Abstract
Background and Objectives
We compared digital speech and language features of patients with amnestic Alzheimer disease (aAD) or logopenic variant primary progressive aphasia (lvPPA) in a biologically confirmed cohort and related these features to neuropsychiatric test scores and CSF analytes.
Methods
We included patients with aAD or lvPPA with CSF (phosphorylated tau ([p-tau]/β-amyloid [Aβ] ≥0.09, and total tau/Aβ ≥0.34) or autopsy confirmation of AD pathology and age-matched healthy controls (HC) recruited at the Frontotemporal Degeneration Center of the University of Pennsylvania for a cross-sectional study. We extracted speech and language variables with automated lexical and acoustic pipelines from participants' oral picture descriptions. We compared the groups and correlated distinct features with clinical ratings and CSF p-tau levels.
Results
We examined patients with aAD (n = 44; age 62 ± 8 years; 24 women; Mini-Mental State Examination [MMSE] score 21.1 ± 4.8) or lvPPA (n = 21; age 64.1 ± 8.2 years; 11 women; MMSE score 23.0 ± 4.2) and HC (n = 28; age 65.9 ± 5.9 years, 15 women; MMSE score 29 ± 1). Patients with lvPPA produced fewer verbs (10.5 ± 2.3; p = 0.001) and adjectives (2.7 ± 1.3, p = 0.019) and more fillers (7.4 ± 3.9; p = 0.022) with lower lexical diversity (0.84 ± 0.1; p = 0.05) and higher pause rate (54.2 ± 19.2; p = 0.015) than individuals with aAD (verbs 12.5 ± 2; adjectives 3.8 ± 2; fillers 4.9 ± 4.5; lexical diversity 0.87 ± 0.1; pause rate 45.3 ± 12.8). Both groups showed some shared language impairments compared with HC. Word frequency (MMSE score: β = −1.6, p = 0.009; Boston Naming Test [BNT] score: β = −4.36, p < 0.001), adverbs (MMSE score: β = −1.9, p = 0.003; BNT score: β = −2.41, p = 0.041), pause rate (MMSE score: β = −1.21, p = 0.041; BNT score: β = −2.09, p = 0.041), and word length (MMSE score: β = 1.75, p = 0.001; BNT score: β = 2.94, p = 0.003) were significantly correlated with both MMSE and BNT scores, but other measures were not correlated with MMSE and/or BNT score. Prepositions (r = −0.36, p = 0.019), nouns (r = −0.31, p = 0.047), speech segment duration (r = −0.33, p = 0.032), word frequency (r = 0.33, p = 0.036), and pause rate (r = 0.34, p = 0.026) were correlated with patients' CSF p-tau levels.
Discussion
Our measures captured language and speech differences between the 2 phenotypes that traditional language-based clinical assessments failed to identify. This work demonstrates the potential of natural speech in reflecting underlying variants with AD pathology.
Speech production is a complex behavior involving coordinated activation of multiple brain regions. Thus, examining speech production provides potential opportunities to identify neurodegenerative disease markers that are sensitive to specific phenotypes. Because Alzheimer disease (AD) accounts for up to 80% of patients with dementia,1 much attention has been paid to cognitive and linguistic profiling of AD. Language produced by patients with amnestic AD (aAD) has been found to be “empty” with an abundance of nonspecific words, circumlocutions, and sparse content.2
Logopenic variant primary progressive aphasia (lvPPA) is 1 of the PPA variants3,4 that is an atypical, nonamnestic manifestation of AD,3,5-9 with a majority of autopsied cases associated with underlying AD pathology.9,10 Since the identification of this PPA variant, many studies have been dedicated to characterizing its language and speech features compared to other variants of PPA.11,12 Previous studies have shown that patients with lvPPA speak slowly with impaired lexical access13 and have poor phonemic discrimination14 with limited auditory-verbal short-term memory, naming impairment,4,5 and dysfluencies.12
Previous studies of patients with neurodegeneration suggest that language and speech features are useful as a screening tool15-17 because speech samples are easy to collect noninvasively and are sensitive to cognitive impairments. Despite the shared pathology of aAD and lvPPA, previous studies have focused on the linguistic profiling of these 2 syndromes separately. With few comparative studies, an important gap remains in the literature. Most previous quantitative work has focused on measures such as the Boston Naming Test (BNT) that assess lexical retrieval during confrontation naming of an object. However, descriptions of natural, connected speech in aAD and lvPPA are frequently informal. In this study, we identified similarities and differences between patients with aAD and lvPPA with biological confirmation of underlying AD pathology by analyzing digitized, natural speech samples with reliable and reproducible automated methods. From previous studies, we hypothesized that patients with lvPPA would produce more dysfluent speech with more limited lexical content than those with aAD. We also hypothesized that patients with aAD and those with lvPPA would share some linguistic features, including decreased speech production. We associated language and speech variables with clinical test scores and CSF analytes for additional validation and specific mechanistic clarification.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board of the Hospital of the University of Pennsylvania approved the study of human participants, and all participants agreed to participate in the study by written consent. All digital data were stored in secured Health Insurance Portability and Accountability Act–compliant servers and handled by personnel trained in personal identifiable information protection.
Participants
We examined oral picture descriptions that were produced by 93 participants who were recruited in the Department of Neurology at the Hospital of the University of Pennsylvania from early 2000 to early 2019. All patients were assessed by experienced neurologists (M.G., D.J.I.) following published diagnostic criteria,18,19 and their clinical phenotypes were reviewed in a consensus conference. To support the diagnosis, participants underwent comprehensive neuropsychological assessments with the National Alzheimer's Coordinating Center Uniform Data Set version 320 and the Rey Complex Figure Test.21 Of 152 patients whose clinical phenotype was either AD (n = 114) or lvPPA (n = 38), we included only 44 participants with aAD and 21 patients with lvPPA who had AD pathology at autopsy (n = 15; 4 with lvPPA and 11 with AD) or who met the criteria of underlying AD pathology on CSF analyte levels (n = 50; 17 with lvPPA and 33 with AD; phosphorylated tau [p-tau]/β-amyloid42 [Aβ] ≥0.0922 and total tau/Aβ ≥0.3423). Five patients with aAD who had a CSF Aβ value >192 pg/mL24 were still included in the analysis because both their p-tau/Aβ ratio and total tau/Aβ ratio met the criteria. Patients with aAD or lvPPA who did not meet both cutoffs, did not have CSF or autopsy data, or did not have AD pathology at autopsy were excluded from the analysis. Thirteen patients of 15 with autopsy data had a high probability of having AD pathologic change on the basis of established ABC scoring.25 Four patients with posterior cortical atrophy and 3 patients with nonamnestic mild cognitive impairment who did not meet the criteria for lvPPA18 were excluded from the analysis. None of the participants included in the study had other neurologic, psychiatric, or medical conditions that could affect cognition. Twenty-eight age-matched elderly healthy controls (HC) who did not have cognitive impairment were included as a control group.
Data Collection
We digitally recorded the participants' descriptions of the Cookie Theft picture from the Boston Diagnostic Aphasia Examination.26 Descriptions were a 1 minute long. Recordings were orthographically transcribed by a linguist and trained annotators. Transcribing a 1-minute speech sample usually took 5 to 7 minutes, and our usual transcription interrater agreement rate was between 93% and 94%. The earliest recording of each participant was analyzed with our lexical and acoustic pipelines, as described below.
Lexical Pipeline
We automatically tagged the part-of-speech (POS) category of all tokens using spaCy,27 which is a Python package for natural language processing, with its large language model (en_core_web_lg) for English. The number of tense-inflected verbs was calculated by summing the number of modal auxiliaries and past-tense and present-tense verbs. Dysfluency markers, including fillers, repetitions, and partial words, were counted separately. The count of each POS category, tense-inflected verbs, and dysfluency markers was converted to counts per 100 words to control for the total number of words per participant.
We rated each word for concreteness,28 semantic ambiguity,29 frequency,30 age at acquisition,31 and familiarity31 using published norms. Word length by the number of phonemes for each word was calculated with the Carnegie Mellon University Pronouncing Dictionary32 using the Natural Language Toolkit package33 in Python. We calculated the mean scores of these measures for content words (nouns, verbs, adjectives, and adverbs) per participant.
Last, we measured lexical diversity using the moving-average type-token ratio,34 which has been described as one of the most reliable measures for calculating lexical diversity.35 The window length was set at 15 words. We also experimented with larger windows (20-word and 25-word windows). Because the results remained the same, we reported only results from 15-word windows. Hereafter, the measures from the lexical pipeline were referred to as language measures. Detailed descriptions of the lexical pipeline and validation of the POS tagging accuracy have previously been published.36
Acoustic Pipeline
We used an in-house speech activity detector to segment audio recordings into speech segments and silent pauses. We visually reviewed the segments. Nonspeech segments at the beginning and end of each recording and interviewer's prompts were excluded from the analysis.
Using the speech activity detector output, we calculated duration-related measurements, including mean speech segment duration, mean pause segment duration, percent of speech, and pause rate per minute. We summed the number of syllables of all words from the published norm32 and computed articulation rate as the number of syllables per second.
In addition, we pitch-tracked all speech segments with Praat.37 To normalize physiologic differences in pitch (f0), we converted the pitch values from Hertz to semitones using the 10th pitch percentile of each participant as a baseline: semitones = 12 × log2(f0/baseline f0). We used the converted 90th percentile as a measure of the pitch range of each speaker. Hereafter, the measures from the acoustic pipeline were referred to as speech measures. Detailed descriptions of the acoustic pipeline have been published previously,38 and the list of all analyzed features is included in eTable 1, links.lww.com/WNL/B1000.
CSF Analysis
Forty-two (30 with aAD and 12 with lvPPA) patients had CSF biomarkers collected within 1 year of the Cookie Theft recording, including Aβ and p-tau. CSF was analyzed with 2 platforms, Luminex xMAP or Innotest ELISA, which was then transformed to the Luminex scale.39 We previously related CSF p-tau levels directly to cerebral tau burden in our autopsy cohort.39 To determine the association of language and speech features with in vivo measures of pathology, we examined the relationship between our language and speech variables and the 2 CSF biomarkers of Aβ and p-tau. Only 1 HC had CSF biomarkers in this dataset.
Statistical Methods
To compare the groups, we tested whether requirements for parametric tests were met with a Levene test. If the data met the requirements for parametric tests, we performed an analysis of variance. If not, we performed a Kruskal-Wallis test. We visually assessed residuals of the models to ensure that the data were suitable for linear modeling. When a group difference was significant, we in addition performed a post hoc pairwise t test or pairwise Wilcoxon rank-sum test for pairwise group comparisons, adjusting p values for multiple group comparisons (n = 3) with the false discovery rate. We reported the effect size of each group comparison using the Cohen d.
Patients' language and speech variables were z scored using the mean and SD of the HC. These z scores were used for visualization and linear regressions to estimate relations of our language variables and clinical ratings. We did not use z scores to determine significant group differences with Z tests because the test statistic in some variables did not follow a normal distribution.
The z scored variables that showed significant group differences were associated with patients' clinical assessments to investigate the relations of our language and speech features to clinical ratings of cognitive, language, and memory impairment. Analyzed clinical assessments included the MMSE, BNT, Rey complex figure copy and delayed recall, Craft Story delayed recall, and forward digit span scores. To examine potential interactions, we included phenotype as an interaction term (clinical ratings ~ language or speech variable × phenotype). The p values were adjusted with the false discovery rate.
To validate our findings with levels of specific CSF biomarkers, we correlated patients' CSF analyte levels with the language and speech variables using Pearson correlation tests. CSF p-tau levels were log-transformed to normalize the data. We also checked whether patients' clinical phenotype and the time difference between Cookie Theft recording and CSF sample collection were significant factors with linear regression models. Because the 2 factors were not significant, we reported only the results of simple correlations to simplify the models. All statistical analyses were performed with R version 4.1.0 and RStudio version 1.4.1717 (R Core Team, Vienna, Austria).
Data Availability
Anonymized data will be shared on request from any qualified investigator for purposes of validation or replication of study methods.
Results
Participant Characteristics
Table 1 shows the demographic and clinical characteristics of the participants. The 3 groups did not differ in age, sex, or education level. The patient groups did not differ from each other in disease duration, CSF biomarkers, and most clinical ratings except the Rey complex figure and forward digit span scores. Patients with aAD were more impaired in both Rey complex figure copy and delayed recall because these patients had the amnestic variant of AD. On the other hand, patients with lvPPA were more impaired on forward digit span, and this was in line with our previous observation.9
Table 1.
Demographic and Clinical Characteristics of Participants
Differences Between Patients With aAD and Patients With lvPPA
The group means of all language and speech variables are summarized in eTable 2, links.lww.com/WNL/B1000. Patients with lvPPA produced fewer tense-inflected verbs compared to those with aAD (p = 0.001,  = 0.94) and HC (p = 0.048,
= 0.94) and HC (p = 0.048,  = 0.61; Figure1A). The tense-inflected verb counts of patients with aAD did not significantly differ from those of HC (p = 0.124,
= 0.61; Figure1A). The tense-inflected verb counts of patients with aAD did not significantly differ from those of HC (p = 0.124,  = 0.4). Patients with lvPPA showed lower lexical diversity than those with aAD (p = 0.05,
= 0.4). Patients with lvPPA showed lower lexical diversity than those with aAD (p = 0.05,  = 0.52) and HC (p = 0.005,
= 0.52) and HC (p = 0.005, 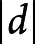 = 1.02), yet patients with aAD did not differ from HC (p = 0.149,
= 1.02), yet patients with aAD did not differ from HC (p = 0.149,  = 0.39; Figure1A). Larger windows yielded similar results (20-word window: lvPPA vs aAD p = 0.047,
= 0.39; Figure1A). Larger windows yielded similar results (20-word window: lvPPA vs aAD p = 0.047,  = 0.53 and lvPPA vs HC p = 0.004,
= 0.53 and lvPPA vs HC p = 0.004,  = 1.02; 25-word window: lvPPA vs aAD p = 0.052,
= 1.02; 25-word window: lvPPA vs aAD p = 0.052,  = 0.52 and lvPPA vs HC p = 0.004,
= 0.52 and lvPPA vs HC p = 0.004,  = 1). Patients with lvPPA produced fewer adjectives than those with aAD (p = 0.019,
= 1). Patients with lvPPA produced fewer adjectives than those with aAD (p = 0.019,  = 0.66) and HC (p < 0.001,
= 0.66) and HC (p < 0.001,  = 1.72); Patients with aAD also produced fewer adjectives than HC (p = 0.003,
= 1.72); Patients with aAD also produced fewer adjectives than HC (p = 0.003,  = 0.75; Figure 1A). Thus, both patient groups were impaired in their adjective production, but patients with lvPPA were more severely impaired compared with those with aAD.
= 0.75; Figure 1A). Thus, both patient groups were impaired in their adjective production, but patients with lvPPA were more severely impaired compared with those with aAD.
Figure 1. Speech Differences Between lvPPA and aAD.

For ease of visualization, z-scored values compared to HC mean and standard deviation are plotted (A and B). Horizontal black line indicates the mean of the healthy controls (HC). aAD = amnestic Alzheimer disease, lvPPA = logopenic variant primary progressive aphasia.
Patients with lvPPA also produced more fillers than patients with aAD (p = 0.022,  = 0.6) and HC (p = 0.01,
= 0.6) and HC (p = 0.01,  = 1.06; Figure 1B), while patients with aAD did not differ significantly from HC (p = 0.383,
= 1.06; Figure 1B), while patients with aAD did not differ significantly from HC (p = 0.383,  = 0.23). Patients with lvPPA showed a higher pause rate than those with aAD (p = 0.015,
= 0.23). Patients with lvPPA showed a higher pause rate than those with aAD (p = 0.015,  = 0.55) and HC (p < 0.001,
= 0.55) and HC (p < 0.001,  = 1.58; Figure 1B). The pause rate of patients with aAD was also higher than that of HC (p < 0.001,
= 1.58; Figure 1B). The pause rate of patients with aAD was also higher than that of HC (p < 0.001,  = 1.34). Last, patients with lvPPA produced more partial words than HC (p = 0.042,
= 1.34). Last, patients with lvPPA produced more partial words than HC (p = 0.042,  = 0.61), but they did not differ significantly from those with aAD (p = 0.147,
= 0.61), but they did not differ significantly from those with aAD (p = 0.147,  = 0.3), and patients with aAD did not differ from HC (p = 0.313,
= 0.3), and patients with aAD did not differ from HC (p = 0.313,  = 0.24). Thus, patients with lvPPA produced an abnormal number of partial words, while speakers with aAD did not.
= 0.24). Thus, patients with lvPPA produced an abnormal number of partial words, while speakers with aAD did not.
Impaired Language and Speech Features in Both aAD and lvPPA
Figure 2 shows all language and speech features in which both patient groups differed from HC. Both patient groups produced fewer prepositions and nouns than HC; patients produced shorter speech segments than HC, and their percent of speech time of the total time was also lower than that of HC (p < 0.001,  > 0.8 for all comparisons). Both groups' content words were shorter, more frequent (length and frequency: p < 0.001,
> 0.8 for all comparisons). Both groups' content words were shorter, more frequent (length and frequency: p < 0.001,  > 0.8), acquired earlier (lvPPA: p < 0.001,
> 0.8), acquired earlier (lvPPA: p < 0.001,  = 0.95; aAD: p < 0.001,
= 0.95; aAD: p < 0.001,  = 0.56), and more concrete (lvPPA: p = 0.028,
= 0.56), and more concrete (lvPPA: p = 0.028,  = 0.7; aAD: p = 0.002,
= 0.7; aAD: p = 0.002,  = 0.86) than those of HC. Both patient groups produced more adverbs (lvPPA: p = 0.029,
= 0.86) than those of HC. Both patient groups produced more adverbs (lvPPA: p = 0.029,  = 0.84; aAD: p = 0.029,
= 0.84; aAD: p = 0.029,  = 0.73) and repetitions (lvPPA: p < 0.001,
= 0.73) and repetitions (lvPPA: p < 0.001,  = 1.39; aAD: p < 0.001,
= 1.39; aAD: p < 0.001,  = 0.79) than HC. Patients also spoke more slowly (lvPPA: p < 0.001,
= 0.79) than HC. Patients also spoke more slowly (lvPPA: p < 0.001,  = 1.01; aAD: p < 0.001,
= 1.01; aAD: p < 0.001,  = 0.57), and they produced fewer words in total than HC (lvPPA: p = 0.032,
= 0.57), and they produced fewer words in total than HC (lvPPA: p = 0.032,  = 0.7; aAD: p = 0.007,
= 0.7; aAD: p = 0.007,  = 0.73).
= 0.73).
Figure 2. Impaired Language and Speech Measures in Both lvPPA (Green) and aAD (Yellow).
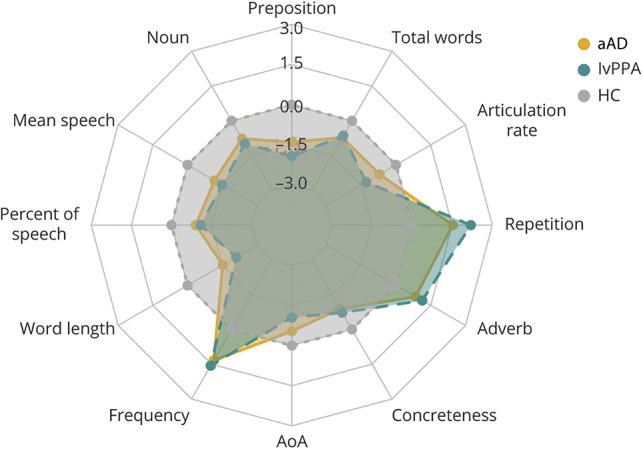
Only speech measures that were significantly different from those of healthy controls (HC) (gray) are plotted, and measures were standardized on the basis of the HC mean and SD. Numbers in blue indicate z-scored values based on HC. aAD = amnestic Alzheimer disease; AoA = age of acquisition; lvPPA = logopenic variant primary progressive aphasia.
Relationships to Clinical Measures
Figure 3 illustrates relationships between the speech and language features and 2 clinical ratings: MMSE and BNT. Only 1 variable showed a significant interaction with phenotype. Patients with aAD who had lower MMSE scores produced more adverbs (β = −1.9, p = 0.003), yet patients with lvPPA who had lower MMSE scores produced fewer adverbs (β = 2.67, p = 0.015).
Figure 3. Results of Linear Regression Models of Language and Speech Measures in Patients With MMSE and BNT Scores.

Standardized estimated coefficients (x-axis) are plotted with colors: positive coefficients in blue and negative coefficients in red. The p values are adjusted with the false discovery rate. AoA = age of acquisition; BNT = Boston Naming Test; MMSE = Mini-Mental State Examination. *p < 0.05, **p < 0.01, ***p < 0.001.
MMSE score was significantly related to 5 language and speech variables. Patients with lower MMSE scores produced more frequent (β = −1.6, p = 0.009) and shorter (β = 1.75, p = 0.001) content words, paused more frequently (β = −1.21, p = 0.041), and produced more adverbs (β = −1.9, p = 0.003) and partial words (β = −1.52, p = 0.021).
BNT score was significantly associated with 10 variables. Patients with lower BNT scores produced frequent words (β = −4.36, p < 0.001), produced many adverbs (β = −2.41, p = 0.041), and had a high pause rate (β = −2.09, p = 0.041). Patients with lower BNT scores also produced fewer prepositions (β = 2.42, p = 0.048) and nouns (β = 5.18, p = 0.003). Patients with lower BNT scores showed a low percent of speech produced during the picture description (β = 2.4, p = 0.045), and they produced shorter (β = 2.94, p = 0.003), less concrete (β = 4.24, p = 0.003), and earlier-acquired (β = 4.69, p = 0.003) content words with shorter speech segments (β = 3.89, p = 0.041).
The other clinical ratings, namely the Rey complex figure copy and delayed recall, the Craft Story delayed recall, and the forward digit span, and their interaction with patients' phenotype were not significantly related to our language/speech measures after p value adjustments for multiple comparisons.
CSF Results
Forty-two (30 with aAD and 12 with lvPPA) patients had CSF biomarkers, including p-tau, collected within 1 year of the Cookie Theft recording (mean interval 4.7 ± 3.5 months). This subset did not differ demographically or clinically from the larger group of patients. The aAD and lvPPA subset groups did not differ in age (p = 0.64), sex (p = 0.99), education (p = 0.82), disease duration (p = 0.75), or time difference between CSF sample collection and the Cookie Theft recording (p = 0.43).
Patients' CSF p-tau level correlated with lower preposition counts (r = −0.36, p = 0.019), lower noun counts (r = −0.31, p = 0.047), and higher word frequency (r = 0.33, p = 0.036; Figure 4A). In addition, patients' p-tau levels were correlated with a higher pause rate (r = 0.34, p = 0.026) and shorter mean speech segment durations (r = −0.33, p = 0.032; Figure 4B). The other measures (adverbs, partial words, tense-inflected verbs, articulation rate, lexical diversity, total words, fillers, repetitions, adjectives, percent of speech, word length, concreteness, age at acquisition) were not related to patients' p-tau levels. Aβ alone did not significantly correlate with any of the language measures.
Figure 4. Significant Correlations of the Speech Variables and CSF p-tau Levels (A and B).

aAD = amnestic Alzheimer disease; lvPPA = logopenic variant primary progressive aphasia; P-tau = phosphorylated tau.
Discussion
LvPPA is most frequently associated with underlying AD pathology, but direct comparison of lvPPA with aAD has been reported rarely. Patients with lvPPA have been compared to patients with the other types of PPA, who generally have frontotemporal lobar degeneration pathology.11,12 While it may be clear that patients with lvPPA differ from patients with the other PPA variant due to different pathology, it remains to be seen how patients with nonamnestic lvPPA differ from patients with aAD who have the same pathology. To fill this gap in the literature, the current study focuses on characterizing the language similarities and differences between lvPPA and aAD in a biologically confirmed cohort. To optimize reliability and reproducibility, we used fully automated lexical and acoustic analyses to characterize language and speech markers of AD pathology. We expected that patients with nonamnestic lvPPA would produce more dysfluent speech and have limited vocabulary compared to patients with aAD because of the phenotypic characteristics of lvPPA. Results confirmed that patients with lvPPA produced fewer adjectives and tense-inflected verbs with lower lexical diversity than patients with aAD and HC. Patients with lvPPA also paused more frequently and produced more fillers and partial words than HC and patients with aAD. It is important to note that the patient groups did not differ in brief language-based clinical assessments such as animal and letter fluency tasks or the Craft Story delayed recall test, even though they differed on some of our language and speech measures, highlighting the importance of monitoring the language and speech characteristics of these patients. We also found that both patient groups shared impairments in some language and speech features relative to HC. For example, both patient groups produced more adverbs, including words like “there” and “here,” but fewer prepositions and nouns than HC. In addition, patients' content words were acquired earlier, shorter, more frequent, and less concrete than those of HC. Patients produced more repetitions and fewer total words with a slower articulation rate and a shorter mean speech duration than HC. Some of these variables were significantly related to clinical test scores and p-tau levels in CSF. We discuss these findings below.
The patient groups significantly differed on 6 language and speech measures: pause rate, partial words, fillers, adjectives, tense-inflected verbs, and lexical diversity. Fillers, adjectives, tense-inflected verbs, and lexical diversity were significantly more impaired in patients with lvPPA than those with aAD, emphasizing the deficits in lexical retrieval and poor fluency in these patients. However, these language and speech features distinguishing between lvPPA and aAD were not related to any of the clinical ratings that we examined, including MMSE, BNT, the Rey complex figure, the Craft Story delayed recall, and forward digit span. It is important to note that the mechanism thought to subserve retrieval of a single word in response to a stimulus picture or recall of episodic memory appears to differ from lexical retrieval during natural connected speech.40 It is thus critical to monitor these speech features because they are not easily explained by more general and commonly used clinical measures.
The result that tense-inflected verb production differed between lvPPA and aAD has rarely been reported. This finding seems to suggest that patients with lvPPA produced fewer complete sentences, assuming that there was at least 1 tense-inflected verb per inflection phrase. This would be consistent with the observation that patients with lvPPA who had limited auditory-verbal short-term memory produced briefer sentences.3,4 Frequent fillers in lvPPA were in line with previous observations and consistent with their limited lexical retrieval.12,13,18 Lower adjective counts in patients with aAD compared to HC have been previously reported,41 yet we further showed that adjective production was more impaired in nonamnestic lvPPA than in aAD. Lexical diversity has frequently been examined in the AD literature,15,16,42 and previous studies have found that the lexical diversity of patients with aAD was lower than that of HC. We showed that lexical diversity was even lower in lvPPA than in aAD. The fact that patients with lvPPA and aAD significantly differed on these measures suggests that our language and speech variables may capture subtle but unique phenotypic differences between lvPPA and aAD. In addition, traditional clinical ratings are relatively insensitive to these linguistic features. In addition, none of the 6 variables except pause rate correlated with CSF p-tau. This may suggest that these markers are related more narrowly to the phenotype. Further studies, including the anatomic distribution of pathology in an autopsy cohort with quantitative measures of pathologic burden, may help shed light on this issue.
Some language and speech measures were related to MMSE and BNT scores but not to the other clinical ratings, including the Craft Story delayed recall, Rey figure copy and delayed recall, and forward digit span. Because the Rey figure copy is not worse in lvPPA than aAD, a visual-perceptual deficit leading to difficulty perceiving the stimulus picture is unlikely to account for the observed distinct speech and language deficits in lvPPA. Pause rate showed more impairment in lvPPA than aAD. This might indicate word-finding difficulty in lvPPA that could provoke frequent pausing to recall an appropriate word from the lexicon. It could also be that patients with lvPPA spoke slowly—these patients' articulation rate was lower than that of HC—due to their difficulty in retrieving words to generate utterances. Pause rate was significantly related to both MMSE score, an indicator of general cognitive impairment, and BNT score, a measure of confrontation naming. Elevated pause rate therefore may reflect in part both patients' word-finding difficulties and their general cognitive impairments. Partial word count, which was impaired only in lvPPA, correlated only with MMSE score but not BNT score, suggesting that it reflected in part disease severity and general cognitive impairments of patients with lvPPA but not impaired object naming.
Word-finding difficulty in aAD and lvPPA has previously been noted13,43-47; studies have shown that patients had impairments in auditory-verbal short-term memory and could not recall the phonologic form of a word. However, comparative studies have not been reported to examine whether both patients with aAD and patients with nonamnestic lvPPA would show word-finding difficulty to a similar degree during natural speech. In our study, both patient groups produced content words that were more abstract, acquired earlier, more frequent and shorter than those of HC, suggesting that they had difficulties in retrieving the full spectrum of lexical items needed to describe the picture. Word frequency and length were significantly related to both MMSE and BNT scores, which suggests that these lexical measures reflect in part patients' disease severity and difficulties in lexical retrieval during confrontation naming. On the other hand, concreteness and age at acquisition were significantly associated only with BNT score, indicating that these may be more sensitive to word-finding difficulty in patients. Patients with lvPPA and aAD did not significantly differ on these measures, confirming that some degree of word-finding difficulty is present in both aAD and nonamnestic lvPPA.
Adverb counts were greater in patients compared to HC. This may be related to patients frequently using proadverbs, including “here” and “there,” which replaced locational prepositional phrases. Patients typically produced utterances like “Mom is standing here,” for example, when HC produced “Mom is standing in front of the sink.” Elevated adverb counts were associated with low BNT scores, suggesting that greater adverb use reflected patients' difficulties in naming specific locations during natural speech. Patients' difficulty in producing locational phrases was also partly reflected in the decreased preposition counts compared to HC, which was also related to BNT scores. On the contrary, patients' pronoun counts did not differ from those of HC; thus, increased adverbs and decreased prepositions seem to support the inference that patients had relatively more difficulty naming locations than naming objects. Additional work is needed to determine whether these language markers are related to temporal propositions and other features in connected speech.
Some of our language and speech variables correlated with CSF p-tau levels but not with Aβ. This finding is in line with previous findings that patients' cognitive impairment is generally not related to Aβ levels but to accumulation of p-tau.48-50 Speech production is one of the most essential daily functions of humans, which needs to be taken into consideration in AD clinical trials and may serve in monitoring response to treatment. Because our automated procedures for collecting speech features are highly reliable and reproducible, investigations of speech variables as secondary outcome measures should be considered in disease-modifying trials targeting tau.
It is a strength of our study that we directly compared language and speech features in patients with aAD and lvPPA with biological evidence of underlying AD pathology. We examined natural connected speech quantitatively using automated analyses of digitized speech samples. We inspected differences and similarities among these groups and showed that our variables could capture subtle linguistic differences between the 2 phenotypes, and traditional cognitive measures appear to be insensitive to some of the features that distinguish aAD and lvPPA. These methods may be useful in monitoring disease progression and response to therapeutic interventions because collecting 1-minute speech samples is easy, highly reproducible, and inexpensive and can be done remotely compared to collection of other biomarkers. Our ongoing projects are currently testing the value of these language and speech features in longitudinal datasets and developing machine learning classifiers for distinguishing patients with AD pathology from those with other types of neurodegenerative changes such as frontotemporal lobar degeneration.
Limitations of this study include the assessment of relatively small samples, the difficulty obtaining train-test generalizability data in rare lvPPA and early-onset aAD cases such as these, the use of a single stimulus picture to elicit the speech sample, and the absence of high-resolution MRI data to assess the anatomic associates of these linguistic features. In addition, we had only 12 patients with lvPPA with CSF biomarkers within 1 year of the picture description data collection, so we were not able to examine the relations between CSF biomarkers and the language/speech variables for each group. Future study with more CSF data will be needed to explore each group's relations between language/speech measures and CSF biomarkers. Last, because we had only 1 HC with CSF data, we were not able to determine whether our language and speech measures are able to distinguish HC with positive CSF AD biomarkers from those with negative CSF AD biomarkers. The relation between CSF biomarkers and language/speech variables in HC will need to be studied further in future research.
We implemented automated methods to analyze acoustic and lexical characteristics of the natural speech of patients with aAD and lvPPA. We identified language and speech markers that differed between the groups. We also found language and speech markers that were shared between these 2 AD phenotypes. This work demonstrates the potential of natural speech to reflect underlying AD pathology while distinguishing between specific phenotypes with the same pathology. Considering the cost-effectiveness and reliability of speech data, such markers could contribute to monitoring of patients for AD clinical trials in a more precise and inclusive way.
Acknowledgment
The authors thank the patients and caregivers who participated in this study.
Glossary
- aAD
amnestic AD
- Aβ
β-amyloid42
- AD
Alzheimer disease
- BNT
Boston Naming Test
- lvPPA
logopenic variant PPA
- p-tau
phosphorylated tau
- POS
part-of-speech
- PPA
primary progressive aphasia
Appendix. Authors

Footnotes
Editorial, page 137
Study Funding
This study was funded by grants from the NIH (AG066597, AG054519, NS109260, P30 AG072979, AG073510-01), Alzheimer's Association (AACSF-18-567131, AARF-D-619473, AARF-D-619473-RAPID, AARF-21-851126), and the Department of Defense (W81XWH-20-1-0531).
Disclosure
M. Grossman participates in clinical trials sponsored by Alector, Eisai, and Biogen that are unrelated to this study. He also receives research support from Biogen and Avid that is unrelated to this study and research support from NIH. M. Liberman serves on the Scientific Advisory Board for Baidu Research, USA, and is a coeditor of the Annual Review of Linguistics. All other authors (S. Cho, K.A.Q. Cousins, S. Shellikeri, S. Ash, N. Nevler, D.J. Irwin) report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Alzheimer's Association. 2020 Alzheimer's Disease Facts and Figures. 2020. Accessed May 9, 2022. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf [Google Scholar]
- 2.Nicholas M, Obler L, Albert M, Goodglass H. Lexical retrieval in healthy aging. Cortex. 1985;21(4):595-606. doi: 10.1016/S0010-9452(58)80007-6 [DOI] [PubMed] [Google Scholar]
- 3.Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol. 2010;23(6):633-637. doi: 10.1097/WCO.0b013e32833fb93e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiol Aging. 2012;33(4):744-752. doi: 10.1016/j.neurobiolaging.2010.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gefen T, Gasho K, Rademaker A, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135(5):1554-1565. doi: 10.1093/brain/aws076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyton CE, Britton AK, Hodges JR, Halliday GM, Kril JJ. Distinctive pathological mechanisms involved in primary progressive aphasias. Neurobiol Aging. 2016;38:82-92. doi: 10.1016/j.neurobiolaging.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 9.Giannini LAA, Irwin DJ, Mcmillan CT, et al. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology. 2017;88(24):2276-2284. doi: 10.1212/WNL.0000000000004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann Neurol. 2018;84(5):729-740. doi: 10.1002/ana.25333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nevler N, Ash S, Irwin DJ, Liberman M, Grossman M. Validated automatic speech biomarkers in primary progressive aphasia. Ann Clin Transl Neurol. 2019;6(1):4-14. doi: 10.1002/acn3.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ash S, Evans E, O'Shea J, et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013;81(4):329-336. doi: 10.1212/WNL.0b013e31829c5d0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069-2088. doi: 10.1093/brain/awq129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JCS, Jiang J, Bond RL, et al. Impaired phonemic discrimination in logopenic variant primary progressive aphasia. Ann Clin Transl Neurol. 2020:1-6. doi: 10.1002/acn3.51101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser K, Meltzer JA, Rudzicz F. Linguistic features identify Alzheimer's disease in narrative speech. J Alzheimers Dis. 2016;49(2):407-422. doi: 10.3233/JAD-150520 [DOI] [PubMed] [Google Scholar]
- 16.Bucks RS, Singh S, Cuerden JM, Wilcock GK. Analysis of spontaneous, conversational speech in dementia of Alzheimer type: evaluation of an objective technique for analysing lexical performance. Aphasiology. 2000;14(1):71-91. doi: 10.1080/026870300401603 [DOI] [Google Scholar]
- 17.Rentoumi V, Paliouras G, Danasi E, et al. Automatic detection of linguistic indicators as a means of early detection of Alzheimer's disease and of related dementias: a computational linguistics analysis. In: 8th IEEE International Conference on Cognitive Infocommunications. 2017;2017):33-38. doi: 10.1109/CogInfoCom.2017.8268212 [DOI]
- 18.Gorno-Tempini ML, Hillis A, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32(4):351-358. doi: 10.1097/WAD.0000000000000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lezak M, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press; 1983. [Google Scholar]
- 22.Irwin D, McMillan CT, Toledo JB, et al. Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Arch Neurol. 2012;69(8):1018-1025. doi: 10.1001/archneurol.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lleó A, Irwin DJ, Illán-Gala I, et al. A 2-step cerebrospinal algorithm for the selection of frontotemporal lobar degeneration subtypes. JAMA Neurol. 2018;75(6):738-745. doi: 10.1001/jamaneurol.2018.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cousins KAQ, Irwin DJ, Wolk DA, et al. ATN status in amnestic and non-amnestic Alzheimer's disease and frontotemporal lobar degeneration. Brain. 2020;143(7):2295-2311. doi: 10.1093/brain/awaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodglass H, Kaplan E, Weintraub S. Boston Diagnostic Aphasia Examination. Lea & Febiger; 1983. [Google Scholar]
- 27.Honnibal M, Johnson M. An improved non-monotonic transition system for dependency parsing. In: EMNLP 2015: Conference on Empirical Methods in Natural Language Processing; 2015:1373-1378. doi: 10.18653/v1/d15-1162 [DOI]
- 28.Brysbaert M, Warriner AB, Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Behav Res Methods. 2014;46(3):904-911. doi: 10.3758/s13428-013-0403-5 [DOI] [PubMed] [Google Scholar]
- 29.Hoffman P, Lambon Ralph MA, Rogers TT. Semantic diversity: a measure of semantic ambiguity based on variability in the contextual usage of words. Behav Res Methods. 2013;45(3):718-730. doi: 10.3758/s13428-012-0278-x [DOI] [PubMed] [Google Scholar]
- 30.Brysbaert M, New B. Moving beyond Kučera and Francis: a critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav Res Methods. 2009;41(4):977-990. doi: 10.3758/BRM.41.4.977 [DOI] [PubMed] [Google Scholar]
- 31.Brysbaert M, Mandera P, Keuleers E. Word prevalence norms for 62,000 English lemmas. Behav Res Methods. 2019;51(2):467-479. [DOI] [PubMed] [Google Scholar]
- 32.Carnegie Mellon Speech Group. The Carnegie Mellon University Pronouncing Dictionary. 2014. Accessed November 16, 2019. speech.cs.cmu.edu/cgi-bin/cmudict. [Google Scholar]
- 33.Loper E, Bird S. NLTK: the Natural Language Toolkit. In: ETMTNLP ’02: Proceedings of the ACL-02 Workshop on Effective Tools and Methodologies for Teaching Natural Language Processing and Computational Linguistics. Philadelphia, PA: Association for Computational Linguistics. 2002;63-70. [Google Scholar]
- 34.Covington MA, McFall JD. Cutting the gordian knot: the moving average type-token ratio (MATTR). J Quant Linguist. 2010;17(2):94-100. [Google Scholar]
- 35.Fergadiotis G, Wright HH, Green SB. Psychometric evaluation of lexical diversity indices: assessing length effects. J Speech Lang Hear Res. 2015;58:840-852. doi: 10.1044/2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho S, Nevler N, Ash S, et al. Automated analysis of lexical features in frontotemporal degeneration. Cortex. 2021;137:215-231. doi: 10.1016/j.cortex.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boersma P, Weenink D. Praat: Doing Phonetics by Computer. 2019. Accessed December 3, 2021. https://www.fon.hum.uva.nl/praat/ [Google Scholar]
- 38.Nevler N, Ash S, Jester C, Irwin DJ, Liberman M, Grossman M. Automatic measurement of prosody in behavioral variant FTD. Neurology. 2017;89(7):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin DJ, Lleó A, Xie SX, et al. Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Ann Neurol. 2017;82(2):247-258. doi: 10.1002/ana.24996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levelt WJM. Speaking: From Intention to Articulation. MIT Press; 1989. [Google Scholar]
- 41.Croisile B, Ska B, Brabant MJ, et al. Comparative study of oral and written picture description in patients with Alzheimer's disease. Brain Lang. 1996;53(1):1-19. doi: 10.1006/brln.1996.0033 [DOI] [PubMed] [Google Scholar]
- 42.Kavé G, Goral M. Word retrieval in picture descriptions produced by individuals with Alzheimer's disease. J Clin Exp Neuropsychol. 2016;38(9):958-966. doi: 10.1080/13803395.2016.1179266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buxbaum LJ, Schwartz MF, Carew TG. The role of semantic memory in object use. Cogn Neuropsychol. 1997;14(2):219-254. doi: 10.1080/026432997381565 [DOI] [Google Scholar]
- 44.Ochipa C, Rothi LJG, Heilman KM. Conceptual apraxia in Alzheimer's disease. Brain. 1992;115(4):1061-1071. doi: 10.1093/brain/115.4.1061 [DOI] [PubMed] [Google Scholar]
- 45.Adlam ALR, Bozeat S, Arnold R, Watson P, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer's disease. Cortex. 2006;42(5):675-684. doi: 10.1016/S0010-9452(08)70404-0 [DOI] [PubMed] [Google Scholar]
- 46.Giffard B, Desgranges B, Nore-Mary F, et al. The nature of semantic memory deficits in Alzheimer's disease: new insights from hyperpriming effects. Brain. 2001;124(8):1522-1532. doi: 10.1093/brain/124.8.1522 [DOI] [PubMed] [Google Scholar]
- 47.Venneri A, McGeown WJ, Hietanen HM, Guerrini C, Ellis AW, Shanks MF. The anatomical bases of semantic retrieval deficits in early Alzheimer's disease. Neuropsychologia. 2008;46(2):497-510. doi: 10.1016/j.neuropsychologia.2007.08.026 [DOI] [PubMed] [Google Scholar]
- 48.Huber CM, Yee C, May T, Dhanala A, Mitchell CS. Cognitive decline in preclinical Alzheimer's disease: amyloid-beta versus tauopathy. J Alzheimers Dis. 2018;61(1):265-281. doi: 10.3233/JAD-170490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13(1):179-189. doi: 10.1016/0197-4580(92)90027-U [DOI] [PubMed] [Google Scholar]
- 50.Engelborghs S, Maertens K, Vloeberghs E, et al. Neuropsychological and behavioural correlates of CSF biomarkers in dementia. Neurochem Int. 2006;48(4):286-295. doi: 10.1016/j.neuint.2005.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified investigator for purposes of validation or replication of study methods.



