Abstract
Background and Objectives
To examine whether the brain biomarkers total-tau (T-tau), glial fibrillary acidic protein (GFAP), and β-amyloid (Aβ) isomers 40 and 42 in plasma relate to the corresponding concentrations in CSF, blood-brain barrier integrity, and duration of postconcussion syndrome (PCS) due to repetitive head impacts (RHIs) in professional athletes.
Method
In this cross-sectional study, professional athletes with persistent PCS due to RHI (median of 1.5 years after recent concussion) and uninjured controls were assessed with blood and CSF sampling. The diagnosis of PCS was based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition). The athletes were enrolled through information flyers about the study sent to the Swedish Hockey League (SHL) and the SHL Medicine Committee. The controls were enrolled through flyers at University of Gothenburg and Sahlgrenska University Hospital, Sweden. The participants underwent lumbar puncture and blood assessment at Sahlgrenska University Hospital. The main outcome measures were history of RHI and PCS severity (PCS >1 year vs PCS <1 year) in relation to plasma and CSF concentrations of T-tau, GFAP, Aβ40, and Aβ42. Plasma T-tau, GFAP, Aβ40, and Aβ42 were quantified using an ultrasensitive assay technology.
Results
A total of 47 participants (28 athletes [median age 28 years, range 18–52] with persistent PCS due to RHI and 19 controls [median age, 25 years, range 21–35]) underwent paired blood and CSF sampling. T-tau, Aβ40, and Aβ42 concentrations measured in plasma did not correlate with the corresponding CSF concentrations, while there was a correlation between plasma and CSF levels of GFAP (r = 0.45, p = 0.020). There were no significant relationships between plasma T-tau, GFAP, and blood-brain barrier integrity as measured by the CSF:serum albumin ratio. T-tau, GFAP, Aβ40, and Aβ42 measured in plasma did not relate to PCS severity. None of the markers measured in plasma correlated with number of concussions, except decreased Aβ42 in those with higher number of concussions (r = −0.40, p = 0.04).
Discussion
T-tau, GFAP, Aβ40, and Aβ42 measured in plasma do not correspond to CSF measures and may have limited utility for the evaluation of the late effects of RHI, compared with when measured in CSF.
Classification of Evidence
This study provides Class III evidence that in professional athletes with postconcussion symptoms, plasma concentrations of T-tau, GFAP, Aβ40, and Aβ42 are not informative in the diagnosis of late effects of repetitive head injuries.
Accumulating evidence suggests that athletes who have been exposed to repetitive head impacts (RHIs), including clinical concussions as well as subconcussive impacts, may develop behavioral, cognitive, and emotional symptoms, persisting for months to years, referred to as postconcussion syndrome (PCS).1 Our understanding of the pathophysiologic consequences of RHI is based on postmortem studies showing various histopathologic changes, including axonal damage, blood-brain barrier (BBB) disruption, astrogliosis, and amyloid deposition.2,3 Signs of axonal injury, astrogliosis, and amyloid dysmetabolism could be quantified by measuring total-tau (T-tau), glial fibrillary acidic protein (GFAP), and β-amyloid (Aβ) in CSF, respectively.4,5 In a study, CSF T-tau was significantly elevated in athletes with history of multiple concussions compared with controls.6 In the same study, higher CSF T-tau was also associated with reduced fractional anisotropy in several white matter tracts, including the cingulum bundle and corpus callosum. Increased CSF T-tau and GFAP have been reported in boxers 7–10 days after a bout compared with the levels after 3 months of rest from boxing.7 In the same study, CSF Aβ40 and Aβ42 were also measured and there were no significant differences between the levels at 7–10 days after a bout compared with the levels after 3 months of rest.7 In a previously published pilot study, we found that athletes with PCS due to RHI have reduced CSF Aβ42.8 In an expanded cohort including patients from the pilot study, we performed a detailed characterization of amyloid metabolism and astrogliosis in CSF. We found decreased CSF concentrations of Aβ38, Aβ40, and Aβ42, with the largest reduction in Aβ42 in athletes with persistent PCS. In the same study, we also measured CSF GFAP and found increased GFAP in athletes with PCS as compared with controls.9 A drawback of the previous studies was that it required lumbar puncture (LP) to measure CNS-derived proteins in CSF, which is an invasive procedure and not always readily available. By contrast, blood is easier to access and could be stored for long-term analysis. However, the concentration of CNS-derived proteins in the peripheral blood is very low compared with CSF because of biologically plausible reasons. The current notion is that the levels of CNS-derived proteins may increase in peripheral blood when there is disruption of the BBB integrity.10 TBI causes disruption of the BBB integrity.11 Clinically, the CSF:serum albumin ratio is commonly used as a surrogate marker of BBB integrity.12
Recent advances in the immunoassay technology have made it possible to quantify T-tau, GFAP, and Aβ species in blood with high analytic sensitivity.13,14 In this current study, we have recruited additional participants to the pilot cohort and measured T-tau, GFAP, Aβ40, and Aβ42 in plasma using ultrasensitive assays. Thus, the primary research questions in this study were (1) to assess whether T-tau, GFAP, Aβ40, and Aβ42 measured in plasma would correlate with levels in CSF, hypothesizing that plasma concentrations would correlate with CSF; (2) to examine the relationship between plasma T-tau, GFAP, Aβ40, and Aβ42 to BBB disruption as reflected by the CSF:serum albumin ratio, hypothesizing that higher plasma biomarker concentrations would correlate with the increased CSF:serum albumin ratio; and (3) to assess whether plasma T-tau, GFAP, Aβ40, and Aβ42 would be altered in athletes with RHI, hypothesizing that athletes with RHI would have increased plasma T-tau and GFAP as well as reduced Aβ42 compared with controls.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The ethics committee at the University of Gothenburg, Sweden, approved the study. All participants gave written informed consent.
Study Population
This cross-sectional study involved professional athletes with PCS due to RHI and healthy controls who all underwent venipuncture and LP. The participants were enrolled between September 2014 and June 2016 at the Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. The 28 athletes and 19 controls have also been part of previous reports.8,9,15 Inclusion criteria for the RHI group were (1) 18 years or older; (2) persistent postconcussive symptoms (for more than 3 months after repetitive concussions; (3) no contraindications to LP—decreased platelet count (<50 × 109/L), focal neurologic sign, papilledema, reduced consciousness, or infection at puncture site; (4) no evidence of structural damage on MRI (T1/T2 and fluid-attenuated inversion recovery sequences); and (5) no known history of other neurologic disorders such as multiple sclerosis or seizures. The inclusion criteria for controls were (1) 18 years or older, (2) no history of concussion or head injury, regardless of cause, (3) no contraindications to LP (as detailed above), and (4) no known history of other neurologic disorders such as multiple sclerosis or seizures.
The athletes were enrolled through information flyers about the study sent to the Swedish Hockey League (SHL) and the SHL Medicine Committee. The athletes who fulfilled the above criteria and were willing to partake in the study, either reached out to the teams' physicians to be referred or contacted the study team directly. The controls were enrolled through flyers at the University of Gothenburg and Sahlgrenska University Hospital. The controls were asked during screening whether they have had or recalled having had a history of head trauma or concussion. Only participants who answered “no” to having a prior concussion were included.
Study Rationale and Sample Size Justification
All participants who met the inclusion criteria and consented to participate were included. The data on T-tau, GFAP, Aβ40, and Aβ42 measured in blood (plasma or serum) and their correlation to CSF and BBB have not been published previously. The 28 athletes and 19 controls presented here have also been part of previous reports.8,9,15 In a pilot study,15 we measured T-tau, GFAP, and Aβ42 in CSF of 16 athletes and 15 controls (also included in the current article). In the subsequent report, we replicated and did a detailed characterization of Aβ metabolism in CSF of the 28 athletes and 19 controls (the same as this study).9 A detailed summary of the studies related to the SHL is provided (eAppendix 1, links.lww.com/WNL/C79). The rationale for this study, as also mentioned in the introduction section, was to assess the relationship between these recently developed ultrasensitive blood assays and their relationship to CSF and BBB integrity. This is important from both clinical and biochemical standpoint.
In regards to the sample size, we detected significant differences in the levels of these biomarkers between the RHI and control group with both lower (almost half of the current sample size) and with the same sample size.8,9 Thus, based on the CSF differences in this cohort, we hypothesized that these biomarkers measured in blood of these athletes would perform similar to CSF.
Diagnosis of Concussion and Severity
Concussion severity was graded according to the sports-related concussion guidelines, which is based on the number of days it takes for an athlete to return to play.16 The athletes who displayed clinical signs of concussion during the game were removed from the game and followed a graded return to play protocol.16 The diagnosis of PCS was based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).17 Athletes who had remained symptomatic for more than a year had chosen to resign from professional play on recommendation by the team physicians. The teams' physicians were present at all regular season games, documenting signs and symptoms of concussion and physical examination findings in the event of a concussion. The teams' physicians also recorded the date when an athlete had completely recovered from his concussion and was able to return to play. All athletes were assessed with the Rivermead Post-Concussion Symptom Questionnaire (RPQ)18 and lifetime number of clinical concussions, documented by the teams' physicians. At enrollment, the RHI group also underwent assessment with RPQ, including total number of clinical concussion assessment, which was documented by the teams' physicians and affirmed by the athlete as concussion. The RHI group was also assessed with RPQ at the end of the study. One of the athletes reported regular usage of central stimulant for attention deficit hyperactivity disorder. None of the participants reported other regular medications.
Main Outcome Measures
The main outcome measures were history of RHI and PCS severity (duration of PCS ≤1 year vs >1 year) in relation to plasma and CSF concentrations of T-tau, GFAP, Aβ40, and Aβ42.
Biochemical Procedures
Lumbar Puncture
LP was performed in the lateral decubitus position, through L3-L4 or L4-L5, between 10:00 am to 2:00 pm. Twenty-gauge, atraumatic needles were used for all the LPs. A total of 8–10 mL CSF was collected in a single polypropylene tube from each participant. The CSF was gently mixed, and a cell count was performed to exclude blood contamination. Thereafter, the CSF was centrifuged (2,000g at 4°C for 10 minutes), and the supernatant was aliquoted in 0.5 mL portions in polypropylene screw cap cryo tubes that were stored at −80°C pending analysis.
CSF Analyses
CSF T-tau, GFAP, Aβ40, and Aβ42 concentration were measured using ELISAs described previously.8,9,19
CSF:Serum Albumin Ratio
Serum and CSF albumin concentrations were measured by immunonephelometry on a Beckman IMMAGE Immunochemistry System (Beckman Instruments, Beckman Coulter, Brea, CA). The CSF:serum albumin ratio was calculated as CSF albumin (mg/L)/serum albumin (g/L).
Plasma Procedure
Blood samples were collected by venipuncture into EDTA tubes and centrifuged within 20–60 minutes. Plasma samples were aliquoted and stored at −80°C pending biochemical analysis. Plasma concentrations of T-tau (Human Total-Tau 2.0, #190), GFAP (GFAP Discovery, #102336), and Aβ40 and Aβ42 (Human Neurology 3-Plex A, #101995) were measured using the Single Molecule Array technology (Quanterix, Billerica, MA).13 All analytes had an average coefficient of variance <10%.
All samples were analyzed at the same time using the same batch of reagents by certified laboratory technicians blind to clinical information.
Biofluid-Based Biomarker Source of Origin and Clinical Interpretation
T-tau is a microtubule-associated protein predominantly expressed in short cortical unmyelinated axons.20 GFAP is manly found in astroglial cells and is a marker of astroglial activation.21 The amyloid deposition or plaques seen in TBI are predominantly composed of 42 amino acid–long and aggregation-prone Aβ42, which are also seen in Alzheimer disease (AD).22,23 By contrast, Aβ40 and Aβ42 measured in plasma have been shown to be increased in AD.24 It is worth pointing out that for GFAP, the assays work equally on plasma and serum, while for T-tau, Aβ40, and Aβ42, the levels are lower in serum compared with plasma.25 Thus, plasma is a better source for quantification of T-tau and Aβ40 and Aβ42, which is the rationale for the quantification of these biomarkers in plasma instead of serum in this study.
Statistical Analysis
The correlations between the plasma and CSF levels of the biomarkers were assessed using Spearman rank correlation. We used the χ2 test for assessing the categorical variables between the RHI group and controls. We used the Mann-Whitney U test for comparison between the RHI group and controls. The Kruskal-Wallis analysis of variance was used to compare continuous variables between the groups. All tests were 2-sided, and statistical significance was determined at p < 0.05. The statistical analyses were performed in GraphPad Prism 8 (GraphPad, La Jolla, CA) and R (v.3.0.3, The R Foundation for Statistical Computing).
Data Availability
The data supporting the findings are available on request from the corresponding author.
Results
Demographic Characteristics
Twenty-eight athletes with RHI (assessed with paired CSF and plasma, a median of 1.5 years since most recent concussion) and 19 neurologically healthy controls were enrolled prospectively (Table 1). Of 28 athletes with RHI, 9 of the athletes returned to play or were symptom-free within 1 year, while 19 had PCS >1 year and had resigned from the game because of persistent symptoms. As previously reported, athletes in the PCS >1 year group had higher RPQ score and number of concussion.9 Age, sex, and race did not differ significantly between the athletes with RHI and controls (Table 1).
Table 1.
Demographic and Clinical Characteristics of Professional Athletes and Controls
Plasma Biomarkers in Relation to CSF and BBB Integrity
Plasma GFAP correlated with CSF (r = 0.46, p = 0.003), while no significant correlations were found between plasma and CSF T-tau, Aβ40, and Aβ42 (Figure 1A). T-tau, GFAP, and Aβ40 measured in plasma did not significantly correlate with the CSF:serum albumin ratio; however, plasma Aβ42 correlated with the CSF:serum albumin ratio (Figure 1). Furthermore, in the control group, plasma Aβ40 and Aβ42 correlated with the CSF:serum albumin ratio (Figure 1).
Figure 1. Correlations Between Plasma and CSF and BBB Integrity.
Panel A shows the correlations between plasma T-tau, GFAP, Aβ40, and Aβ42 and corresponding CSF. Panel B shows the relationship between plasma biomarkers and CSF:serum albumin ratio. The correlations were tested using Spearman sign rank correlation (r). The fitted line including the 95% CI is from a linear regression. Aβ = β-amyloid; GFAP = glial fibrillary acidic protein; RHI = repetitive head impact; T-tau = total-tau.
Plasma Biomarkers in Relation to Diagnosis of RHI and RTP
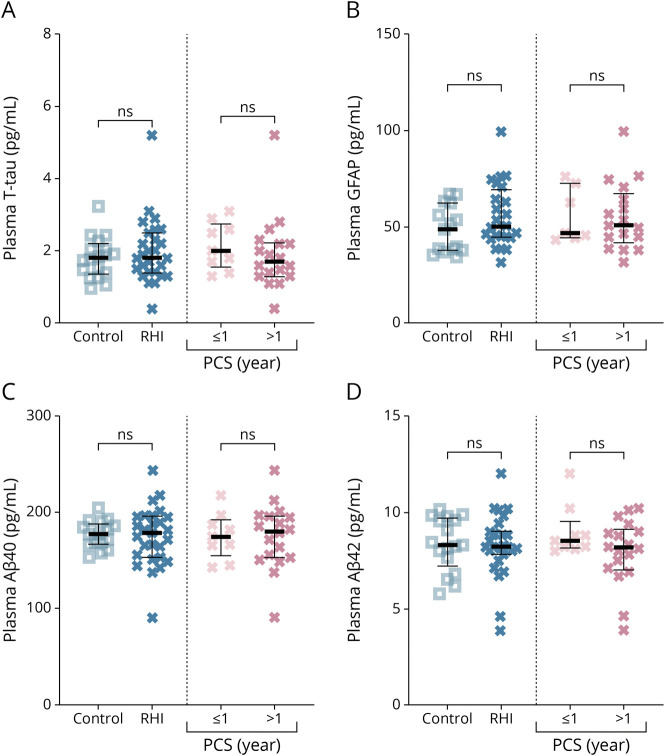
Plasma concentrations of T-tau, GFAP, Aβ40, and Aβ42 did not differ significantly between athletes with RHI and controls or between those who could return to play vs those who had resigned because of persistent PCS (Figure 2).
Figure 2. Plasma Biomarkers in Relation to Diagnosis of RC and Duration of PCS.
(A–D) show the differences in plasma concentrations of the biomarker in athletes with exposure to RHI vs controls and PCS duration. Aβ = β-amyloid; GFAP = glial fibrillary acidic protein; n.s. = not significant; PCS = postconcussive syndrome; RHI = repetitive head impact; T-tau = total-tau.
Plasma Biomarkers in Relation to Injury Severity and Number of Concussions
There were no correlations between T-tau, GFAP, Aβ40, or Aβ42 and total RPQ score (Figure 3A). There were no correlations between plasma T-tau, GFAP, and Aβ40 and number of clinically evident concussions, except decreased plasma Aβ42 correlating with higher number of concussions (r = −0.40, p = 0.04; Figure 3B).
Figure 3. Plasma Biomarkers in Relation to Postconcussion Symptoms Severity and Number of Concussions.
Panel A shows the relationship between plasma levels of the biomarkers and total RPQ scores. Panel B shows the relationship between plasma levels of the biomarkers and number of lifetime concussions. The correlations were tested using Spearman sign rank correlation (r). The fitted lines including 95% CIs are from linear regression models. Aβ = β-amyloid; GFAP = glial fibrillary acidic protein; RPQ = Rivermead Post-Concussion Symptoms Questionnaire; T-tau = total-tau.
Classification of Evidence
This study provides Class III evidence that in professional athletes with postconcussion symptoms, plasma concentrations of T-tau, GFAP, Aβ40, and Aβ42 are not informative in the diagnosis of late effects of repetitive head injuries.
Discussion
In this study, we found that (1) T-tau, Aβ40, and Aβ42 measured in plasma do not correlate significantly with the levels in CSF, while plasma levels of GFAP correlated with CSF levels; (2) there were no significant correlations between plasma concentrations of these biomarkers and BBB integrity, except for a correlation between Aβ42 and CSF:serum albumin ratio; and (3) plasma T-tau, GFAP, Aβ40, and Aβ42 showed no significant associations with the duration of PCS due to RHI.
We observed a moderate correlation between plasma and CSF GFAP, while there were no significant correlations between plasma T-tau, Aβ40, and Aβ42 and corresponding CSF levels, suggesting that plasma GFAP, to a certain degree, reflects CSF, while plasma assays for T-tau, Aβ40, and Aβ42 may not reflect CSF or brain pathophysiology. The moderate or weak correlations seen here could have several explanations, including faster clearance of these biomarkers from the blood, variance because of different integrity of the BBB among participants, or be related to the analytical performance of the blood assays used. There are no existing studies in which individuals with a history of RHI and healthy controls have been assessed with paired CSF and blood sampling, restricting direct comparison of these results. These findings suggest that CSF may be a better fluid source of quantification of GFAP, Aβ40, and Aβ42 in assessing signs of astroglial injury or amyloid metabolism in those with a history of RHI.
Paired CSF and blood sampling also allow for assessment of blood biomarkers in relation to BBB integrity. The CSF:serum albumin ratio is used as a surrogate marker of BBB integrity.12 There were no associations between blood concentrations of T-tau, GFAP, and CSF:serum albumin ratio, suggesting that (1) these biomarkers may be released into the bloodstream independent of BBB integrity, (2) that CSF:serum albumin ratio may not be a sensitive enough measure of BBB function, or that (3) these biomarkers may be released into the blood through other mechanisms, such as through the CSF and arachnoid villi or the glymphatic system.26,27 Of note, correlations were seen between Aβ40 and Aβ42 and CSF:serum albumin ratio in the control group but not RHI group. This is an interesting finding, which needs further replication in a larger cohort of healthy controls.
Increased concentrations of CSF T-tau have previously been seen in acute samples from boxers with RHI.7 In the context of chronic TBI, a recent study found no difference in plasma T-tau in National Football League players with a history of RHI compared with controls.28 In direct comparison, herein, T-tau measured neither in CSF or plasma differed between RHI and controls. These findings suggest that both CSF and plasma T-tau have limited utility as a biomarker for the late effects of RHI.
GFAP measured in blood has been assessed in numerous studies of acute TBI, where GFAP concentration was increased in those with intracranial bleeding.29-31 In the context of sports-related concussion, increased levels of GFAP measured within 48 hours after a concussion have been seen in collegiate athletes.32,33 Plasma GFAP has not been assessed as a biomarker in the chronic phase or in the setting of professional athletes who have had RHI. In contrast to CSF GFAP measured in this cohort,8 plasma GFAP did not relate to diagnosis of RHI or injury severity, suggesting that plasma GFAP is less sensitive than CSF GFAP to detect astrogliosis in the chronic phase of RHI.
Experimental and postmortem studies suggest that athletes who have had RHI may develop brain amyloid deposition.2,3,34 In direct comparison with these studies, we previously observed decreased CSF Aβ40 and Aβ42 in this cohort,8 with the highest effect size seen for Aβ42—a principal component of amyloid plaque seen in neurodegenerative diseases.22,23 Unlike CSF Aβ measured in this cohort,8 Aβ measured in plasma did not differ between athletes with RHI and controls, suggesting that plasma Aβ is not as sensitive as CSF in detecting amyloid dysmetabolism.
One limitation of this study is the rather modest sample size. Nevertheless, it is difficult to motivate professional athletes or healthy individuals to undergo LP. Furthermore, we did not have longitudinal blood and CSF data to investigate the precise relationship between PCS and RHI. Finally, there is always risk of selection bias. The participants enrolled in this study were predominantly White and male, which may not be representative of all athletes who develop long-term PCS. A previous study has shown that serum GFAP levels may differ between White and Black athletes,35 which we could not assess herein.
These results suggest that T-tau, GFAP, Aβ40, and Aβ42 measured in plasma do not correspond to CSF measures and may have limited utility for the evaluation of the late effects of RHI, compared with when measured in CSF.
Acknowledgment
The authors thank the study participants and the Swedish Hockey League, Swedish Hockey League Medicine Committee, and the teams' medical staff. Also, they thank Celia Hök Fröhlander (MSN) for assisting with the fluid sampling and coordination of the study participants to the Neurochemistry Laboratory at Sahlgrenska University Hospital, Mölndal, Sweden.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- GFAP
glial fibrillary acidic protein
- LP
lumbar puncture
- PCS
postconcussive syndrome
- RHI
repetitive head impact
- RPQ
Rivermead Post-Concussion Symptom Questionnaire
- SHL
Swedish Hockey League
- TBI
traumatic brain injury
- T-tau
total-tau
Appendix. Authors

Footnotes
See page e427
Class of Evidence: NPub.org/coe
Study Funding
The study was funded by the Swedish Alzheimer Foundation (grant AF-742881), Hjärnfonden, Sweden (grant FO2017-0243), and the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (grant ALFGBG-715986).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Blennow K, Brody DL, Kochanek PM, et al. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084. [DOI] [PubMed] [Google Scholar]
- 2.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270-303. [DOI] [PubMed] [Google Scholar]
- 3.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American Football. JAMA. 2017;318(4):360-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahim P, Gill JM, Blennow K, Zetterberg H. Fluid biomarkers for chronic traumatic encephalopathy. Semin Neurol. 2020;40(4):411-419. [DOI] [PubMed] [Google Scholar]
- 5.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63(9):1277-1280. [DOI] [PubMed] [Google Scholar]
- 6.Taghdiri F, Multani N, Tarazi A, et al. Elevated cerebrospinal fluid total tau in former professional athletes with multiple concussions. Neurology. 2019;92(23):e2717-e2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One. 2012;7(4):e33606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahim P, Tegner Y, Gustafsson B, et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol. 2016;73(11):1308-1315. [DOI] [PubMed] [Google Scholar]
- 9.Shahim P, Tegner Y, Marklund N, et al. Astroglial activation and altered amyloid metabolism in human repetitive concussion. Neurology. 2017;88(15):1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uher T, McComb M, Galkin S, et al. Neurofilament levels are associated with blood-brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler. 2021;27(2):220-231. [DOI] [PubMed] [Google Scholar]
- 11.Cash A, Theus MH. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int J Mol Sci. 2020;21(9):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skillbäck T, Delsing L, Synnergren J, et al. CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol Aging. 2017;59:1-9. [DOI] [PubMed] [Google Scholar]
- 13.Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28(6):595-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahim P, Politis A, van der Merwe A, et al. Time course and diagnostic utility of NfL, tau, GFAp, and UCH-L1 in subacute and chronic TBI. Neurology. 2020;95(6):e623-e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahim P, Politis A, van der Merwe A, et al. Neurofilament light as a biomarker in traumatic brain injury. Neurology. 2020;95(6):e610-e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport—the 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM R. 2013;5:255-279. [DOI] [PubMed] [Google Scholar]
- 17.Lagarde E, Salmi LR, Holm LW, et al. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. JAMA Psychiatry. 2014;71(9):1032-1040. [DOI] [PubMed] [Google Scholar]
- 18.Cifu DX, Walker WC, West SL, et al. Hyperbaric oxygen for blast-related post-concussion syndrome: 3-month outcomes. Ann Neurol. 2014;75(2):277-286. [DOI] [PubMed] [Google Scholar]
- 19.Rosengren LE, Wikkelsø C, Hagberg L. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of adults. J Neurosci Methods. 1994;51(2):197-204. [DOI] [PubMed] [Google Scholar]
- 20.Trojanowski JQ, Schuck T, Schmidt ML, Lee VM. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989;37(2):209-215. [DOI] [PubMed] [Google Scholar]
- 21.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43(2):429-435. [DOI] [PubMed] [Google Scholar]
- 22.Zetterberg H, Lautner R, Skillbäck T, et al. CSF in Alzheimer's disease. Adv Clin Chem. 2014;65:143-172. [DOI] [PubMed] [Google Scholar]
- 23.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87(17):1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashton NJ, Suárez-Calvet M, Karikari TK, et al. Effects of pre-analytical procedures on blood biomarkers for Alzheimer's pathophysiology, glial activation, and neurodegeneration. Alzheimers Dement (Amst). 2021;13(1):e12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalm M, Boström M, Sandelius Å, et al. Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood-brain barrier permeability. Brain Res. 2017;1668:12-19. [DOI] [PubMed] [Google Scholar]
- 28.Alosco ML, Tripodis Y, Jarnagin J, et al. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement (Amst). 2017;7:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill J, Latour L, Diaz-Arrastia R, et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology. 2018;91(15):e1385-e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazarian JJ, Biberthaler P, Welch RD, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018;17(9):782-789. [DOI] [PubMed] [Google Scholar]
- 32.Pattinson CL, Meier TB, Guedes VA, et al. Plasma biomarker concentrations associated with return to sport following sport-related concussion in collegiate athletes—a Concussion Assessment, Research, and Education (CARE) consortium study. JAMA Netw Open. 2020;3(8):e2013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier TB, Huber DL, Bohorquez-Montoya L, et al. A prospective study of acute blood-based biomarkers for sport-related concussion. Ann Neurol. 2020;87(6):907-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886-899. [DOI] [PubMed] [Google Scholar]
- 35.Asken BM, Bauer RM, DeKosky ST, et al. Concussion BASICS II: baseline serum biomarkers, head impact exposure, and clinical measures. Neurology. 2018;91(23):e2123-e2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings are available on request from the corresponding author.