Abstract
Supplementary motor area (SMA) syndrome is a typically transient condition resulting from damage to the medial premotor cortex. The exact mechanism of recovery remains unknown but is traditionally described as a process involving functional compensation by the contralateral SMA through corpus callosal fibers. The purpose of this case study is to highlight a distinct extracallosal mechanism of functional recovery from an SMA syndrome in a patient with agenesis of the corpus callosum (ACC). We present the clinical presentation and perioperative functional neuroimaging features of a 16-year-old patient with complete ACC who exhibited recovery from an SMA syndrome resulting from surgical resection of a right-sided low-grade glioma. Preoperative fMRI revealed anatomically concordant activation areas during finger and toe tapping tasks bilaterally. Three months after surgery, the patient had fully recovered, and a repeat fMRI revealed shift of the majority of the left toe tapping area from the expected contralateral hemisphere to the ipsilateral left paracentral lobule and SMA. The fMRI signal remodeling observed in this acallosal patient suggests that within-hemisphere plasticity of the healthy hemisphere may constitute an alternative critical process in SMA syndrome resolution and challenges the traditional view that transcallosal fibers are necessary for functional recovery.
Pearls
Supplementary motor area (SMA) syndrome is a condition resulting from unilateral injury to the medial aspect of the premotor cortex.
The classical SMA syndrome is transient and is characterized by contralateral hemiparesis and hemiapraxia. Language deficits are commonly observed in dominant hemisphere SMA injuries.
The most widely accepted theory attributes functional recovery to compensation by the intact contralateral SMA through commissural fibers of the corpus callosum (CC).
Oy-sters
SMA syndrome should always be considered after medial premotor cortex injury. Even in patients exhibiting profound neurologic deficits, a significant recovery is expected.
In patients with agenesis of the CC (ACC), recovery from SMA syndrome remains possible through extracallosal compensatory mechanisms. Thus, ACC may not preclude surgical removal of lesions within the SMA.
Case Report
A 16-year-old right-handed girl with complete ACC was referred to the outpatient clinic for a 7-year history of refractory headaches. Initial neurologic examination was normal. Brain MRI confirmed the complete ACC and revealed a nonenhancing lesion confined within the right SMA suggestive of a low-grade glioma (LGG) (Figure 1A). A task-based fMRI was performed (methodology described in eMethods in the Supplement, links.lww.com/WNL/C57) and revealed the location of finger and toe tapping activations within the expected regions of the contralateral sensorimotor cortex, but no SMA activity (Figure 1B and C). Language tasks disclosed a diffuse bilateral speech representation including the SMA on both sides. Considering the absence of CC fibers and the presumed lower likelihood of postoperative functional compensation, the risk of permanent deficits after resection of the SMA was deemed non-negligible. However, because of the poor outcome often associated with unresected LGG, a surgical treatment was favored. Cortical stimulation–guided gross total resection of the SMA tumor was performed.
Figure 1. Preoperative Structural and Motor Task–Based fMRI.
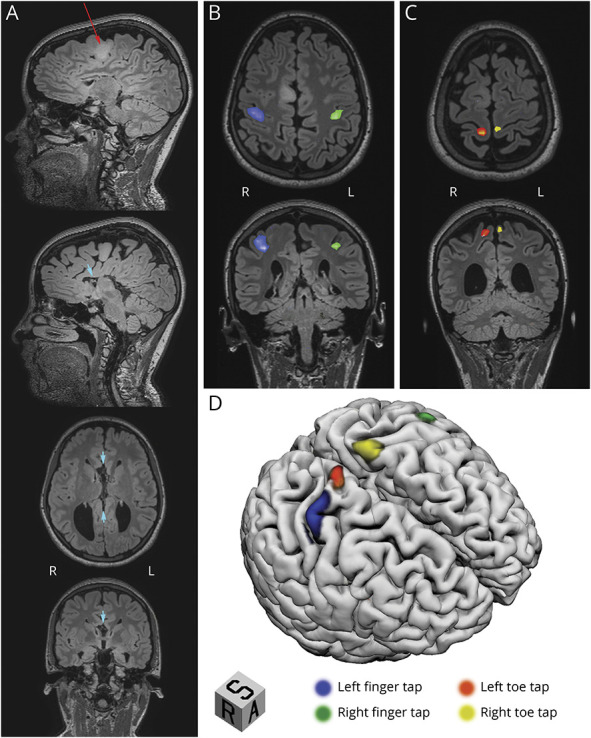
(A, upper item) Sagittal T2 weighting of FLAIR MRI sequences showing a 2.5cm hyperintense infiltrative tumor confined within the right supplementary motor area (SMA; red arrow). (A, lower 3 items) Sagittal, axial, and coronal T2 weighting of FLAIR MRI sequences highlighting the absence of the corpus callosum (CC; short cyan arrows). (B and C) Axial and coronal fMRI showing blood-oxygen-level-dependent (BOLD) activations of the middle portion of the contralateral primary sensorimotor cortex during finger tapping (B) and the superior aspect of the contralateral primary motor cortex during toe tapping (C). (D) T1 surface–based 3D reconstruction with superimposed regions activated during motor tasks revealed anatomically concordant functional areas.
After surgery, the patient developed a left-sided tonus-sparing 3/5 hemiparesis and significant hemiapraxia, consistent with a motor SMA syndrome. At the 3-month follow-up, she had fully recovered, and a repeat MRI showed no tumor recurrence (Figure 2A). Repeat task-based fMRI revealed expected finger tapping and right toe tapping areas, with the latter expanding to also encompass the left SMA (Figure 2B). Surprisingly, a considerable portion of the area activated by left toe tapping had shifted to the left paracentral lobule and the adjacent SMA (Figure 2C).
Figure 2. Structural and Motor Task–Based fMRI 3 Months After Surgery.

Clinically, the patient had completely recovered from the supplementary motor area (SMA) syndrome. (A) Sagittal T2 weighting of FLAIR images revealing gross total resection of the tumor-infiltrated SMA with sparing of the paracentral lobule (red arrow). The absence of the corpus callosum (CC) is notable above the frontal horn and body of the lateral ventricle (short cyan arrow). (B and C) Axial and coronal fMRI. (B) Blood-oxygen-level-dependent (BOLD) signals matched the location of the preoperative activations during finger tapping. (C) Activation maps revealed the anterior expansion of the right toe tapping area (now incorporating most of the SMA) and the contralateral transfer of a major part of the area activated by left toe tapping to the left paracentral lobule and SMA. (D) T1 surface–based 3D reconstruction with overlaid task-activated regions highlighting the expansion of right toe tapping function and the contralateral transfer of the left toe tapping area. The resection cavity is discernible at the medial aspect of the right premotor cortex.
Discussion
The SMA is responsible for several key motor functions, including planning, initiation, and execution of voluntary movements as well as word production and articulation on the language-dominant hemisphere. Unilateral damage to the medial premotor cortex often results in the well-described SMA syndrome, a condition characterized by tonus-sparing contralateral motor weakness and hemiapraxia coupled with language impairments in dominant hemisphere injuries.1 A hallmark feature of SMA syndrome is the complete or near-complete resolution of symptoms within weeks to months of onset.2 Although the transient nature of the observed deficits remains incompletely understood, cumulative evidence suggests that neurologic recovery relies on the presence of an intact CC.3,4 Yet, our patient with ACC fully recovered, suggesting that extracallosal mechanisms may be involved in SMA syndrome resolution.
Recovery from SMA syndrome has traditionally been explained by a process beginning after the cortical insult and involves a functional compensation enabled by fibers of the CC connecting the damaged ipsilateral lateral premotor area to the contralateral SMA and premotor area of the healthy hemisphere.2,5,6 The exact compensatory mechanism of these commissural fibers remains incompletely understood but has been attributed to excitatory interhemispheric connections favoring functional activation of the homologous SMA and adjacent regions.2,5,6 Although the aforementioned compensation is the most widely accepted mechanism of recovery, other processes have been proposed. Transcranial magnetic stimulation studies have shown that transcallosal inhibitory fibers may be present between homologous motor areas.3,7 After unilateral injury, the presumed tonic inhibition is thought to be released, ultimately leading to functional hyperactivation of the undamaged contralateral region.3,7 Other reports have suggested that, in patients with slowly progressive unilateral SMA tumors, a CC-mediated gradual switch of function to the contralateral healthy SMA may enable recovery after surgical resection of the lesion.3 These theories postulate that the contralateral SMA acts as a functional substitute for its injured counterpart and relies on CC-associated mechanisms.
The recovery observed in the current patient without a CC suggests that alternative pathophysiologic processes may be implicated in functional remodeling. Before surgery, our patient did not exhibit SMA activation during motor tasks, a finding that is consistent with previous studies.8 After surgery, the left toe tapping area was mainly detected in the left paracentral lobule and SMA, suggesting the transfer of function to the contralateral side. The unexpected left-sided SMA activation induced by left toe tapping suggests the presence of extracallosal within-hemisphere processes in functional reorganization, which may have resulted from a compensatory increase in activation from a previously undetected blood-oxygen-level-dependent signal. This idea is consistent with previous fMRI studies in patients with an intact CC, which revealed a similar pattern of SMA activation ipsilateral to limb movements after surgery.9 Indeed, few studies have reported an increase in intrahemispheric functional connectivity between sensorimotor nodes of the healthy hemisphere during SMA recovery.2 Furthermore, unilateral motor movements have been shown to be represented bilaterally in the SMA but exhibit contralateral predominance.10,11 Thus, unilateral SMA damage may uncover a functionally silent, albeit present, network in the uninjured contralateral SMA, capable of supplanting the function of the injured cortex. This process may be facilitated by plastic functional changes within the contralateral intact hemisphere.
The current study provides unique information regarding a distinct mechanism of recovery in SMA syndrome independent of the CC. It is worth mentioning that patients with ACC commonly develop strong extracallosal interhemispheric connections that travel through the anterior commissure, the posterior commissure, or subcortical circuits (amygdalar, hippocampal, and/or cerebellar circuits). These compensatory connections are potentially capable of taking over the function of the CC and may have played a role in the observed functional reorganization.12,13 However, their contribution does not preclude that intrahemispheric plasticity of the healthy hemisphere was observed in our patient, and that similar within-hemisphere remodeling may be involved in SMA syndrome recovery in patients with an intact CC.
The favorable outcome seen in the current case suggests that resection of the SMA may be considered in patients with ACC. These findings may help address concerns regarding the safety of SMA resection in ACC and facilitate surgical decision making and patient counseling. In addition, the functional restructuration observed despite the absence of CC suggests that unilateral within-hemisphere plasticity may constitute a previously overlooked critical process in SMA syndrome recovery and raises the question as to whether corpus callosal fibers are in fact requisite to overcome the functional deficits related to SMA injury.
Appendix. Authors

Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Quirarte JA, Kumar VA, Liu HL, Noll KR, Wefel JS, Lang FF. Language supplementary motor area syndrome correlated with dynamic changes in perioperative task-based functional MRI activations: case report. J Neurosurg. 2020;134(6):1-5. [DOI] [PubMed] [Google Scholar]
- 2.Vassal M, Charroud C, Deverdun J, et al. Recovery of functional connectivity of the sensorimotor network after surgery for diffuse low-grade gliomas involving the supplementary motor area. J Neurosurg. 2017;126(4):1181-1190. [DOI] [PubMed] [Google Scholar]
- 3.Potgieser ARE, de Jong BM, Wagemakers M, Hoving EW, Groen RJM. Insights from the supplementary motor area syndrome in balancing movement initiation and inhibition. Front Hum Neurosci. 2014;8:960-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chivukula S, Pikul BK, Black KL, Pouratian N, Bookheimer SY. Contralateral functional reorganization of the speech supplementary motor area following neurosurgical tumor resection. Brain Lang. 2018;183:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oda K, Yamaguchi F, Enomoto H, Higuchi T, Morita A. Prediction of recovery from supplementary motor area syndrome after brain tumor surgery: preoperative diffusion tensor tractography analysis and postoperative neurological clinical course. Neurosurg Focus 2018;44(6):E3-E6. [DOI] [PubMed] [Google Scholar]
- 6.Baker CM, Burks JD, Briggs RG, et al. The crossed frontal aslant tract: a possible pathway involved in the recovery of supplementary motor area syndrome. Brain Behav. 2018;8(3):e00926-e00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125(pt 8):1896-1907. [DOI] [PubMed] [Google Scholar]
- 8.Krainik A, Lehéricy S, Duffau H, et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001;57(5):871-878. [DOI] [PubMed] [Google Scholar]
- 9.Acioly MA, Cunha AM, Parise M, Rodrigues E, Tovar-Moll F. Recruitment of contralateral supplementary motor area in functional recovery following medial frontal lobe surgery: an fMRI case study. J Neurol Surg A, Cent Eur Neurosurg. 2015;76(6):508-512. [DOI] [PubMed] [Google Scholar]
- 10.Chung GH, Han YM, Jeong SH, Jack CR. Functional heterogeneity of the supplementary motor area. AJNR Am J Neuroradiol. 2005;26(7):1819-1823. [PMC free article] [PubMed] [Google Scholar]
- 11.Sailor J, Meyerand ME, Moritz CH, et al. Supplementary motor area activation in patients with frontal lobe tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 2003;24(9):1837-1842. [PMC free article] [PubMed] [Google Scholar]
- 12.Siffredi V, Farouj Y, Tarun A, et al. Large-scale functional network dynamics in human callosal agenesis: increased subcortical involvement and preserved laterality. NeuroImage. 2021;243:118471. [DOI] [PubMed] [Google Scholar]
- 13.Tovar-Moll F, Monteiro M, Andrade J, et al. Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci U S A. 2014;111(21):7843-7848. [DOI] [PMC free article] [PubMed] [Google Scholar]


