Abstract
Background and Objectives
International evidence shows that patients treated at nonurban hospitals experience poorer access to key stroke interventions. Evidence for whether this results in poorer outcomes is conflicting and generally based on administrative or voluntary registry data. The aim of this study was to use prospective high-quality comprehensive nationwide patient-level data to investigate the association between hospital geography and outcomes of patients with stroke and access to best-practice stroke care in New Zealand.
Methods
This is a prospective, multicenter, nationally representative observational study involving all 28 New Zealand acute stroke hospitals (18 nonurban) and affiliated rehabilitation and community services. Consecutive adults admitted to the hospital with acute stroke between May 1 and October 31, 2018, were captured. Outcomes included functional outcome (modified Rankin Scale [mRS] score shift analysis), functional independence (mRS score 0–2), quality of life (EuroQol 5-dimension, 3-level health-related quality of life questionnaire), stroke/vascular events, and death at 3, 6, and 12 months and proportion accessing thrombolysis, thrombectomy, stroke units, key investigations, secondary prevention, and inpatient/community rehabilitation. Results were adjusted for age, sex, ethnicity, stroke severity/type, comorbid conditions, baseline function, and differences in baseline characteristics.
Results
Overall, 2,379 patients were eligible (mean [SD] age 75 [13.7] years; 51.2% male; 1,430 urban, 949 nonurban). Patients treated at nonurban hospitals were more likely to score in a higher mRS score category (greater disability) at 3 (adjusted odds ratio [aOR] 1.28, 95% CI 1.07–1.53), 6 (aOR 1.33, 95% CI 1.07–1.65), and 12 (aOR 1.31, 95% CI 1.06–1.62) months and were more likely to have died (aOR 1.57, 95% CI 1.17–2.12) or experienced recurrent stroke and vascular events at 12 months (aOR 1.94, 95% CI 1.14–3.29 and aOR 1.65, 95% CI 1.09–2.52). Fewer nonurban patients received recommended stroke interventions, including endovascular thrombectomy (aOR 0.25, 95% CI 0.13–0.49), acute stroke unit care (aOR 0.60, 95% CI 0.49–0.73), antiplatelet prescriptions (aOR 0.72, 95% CI 0.58–0.88), ≥60 minutes of daily physical therapy (aOR 0.55, 95% CI 0.40–0.77), and community rehabilitation (aOR 0.69, 95% CI 0.56–0.84).
Discussion
Patients managed at nonurban hospitals experience poorer stroke outcomes and reduced access to key stroke interventions across the entire care continuum. Efforts to improve access to high quality stroke care in nonurban hospitals should be a priority.
Globally, stroke is a leading cause of death and disability.1 Organized stroke care in both the acute and rehabilitation settings has been shown to result in a greater likelihood of patient survival, independence, and return to home.2
New Zealand has a population of 4.9 million people dispersed over an area of 268,021 km, ranking 126th in population density globally. Health care is provided predominantly via a publicly funded single-payer universal coverage system with a small private sector offering elective surgeries and ambulatory specialist consultations. As a result, all acute stroke services are offered via the government-funded public health sector at 28 acute hospitals. Copayments are required for general practitioner visits, most ambulance services, and medications (NZ $5 per prescription). Public hospital services, including postdischarge community rehabilitation and specialist follow-up, are free of charge without copayments. Funding is allocated according to the population served by each of 20 health districts. Additional subsidies are provided to rural hospitals with low population density and where there are higher proportions of older people or people of indigenous Māori ethnicity. Last, there is a tertiary adjuster allocated to centers providing high-cost tertiary services.
Departures from best-practice stroke care in smaller, nonurban New Zealand hospitals have been accepted when a small population is dispersed over a wide geographic area.3 For example, these hospitals have not been required to have a geographically designated stroke unit or stroke-specific rehabilitation service, with stroke care provided either by general teams or by non–ward-based mobile stroke teams that generally comprise a physician, a specialty nurse, and often non–stroke team–specific allied health clinicians. Within these settings, patients are often treated by clinicians without specific training in stroke care. It is unclear to what degree, if any, these compromises affect patient outcomes.
In recent years, authors of New Zealand stroke service surveys have found that significant regional variations in the implementation of best-practice care continue to exist, despite significant work that has gone into implementing best-practice stroke care.4,5 International research reports that patients admitted to nonurban hospitals have poorer access to key stroke interventions compared to those admitted to urban hospitals,6,7 although there is conflicting evidence regarding the impact of hospital geographic location on patient outcomes.6,8,9 The aim of this study was to determine the extent of stroke care access inequities and the degree to which this affects patient outcomes in New Zealand.
Methods
Reducing Ethnic and Geographic Inequities to Optimise New Zealand Stroke Care (REGIONS Care) is a multipart nationwide prospective observational study designed to assess the impact of geography and ethnicity on stroke outcomes and access to best-practice care. It involves a comprehensive nationwide stroke dataset with a subset of patients recruited to undergo extended follow-up, data linkage with health administrative data, focus groups, and surveys. Here, we report the results of the analysis based on geographic location (urban or nonurban). Full study methods, including prospective sample size calculations and analysis plan, have been described elsewhere.10
Study Sample
This study involved all 28 New Zealand hospitals and associated rehabilitation and community services caring for patients with acute stroke. All adult patients admitted to hospital between May 1 and July 31, 2018, with a discharge diagnosis of stroke were captured. After this date, we continued consecutive patient recruitment until hospitals achieved a minimum sample size of 150 (thrombectomy centers) or 100 (all other centers) or until October 31, 2018, whichever occurred first. We grouped hospitals into urban and nonurban centers, defining urban as any hospital located within a 30-minute drive (<25 km) of an urban area comprising a population of >100,000 people. The study was powered to detect a 10% difference in favorable outcome at 90% power with α = 0.05 between groups up to 12 months.
Patients with TIA or other nonstroke diagnoses, including thrombolysed stroke mimics, and people <18 years of age were excluded. For any given individual patient, only the initial admission during the study period was counted as an index event; any subsequent admissions were considered outcome events.
Data Collection
Baseline data included patient demographics, vascular risk factors, premorbid level of function, employment status, domiciliary information, disability at hospital admission, arrival mode, arrival time from symptom onset, and stroke characteristics. Ethnicity was determined by self-identification. Postadmission data included in-hospital interventions and services, investigations, and therapies up to 3 months after admission, follow-up appointments up to 12 months, and outcome variables as described below. All patients were invited at 3 months to consent to further follow-up assessments at 6 and 12 months until a preset center sample size target was reached.
Outcomes
Main outcome measure was modified Rankin Scale (mRS) score at 3 months using an ordinal shift analysis.10 Additional poststroke outcomes assessed were mRS score shift analysis at 6 and 12 months; dichotomized mRS score into favorable outcome (mRS score 0–2) and unfavorable outcome (mRS score 3–6); EuroQol 5-dimension, 3-level health-related quality of life questionnaire (EQ-5D-3L) scores; stroke recurrence; vascular events; readmission; and death at 3, 6, and 12 months.
Stroke care access measures were stroke thrombolysis and endovascular thrombectomy, including associated time delays both before hospital admission and in hospital, acute stroke unit care, timely assessment by key members of an interdisciplinary stroke team, relevant investigations to determine stroke etiology, early mobilization within 48 hours, swallow assessment within 6 and 24 hours, guideline-based deep vein thrombosis prophylaxis, early prescription of antithrombotics, prescription of best medical management (tailored to stroke diagnosis and cause) by time of discharge, timely access to a therapist, therapist contact time during inpatient, and community rehabilitation.
Data Analysis
All data were analyzed in Stata/IC 16.0 (StataCorp, College Station, TX). We used descriptive statistics to summarize patient baseline characteristics using proportions for dichotomous, means, and SDs for continuous variables and medians and interquartile ranges for nonnormally distributed continuous variables. We used the Pearson χ2 test to compare dichotomous, t test for normally distributed continuous, and Wilcoxon rank-sum test for nonnormally distributed continuous baseline variables between hospital locations. Logistic regression, ordinal logistic regression (including mRS score shift analysis), and linear regression were used to assess associations between hospital geographic location and dichotomous, ordinal, and continuous outcomes, respectively, checking for normal distribution of the residuals. We initially conducted univariable analyses for all outcome variables. Multivariable models were controlled for known confounders, including age, ethnicity, stroke severity, stroke type, and premorbid level of function. Baseline characteristics that differed between groups by p < 0.1 were also included in the multivariable models. For specific service access outcomes, we included additional variables known to affect intervention access that are outside the control of the hospital service such as hospital arrival time and mode when considering reperfusion therapy access. We then backward eliminated covariates starting with differences in baseline characteristics, followed by specific covariates added for the model in question, and finishing with known confounders. We eliminated covariates only if the effect on the odds ratio was <0.1 and model fit was either improved or unaffected. In general, we aimed for the lowest number of covariates and best model fit without removing covariates that significantly affected the overall result. We checked for interaction effects between hospital location and ethnicity.
Standard Protocol Approvals, Registrations, and Patient Consents
The Health Research Council of New Zealand (HRC 17/037) funded this study. The study received ethics approval from the Central Region Health and Disability Ethics Committee (17CEN164). Routine clinical patient data collection up to 3 months after discharge was classified as a clinical audit, and the ethics committee waived the need for individual patient consent. We consented patients at their routine 3-month follow-up for subsequent follow-up because this was outside of usual care.
Data Availability
Deidentified individual participant data, data dictionary, protocol, and consent forms can be requested via the corresponding author and will be available once all results from the study have been published, assuming that appropriate ethics approval is obtained.
Results
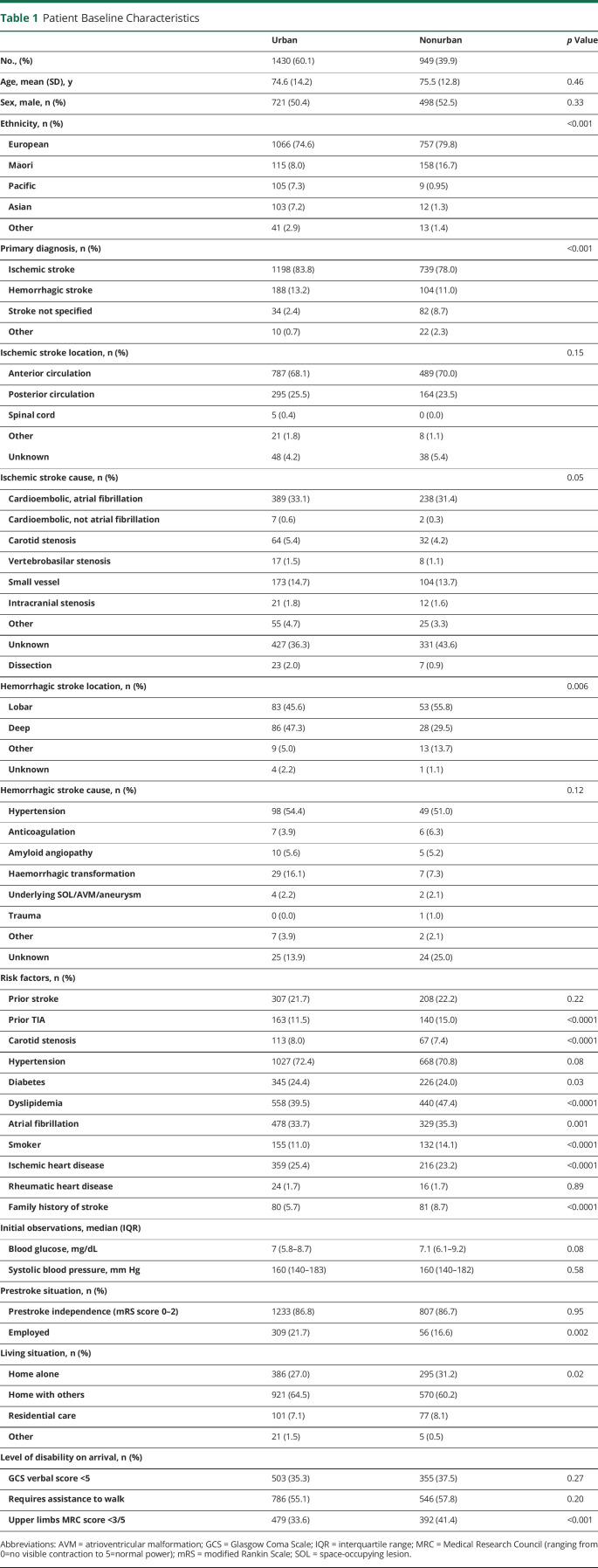
Overall, 2,379 consecutive eligible patients (mean [SD] age 75 [13.7] years; 51.2% male; 76.6% European; 11.5% Māori; 4.8% Pacific; 81.5% ischemic stroke and 12.3% hemorrhagic stroke) were included during the study period: 1,430 (60.1%) presented to an urban hospital and 949 (39.9%) to a nonurban hospital (Table 1).
Table 1.
Patient Baseline Characteristics
Impact of Hospital Location on Outcomes
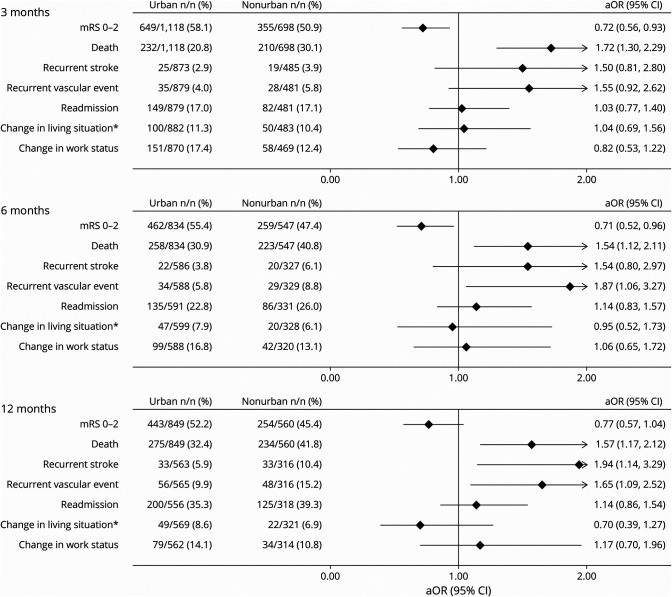
Patients treated at nonurban hospitals were more likely to score in a higher mRS (greater disability) score category at 3 (adjusted odds ratio [aOR] 1.28, 95% CI 1.07–1.53), 6 (aOR 1.33, 95% CI 1.07–1.65), and 12 (aOR 1.31, 95% CI 1.06–1.62) months (Figure 1) and had lower odds of a dichotomized favorable outcome (mRS score 0–2) at 3 months (aOR 0.72, 95% CI 0.56–0.93) and 6 months (aOR 0.71, 95% CI 0.52–0.96). At 12 months, the difference did not quite reach statistical significance (aOR 0.77, 95% CI 0.57–1.04) (Figure 2).
Figure 1. mRS Score Shift Analysis at 3, 6, and 12 months.
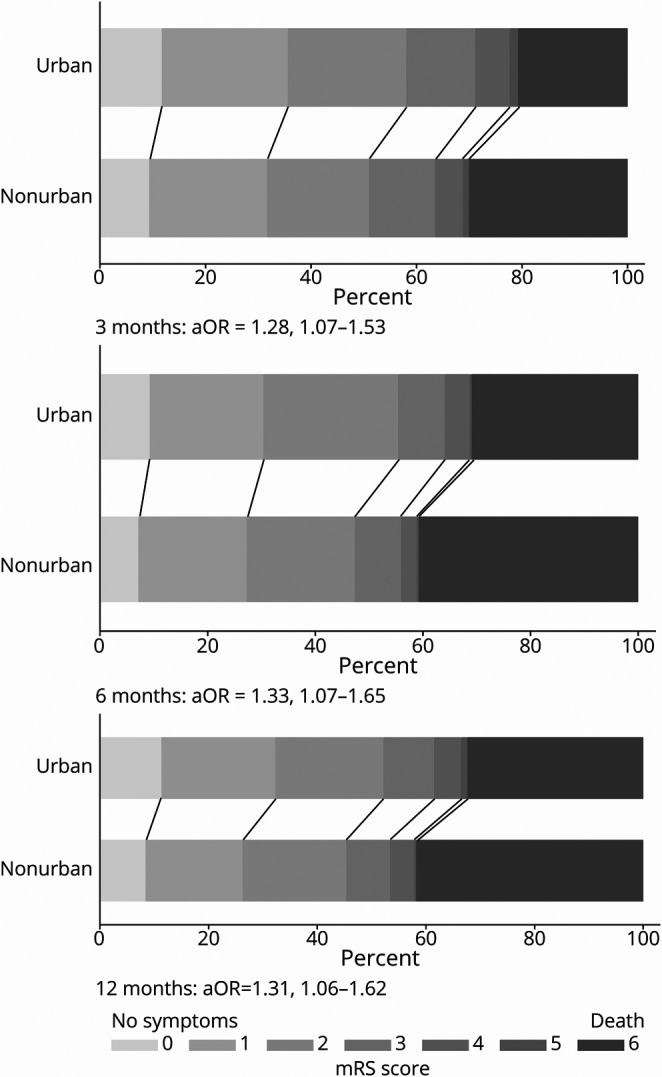
aOR = adjusted odds ratio; mRS = modified Rankin Scale. All outcomes were adjusted for premorbid level independence, age, sex, ethnicity, stroke severity, and baseline characteristic differences of p < 0.1
Figure 2. Urban Versus Nonurban Comprehensive Stroke Outcomes.
aOR = adjusted odds ratio; mRS = modified Rankin Scale. All outcomes were adjusted for premorbid level independence, age, sex, ethnicity, stroke severity, and baseline characteristic differences of p < 0.1. Covariates were backward eliminated if removal did not substantially affect the odds ratio, aiming to minimize number of covariates and to optimize model fit. *Change in living situation refers to a new move to a care facility, move from independent living to the home of a family member or other caregiver, or move by a family member or caregiver into the patient's home to provide care.
Patients in nonurban hospitals had greater odds of recurrent vascular events at 6 months (aOR 1.87, 95% CI 1.06–3.27) and 12 months (aOR 1.65, 95% CI 1.09–2.52) (Figure 2) and higher odds of recurrent stroke at 12 months (aOR 1.94, 95% CI 1.14–3.29). Patients treated at nonurban hospitals also had higher odds of death at all 3 time points; however, there was no significant difference in hospital readmission at any of the 3 time points.
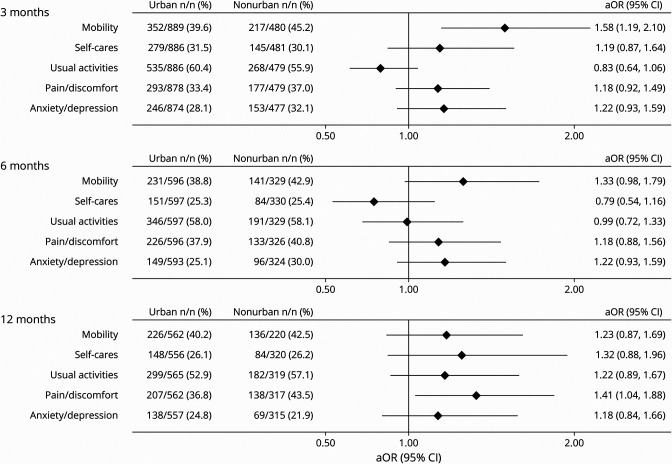
For quality of life, measured with the EQ-5D-3L, patients treated at nonurban hospitals had a greater odds of reporting difficulties with their mobility at 3 months (aOR 1.58, 95% CI 1.19–2.10) and pain at 12 months (aOR 1.41, 95% CI 1.04–1.88) (Figure 3). The EuroQuol Vertical Visual Analogue Scale score was significantly lower for patients treated at nonurban hospitals at 3 months (−3.21, 95% CI −5.85 to −0.56), and the summary index scores including deceased patients were significantly lower at all 3 time points for patients treated at nonurban hospitals (3 months: −0.05, 95% CI −0.08 to −0.02, p < 0.0001; 6 months: −0.05, 95% CI −0.08 to −0.02, p = 0.003; 12 months: −0.04, 95% CI −0.08 to −0.01, p = 0.01).
Figure 3. Urban vs Nonurban Quality of Life (EQ-5D-3L): Reporting Any Problems.
aOR = adjusted odds ratio; EQ-5D-3L = EuroQol 5-dimension, 3-level health-related quality of life questionnaire. All outcomes were adjusted for premorbid level independence, age, ethnicity, stroke severity, and baseline characteristic differences of p < 0.1. Covariates were backward eliminated if removal did not substantially affect the odds ratio, aiming to minimize number of covariates and to optimize model fit).
Impact of Hospital Location on Access to Clinical Guideline-Recommended Stroke Interventions
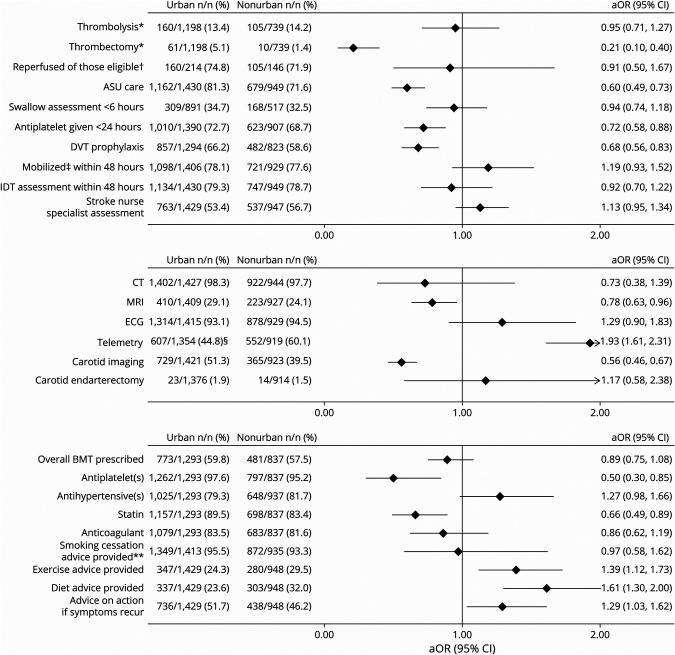
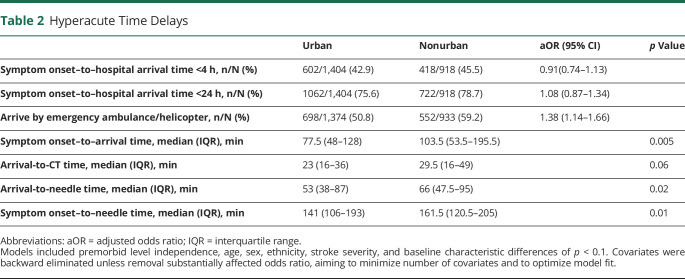
There was no difference in patients with ischemic stroke treated with IV thrombolysis between nonurban hospitals (14.2%) and urban hospitals (13.4%) (Figure 4). In contrast, access to endovascular thrombectomy at nonurban hospitals was lower (aOR 0.25, 95% CI 0.13–0.49). Median onset-to-door time, door-to-needle time, and onset-to-needle time were all significantly longer at nonurban hospitals (Table 2).
Figure 4. Urban vs Nonurban Access to Stroke Interventions/Car.
All outcomes were adjusted for premorbid level of independence, age, sex, ethnicity, stroke severity, baseline characteristic differences of p < 0.1, and intervention-specific covariates such as time delay to reach hospital, mode of transport for reperfusion therapies, and palliation within 24 hours for early mobilization and allied health input. Covariates were backward eliminated unless removal substantially affected odds ratio, aiming to minimize number of covariates and to optimize model fit. Stroke nurse specialist assessment refers to a stroke clinical nurse specialist review of the patient on the ward while an inpatient. Best medical therapy (BMT) refers to antiplatelet(s), statins, and antihypertensives for patients with noncardioembolic ischemic stroke, antihypertensives for patients with intracerebral hemorrhage attributed to hypertension, and anticoagulation for patients with cardioembolic stroke unless any contraindications were documented. aOR = adjusted odds ratio; ASU = acute stroke unit; DVT = deep vein thrombosis; IDT = interdisciplinary team; IVT = IV thrombolysis. *Denominator for these analyses consists of only those patients with a primary diagnosis of ischemic stroke. †Reperfused of those eligible refers to patients undergoing thrombolysis and/or thrombectomy among those who presented within the require time window and did not have appropriate exclusion criteria; ‡Mobilized refers to any activity out of bed. **Analysis was limited to current smokers at the time of presentation. §The higher rate of telemetry at nonurban hospitals is likely related to the preference of one of the largest New Zealand tertiary centers to perform serial 12-lead ECGs for atrial fibrillation detection.
Table 2.
Hyperacute Time Delays
Other differences in nonurban hospitals included reduced care provided in an acute stroke unit (aOR 0.60, 95% CI 0.49–0.73), provision of antiplatelet medications within 24 hours of admission (aOR 0.72, 95% CI 0.58–0.88), appropriate deep vein thrombosis prophylaxis (aOR 0.68, 95% CI 0.56–0.83), provision of antiplatelet medications (aOR 0.50, 95% CI 0.30–0.85), and provision of statins (aOR 0.66, 95% CI 0.49–0.89) as secondary vascular prevention before discharge (Figure 4).
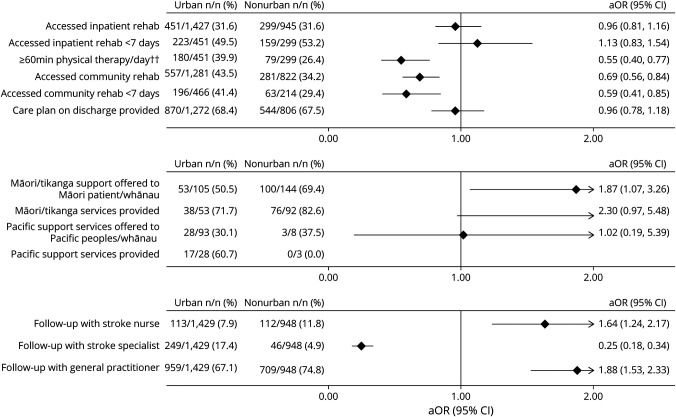
Compared with those presenting to urban centers, patients treated at nonurban hospitals were less likely to access a minimum of 1 hour of physical therapy per working day during inpatient rehabilitation (aOR 0.55, 95% CI 0.40–0.77), although access was suboptimal in both groups (26.4% and 39.9%, respectively). Patients treated at nonurban hospitals were also less likely to access community rehabilitation after hospital discharge (aOR 0.69, 95% CI 0.56–0.84) and less likely to access it within 7 days of discharge (aOR 0.59, 95% CI 0.41–0.85) (Figure 5).
Figure 5. Urban vs Nonurban Access to Rehabilitation and Follow-Up Care.
All outcomes were adjusted for premorbid level of independence, age, sex, ethnicity, stroke severity, baseline characteristic differences of p < 0.1, and intervention-specific covariates such as time delay to reach hospital, mode of transport for reperfusion therapies, and palliation within 24 hours for early mobilization and allied health input. Covariates were backward eliminated unless removal substantially affected odds ratio, aiming to minimize number of covariates and to optimize model fit. Follow-up with stroke nurse refers to postdischarge follow-up appointment with a stroke clinical nurse. aOR = adjusted odds ratio; Tikanga = Māori customary practices; whānau = immediate or extended family in Māori language. ††Weekdays only.
The odds of Māori patients being offered cultural support service while in hospital were higher in nonurban hospitals (aOR 1.87, 95% CI 1.07–3.26). There was no difference found for Pacific patients being offered Pacific support services between urban and nonurban hospitals (aOR 1.02, 95% CI 0.19–5.39).
Discussion
This prospective observational study provides new evidence on the relationship between hospital location and stroke care access and outcome using a uniquely comprehensive methodology that captures national consecutive stroke census data spanning care provision from hyperacute care to community care with follow-up to 12 months. We found that patients presenting to nonurban hospitals experience poorer poststroke functional independence at 3, 6, and 12 months, as well as higher stroke and vascular event recurrence and mortality at most time points. Hospital readmission rates were unaffected, and quality of life was less profoundly affected, although the latter is likely due to survival bias because we found higher mortality rates in the nonurban centers compared to urban centers at the time these outcomes were collected. In addition, we found inequitable access to many best-practice stroke care interventions for patients managed in nonurban hospitals.
Exploring differences in service access is important because it offers potential opportunities to focus service improvement efforts to achieve equitable outcomes despite geographic challenges. We found disparities in service access across many aspects of stroke care, some of which have previously been reported by others, while others represent novel findings.6,8,11 In contrast to other studies,6,7,9 we found equal access to IV thrombolysis regardless of geography. A recent study from Australia reported a significant disparity in thrombolysis rates between urban (12.7%) and rural (7.5%) hospitals.9 Equitable access in New Zealand is likely due to advanced telestroke networks and a mandatory national reperfusion register implemented in 2015 with quarterly central reporting.12,13 However, endovascular thrombectomy remains less accessible in nonurban settings. A New Zealand–wide service improvement program has just been approved to address this.14
Two prior large international studies found no difference in prescriptions for secondary vascular prevention medications by geography.8,15 Our study found fewer prescriptions of antiplatelet agents and statins in nonurban hospitals, along with reduced access to acute stroke unit care, deep vein thrombosis prophylaxis, and carotid imaging. These areas present further opportunities to improve patient care to achieve better outcomes. It is important to note that many assessed interventions were equally accessed at nonurban centers, indicating that equity is achievable. Our study provides guidance to stroke teams on where to focus their improvement efforts.
In contrast to previous studies,8,16 we found equal access to inpatient rehabilitation facilities across the country. However, a significant disparity in the amount of therapy patients receive in nonurban hospitals was observed, and the overall intensity of therapy across the country is very low. Research has shown a dose-dependent relationship between therapy and functional recovery after stroke.17 This study has shown that services are currently falling well short of the recommended 2-hour daily physical therapy minimum,18 regardless of geographic location, raising concerns about how much benefit patients are actually receiving from their rehabilitation. The New Zealand National Stroke Network recently published a rehabilitation strategy and action plan to address this issue, and this study provides further guidance directing these efforts to focus on increasing intensity of therapy and access to community rehabilitation services.19,20
Nonurban hospitals performed better than urban hospitals in providing in-hospital cultural support for indigenous Māori patients. In New Zealand and other bicultural and multicultural countries, the provision of culturally responsive stroke care is crucial, involving the integration of cultural practices, values, and concepts into service delivery.3 Māori have identified a lack of cultural concordance as a barrier to accessing health care.21 New Zealand urban hospitals could benefit from understanding how nonurban centers are enhancing access to cultural support and culturally appropriate care for Māori patients. Addressing such barriers is of great importance because Māori, much like many other marginalized indigenous populations around the globe, experience overall worse health outcomes, generally attributed to the consequences of long-standing, entrenched structural inequities linked to a history of colonization.22,23 As part of our wider research program, we have recently reported and discussed in much greater detail the issues faced by Māori people with stroke, which include not only significantly worse stroke outcomes but also poorer access to several key stroke interventions.24 Results of the present study, which focused on geographic inequities, controlled for ethnicity, confirming that ethnicity alone cannot explain the geographic inequities we have identified. However, given that Māori often reside rurally, they will frequently face both ethnic and geographic inequities.
Service improvements to reduce geographic inequities may involve targeted education and resource investment to increase the number of stroke experts in nonurban centers. However, achieving optimal care in small general hospital settings may remain challenging considering potential financial inefficiencies, disincentives, and recruitment challenges. Alternatively, centralization of stroke services could be considered. Such hub-and-spoke models have been used successfully in the United Kingdom, with reduced mortality rates, reduced hospital length of stay, and improved patient access to important stroke interventions.25,26 Some patients, however, may still prefer being treated close to home, especially when the distance to a tertiary center is several hours by car. Qualitative research undertaken as part of this wider study will help provide insights into patient preferences as to whether further centralization of stroke services should be explored. From a health care modeling perspective, keeping patients close to home has the added advantage of ensuring that sufficient case volumes remain in regional hospitals to maintain overall viability of nonurban health care facilities. This may be more important in low-population-density countries such as New Zealand, Australia, and Scotland, where disestablishing smaller community hospitals might mean hours of patient travel times for routine hospital care. In these settings, a virtual centralization through telehealth systems as part of strong regional clinical networks extending into the rehabilitation and community phases of care may offer the best opportunities for widespread improvement. We have demonstrated that such collaborative regional clinical networks, along with mandated nationwide quality initiatives, achieve geographic equity in thrombolysis access.12,13
Limitations of this study include its observational nature and associated risk of potential residual confounding, particularly because some baseline characteristics differed between study groups. The difference in ethnicity by hospital location is not unexpected because nearly 80% of Pacific peoples live in the Auckland and Wellington urban regions27 and a higher proportion of Māori live in nonurban areas compared with the total New Zealand population.28 This may also explain observed differences in stroke risk factors given that previous research has shown that Māori have higher rates of obesity, smoking, hypertension, and diabetes.29-31 However, we did not find a significant interaction effect between hospital location and ethnicity, and all models were adjusted for ethnicity and other potential confounders, including any differences in baseline characteristics. We were unable to capture socioeconomic status in this study. However, the New Zealand health system provides universal free public hospital health coverage; thus, finances are likely less of an access barrier than in other health systems. Last, in light of the multiple comparisons assessing access to various stroke interventions, these findings should be interpreted with a degree of caution and viewed primarily as exploratory, especially when CIs are large or only trends were identified.
Strengths of this study are the inclusion of all New Zealand acute stroke services and consecutive patient recruitment, allowing a census dataset and eliminating selection bias while offering excellent representation from both urban and nonurban hospitals. Data collection across the care continuum, including postdischarge community care and 12-month follow-up, achieved in 91.5%, represents a further key strength. Previous research assessing the effect of hospital location has focused primarily on the acute stroke phase of care and association with early patient outcomes, reporting conflicting results.6,8,32 Looking at the whole continuum of care and outcomes up to 12 months after stroke has allowed us to also explore differences in access to inpatient rehabilitation and community care, which are likely relevant to patients and may affect longer-term outcomes.
Patients treated in nonurban hospitals experience poorer outcomes in terms of functional independence, death, recurrent strokes, and vascular events and have poorer access to best-practice stroke care both in hospital and after discharge. This research highlights specific areas for targeted improvement that will likely need to involve new models of care, in addition to focused resource investment and education. A full health economics analysis to determine additional resource investment requirements and cost-utility is underway.
Glossary
- aOR
adjusted odds ratio
- mRS
modified Rankin Scale
- REGIONS Care
Reducing Ethnic and Geographic Inequities to Optimise New Zealand Stroke Care
Appendix. Authors
Study Funding
Funding provided by the Health Research Council of New Zealand (HRC 17/037 $1.2 mil).
Disclosures
S. Thompson has nothing to disclose. P.A. Barber receives funding from the Neurologic Foundation of New Zealand. J. Gommans has nothing to disclose. D. Cadilhac reports grants from the Health Research Council of New Zealand, National Health and Medical Research Council, Medtronic, Amgen, Stroke Foundation, Academy of Science, Heart Foundation, CSIRO, Victorian Agency for Health Innovation, Boehringer Ingelheim, Melbourne Health, National Institute for Health Research UK, Western Australian government, and South Australian government. A. Davis and J. Fink have nothing to disclose. M. Harwood was an appointed member of Waitemata District Health Board for 2016 to 2019. W. Levack has nothing to disclose and H. McNaughton have nothing to disclose. V. Feigin holds other grants from the Health Research Council. G. Abernethy, J. Girvan, H. Denison, M. Corbin, J. Kim, and A. Wilson have nothing to disclose. J. Douwes holds other Health Research Council grants. A. Ranta holds other grants from the Health Research Council and receives funding from the New Zealand Ministry of Health. Go to Neurology.org/N for full disclosures.
References
- 1.GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439-458. doi: 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stroke Unit Trialists Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;9:CD000197. doi: 10.1002/14651858.CD000197.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroke Foundation, New Zealand Guidelines Group. New Zealand clinical guidelines for stroke management 2010. 2010. Accessed March 22, 2021. health.govt.nz/system/files/documents/publications/nzclinicalguidelinesstrokemanagement2010activecontents.pdf.
- 4.Child N, Fink J, Jones S, Voges K, Vivian M, Barber PA. New Zealand national acute stroke services audit: acute stroke care delivery in New Zealand. N Z Med J. 2011;125(1358):44-51. [PubMed] [Google Scholar]
- 5.McNaughton H, McRae A, Green G, Abernethy G, Gommans J. Stroke rehabilitation services in New Zealand: a survey of service configuration, capacity and guideline adherence. N Z Med J. 2014;127(1402):10-19. [PubMed] [Google Scholar]
- 6.Hammond G, Luke AA, Elson L, Towfighi A, Joynt Maddox KE. Urban-rural inequities in acute stroke care and in-hospital mortality. Stroke. 2020;51(7):2131-2138. doi: 10.1161/STROKEAHA.120.029318 [DOI] [PubMed] [Google Scholar]
- 7.Gonzales S, Mullen MT, Skolarus L, Thibault DP, Udoeyo U, Willis AW. Progressive rural–urban disparity in acute stroke care. Neurology. 2017;88(5):441-448. doi: 10.1212/WNL.0000000000003562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koifman J, Hall R, Li S, et al. The association between rural residence and stroke care and outcomes. J Neurol Sci. 2016;363:16-20. doi: 10.1016/j.jns.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 9.Dwyer M, Francis K, Peterson GM, et al. Regional differences in the care and outcomes of acute stroke patients in Australia: an observational study using evidence from the Australian Stroke Clinical Registry (AuSCR). BMJ Open. 2021:11e040418. doi: 10.1136/bmjopen-2020-040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranta A, Thompson S, Harwood MLN, et al. Reducing Ethnic and Geographic Inequities to Optimise New Zealand Stroke Care (REGIONS Care): protocol for a nationwide observational study. JMIR Res Protoc. 2021;10(1):e25374. doi: 10.2196/25374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phipps MS, Jia H, Chumbler NR, et al. Rural-urban differences in inpatient quality of care in US veterans with ischemic stroke: rural-urban differences in VA inpatient stroke quality. J Rural Health. 2014;30(1):1-6. doi: 10.1111/jrh.12029 [DOI] [PubMed] [Google Scholar]
- 12.Hedlund F, Leighs A, Barber PA, Lundström E, Wu TY, Ranta A. Trends in stroke reperfusion treatment and outcomes in New Zealand. Intern Med J. 2020;50(11):1367-1372. doi: 10.1111/imj.14682 [DOI] [PubMed] [Google Scholar]
- 13.Ranta A, Lanford J, Busch S, et al. Impact and implementation of a sustainable regional telestroke network: telestroke outcome and implementation. Intern Med J. 2017;47(11):1270-1275. doi: 10.1111/imj.13557 [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health. Stroke clot retrieval: a national service improvement programme action plan. 2021. Accessed February 25, 2021. health.govt.nz/publication/stroke-clot-retrieval-national-service-improvement-programme-action-plan.
- 15.Rodriguez D, Cox M, Zimmer LO, et al. Similar secondary stroke prevention and medication persistence rates among rural and urban patients: secondary stroke prevention. J Rural Health. 2011;27(4):401-408. doi: 10.1111/j.1748-0361.2010.00352.x [DOI] [PubMed] [Google Scholar]
- 16.Jia H, Cowper DC, Tang Y, Litt E, Wilson L. Postacute stroke rehabilitation utilization: are there differences between rural-urban patients and taxonomies? Stroke rehabilitation and rural-urban taxonomy. J Rural Health. 2012;28(3):242-247. doi: 10.1111/j.1748-0361.2011.00397.x [DOI] [PubMed] [Google Scholar]
- 17.Foley N, McClure JA, Meyer M, Salter K, Bureau Y, Teasell R. Inpatient rehabilitation following stroke: amount of therapy received and associations with functional recovery. Disabil Rehabil. 2012;34(25):2132-2138. doi: 10.3109/09638288.2012.676145 [DOI] [PubMed] [Google Scholar]
- 18.Stroke Foundation. Clinical Guidelines for Stroke Management 2017–Chapter 5 of 8: Rehabilitation v5.4. 2019. Accessed March 22, 2021. informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management. [Google Scholar]
- 19.Green G, Maddula M. New Zealand stroke rehabilitation: a strategy. 2018. Accessed April 16, 2020. strokenetwork.org.nz/new-zealand-stroke-rehabilitation–a-strategy.
- 20.National Stroke Network (NZ)–Rehabilitation Working Group. Take action for stroke rehabilitation. 2020. Accessed May 26, 2021. cdn-flightdec.userfirst.co.nz/uploads/sites/strokenetwork/files/Rehabilitation_Action_Plan/Stroke_Rehabilitation_Action_Plan_DEC_2020_FINAL.pdf.
- 21.Mauri Ora Associates. He ritenga whakaaro: Māori experiences of health services. 2009. Accessed February 15, 2021. mauriora.co.nz/file/He-Ritenga-Whakaaro.pdf.
- 22.Hobbs M, Ahuriri-Driscoll A, Marek L, Campbell M, Tomintz M, Kingham S. Reducing health inequity for Māori people in New Zealand. Lancet. 2019;394(10209):1613-1614. doi: doi: 10.1016/S0140-6736(19)30044-3 [DOI] [PubMed] [Google Scholar]
- 23.King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. 2009;374(9683):76-85. [DOI] [PubMed] [Google Scholar]
- 24.Thompson SG, Barber AP, Gommans JH, et al. The impact of ethnicity on stroke care access and patient outcomes: a New Zealand nationwide observational study. Lancet Reg Health West Pac. 2022;20:100359. doi: 10.1016/j.lanwpc.2021.100359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris S, Hunter RM, Ramsay AIG, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ. 2014;349:g4757. doi: 10.1136/bmj.g4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris S, Ramsay AIG, Boaden RJ, et al. Impact and sustainability of centralising acute stroke services in English metropolitan areas: retrospective analysis of hospital episode statistics and stroke national audit data. BMJ. 2019;364:l1. doi: 10.1136/bmj.l1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry for Pacific Peoples. Pacific People in NZ. 2020. Accessed February 15, 2021. mpp.govt.nz/pacific-people-in-nz/.
- 28.Massey University. Environmental health indicators New Zealand: urban-rural profile. Accessed February 11, 2021. ehinz.ac.nz.
- 29.Feigin V, Carter K, Hackett M, et al. Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002-2003. Lancet Neurol. 2006;5(2):130-139. doi: 10.1016/S1474-4422(05)70325-2 [DOI] [PubMed] [Google Scholar]
- 30.Feigin VL, Krishnamurthi RV, Barker-Collo S, et al. 30-Year trends in stroke rates and outcome in Auckland, New Zealand (1981-2012): a multi-ethnic population-based series of studies. PLoS One. 2015:10e0134609. doi: 10.1371/journal.pone.0134609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranta A, Thompson S, Harwood M, et al. NZ Māori and Pacific peoples experience poorer long-term outcome following stroke in New Zealand. Int J Stroke. 2020;15(IS):3-752. doi: 10.1177/1747493020963387 [DOI] [Google Scholar]
- 32.Otite FO, Akano EO, Akintoye E, et al. Rural–urban disparities in intracerebral hemorrhage mortality in the USA: preliminary findings from the national inpatient sample. Neurocrit Care. 2020;32(3):715-724. doi: 10.1007/s12028-020-00950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified individual participant data, data dictionary, protocol, and consent forms can be requested via the corresponding author and will be available once all results from the study have been published, assuming that appropriate ethics approval is obtained.