Abstract
Background
The most appropriate diagnostic tests for the assessment of the uterine cavity in patients undergoing standard infertility evaluation in daily practice remain unclear. Routine hysteroscopic uterine cavity evaluation before an in vitro fertilization-embyo transfer (IVF-ET) cycle is not a uniformly accepted procedure. However, cervical findings associated with difficult ET have rarely been reported in previous hysteroscopic studies. The main objective of this study was to examine the relationship between cervical finding under flexible outpatient hysteroscopy (OH) and difficult ET.
Methods
A cohort clinical study was conducted with 650 patients undergoing their first in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) from January 2010 to December 2017. Of them, 605 women with available embryos underwent transfer cycles. Outpatient hysteroscopies were scheduled during the follicular phase of the menstrual cycle, 1–3 months before starting IVF/ICSI treatment.
Results
Among the 650 women who underwent transvaginal sonography (TVS) and OH, abnormal sonographic findings were observed in 51 women (7.8%) in which submucous myoma, endometrial polyp, and endometrial hyperplasia were the most common. Abnormal hysteroscopic intracavitary findings were observed in 158 cases (24.3%) in which endometrial polyp, submucous myoma, and intrauterine adhesions were the most common. These results showed that TVS was specific (100%) but not sensitive (32.3%) compared with OH. Embryo transfer (ET) was difficult in 25% of women with cervical stenosis (CS) or tortuous cervical canal (TC) and was significantly more difficult in women in the TC group (30.7%) than in the CS group (19.6%).
Conclusion
OH can identify cervical charactistics associated with a high incidence of difficult ET.
Keywords: Cervical stenosis, Tortuous cervical canal, Difficult embryo transfer, Intrauterine pathologies, In vitro fertilization, Outpatient hysteroscopy
At a glance commentary
Scientific background on the subject
Routine hysteroscopic uterine cavity evaluation before an in vitro fertilization-embyo transfer (IVF-ET) cycle is not a uniformly accepted procedure. However, little or no studies validate the efficacy of OH in predicting ET difficulty. Cervical findings associated with difficult ET have rarely been reported in previous hysteroscopic studies.
What this study adds to the field
Outpatient hysteroscopy can identify cervical lesions associated with difficult ET in women undergoing initial IVF/ICSI procedures. Cervical dilatation and mock ET in patients with cervical lesions result in easier subsequent ultrasound-guided ET. Routine OH prior to IVF can be cost-effective in avoiding difficult ET.
Outpatient hysteroscopy (OH), which enables direct visualization of the uterine cavity without anesthesia, has been recommended as a routine procedure in assessing the causes of infertility [1]. OH of infertile women with normal transvaginal sonography (TVS) findings or who have failed previous in vitro fertilization–embryo transfer (IVF-ET) cycles have shown that the incidence of abnormalities of the uterine cavity (such as polyps, myoma, and adhesions) is relatively high (approximately 20–40%) [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. Moreover, correction of these OH-detected abnormalities markedly improves outcomes in these patients [16,17].
Two randomized controlled trials reported that OH in women with two or more failed IVF treatment cycles increased pregnancy rates by up to 13% [9,10]. However, routine hysteroscopy for uterine cavity evaluation before an IVF-ET cycle is not a uniformly accepted procedure [18].
The ET technique has a major impact on the pregnancy rates of assisted reproductive technologies, with poor execution estimated result in 30% of IVF failures [19]. Guidelines have been developed for the performance of the major steps of ET [20]. ET is frequently difficult due to forbidding cervix [21], including tortuosity and/or stenosis of the cervical canal. Unfortunately, the ease of the ET procedure in individual patients is largely unpredictable. The only diagnostic tool available to date is mock transfer, but its reliability is questionable.
Little or no studies validate the efficacy of OH in predicting ET difficulty. Recently, Larue et al. conducted an observational study to detect anatomical anomalies by assessing the cervix and uterus using TVS and rigid hysteroscopy [22]. They found that the most common anatomical characteristics associated with difficult ET were abnormal crypts in the cervical canal (86%) and tortuosity of the cervical canal (68%). The findings by Larue et al. were consistent with our previous clinical observation regarding the difficulty of ET. This retrospective cohort study evaluated the relationship between OH findings and the difficulty of ET. OH was used to assess the anatomical state of the cervix and determine the feasibility of predicting difficult embryo transfer (ET).
Methods
Participants
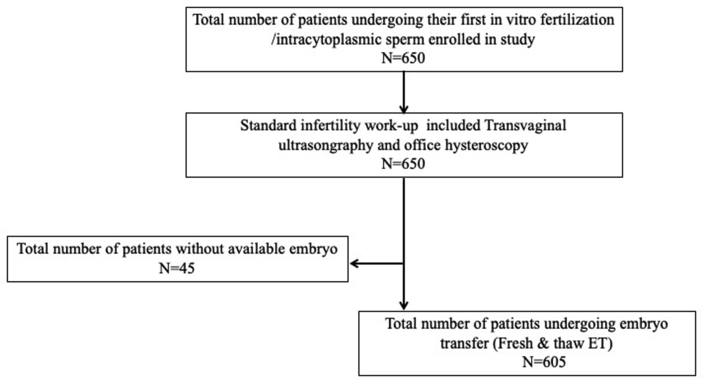
This study included all women undergoing their first IVF/intracytoplasmic sperm injection (ICSI) by K.C. Lan service at our institution between January 2010 and December 2017. All IVF/ICSI couples underwent the standard infertility work-up, consisting of medical history, physical examination, hormone status, semen analysis, TVS, andOH [Fig. 1].
Fig. 1.
Flow chart of study population.
The rationale involving OH is as follows: OH should be part of the infertility workup before ART, even in patients with normal HSG and/or TVS [1]. In addition, mock ET can be performed by OH better than traditional blind sounding and patients less suffering.
TVS of the uterine cavity was performed to evaluate the regularity of the endometrial lining and to measure the double layer of the endometrium in both the sagittal and transverse planes, thereby determining the presence of features associated with predefined abnormalities (polyps, submucous myoma, adhesions, or septa).
Hysteroscopy procedure and uterine cavity assessment
OH were scheduled during the follicular phase of the menstrual cycle, 1–3 months before starting IVF/ICSI treatment. The cervix was cleaned with povidone-iodine solution. No premedication or local anesthetic was administered.
All OH procedures were performed by a single examiner (K.C. Lan) with the patient in a semi-recumbent position in an electronic gynecological examination chair. Panoramic hysteroscopy was performed using a 3.8-mm diameter flexible hysteroscope (HYF–V, Olympus, Tokyo, Japan) without mechanical or drug-induced dilatation. Images of the cervical canal and the uterine cavity were viewed on a high-resolution color television monitor. Normal saline 0.9% was used as the distending medium and was propelled by an electronic pump (Endomat, Storz, Tuttlingen, Germany), with intrauterine pressure maintained at 45 mmHg. Illumination was provided by a halogen light source with a fiber-optic cable. After hysteroscopy, the cervical canal, tubal ostium, uterine cavity, and endometrium were examined. Any detected pathologies were recorded.
Women with any of these intracavitary abnormalities underwent subsequent diagnostic or therapeutic hysteroscopy to resolve these abnormalities prior to starting treatment for infertility. In case of the operator could not accomplish the OH examination due to cervical stenosis (CS) or tortuous cervical canal (TC), therapeutic hysteroscopy was also scheduled for cervical dilatation and mock ET by sounding under general anesthesia later. If polyps or myomas were detected, the diagnosis was confirmed histopathologically. Hysteroscopic findings for characteristic chronic endometritis include the presence of local or diffuse hyperemia, edema of the stroma, and presence of micropolyps [17].
We defined the following conditions during the procedure or post-procedure 1 week as a hysteroscopic-related complication: severe uterine pain cannot tolerate, massive vaginal bleeding, pelvic inflammatory disease, uterine perforation, shock, or pulmonary emboli.
The passage through the cervical canal studied was marked by three reference points: the internal and external os of the cervix, and a virtual reference point defined as the midpoint between the two orifices. The passage through the cervical canal was considered as direct when the three reference points were aligned, and as tortuous when the reference points were not aligned [22].
Tortuous cervical canal (TC) and cervical stenosis (CS)
In our study, tortuous cervical canal (TC) was defined as hysteroscopic images of a TC with complex crypts and ridges [Fig. 2].
Fig. 2.
Hysteroscopic images, showing various characteristics of tortuous cervical canals, including with crypts and ridges.
Cervical stenosis (CS) was defined as following condition:
-
1.
A flexible hysteroscope fails to enter the external os (less than 3.8-mm in diameter) during the initial outpatient procedure.
-
2.
The affected part of the cervical canal or the entire canal from the external to the internal os [23] but without a TC.
Embryo transfer (ET)
The laboratory facilities, clinical strategy, and protocol for controlled ovarian hyperstimulation have been described [24,25]. Briefly, ETs were performed on day 3 or 5 (blastocyst) of development. Patients were placed in the lithotomy position and then exposed to the cervix with a speculum.
All ET procedures were performed by a single operator (K.C. Lan). Transabdominal ultrasound was performed by an assisted staff, with the bladder full, to visualize the endometrial lining and the endocervical angle. A Labotect (Labotect, GmbH, Germany) or Kitazato (Kitazato, Tokyo, Japan) catheter loaded with the embryos was inserted into the cervical os through the endocervical canal and into the uterine cavity. The embryos, suspended in 20 μL of transfer medium in a syringe, were gently injected into the uterine cavity by the embryologist, and the catheter was slowly withdrawn a few seconds later. After microscopic inspection of the catheter for retained embryos, the speculum was removed. The patient remained in supine position for 15 min.
ET difficulty
The ET procedure was defined as difficult if (i) it required additional instrumentation, such as a tenaculum; (ii) if it required additional time (more than 3min) or (iii) effort or firmer catheters; or (iv) if any resistance to negotiating the internal cervical os was encountered.
ET was defined as severely difficult if all four factors were present [19,26].
Statistics
Categorical variables (reported as proportions) were compared using the chi-square or Fisher's exact test, where appropriate. Accuracy was represented using sensitivity (true positive/true positive + false negative) and specificity (true negative/true negative + false positive). Continuous variables are expressed as mean ± standard deviation (SD). All p-values were two-sided with p < 0.05 considered statistically significant. The power was larger than 90% when we recruited 402 patients to detect a difference of proportion of 0.282 between the cervical tortuous group and the normal group, using the two-sided t-test with a significance level of 5%.
Results
A total of 650 women were scheduled for hysteroscopy at our institute prior to their first IVF/ICSI treatment cycle [Fig. 1]. The demographic characteristics and causes of infertility are shown in Table 1. The mean patient age was 35.7 ± 4.2 years. Forty-six (7.1%) patients failed OH because of marked CS and/or a tortuous canal. No procedure was aborted due to a patient's inability to tolerate the procedure, and none of the women experienced early or late complications of OH.
Table 1.
Demographic characteristics of the 650 women who underwent initial IVF/ICSI.
| Number who underwent IVF | 344 |
| Number who underwent ICSI | 306 |
| Age of female partner (years) | 35.7 ± 4.2 (21–48). |
| Body mass index (kg/m2) | 22.5 ± 3.4 (15.6–40.7) |
| Infertility (No) | |
| Primary | 368 |
| Secondary | 382 |
| Duration of infertility (years) | 3.9 ± 2.9 (1–20) |
| No. of mature or approaching mature oocytes retrieved | 6.6 ± 3.8 (1–22) |
| Endometrial thickness on day of hCG (mm) | 1.3 ± 0.2 |
| Estradiol (pg/mL) on hCG day | 2157.7 ± 1369.9 |
| Progesterone (pg/mL) on hCG day | 0.9 ± 0.6 |
| Number of indications | |
| Tubal factor | 108 |
| Male | 211 |
| Endometriosis | 33 |
| Ovulatory factor | 128 |
| Unexplained and others | 68 |
| Combined factors | 102 |
| Normal fertilization rate | 79.8% |
| Number of embryos transferred | 2.1 ± 0.7 (1–4) |
| Clinical pregnancy rate/transfer cycle | 40.2% (243/605) |
| Implantation rate | 26.0% (312/1198) |
Note: Values are mean ± SD (range), number, or proportion.
Abnormal sonographic findings were observed in 51 women (7.8%) in which submucous myoma, endometrial polyp, and endometrial hyperplasia were the most common. Abnormal hysteroscopic intacavitary findings were observed in 158 cases (24.3%) in which endometrial polyp, submucous myoma, and intrauterine adhesions were the most common. These results showed that TVS was specific (100%), but not sensitive (32.3%) compared to OH [Table 2] in the detection of major differences in endometrial polyps.
Table 2.
Transvaginal sonography (TVS) and hysteroscopic intracavitary findings in the 650 first IVF/ICSI women included in this study.
| Findings | TVS (n = 650) | Hysteroscopy (n = 650) |
|---|---|---|
| Endometrial polyps | 11(1.7%) | 88 (13.5%) |
| Submucosal myoma | 21 (3.2%) | 21 (3.2%) |
| Mullerian anomalies | 4 (0.6%) | 8 (1.2%) |
| Adhesions | 4 (0.6%) | 11(1.7%) |
| Cesearn section scar | 2 (0.3%) | 7 (1.1%) |
| Endometrial. hyperplasia. | 7 (1.1%) | 7 (1.1%) |
| Endometritis | 0 | 3 (0.5%) |
| Combined lesions | 2 (0.3%) | 13 (2.0%) |
| Total findings | 51 (7.8%)a | 158 (24.3%) |
Sensitivity, 32.3%; specificity, 100% versus hysteroscopy.
Hysteroscopic intracavitary pathologies were observed in 158 patients, including endometrial polyps in 88 patients (13.5%), submucosal myoma in 21 (3.2%), Mullerian duct anomalies in eight (1.2%), intrauterine adhesions in 11 (1.7%), chronic endometritis in 3 (0.5%), Cesearn section scar in 7 (1.1%), and endometrial hyperplasia in 7 (1.1%). The remaining 13 (2.0%) patients had more than one abnormality.
As shown in Fig. 1 and 45 patients had no available embryos for transfer and 605 underwent ET. Table 3A shows the cervical evaluation by hysteroscopy findings in these 605 ET patients. Intracavitary abnormal findings, but normal cervix, CS, and tortous cervical canal were observed in 147 (24.3%), 56 (8.6%), and 52 (6.6%) patients, respectively.
Table 3A.
Cervical evaluation by Hysteroscopy in the 605 women with embryos transfer included in this study.
| No. of unremarkable finding | 350 (57.8%) |
|---|---|
| No of abnormal Intracavity finding but with normal cervix | 147 (24.3%) |
| No. of Cervical stenosis | 56 (9.3%) |
| No. of Tortuous Cervival canal | 52 (8.6%) |
The relationship between hysteroscopy findings and transfer difficulty is shown in Table 3B. Patients with CS or TC, including the aforementioned OH failure, all had received ET. Among the remaining 605 transfer cycles, 39 (6.4%) were classified as difficult. Difficult ET was significantly more frequent in patients with CS or tortous cervical canal (25%) than in patients with intracavity lesions (2.0%) and normal patients (2.6%). Difficult ET was also significantly more frequent in patients with TC (30.7%) than in patients with CS (19.6%), but the frequency of severely difficult ET in these two groups was similar (11.6% vs. 14.2%).
Table 3B.
ET Findings of the 605 hysteroscopies included in this study.
| Normal | Abnormal Intracavity finding but with normal cervix | Cervix stenosis | Tortuous cervical canal | |
|---|---|---|---|---|
| Number of patients | 350 | 147 | 56 | 52 |
| Difficult ET | 2.6% (9) | 2.0% (3) | 19.6% (11) a | 30.7% (16) a,b |
| Severely difficult ET | 1.1% (4) | 0.7% (1) | 14.2% (8) a | 11.6% (6) a |
(n): number of patients.
a, bp < 0.05.
Compared with the normal and intrauterine groups.
Compared with the cervical stenosis group.
Discussion
The most appropriate diagnostic tests for the assessment of the uterine cavity in patients undergoing standard infertility evaluation in daily practice remain unclear. Although TVS and hysteroscopy are both utilized, hysteroscopy is generally considered the gold standard in the diagnosis of intrauterine pathology [11,27]. It is therefore necessary to identify subgroups that may benefit most from OH.
The present study found that TVS did not reveal any of the predefined intracavitary abnormalities revealed by hysteroscopy in 107 (16.5%) patients. The prevalence of such unsuspected intrauterine abnormalities, diagnosed by hysteroscopy prior to IVF, ranges from 20% to 45% [8,[13], [14], [15]], which is much higher than the 11% reported in a previous study [12]. These discrepancies may be due to differences in study design, patient inclusion criteria, and types of intrauterine pathologies included. Furthermore, differences in the prevalence of abnormalities may be due to variations in the interpretation of hysteroscopy findings. The most frequently detected abnormality in the current study was endometrial polyps, consistent with previous findings. However, the incidence of this abnormality (13.5%) was higher than that previously reported [1,3,6,12]. The effects on fertility of polyp number, size, and location remain unclear, as well as the effects of polypectomy [28]. To date, there is little evidence showing whether correction of endometrial polyps improves IVF outcomes.
We had 11 patients with IUA. Hysteroscopy is currently considered the gold standard treatment because it allows simultaneous diagnosis and treatment [29]. Currently, the treatment of IUA relies on the mechanical methods of surgical lysis and the barrier applied between to prevent adhesion reformation. Repeated hysteroscopy with adhesion prevention is still the gold standard and a known way to attain success in IUA treatment. As IUA has been questioned not merely as a surgical disease, sound knowledge of endometrial regeneration and adhesion prevention are needed to find better treatment strategies [27]. However, subfertile patients with Asherman's syndrome undergoing adhesiolysis should be appropriately informed about the risk of associated life-threatening complications and preterm delivery [30].
The ease of the ET procedure has been reported to influence its success [19]. There is no universal definition of difficult ET, complicating efforts to accurately compare subjective evaluations among studies [19,31]. The prevalence of difficult ET has been reported to be approximately 7% [19], similar to our finding. Compared with past OH studies, the present study highlights cervical findings associated with difficult ET. Strategies to overcome anatomic impediments to hysteroscopy have been proposed, but the study did not assess the relationship between overcoming these impediments and ET difficulty [23]. The anatomic features causing difficult transfer detected during assessments of the cervix and uterus byTVS, rigid hysteroscopy, and mock transfer [22], were similar to those detected using flexible OH in our study.
The prevalence of CS was recently reported to be 29.9% [23]. Using OH, we found that 108 (16.6%) patients had cervical lesions, including tight internal or external cervical os projecting ridges within the cervical canal, TC, or an acutely anteflexed or retroflexed uterus. ET can be complicated by tortuosity and/or stenosis of the cervical canal. In contrast to OH, cervical ultrasound elastography is less effective in predicting the ease of ET [32], and our results suggested that ultrasound was less effective in predicting cervical lesions.
Our results indicate that the rate of difficult ET is significantly higher in patients with cervical lesions than in those without them, especially in those with TC. Indeed, to optimize IVF outcomes, women in our infertility clinic have regularly undergone OH prior to IVF since 2011. Amelioration of cervical canal obstruction and optimization of the ET procedure has reduced the incidence of very difficult ET in patients with TC, making its incidence similar to that in patients in the CS subgroup.
Patients found to have CS or TC can undergo a destructive or invasive procedure, such as cervical canal resection by shaving or morcellation [33,34]. Our center does not favor these procedures. Rather, patients in our center with CS or TC are treated with cervical dilatation and mock ET, with or without the application of tenaculum and different hardness ET catheter. We found that these procedures lead to easier subsequent ultrasound-guided ET.
Two recent high-quality trials demonstrated that routine hysteroscopy did not improve live birth rates in infertile women undergoing initial IVF treatment or in those with a history of unsuccessful IVF treatment cycles but normal TVS findings [[35], [36], [37]]. However, an increasing number of experimental and clinical studies have emphasized the importance of uterine and intrauterine pathology for spontaneous and post-ART fertility [38]. Office hysteroscopy is a feasible, non-invasive and highly effective diagnostic and therapeutic procedure for allowing the resolution of female infertility related to these pathologies [39]. A systematic review and meta-analysis found moderate evidence that hysteroscopy increases pregnancy rate if performed before IVF, regardless of intrauterine abnormalities [4,16]. Although the efficacy of regular hysteroscopy prior to IVF has been questioned due to lack of definitive evidence, IVF itself is accompanied by psychological, physical, and financial burdens that increase with every failed IVF treatment cycle. Efforts should be made to minimize the number of cycles a patient must undergo, and the balance between the tolerability of OH and the burden that accompany IVF/ICSI must therefore be determined. Moreover, a thorough cost-effectiveness analysis would help physicians in creating an optimal strategy for each patient [40].
Although OH failed in 46 of our patients, recent technical and technological innovations, along with a higher level of experience of the operator, allowed for OH to overcome even severe CS. Thus, this significantly reduces the rate of failed procedures and the need for surgery and general anesthesia. Safety, ease of use, high diagnostic accuracy, and high patient tolerability have made OH an ideal procedure for patients undergoing IVF/ICSI [41]. In evaluating the relationship between cervical lesions and ET, we found that flexible hysteroscopy is a feasible and valid procedure when performed in an outpatient setting without anesthesia [42,43]. However, studies are needed to determine the efficacy of OH in specific subsets of patients undergoing IVF. In addition, randomized trials are needed to confirm the effectiveness of hysteroscopy in avoiding difficult ET.
The clinical application suggests that the OH can be used as a routine assessment tool before IVF treatment and ET. Patients with repeated IVF failures or a history of complicated procedures (such as artificial insemination; Hysterosalpingogram, or contraceptive device insertion) should use hysteroscopy as an assessment tool because these patients are highly suspected for difficult ET if they undergo IVF.
Our study highlighted the application of flexible OH for difficult ET and evaluated the frequency of these characteristics as causes of difficulty during ET by a single expert operator, and to propose a detailed review of this subject that can be used in clinical practice. However, the retrospective design, the conduction in a single center, the small number of patients recruited, and a slightly subjective definition of ET difficulty and cervical findings are limitations of our study. In addition, our study involved only one operatior in order to avoid inter-personal differences.
Conclusion
In conclusion, this study showed that OH can identify cervical lesions associated with difficult ET in women undergoing initial IVF/ICSI procedures. Cervical dilatation and mock ET (with or without the application of tenaculum) in patients with cervical lesions result in easier subsequent ultrasound-guided ET. Routine OH prior to IVF can be cost-effective in avoiding difficult ET.
Ethics approval and consent to participate
Written informed consent was obtained from all patients, and this study was approved by the Ethics Committee of CGMH and the local institutional review board (CGMH201601539B0 and 201601539B0C601).
Funding
This study was supported by the grants CMRPG8G0071-73 and CMRPG8J1131 from the Chang Gung Memorial Hospital.
Conflicts of interest
Drs. Kuo -Chung Lan and Yu-Che Ou and Kuan-Hui Huang have no conflicts of interest or financial ties to disclose.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.El-Mazny A., Abou-Salem N., El-Sherbiny W., Saber W. Outpatient hysteroscopy: a routine investigation before assisted reproductive techniques? Fertil Steril. 2011;95:272–276. doi: 10.1016/j.fertnstert.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Surrey E.S. Should diagnostic hysteroscopy be performed before in vitro fertilization-embryo transfer? J Minim Invasive Gynecol. 2012;19:643–646. doi: 10.1016/j.jmig.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Kilic Y., Bastu E., Ergun B. Validity and efficacy of office hysteroscopy before in vitro fertilization treatment. Arch Gynecol Obstet. 2013;287:577–581. doi: 10.1007/s00404-012-2584-z. [DOI] [PubMed] [Google Scholar]
- 4.Pundir J., Pundir V., Omanwa K., Khalaf Y., El-Toukhy T. Hysteroscopy prior to the first IVF cycle: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:151–161. doi: 10.1016/j.rbmo.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Karayalcin R., Ozyer S., Ozcan S., Uzunlar O., Gurlek B., Moraloglu O., et al. Office hysteroscopy improves pregnancy rates following IVF. Reprod Biomed Online. 2012;25:261–266. doi: 10.1016/j.rbmo.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Elsetohy K.A., Askalany A.H., Hassan M., Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Arch Gynecol Obstet. 2015;291:193–199. doi: 10.1007/s00404-014-3397-z. [DOI] [PubMed] [Google Scholar]
- 7.Bakas P., Hassiakos D., Grigoriadis C., Vlahos N., Liapis A., Gregoriou O. Role of hysteroscopy prior to assisted reproduction techniques. J Minim Invasive Gynecol. 2014;21:233–237. doi: 10.1016/j.jmig.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Karayalcin R., Ozcan S., Moraloglu O., Ozyer S., Mollamahmutoglu L., Batioglu S. Results of 2500 office-based diagnostic hysteroscopies before IVF. Reprod Biomed Online. 2010;20:689–693. doi: 10.1016/j.rbmo.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Demirol A., Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod Biomed Online. 2004;8:590–594. doi: 10.1016/s1472-6483(10)61108-x. [DOI] [PubMed] [Google Scholar]
- 10.Rama Raju G.A., Shashi Kumari G., Krishna K.M., Prakash G.J., Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006;274:160–164. doi: 10.1007/s00404-006-0174-7. [DOI] [PubMed] [Google Scholar]
- 11.Bozdag G., Aksan G., Esinler I., Yarali H. What is the role of office hysteroscopy in women with failed IVF cycles? Reprod Biomed Online. 2008;17:410–415. doi: 10.1016/s1472-6483(10)60226-x. [DOI] [PubMed] [Google Scholar]
- 12.Fatemi H.M., Kasius J.C., Timmermans A., van Disseldorp J., Fauser B.C., Devroey P., et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod. 2010;25:1959–1965. doi: 10.1093/humrep/deq150. [DOI] [PubMed] [Google Scholar]
- 13.Balmaceda J.P., Ciuffardi I. Hysteroscopy and assisted reproductive technology. Obstet Gynecol Clin N Am. 1995;22:507–518. [PubMed] [Google Scholar]
- 14.La Sala G.B., Montanari R., Dessanti L., Cigarini C., Sartori F. The role of diagnostic hysteroscopy and endometrial biopsy in assisted reproductive technologies. Fertil Steril. 1998;70:378–380. doi: 10.1016/s0015-0282(98)00147-2. [DOI] [PubMed] [Google Scholar]
- 15.Hinckley M.D., Milki A.A. 1000 office-based hysteroscopies prior to in vitro fertilization: feasibility and findings. J Soc Laparoendosc Surg. 2004;8:103–107. [PMC free article] [PubMed] [Google Scholar]
- 16.Di Spiezio Sardo A., Di Carlo C., Minozzi S., Spinelli M., Pistotti V., Alviggi C., et al. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta-analysis. Hum Reprod Update. 2016;22:479–496. doi: 10.1093/humupd/dmw008. [DOI] [PubMed] [Google Scholar]
- 17.Miura S.K.S. Effect of insulin and growth hormone on rat uterine RNA synthesis. Proc Soc Exp Biol Med. 1970;133:882–885. doi: 10.3181/00379727-133-34586. [DOI] [PubMed] [Google Scholar]
- 18.Pellicer A., Galliano D. Hysteroscopy before IVF: can it improve outcomes? Lancet. 2016;387:2578–2579. doi: 10.1016/S0140-6736(16)00549-3. [DOI] [PubMed] [Google Scholar]
- 19.Kava-Braverman A., Martinez F., Rodriguez I., Alvarez M., Barri P.N., Coroleu B. What is a difficult transfer? Analysis of 7,714 embryo transfers: the impact of maneuvers during embryo transfers on pregnancy rate and a proposal of objective assessment. Fertil Steril. 2017;107:657–663.e1. doi: 10.1016/j.fertnstert.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Practice Committee of the American Society for Reproductive Medicine Electronic address: ASRM@asrm.org, practice committee of the American society for reproductive M. Performing the embryo transfer: a guideline. Fertil Steril. 2017;107:882–896. doi: 10.1016/j.fertnstert.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Noyes N., Licciardi F., Grifo J., Krey L., Berkeley A. In vitro fertilization outcome relative to embryo transfer difficulty: a novel approach to the forbidding cervix. Fertil Steril. 1999;72:261–265. doi: 10.1016/s0015-0282(99)00235-6. [DOI] [PubMed] [Google Scholar]
- 22.Larue L., Keromnes G., Massari A., Roche C., Bouret D., Cassuto N.G., et al. Anatomical causes of difficult embryo transfer during in vitro fertilization. J Gynecol Obstet Hum Reprod. 2017;46:77–86. doi: 10.1016/j.jgyn.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Bettocchi S., Bramante S., Bifulco G., Spinelli M., Ceci O., Fascilla F.D., et al. Challenging the cervix: strategies to overcome the anatomic impediments to hysteroscopy: analysis of 31,052 office hysteroscopies. Fertil Steril. 2016;105:e16–e17. doi: 10.1016/j.fertnstert.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Tsai Y.R., Huang F.J., Lin P.Y., Kung F.T., Lin Y.J., Lin Y.C., et al. Progesterone elevation on the day of human chorionic gonadotropin administration is not the only factor determining outcomes of in vitro fertilization. Fertil Steril. 2015;103:106–111. doi: 10.1016/j.fertnstert.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Lan K.C., Huang F.J., Lin Y.C., Kung F.T., Hsieh C.H., Huang H.W., et al. The predictive value of using a combined Z-score and day 3 embryo morphology score in the assessment of embryo survival on day 5. Hum Reprod. 2003;18:1299–1306. doi: 10.1093/humrep/deg239. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar M.A., Netherton R., Majumder K., Edi-Osagie E., Sajjad Y. Methods employed to overcome difficult embryo transfer during assisted reproduction treatment. Arch Gynecol Obstet. 2015;292:255–262. doi: 10.1007/s00404-015-3657-6. [DOI] [PubMed] [Google Scholar]
- 27.Yen C.F., Chou H.H., Wu H.M., Lee C.L., Chang T.C. Effectiveness and appropriateness in the application of office hysteroscopy. J Formos Med Assoc. 2019;118:1480–1487. doi: 10.1016/j.jfma.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Kodaman P.H. Hysteroscopic polypectomy for women undergoing IVF treatment: when is it necessary? Curr Opin Obstet Gynecol. 2016;28:184–190. doi: 10.1097/GCO.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 29.Di Guardo F., Della Corte L., Vilos G.A., Carugno J., Torok P., Giampaolino P., et al. Evaluation and treatment of infertile women with Asherman syndrome: an updated review focusing on the role of hysteroscopy. Reprod Biomed Online. 2020;41:55–61. doi: 10.1016/j.rbmo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N., Takeuchi R., Izuchi D., Yuge N., Miyazaki M., Yasunaga M., et al. Hysteroscopic adhesiolysis for patients with Asherman's syndrome: menstrual and fertility outcomes. Reprod Med Biol. 2013;12:159–166. doi: 10.1007/s12522-013-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips J.A., Martins W.P., Nastri C.O., Raine-Fenning N.J. Difficult embryo transfers or blood on catheter and assisted reproductive outcomes: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2013;168:121–128. doi: 10.1016/j.ejogrb.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Stanziano A., Caringella A.M., Cantatore C., Trojano G., Caroppo E., D'Amato G. Evaluation of the cervix tissue homogeneity by ultrasound elastography in infertile women for the prediction of embryo transfer ease: a diagnostic accuracy study. Reprod Biol Endocrinol. 2017;15:64. doi: 10.1186/s12958-017-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salari B.W., Bhagavath B., Galloway M.L., Findley A.D., Yaklic J.L., Lindheim S.R. Hysteroscopic morcellator to overcome cervical stenosis. Fertil Steril. 2016;106:e12–e13. doi: 10.1016/j.fertnstert.2016.07.1091. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y.H., Hwang J.L., Huang L.W., Seow K.M., Chen H.J., Tzeng C.R. Efficacy of hysteroscopic cervical resection for cervical stenosis. J Minim Invasive Gynecol. 2013;20:836–841. doi: 10.1016/j.jmig.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Smit J.G., Kasius J.C., Eijkemans M.J.C., Koks C.A.M., van Golde R., Nap A.W., et al. Hysteroscopy before in-vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet. 2016;387:2622–2629. doi: 10.1016/S0140-6736(16)00231-2. [DOI] [PubMed] [Google Scholar]
- 36.El-Toukhy T., Campo R., Khalaf Y., Tabanelli C., Gianaroli L., Gordts S.S., et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): a multicentre, randomised controlled trial. Lancet. 2016;387:2614–2621. doi: 10.1016/S0140-6736(16)00258-0. [DOI] [PubMed] [Google Scholar]
- 37.Smit J.G., Kasius J.C., Eijkemans M.J., Koks C.A., Van Golde R., Oosterhuis J.G., et al. The inSIGHT study: costs and effects of routine hysteroscopy prior to a first IVF treatment cycle. A randomised controlled trial. BMC Wom Health. 2012;12:22. doi: 10.1186/1472-6874-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galliano D., Bellver J., Diaz-Garcia C., Simon C., Pellicer A. ART and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update. 2015;21:13–38. doi: 10.1093/humupd/dmu047. [DOI] [PubMed] [Google Scholar]
- 39.Chiofalo B., Palmara V., Vilos G.A., Pacheco L.A., Lasmar R.B., Shawki O., et al. Reproductive outcomes of infertile women undergoing "see and treat" office hysteroscopy: a retrospective observational study. Minim Invasive Ther Allied Technol. 2019;30:147–153. doi: 10.1080/13645706.2019.1705352. [DOI] [PubMed] [Google Scholar]
- 40.Kasius J.C., Eijkemans R.J., Mol B.W., Fauser B.C., Fatemi H.M., Broekmans F.J. Cost-effectiveness of hysteroscopy screening for infertile women. Reprod Biomed Online. 2013;26:619–626. doi: 10.1016/j.rbmo.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Bahadur A., Malhotra N., Singh N., Gurunath S., Mittal S. Comparative study on the role of diagnostic hysteroscopy in evaluation of the uterine cavity prior to in vitro fertilization in a developing country. Arch Gynecol Obstet. 2013;288:1137–1143. doi: 10.1007/s00404-013-2842-8. [DOI] [PubMed] [Google Scholar]
- 42.Wang C.J., Mu W.C., Yuen L.T., Yen C.F., Soong Y.K., Lee C.L. Flexible outpatient hysterofibroscopy without anesthesia: a feasible and valid procedure. Chang Gung Med J. 2007;30:256–262. [PubMed] [Google Scholar]
- 43.Kuroda K., Kitade M., Kikuchi I., Kumakiri J., Matsuoka S., Tokita S., et al. A new instrument: a flexible hysteroscope with narrow band imaging system: optical quality comparison between a flexible and a rigid hysteroscope. Minim Invasive Ther Allied Technol. 2011;20:263–266. doi: 10.3109/13645706.2010.548935. [DOI] [PubMed] [Google Scholar]