Abstract
Background
We examine the effects of low energy shock wave (LESW) on bladder and mitochondrial function in a rat model of HCl induced cystitis, and the influence of dynamic bladder filling volume on LESW responses. Dysregulation of mitochondria function may impact the urothelial barrier and contribute to bladder dysfunction in patients with Interstitial cystitis/bladder pain syndrome (IC/BPS).
Methods
Female Sprague–Dawley rats underwent urethral catheterization and intravesical instillation of 0.2 ml of 0.4 N HCl (N = 32) or 0.2 ml saline (N = 8) kept for 90 s. After HCl instillation, the bladder received LESW treatment while filled with 0 ml, 0.2 ml or 0.4 ml saline or no LESW treatment. Continuous cystometry (CMG) was performed on day 8. The bladder was harvested after CMG for histology and Western blotting.
Results
HCl provoked bladder overactivity, bladder wall inflammation marked by infiltration of mast cells, increased bax/bcl2 ratio consistent with increased TUNEL staining and increased release of mitochondrial-integrity markers (cleaved caspase 3 and Cytochrome c). LESW treatment suppressed HCl provoked bladder overactivity in association with lower inflammatory reaction, mast cells infiltration, and a lower bax/bcl2 ratio also reflected by reduced TUNEL staining and mitochondrial-integrity markers irrespective of the volume of saline in bladder at the time of LESW.
Conclusions
These findings support that antiinflammatory effect of LESW in chemical cystitis is associated with the reversal of the molecular-cellular perturbations in mitochondrial dependent intrinsic apoptotic pathway.
Keywords: HCl, Cystitis, Shock wave, Mitochondria
At a glance of commentary
Scientific background on the subject
The etiology of IC/BPS is still unclear and new treatments are needed to solve this thorny problem. In this study, we investigate the effects of LESW on bladder inflammation and mitochondria apoptotic pathway in a chemical cystitis model.
What this study adds to the field
LESW significantly decreased the induced elevation in the ratio of Bax to Bcl 2 and expression of caspase 3 without altering the expression of cytochrome c. LESW exerts physical energy on inflamed bladder that may attenuate mitochondrial dependent apoptotic pathway and decrease bladder inflammation and overactivity.
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic debilitating condition of the bladder including symptoms of pain, pressure and discomfort in the absence of infection or other identifiable causes [1]. In the United States, the prevalence of IC/BPS is estimated about 2.7%–6.5% for women and 1.9%–4.2% for men aged 18 years or older [2,3]. The prevalence of IC/BPS in Taiwan was 21.8/100,000 in 2002 and increased to 40.2/100,000 in 2013 [4].
Etiology of IC/BPS remains a mystery [5] and is likely to be multifactorial with symptoms overlapping with other common urological diseases. Denudation or thinning of the urothelium associated with infiltration of macrophages, eosinophils, or mast cells is a common histopathological finding in IC/BPS bladder patients [6]. Analysis of biopsy taken from IC/BPS patients found an increase in apoptotic cells, lower expression of proliferation markers and elevation of apoptotic signaling molecules, including Bax, cleaved caspase-3, and Bad [7]. Activation of mast cells and inflammatory cells in bladder wall induces a release of inflammatory mediators to elicit a neuroinflammatory response. Neuroinflammation and increased epithelial permeability that may allow for the influx of urine potassium ion and toxic solutes can provoke C afferent fiber sensitization [5,6].
New treatment for IC/BPS is a great unmet medical need and we are investigating a physical approach of low energy shock wave (LESW), known to exert anti-inflammatory, anti-apoptotic effects, that may improve tissue repair and increased angiogenesis [8,9]. LESW exerts a mechanical shear force via its “cavitation effect” on cell membranes and contents, including mitochondria, which are affected by shock waves that induce changes in ATP production [10] to reduce oxidative stress, and alleviate inflammation in rat models of cystitis [11,12].
LESW has been shown to attenuate apoptosis in H9c2 heart myoblast cell line ischemia/hypoxia (I/H) model as well as infarct border zone in acute myocardial infarction model in rats through the modulation of mitochondria apoptotic pathway [8,9]. Moreover, a recent publication suggested that dysregulation of mitochondria function and alterations in energy metabolism increased susceptibility to ROS generation and apoptosis may impact the urothelial barrier and contribute to bladder dysfunction [13]. We hereby investigate the effects of LESW on bladder inflammation and mitochondria apoptotic pathway in a chemical cystitis model.
Materials and methods
A cystitis rat model
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee before they were executed on 40 female Sprague–Dawley rats (250–300 g). The HCl induced cystitis model was induced with slight modification from previous study [2]. Under isoflurane anesthesia, a polyethylene-50 (PE-50) catheter was inserted into the bladder through the urethral orifice. The bladder was manually emptied of urine, and then instilled with either 0.2 ml of 0.4 N HCl (n = 32) or 0.2 ml of phosphate buffered saline (PBS) for controls (n = 8). The rats were then kept in the supine position for 90 s, following which the bladder was emptied using the catheter.
Low energy shock wave (LESW) treatment (200 shocks, 3 pulses per second at the intensity 0.12 mJ/mm2)
After induction of cystitis, the bladder was instilled with 0 ml, 0.2 ml or 0.4 ml saline (N = 8 for each group) before placing the shock wave applicator (Storz, Germany) directly on the ultrasound transmission gel covered skin area over the bladder with frequency of 3 pulses per second. The magnitude of shock wave intensity (0.12 mJ/mm2) and number (200 shocks) used in our protocol was determined by previous reports [11,12]. One group of cystitis rats (N = 8) not exposed to LESW served as control.
Cystometrogram (CMG)
On Day 8, the animals were anesthetized with urethane (1 g/kg, s.c.). A PE-50 tubing was inserted transurethral into the bladder and the catheter was connected via a three-way stopcock to a pressure transducer and syringe pump for recording intravesical pressure and for infusing saline (0.08 ml/min) into the bladder for eliciting repetitive voiding. The amplitude (the peak pressure minus the basal pressure during each contraction period), pressure threshold (PT, the pressure immediately before the reflex contraction), pressure at baseline (PB, the pressure immediately after the reflex contraction) and intercontraction interval (ICI – the average time interval between contractions of reflex bladder contractions) were recorded. Measurements in each animal represented the average of three to five bladder contractions [12].
Transcardiac perfusion
After CMG, animals were deeply anesthetized for transcardiac perfusion, first with Krebs buffer followed by 4% paraformaldehyde fixative. The animals were then dissected to harvest the bladder and cut into one half for histology, immunohistochemistry, and another half for western blotting.
Histology and immunohistochemistry
Harvested bladder from different animal groups was fixed in buffered 4% formaldehyde for 24–48 h, embedded in paraffin, and stained with hematoxylin and eosin. The HCl-induced inflammatory reaction was graded by a score of 0–3 as follows: 0, no evidence of inflammatory cell infiltrates or interstitial edema; 1, mild (few inflammatory cell infiltrates and little interstitial edema); 2, moderate (moderate amount of inflammatory cell infiltrates and moderate interstitial edema); 3, severe (extensive presence of large amount of inflammatory cell infiltrates and severe interstitial edema) [12].
After deparaffination, mast cell infiltration, fibrosis, and apoptotic cell were assessed by toluidine blue staining (sigma), Masson's trichrome staining (sigma), and TUNEL staining (terminal deoxynucleotidyl-mediated deoxyuridine triphosphate nick end labeling stain; Roche), respectively. The toluidine blue-positive mast cells or trichrome-positive collagen area or TUNEL positive apoptosis cells with a minimal threshold of 500 pixels was quantified in three fields at 200× magnification using a 20 × 10 grid in the eyepiece. Slides were then dehydrated through increasing concentrations of alcohol to xylene and coverslip mounted with Entellan (Merck, Darmstadt, Germany). Each slide was examined under the microscope and 10 randomly chosen areas were selected for quantitative digital image analysis using Image J and Image-Pro Plus 6.1 software.
Western blot analysis for Bax, Bcl-2, cytochrome c, cleaved caspase 3
The procedures for Western Blot analysis were as described previously [10]. Expression of Bax, Bcl-2, cytochrome c, and cleaved caspase 3 were analyzed according to the standard protocol (Amersham Biosciences). The mouse anti-GAPDH monoclonal antibody (1:10,000 dilution; Millipore) and rabbit anti-Bax polyclonal antibody (1:1000 dilution; Cell signaling), rabbit anti-Bcl-2 (1:1000 dilution; Proteintech), rabbit anti-cytochrome c polyclonal antibody (1:1000 dilution; Cell signaling), and rabbit anti-cleaved caspase 3 polyclonal antibody (1:1000 dilution; cell signaling) were used.
Statistical analysis
All data were presented as means ± SE. Parameter values were compared using one-way ANOVA followed by Scheffe test, with P < 0.05 considered significant. All statistical analysis was undertaken using SPSS v.18.0 (IBM Corp., Armonk, NY, USA).
Results
Cystometry
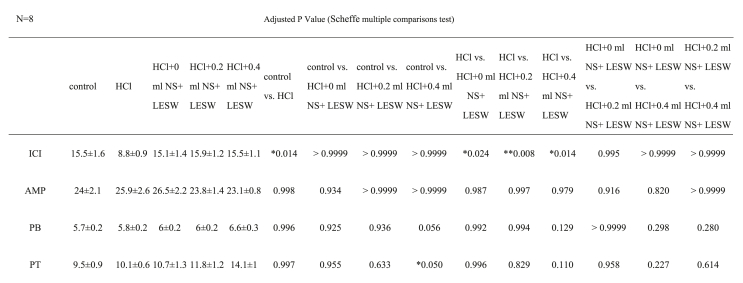
As shown in Table 1 and Fig. 1, on day 8, brief exposure to HCl provoked bladder overactivity marked by 43.2% decrease in ICI compared to the control (PBS instilled) group. However, LESW treatment significantly suppressed the bladder overactivity irrespective of the bladder distension at the time of LESW [ICI 71.7%, 80.7%, and 71.6% increase for bladders in 0 ml, 0.2 ml, and 0.4 ml saline groups, respectively, Table 1]. There was no significant difference in contraction amplitude, pressure threshold or pressure at baseline in the LESW treated or untreated groups.
Table 1.
Effects of 0.4 N HCl with or without low energy shock wave (LESW) on CMG parameters.
Fig. 1.
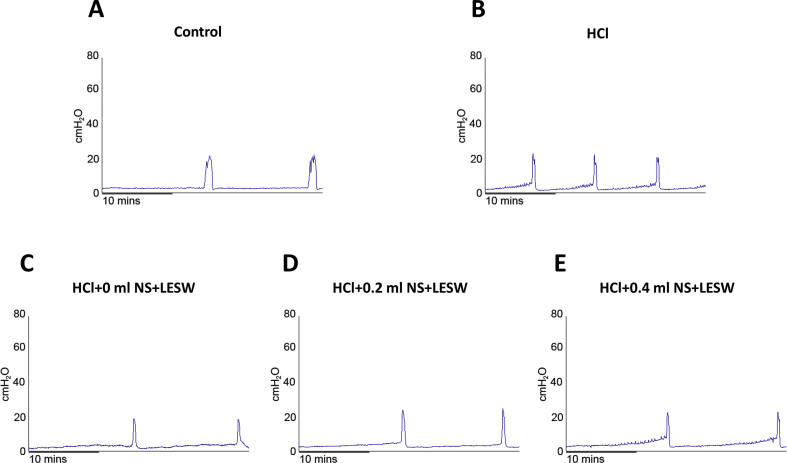
Representative traces of in vivo continuous cystometrogram (CMG) in rats. CMG was performed on day 8 in control rat (A) post HCl treated rat (B), post HCl + LESW (bladder with 0 ml saline) treated rat (C), post HCl + LESW (bladder with 0.2 ml saline) treated rat (D), post HCl + LESW (bladder with 0.4 ml saline) treated rat (E). Intravesical HCl treated rats showed significant reduction in ICI, however, the reduction in ICI was significantly reduced in LESW treated rat.
Histological and immunohistochemistry of urinary bladder
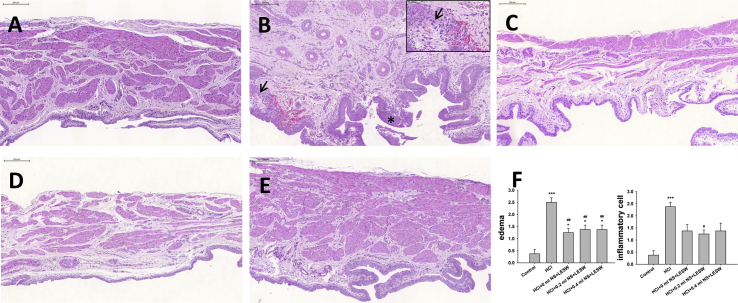
HCl exposure provoked moderate and severe bladder inflammation on day 8 [Fig. 2B] compared to the control group [Fig. 2A]. Higher magnification inset in panel of Fig. 2B displays progressive infiltration of inflammatory cells, and moderate to severe edema in bladder mucosa. Increased presence of circles representing blood vessels in lamina propria of HCl exposed group was absence in LESW treated group. LESW decreased bladder wall inflammation evoked by HCl by 42.0%, 47.5%, and 42.0% for bladder distended with 0 ml, 0.2 ml, and 0.4 ml saline groups, respectively [Fig. 2C–E]. LESW decreased edema score by 50.0%, 44.8%, and 44.8% for bladder distended with 0 ml saline, 0.2 ml saline, and 0.4 ml saline groups, respectively. Bladder distension did not generate statistically significant difference among the LESW treatment groups.
Fig. 2.
Photomicrographs showing the effects of LESW on bladder histology (H–E staining) in rats with HCl-induced cystitis, in control rat (A) post HCl treated rat (B), post HCl + LESW (bladder with 0 ml saline) treated rat (C), post HCl + LESW (bladder with 0.2 ml saline) treated rat (D), post HCl LESW (bladder with 0.4 ml saline) treated rat (E). The bladder of the control rat shows intact urothelium with thin and unremarkable lamina propria (A). Thickening of urothelium (star) and edematous change with accumulation of inflammatory cells and increase of blood vessels (arrowhead) in the lamina propria (arrow) was noted in the bladder with HCl effect (B). The inflammatory reaction was reduced after LESW treatment (Magnification ×100; inlet, magnification ×400, control vs HCl treated rats with or without LESW treated rats, ∗∗∗p < 0.001. HCl vs HCl + LESW treatment rats, #p < 0.05, ##p < 0.01).
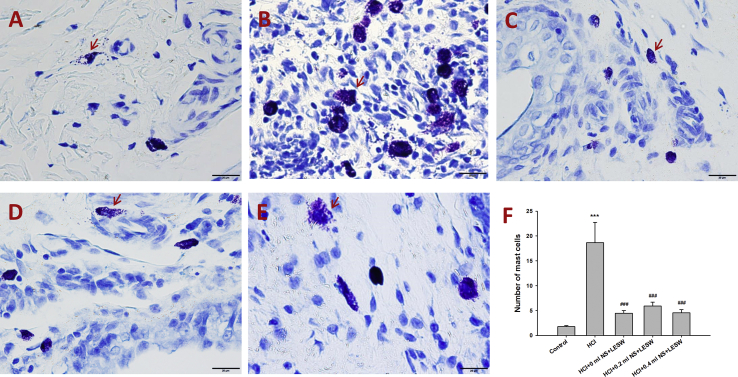
Fig. 3 showed the data on the mast cell counts. Intravesical HCl instillation resulted in an increase in mast cell count than in the normal control group [Fig. 3B]. LESW treatment significantly decreased the mast cell counts by 75.9%, 68.4%, and 75.9% for bladder with 0 ml, 0.2 ml, and 0.4 ml saline groups, respectively [Fig. 3C–E]. Bladder distension did not generate statistically significant difference among the LESW treatment groups.
Fig. 3.
Toluidine blue stain; in control rat (A), post HCl treated rat (B), post HCl + LESW (bladder with 0 ml saline) treated rat (C), post HCl + LESW (bladder with 0.2 ml saline) treated rat (D), post HCl + LESW (bladder with 0.4 ml saline) treated rat (E). A large number of mast cells were activated in HCl treated bladder tissue. Mast cell activation (arrow) was shown by toluidine blue staining in the bladder specimen. Mast cell activation is significantly decreased in that with LESW treatment (Magnification ×400, control vs HCl with or without LESW treated rats, ∗∗∗p < 0.001, HCl vs HCl + LESW treated rats, ###p < 0.001).
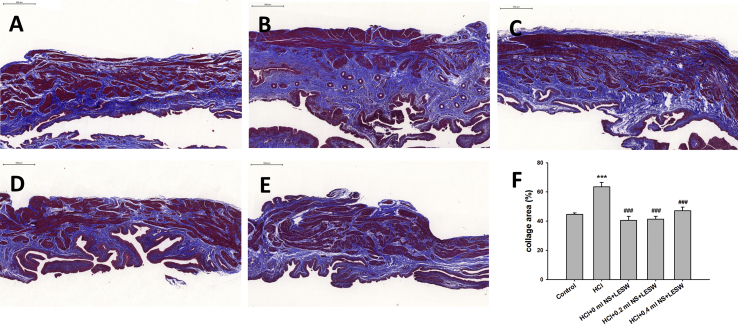
Intravesical HCl resulted in significantly higher density of collagen fibers identified by Masson's trichrome staining against the whole area of tissues [Fig. 4B] than in the normal control group [Fig. 4A]. LESW treatment significantly decreased collagen fibers staining by 36.1%, 35.0%, and 25.7% for bladder with 0 ml, 0.2 ml, and 0.4 ml saline groups, respectively [Fig. 4C–E]. Bladder distension did not generate statistically significant difference among the LESW treatment groups.
Fig. 4.
Masson's trichrome stain; in control rat (A), post HCl treated rat (B), post HCl + LESW (bladder with 0 ml saline) treated rat (C), post HCl + LESW (bladder with 0.2 ml saline) treated rat (D), post HCl + LESW (bladder with 0.4 ml saline) treated rat (E). Intravesical HCl resulted in significantly more percentage of staining area of collagen fibers identified by Masson's trichrome staining against the whole area of tissues than in the normal control group. LESW treatment significantly decreased collagen fibers staining (Magnification ×100, control vs HCl with or without LESW treated rats, ∗∗∗p < 0.001, HCl vs HCl with LESW treated rats, ###p < 0.001).
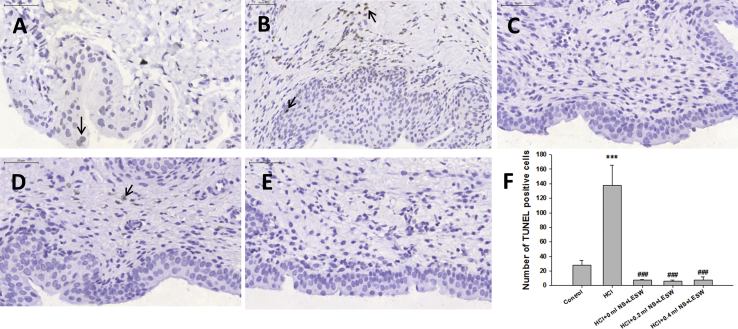
The TUNEL staining of sections of control animals revealed few apoptotic cells [Fig. 5A], which was significantly increased in HCl treated animals [Fig. 5B]. LESW treatment significantly decreased TUNEL staining cells by 94.7%, 95.6%, and 94.3% for bladder with 0 ml saline, 0.2 ml saline, and 0.4 ml saline group, respectively [Fig. 5C–E]. There was no significant difference among the LESW treatment groups.
Fig. 5.
Terminal deoxynucleotidyl-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining of bladder sections of control rat (A), post HCl treated rat (B), post HCl + LESW (bladder with 0 ml saline) treated rat (C), post HCl + LESW (bladder with 0.2 ml saline) treated rat (D), post HCl LESW (bladder with 0.4 ml saline) treated rat (E). Increasing number of apoptotic nuclei was seen in the HCl treated animal. LESW treatment significantly decreased TUNEL staining cells (Magnification ×400, control vs HCl with or without LESW treated rats, ∗∗∗p < 0.001, HCl vs HCl with LESW treated rats, ###p < 0.001).
Bax, Bcl-2, cytochrome c, cleaved caspase 3 expression post HCl with or without LESW treatment
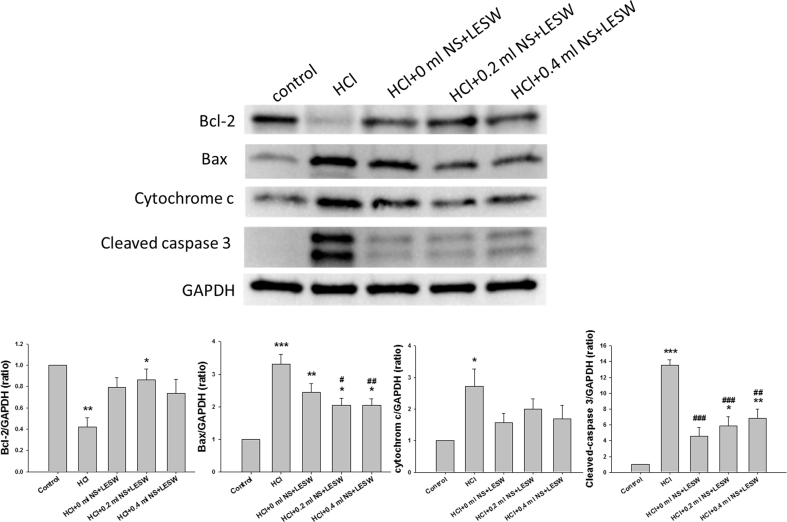
Western blotting demonstrated that relative to the control group, HCl induced statistically significant increase in the ratio of Bax to Bcl2 (control vs HCl, 1: 12.7 ± 4.3, p = 0.004), cytochrome c, and cleaved caspase 3 expression [Fig. 6]. LESW treatment partially reversed the deleterious effects of HCl by decreasing the elevated ratio of Bax to Bcl2 (3.4 ± 0.5, 2.7 ± 0.4, 3.2 ± 0.4, for bladder with 0 ml, 0.2 ml, and 0.4 ml saline, respectively; p < 0.05 vs HCl) with a significantly decrease in Bax expression by 37.9%, and 37.9% for bladder with 0.2 ml saline, and 0.4 ml saline group, and significantly increased expression of Bcl-2 by 104.8% for bladder with 0.2 ml saline. LESW decreased the markers of mitochondrial damage such as cleaved caspase 3 expression by 66.0%, 56.4%, and 49.4% for bladder with 0 ml, 0.2 ml, and 0.4 ml saline groups. Bladder distension did not generate statistically significant difference among the LESW treatment groups.
Fig. 6.
Western blot for Bax, Bcl 2, cytochrome c, cleaved caspase 3, protein expression in control rat, and HCl with or without LESW treated rat (control vs HCl with or without LESW treated rats, ∗<0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, HCl vs HCl with LESW treatment, #p < 0.05, ##p < 0.01, ###p < 0.001).
Discussion
The current study revealed that a brief exposure to HCl provoked severe bladder wall inflammation, fibrosis, mast cells infiltration, apoptosis, and bladder overactivity, which were significantly suppressed by LESW treatment. HCl exposure was associated with increased ratio of Bax to Bcl2 with upregulation of Bax, Cytochrome c, and cleaved caspase 3 expression. LESW treatment significantly decreased the induced elevation in the ratio of Bax to Bcl 2 and expression of caspase 3 without significantly altering the expression of cytochrome c. Our findings suggest that LESW exerts physical energy on inflamed bladder that may attenuate mitochondrial dependent apoptotic pathway and decrease bladder inflammation and overactivity.
Our findings of mast cell infiltration and edema in HCL cystitis corroborates an earlier report of proteomic investigation on this model [14]. Inflammatory microcirculatory changes noted in rat urinary bladder after cyclophosphamide [15] or ketamine [16] appears similar to the remodeling of microvasculature in bladder of untreated HCl group. Histologic evidence in HCl group untreated with LESW is similar to the findings of fluorescein angiography in IC/BPS patient [17] with perivascular infiltrates [18].
The findings of LESW inhibiting fibrosis, and inflammatory cells infiltration in bladder agrees with the recently reported results by Abe et al., where LESW significantly ameliorated left ventricular remodeling and fibrosis, and suppressed the infiltration of neutrophils and macrophages in a rat model of acute myocardial infarction [19]. The effect of LESW on the perturbation of apoptosis markers and mitochondrial integrity markers corroborates the reported downregulation of COX2, IL6, and NGF expression for the attenuation of bladder pain, inflammation and overactivity by LESW in a cyclophosphamide (CYP) induced cystitis model in rats [12]. The effect of LESW on CYP evoked bladder damage is mediated by the suppression of inflammation (decreased expression of IL-12, MMP9, TNF-a, nuclear factor-kB and iNOS) and oxidative stress (decreased NADPH oxidase 1 and NOX-2 expression) is reported by Chen et al. as well [11].
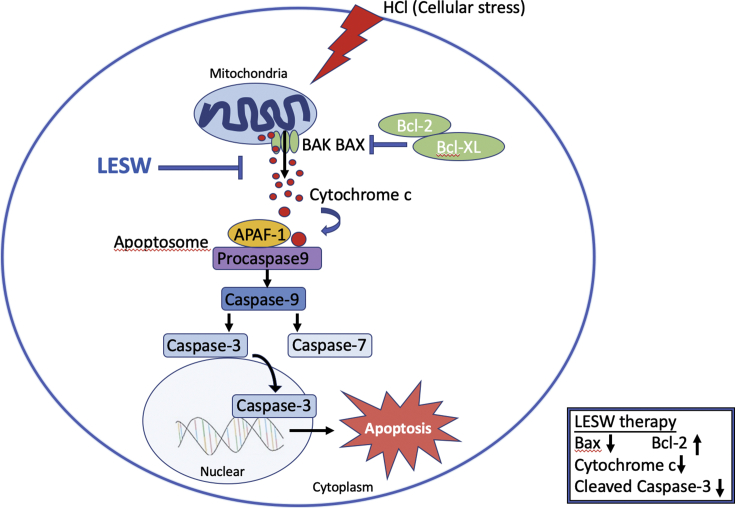
The effect of LESW on the ratio of Bax to Bcl 2 and the measured levels of mitochondrial-integrity markers (cleaved caspase 3 and Cytochrome c) implicates a role for mitochondrial intrinsic apoptotic pathway [20] in histological and functional perturbations of chemical cystitis [Fig. 7]. This pathway is known to trigger in response to a number of cellular stresses inducing tropic factor deprivation, DNA damage, or excessive oxidative damage. The release of cytochrome c, the second mitochondria-derived activator of caspase (SMAC/DIABLO) and OMI/high-temperature requirement protein A2 (HTRA2) from mitochondrial intermembrane space to cytosol through the opening of mitochondrial permeability transition pore (mPTP) promotes apoptosis executioners, procaspase, activation. The opening of mPTP is tightly regulated by pro- and anti-apoptotic BCL-2 family proteins (Bim, Hrk/DP5, Bmf, Puma, Noxa, BID, BAD, NOXA, etc. and BCL-2, BCL-xL, BCL-w, Mcl-1, respectively) [21].
Fig. 7.
A model illustrating LESW treatment attenuates mitochondrial dysfunction in HCl induced cystitis in rats.
It has been suggested that the degree of shock wave transmission in the bladder might be influenced by bladder distensibility. Therefore, we studied LESW effect on bladder distended with 0, 0.2, or 0.4 ml of saline, which represents up to 50% of bladder capacity. Based on our findings in functional, histological and molecular domains, LESW resulted in similar therapeutic effects irrespective of bladder distension. It can be concluded that LESW can be safely delivered to bladder distended with urine at less than 50% of bladder capacity. We recently reported the safety of LESW in a clinical trial on IC/BPS patients refractory to conventional therapy. The delivery of LESW was found to be safe with the potential for a higher grade of pain reduction when bladder was distended with 50–100 ml urine [22].
There are some limitations in this study, namely, the causal relationship between improved bladder function and reduced inflammation is unclear. Although the study demonstrated anti-inflammatory effects associated with improved bladder function by using LESW in HCl induced cystitis as evidence by histological and cystometry studies, the evaluation of inflammatory factors such as IL-1, IL-6,NF-kB,iNOS, COX-2 et al. remain to be determined. Confirmation of an anti-inflammatory effect and the reversal of the mitochondrial dependent intrinsic apoptotic pathway by LESW is warranted in future studies.
Conclusions
In conclusion, our study found LESW attenuation of mitochondrial dependent intrinsic apoptotic pathway, inhibit bladder inflammatory reaction and overactivity in a rat cystitis model. These findings support the application of LESW for the treatment of IC/BPS.
Funding
This research was funded by grants from Ministry of Science and Technology (106-2314-B-182A-122-MY3, 109-2314-B-182A-135-MY3).
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
We acknowledge the support from the conjoint laboratory of Center for Shockwave Medicine and Center for Mitochondrial Research and Medicine, Tissue Engineering, and Kaohsiung Chang Gung Memorial Hospital.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Hanno P.M., Erickson D., Moldwin R., Faraday M.M., American Urological A. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Berry S.H., Elliott M.N., Suttorp M., Bogart L.M., Stoto M.A., Eggers P., et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suskind A.M., Berry S.H., Ewing B.A., Elliott M.N., Suttorp M.J., Clemens J.Q. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141–145. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M.H., Chang K.M., Tsai W.C. Morbidity rate and medical utilization in interstitial cystitis/painful bladder syndrome. Int Urogynecol J. 2018;29:1045–1050. doi: 10.1007/s00192-018-3574-x. [DOI] [PubMed] [Google Scholar]
- 5.Meng E., Hsu Y.C., Chuang Y.C. Advances in intravesical therapy for bladder pain syndrome (BPS)/interstitial cystitis (IC) Low Urin Tract Symptoms. 2018;10:3–11. doi: 10.1111/luts.12214. [DOI] [PubMed] [Google Scholar]
- 6.Jhang J.F., Kuo H.C. Pathomechanism of interstitial cystitis/bladder pain syndrome and mapping the heterogeneity of disease. Int Neurourol J. 2016;20:S95–S104. doi: 10.5213/inj.1632712.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shie J.H., Liu H.T., Kuo H.C. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79:484. e7–13. doi: 10.1016/j.urology.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 8.Yu W., Shen T., Liu B., Wang S., Li J., Dai D., et al. Cardiac shock wave therapy attenuates H9c2 myoblast apoptosis by activating the AKT signal pathway. Cell Physiol Biochem. 2014;33:1293–1303. doi: 10.1159/000358697. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Shen T., Liu B., Dai D., Cai J., Zhao C., et al. Cardiac shock wave therapy attenuates cardiomyocyte apoptosis after acute myocardial infarction in rats. Cell Physiol Biochem. 2018;49:1734–1746. doi: 10.1159/000493616. [DOI] [PubMed] [Google Scholar]
- 10.Liu T., Shindel A.W., Lin G., Lue T.F. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Impot Res. 2019;31:170–176. doi: 10.1038/s41443-019-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.T., Yang C.C., Sun C.K., Chiang H.J., Chen Y.L., Sung P.H., et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am J Transl Res. 2014;6:631–648. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H.J., Lee W.C., Tyagi P., Huang C.C., Chuang Y.C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol Urodyn. 2017;36:1440–1447. doi: 10.1002/nau.23141. [DOI] [PubMed] [Google Scholar]
- 13.Kullmann F.A., McDonnell B.M., Wolf-Johnston A.S., Kanai A.J., Shiva S., Chelimsky T., et al. Stress-induced autonomic dysregulation of mitochondrial function in the rat urothelium. Neurourol Urodyn. 2019;38:572–581. doi: 10.1002/nau.23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyagi P., Chen X., Hayashi Y., Yoshimura N., Chancellor M.B., de Miguel F. Proteomic investigation on chronic bladder irritation in the rat. Urology. 2008;71:536–540. doi: 10.1016/j.urology.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaromi P., Szabo A., Garab D., Bodnar D., Uhercsak G., Boros M., et al. [Experimental studies on microcirculatory inflammatory reactions of the urinary bladder] Magy Seb. 2012;65:184–190. doi: 10.1556/MaSeb.65.2012.4.3. Hungarian. [DOI] [PubMed] [Google Scholar]
- 16.Hung C.H., Hsieh S.W., Chen S.K., Lin C.M. Augmentation enterocystoplasty for patients with ketamine-induced cystitis: an 8-year experience and a review of series. Urol Sci. 2019;30:232–237. [Google Scholar]
- 17.Zimmern P.E., Laub D., Leach G.E. Fluorescein angiography of the bladder: technique and relevance to bladder cancer and interstitial cystitis patients. J Urol. 1995;154:62–65. doi: 10.1016/s0022-5347(01)67225-2. [DOI] [PubMed] [Google Scholar]
- 18.Holm-Bentzen M., Lose G. Pathology and pathogenesis of interstitial cystitis. Urology. 1987;29:8–13. [PubMed] [Google Scholar]
- 19.Abe Y., Ito K., Hao K., Shindo T., Ogata T., Kagaya Y., et al. Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ J. 2014;78:2915–2925. doi: 10.1253/circj.cj-14-0230. [DOI] [PubMed] [Google Scholar]
- 20.Kicinska A., Jarmuszkiewicz W. Flavonoids and mitochondria: activation of cytoprotective pathways? Molecules. 2020;25:3060. doi: 10.3390/molecules25133060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollville E., Romero S.E., Deshmukh M. Apoptotic cell death regulation in neurons. FEBS J. 2019;286:3276–3298. doi: 10.1111/febs.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang Y.C., Meng E., Chancellor M., Kuo H.C. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome-A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol Urodyn. 2020;39:1505–1514. doi: 10.1002/nau.24382. [DOI] [PubMed] [Google Scholar]