 2.53, 95% CI 1.84–3.48; IS: 2.52, 1.79–3.53; HS: 2.80, 1.05–7.43).
Discussion
These findings suggest that the onset of seizures in young stroke survivors is associated with a 2.53 times increased risk of developing dementia.
Classification of Evidence
This study provides Class II evidence that poststroke seizures increase the probability of dementia in young stroke survivors.
Dementia is frequent after a stroke, affecting 1 in 3 stroke survivors. Dementia after stroke is associated with a 2–6 times higher adjusted mortality rate,1,2 a higher rate of stroke recurrence, and a poor functional outcome.3 It is often anticipated that dementia prevalence and burden after stroke are likely to increase because of improved stroke survival and aging.4 Many of the known predictors of dementia after stroke are related to stroke risk factors and the stroke itself, including a history of diabetes, atrial fibrillation, the number and size of stroke lesion, the type of stroke (greater risk for hemorrhagic stroke [HS]), and the stroke severity. Stroke consequences and sequela such as dysphasia, incontinence, and seizures are also independent predictors of dementia occurring after stroke.5
On the other hand, stroke is also associated with an increased risk of seizures. The cumulative incidence of seizure among stroke survivors is up to 12% after a follow-up period of 10 years.6,7 Furthermore, stroke survivors visit the emergency department or are hospitalized for seizures at a rate 10 times higher than the general population.7 Poststroke seizures are associated with poor functional outcomes,8 increased risk of death,9 and a higher rate of stroke recurrence.10,11 More importantly, seizures may be associated with accelerated clinical decline and neuronal loss in patients with dementia.12,13 For example, in a small hospital series of 169 predominantly elderly patients with stroke without preexisting dementia, early seizure was associated with a nearly 4-fold increased risk of developing dementia within 3 years of the index event.14 Our population-based study has evaluated the effect of late-onset seizures on subsequent development of dementia among stroke survivors. If we could confirm in a population-based study the deleterious consequences of seizures on dementia among stroke survivors, this would provide important public health information related to dementia occurring after stroke. Moreover, the investigation of the relationship between dementia and seizures in young stroke survivors (18–60 years) is particularly meaningful given the longer life expectancy and higher social and economic burden. Finally, if poststroke seizures are associated with dementia, this would support the future investigation of antiseizure medications to prevent the onset and progression of dementia following a stroke. We therefore propose to test the hypothesis that among young stroke survivors, poststroke seizures are associated with an increased risk of subsequently developing dementia, using a large nationwide database in the United States. The goal of this study was to examine the association between new onset of seizure and dementia in a population-based sample of young patients with stroke.
Methods
Design and Data Source
We performed a retrospective study using data from January 1, 2005, through December 31, 2014 from the IBM Watson Health MarketScan Commercial Claims and Encounters database. The database consists of reimbursed health care claims that include individual-level and longitudinal data from patients aged 0–64 years covered by diverse private insurance plans across all 50 US states and the District of Columbia. MarketScan contains inpatient and outpatient medical claims, enrollment information of enrollees, and prescription drug claims for approximately 40 million privately insured patients annually. Patients' demographic characteristics include information such as age, sex, and US census regions. Data on race and ethnicity are not available in MarketScan. Detailed information about the database is described elsewhere.15
Standard Protocol Approvals, Registrations, and Patient Consents
This study protocol was submitted to the Pennsylvania State College of Medicine institutional review board and was not considered to be human subject research. All records contained within the database are fully deidentified. Thus, informed consent was waived.
Study Population
We identified patients with stroke, aged ≥18 years, using the International Classification of Diseases, Ninth Revision (ICD-9), during January 1, 2006, through December 31, 2009. Patients with ischemic stroke (IS) were identified using ICD-9 433.x1, 434.x1, and 436 and HS using ICD-9 codes 430, 431, 432.0, 432.1, and 432.9. All participants met the following criteria at baseline: (1) free of dementia, brain tumors, toxin exposure, traumatic brain injury, and conditions listed in eTable 1 (links.lww.com/WNL/C44); (2) having 1-year continuous enrollment before the index stroke diagnosis date and 5 years after; and (3) no seizure within 1 year before the index dates. In all, we identified 23,680 participants with a diagnosis of stroke who met the study inclusion criteria. For participants who developed seizures, we used the first date of seizures diagnosis as the index date and the first date of stroke diagnosis for those who did not develop seizure. Figure 1 summarizes the selection process for participants with stroke.
Figure 1. Study Participants' Flowchart.
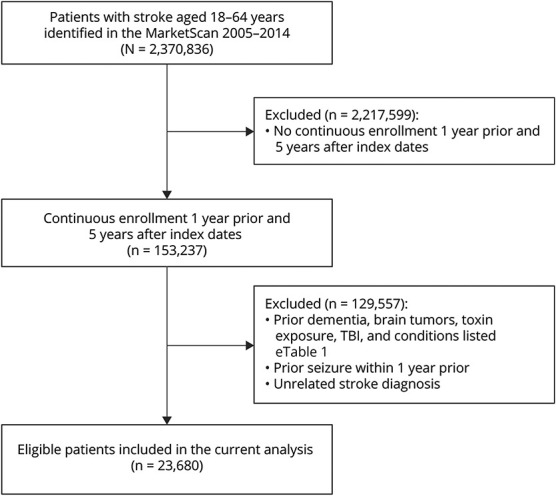
TBI = traumatic brain injury.
Assessment of Exposure
The study exposure of interest was a diagnosis of seizure during the baseline period (January 1, 2006, to December 31, 2009), identified using ICD-9 codes 345.x and 780.39.
Assessment of Outcome
The study outcome of interest was dementia that occurred in the 5-year follow-up after baseline comprised between January 1, 2010, and December 31, 2014. Incident cases of dementia were identified by the presence of relevant ICD-9 codes in the database (eTable 2, links.lww.com/WNL/C44).
Covariates
The following demographic covariates were extracted from the database: age, sex, place of residence, and US census region. Based on the available literature,16 we also extracted the following individual-level data at baseline in the database: presence of diabetes, hypertension, depression, acute myocardial infarction (MI), congestive heart failure (CHF) using ICD-9 (eTable 3, links.lww.com/WNL/C44), and use of antiseizure drugs (eTable 4).
Statistical Analysis
All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC). We used R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) to generate Kaplan-Meier figures. For each participant, we calculated the person-time from the index data to the first occurrence of dementia, inpatient death, end of enrollment, or end of the study period (December 31, 2014), whichever date came first. We performed descriptive analyses using t tests for continuous variables and χ2 tests for categorical variables. We applied a Cox proportional hazards regression model to calculate the hazard ratio (HR) and 95% CI for the risk of dementia by seizure status. We tested the proportional hazards assumption for the full model 2, which was not violated (p = 0.35). In the multivariable models, we adjusted for age (years), sex (male/female), type of residence (rural or urban), region (South, West, Midwest, Northeast, or unknown), diabetes, hypertension, depression, acute MI, CHF (each yes or no), and use of antiseizure drugs (each yes or no). Patients were included regardless of whether they were on antiseizure drugs, which can be prescribed for conditions other than seizures.
To further test the robustness of our results, we conducted a series of sensitivity analyses. First, to reduce potential bias, we conducted the analysis with inverse probability of seizure weighting using the propensity score, which was calculated by including the covariates mentioned above in the final model 2. This approach allowed us to balance baseline data between participants with seizures and those without seizures. Second, we further restricted our study population to healthy individuals by excluding participants with chronic disease such as diabetes, hypertension, depression, acute MI, CHF, and antiseizure drug use. This could potentially reduce the residual confounding due to uneven distribution of these factors across exposure groups. Because the risk of recurrent stroke is not uncommon and could potentially contribute to dementia, we performed a stratified analysis by stroke recurrence status. Similarly, we examined the interaction between age and seizures for the risk of dementia given that the risk of dementia is not uniform across all age groups. Statistical tests were reported as significant at p values less than 0.05. We reported statistically significant association in the Results section.
Data Availability
Although the data are available for others to purchase, the terms of our data use agreement with IBM prohibit us from sharing the MarketScan data with other investigators.
Results
Study Participants
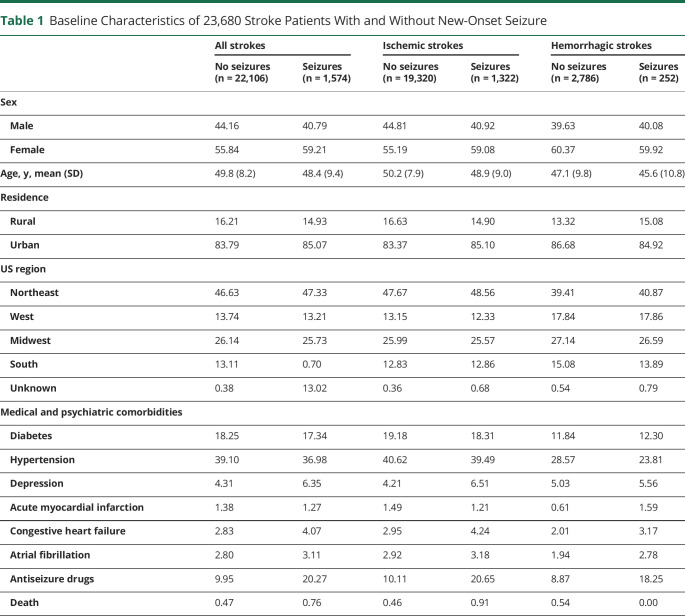
The stroke cohort included a total of 23,680 patients, of whom 20,642 (87.2%) had ISs and 3,038 (12.8%) HSs. Patients with stroke were relatively young (the MarketScan database does not contain individuals aged 65 years and older) with a mean (SD) age of 49.8 (8.3) years for all strokes, 50.2 (8.0) years for ISs, and 47.0 (10.0) years for HSs. The proportions of women were 56.1%, 55.4%, and 60.3% for all strokes, ISs, and HSs, respectively. In general, selected stroke risk factors such as diabetes mellitus and hypertension and medical comorbidities were highly prevalent in the cohort (Table 1).
Table 1.
Baseline Characteristics of 23,680 Stroke Patients With and Without New-Onset Seizure
Seizure Incidence
During the baseline study period (January 1, 2006, to December 31, 2009), seizures developed in 1,574 (6.7%) patients with stroke overall, including 1,322 (6.4%) and 252 (8.3%) with ISs and HSs, respectively (Table 1). More women than men developed seizures for all stroke types (59.21 vs 40.79%) and for ISs (59.08% vs 40.92%). Patients who developed seizures were younger than those who did not develop seizures (all strokes mean age: 48.4 years vs 49.8 years; ISs mean age: 48.9 years vs 50.2 years; HSs: 45.6 years vs 47.1 years). Stroke risk factors such as diabetes and hypertension were equally distributed among patients with seizures and those without seizures. Stroke survivors overall and IS survivors with seizures were more likely to have CHF (all strokes: 4.07% vs 2.83%; ISs: 4.24% vs 2.95%). Depression was more likely to be present in patients who developed seizures than those who did not for all strokes (6.35% vs 4.31%) and ISs (6.51% vs 4.21%). The rate of antiseizure drugs use was higher among those who developed seizures than their counterparts (all strokes: 20.27% vs 9.95%; ISs: 20.65% vs 10.11%; HSs: 18.25% vs 8.87%).
Incidence of Dementia and Association With Seizures After Stroke
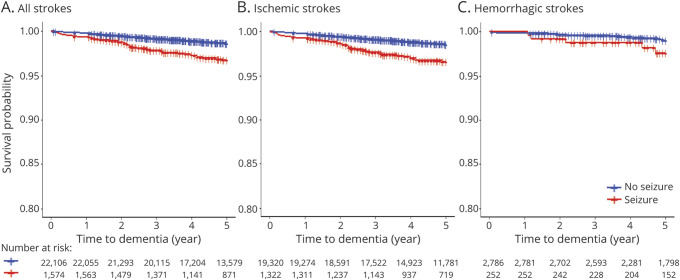
During the 5-year follow-up period (January 1, 2010, to December 31, 2014; mean [SD], 4.4 [1.0] years), we identified 313 cases of dementia (1.3%) in the stroke cohort, including 285 (1.4%) and 28 (0.9%) patients with ISs and HSs, respectively (Table 2, Figure 2).
Table 2.
Hazard Ratios and 95% CIs of Dementia, According to Seizure Status for Total Stroke (N = 23,680)
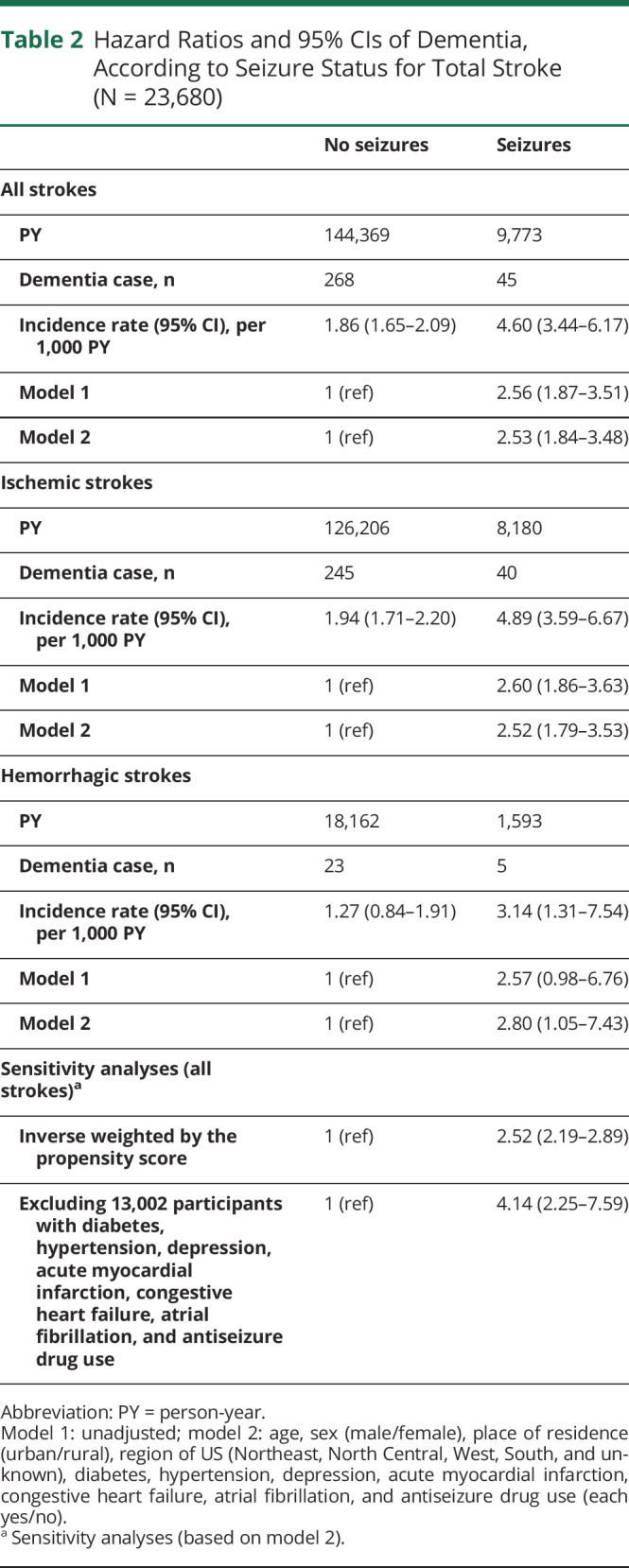
Figure 2. Unadjusted Cumulative Incidence of Dementia in Young Stroke Survivors With and Without Seizures.
(A) All strokes. (B) Ischemic strokes. (C) Hemorrhagic strokes.
An unadjusted Cox proportional hazards analysis found that seizure status was a significant predictor of incident dementia for all strokes (HR: 2.56, 95% CI 1.87–3.51) and ISs (HR: 2.60, 95% CI 1.86–3.63) but not for HSs (HR: 2.57, 95% CI 0.98–6.76). In the entire cohort, the risk of incident dementia associated with seizures remained high (adjusted HR: 2.53, 95% CI 1.84–3.48) after adjusting for age, sex, place of residence (urban/rural), US regions, diabetes, hypertension, depression, acute MI, CHF, atrial fibrillation, and use of antiseizure drugs. Similarly, the adjusted hazard for incident dementia remained high for patients with IS with seizures compared with those without seizures (adjusted HR: 2.52, 95% CI 1.79–3.53). There was also a statistically significant association between seizures and incident dementia in patients with HS after adjustment (adjusted HR: 2.80, 95% CI 1.05–7.43).
In a sensitivity analysis of the entire cohort, compared with those without seizures, and applying inverse weighting by the propensity score on the adjusted model, the association between seizures and incident dementia was attenuated but remained statistically significant (adjusted HR: 2.52, 95% CI 2.19–2.89). In the second sensitivity analysis, excluding 12,807 participants with diabetes, hypertension, depression, acute MI, CHF, and use of antiseizure drugs, the hazard for developing dementia for patients with seizures was 4.14 (95% CI 2.25–7.59) compared with those without seizures. In a stratified analysis (eTables 5 and 6, links.lww.com/WNL/C44), assessing the association between seizures and dementia by stroke recurrence status (number of patients with recurrent strokes = 9,100), the association between seizures and dementia remained statistically significant for recurrent strokes (adjusted HR: 2.25, 95% CI 1.59–3.19) and was attenuated for patients without recurrent strokes (adjusted HR: 1.87, 95% CI 0.80–4.353). There was no significant interaction between seizures and age (dichotomized as <50 years and ≥50 years) with pinteraction = 0.93 (eTable 7, links.lww.com/WNL/C44).
Discussion
In this population-based study of nearly 24,000 young stroke survivors, developing poststroke seizures was associated with a 2.5 times increase in the risk of subsequently developing dementia. Patients with ISs and HSs had a similar risk of developing dementia. The association was unchanged when sensitivity analyses were performed.
To the best of our knowledge, this is the first large-scale study assessing the association between poststroke seizures and the risk of dementia in a group of young stroke patients. Using a small hospital cohort of 169 patients with stroke with a median age of 73 years, the authors concluded that early epileptic seizures were associated with a 3.81 times increased risk of dementia within 3 years of stroke.14 Seizures have been associated with the onset of dementia in older adults without stroke. Late-onset seizures in older veterans drawn from a hospital registry doubled the risk of developing dementia.16 Our study therefore expands on prior studies by providing data in young patients from a large cohort of stroke survivors. These findings are of interest for researchers and clinicians for several reasons. First, stroke increasingly affects young patients.17,18 Stroke survival has also improved; therefore, clinicians are more likely to address the issues of long-term complications of stroke including dementia.4 Second, seizures are common complications of stroke, but seizures are amenable to therapeutic interventions.6,7 The possibility to treat or prevent seizures in patients with stroke has potential implications because seizure control in this population may advert dementia onset or slow down the progression of cognitive impairment in stroke survivors. Third, the economic burden of stroke is higher among young stroke survivors; therefore, interventions targeting young stroke survivors may provide robust economic and societal gains by preventing its long-term effects such as dementia and seizure in this age group.19 Fourth, although early-onset dementia (before age 65 years) is traditionally considered to be related to a single neurodegenerative disease,20,21 the current analysis is a call to increase attention on other causes of dementia including in particular strokes, accounting for recent rise in stroke incidence among young individuals,22,23 and the potential role of underdiagnosed seizures.
The association between seizures and cognition in patients with stroke has multiple facets. Seizures may be markers of overall severity of brain pathology including in stroke patients. They are common complications of stroke with large epidemiologic studies suggesting a cumulative incidence of seizures of 10% or more at 10 years.7 Furthermore, seizures occur at a rate 8–10 times higher in patients with dementia, particularly advanced Alzheimer disease, compared with those without dementia.24,25 Of note, vascular changes are frequently encountered in degenerative dementia pathology, and both vascular and degenerative dementias frequently coexist in the same patient.26 Finally, controlling vascular risk factors may slow down the progress of degenerative dementia.26 In addition, the presence of vascular lesions accelerates neurodegeneration, which in turns increases the risk of seizures. One of the best evidence for neurodegeneration causing seizures comes from animal models. For example, amyloid β brain deposition, a neuropathologic marker of Alzheimer disease, causes aberrant network excitation leading to recurrent.27 Another potential pathophysiologic construct is that vascular lesions may lead to late-onset seizure, which in turn may accelerate the onset of dementia. In support of this hypothesis, it has been shown that subclinical interictal epileptiform discharges in a group of patients with Alzheimer disease were associated with faster cognitive decline.28 In addition, repetitive clinical seizures may lead to permanent brain injury and hence cognitive deficits, particularly in the presence of preexisting brain lesions such as strokes.29 The deleterious effects of seizures on cognition may be mediated by the increase in infarct size with repetitive seizures as observed in animal models; however, whether this is applicable to chronic infarcts remains to be demonstrated.30 Antiseizure drugs may impair cognitive functions and contribute to dementia although the net effect in patients with ongoing seizures is uncertain because seizure control may also improve cognition. The current analysis accounted for the potential role of antiseizure drugs on cognition by accounting for their effects in various analyses (regression models and sensitivity analysis).31 In animal models of Alzheimer disease, chronic epileptiform activities are implicated in the development of aberrant network activities and cognitive impairment.32
The results of the current analysis are in line with this last hypothesis. We included patients with no preexisting dementia who had no seizures for at least 1 year after stroke. A 2.5 times increase in the risk of dementia suggests that seizures independently contributed to impaired brain activities.
This is a retrospective cohort study, which relied on available variables in the database and could not assess the effect of granular data such as the stroke lesion characteristics, stroke etiologies, stroke severity, markers of inflammation including C-reactive protein, electroencephalographic findings, level of seizure control, and dementia subtype. Furthermore, the use of ICD-9–Clinical Modification codes to establish diagnoses could have introduced biases because they are less sensitive than structured diagnoses. This is particularly true for the diagnosis of dementia in patients with stroke, which may be challenging because of decline in previous functional activities. Finally, patients who died early on after stroke or those with severe stroke may have been excluded, potentially underestimating poststroke dementia incidence.
To address this limitation, we excluded patients with preexisting dementia, accounted for seizures that developed a year after the index stroke event, and applied dedicated statistical analysis in a large sample of patients; this careful approach allowed us to provide the most robust results to date of the association between seizure and incident dementia in young patients with stroke.
This population-based study revealed that seizures after a stroke more than double the risk of dementia in young stroke survivors. This observation raises the possibility that systematically screening and treating stroke survivors for seizures may reduce the onset of dementia and have important public health implications. Future studies may address screening strategies to identify individuals at higher risk.
Glossary
- CHF
congestive heart failure
- HR
hazard ratio
- HS
hemorrhagic stroke
- ICD-9
International Classification of Diseases, Ninth Revision
- IS
ischemic stroke
- MI
myocardial infarction
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The authors report no targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Desmond DW, Moroney JT, Sano M, Stern Y. Mortality in patients with dementia after ischemic stroke. Neurology. 2002;59(4):537-543. doi: 10.1212/wnl.59.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Tatemichi TK, Paik M, Bagiella E, Desmond DW, Pirro M, Hanzawa LK. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25(10):1915-1919. doi: 10.1161/01.str.25.10.1915. [DOI] [PubMed] [Google Scholar]
- 3.Moroney JT, Bagiella E, Tatemichi TK, Paik MC, Stern Y, Desmond DW. Dementia after stroke increases the risk of long-term stroke recurrence. Neurology. 1997;48(5):1317-1325. doi: 10.1212/wnl.48.5.1317. [DOI] [PubMed] [Google Scholar]
- 4.Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315-353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006-1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 6.Graham NS, Crichton S, Koutroumanidis M, Wolfe CD, Rudd AG. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke. 2013;44(3):605-611. doi: 10.1161/STROKEAHA.111.000220. [DOI] [PubMed] [Google Scholar]
- 7.Merkler AE, Gialdini G, Lerario MP, et al. Population-based assessment of the long-term risk of seizures in survivors of stroke. Stroke. 2018;49(6):1319-1324. doi: 10.1161/STROKEAHA.117.020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumral E, Uncu G, Donmez I, et al. Impact of poststroke seizures on neurological deficits: magnetic resonance diffusion-weighted imaging study. Eur Neurol. 2013;69(4):200-206. doi: 10.1159/000345360. [DOI] [PubMed] [Google Scholar]
- 9.Zelano J. Prognosis of poststroke epilepsy. Epilepsy Behav. 2020;104(pt B):106273. doi: 10.1016/j.yebeh.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet. 2004;363(9416):1184-1186. doi: 10.1016/S0140-6736(04)15946-1. [DOI] [PubMed] [Google Scholar]
- 11.Larsson D, Farahmand B, Asberg S, Zelano J. Risk of stroke after new-onset seizures. Seizure. 2020;83:76-82. doi: 10.1016/j.seizure.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Forstl H, Burns A, Levy R, Cairns N, Luthert P, Lantos P. Neurologic signs in Alzheimer's disease. Results of a prospective clinical and neuropathologic study. Arch Neurol. 1992;49(10):1038-1042. doi: 10.1001/archneur.1992.00530340054018. [DOI] [PubMed] [Google Scholar]
- 13.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the Alzheimer type. Dementia. 1995;6(5):258-263. doi: 10.1159/000106956. [DOI] [PubMed] [Google Scholar]
- 14.Cordonnier C, Henon H, Derambure P, Pasquier F, Leys D. Early epileptic seizures after stroke are associated with increased risk of new-onset dementia. J Neurol Neurosurg Psychiatry. 2007;78(5):514-516. doi: 10.1136/jnnp.2006.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truven Health Analytics, part of the IBM Watson Health business. The Truven Health MarketScan databases for health services researchers. Accessed February 19, 2020. ibm.com/downloads/cas/6KNYVVQ2.
- 16.Keret O, Hoang TD, Xia F, Rosen HJ, Yaffe K. Association of late-onset unprovoked seizures of unknown etiology with the risk of developing dementia in older veterans. JAMA Neurol. 2020;77(6):710-715. doi: 10.1001/jamaneurol.2020.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bejot Y, Duloquin G, Thomas Q, et al. Temporal trends in the incidence of ischemic stroke in young adults: Dijon stroke registry. Neuroepidemiology. 2021;55(3):239-244. doi: 10.1159/000516054. [DOI] [PubMed] [Google Scholar]
- 18.Khan SU, Khan MZ, Khan MU, et al. Clinical and economic burden of stroke among young, midlife, and older adults in the United States, 2002–2017. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):431-441. doi: 10.1016/j.mayocpiqo.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girotra T, Lekoubou A, Bishu KG, Ovbiagele B. A contemporary and comprehensive analysis of the costs of stroke in the United States. J Neurol Sci. 2020;410:116643. doi: 10.1016/j.jns.2019.116643. [DOI] [PubMed] [Google Scholar]
- 20.Kelley BJ, Boeve BF, Josephs KA. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch Neurol. 2008;65(11):1502-1508. doi: 10.1001/archneur.65.11.1502. [DOI] [PubMed] [Google Scholar]
- 21.Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74(9):1206-1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017;74(6):695-703. doi: 10.1001/jamaneurol.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yahya T, Jilani MH, Khan SU, et al. Stroke in young adults: current trends, opportunities for prevention and pathways forward. Am J Prev Cardiol. 2020;3:100085. doi: 10.1016/j.ajpc.2020.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarmeas N, Honig LS, Choi H, et al. Seizures in Alzheimer disease: who, when, and how common? Arch Neurol. 2009;66(8):992-997. doi: 10.1001/archneurol.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer's disease. Neurology. 1986;36(9):1226-1230. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 26.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4(11):752-759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 27.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66(4):435-440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vossel KA, Ranasinghe KG, Beagle AJ, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer's disease. Ann Neurol. 2016;80(6):858-870. doi: 10.1002/ana.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3(10):608-617. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- 30.Williams AJ, Tortella FC. Neuroprotective effects of the sodium channel blocker RS100642 and attenuation of ischemia-induced brain seizures in the rat. Brain Res. 2002;932(1-2):45-55. doi: 10.1016/s0006-8993(02)02275-8. [DOI] [PubMed] [Google Scholar]
- 31.Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58(8 suppl 5):S21-S26. doi: 10.1212/wnl.58.8_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci USA. 2012;109(42):E2895-E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Although the data are available for others to purchase, the terms of our data use agreement with IBM prohibit us from sharing the MarketScan data with other investigators.