Abstract
Background
Aortic valve stenosis (AS) is a common, lethal cardiovascular disease. There is no cure except the valve replacement at last stage. Therefore, an understanding of the detail mechanism is imperative to prevent and intervene AS. Metabolic syndrome (MetS) is one of the major risk factors of AS whereas fructose overconsuming tops the list of MetS risk factors. However, whether the fructose under physiological level induces AS is currently unknown.
Methods
The human valve interstitial cells (hVICs), a crucial source to develop calcification, were co-incubated with fructose at 2 or 20 mM to mimic the serum fructose at fasting or post-fructose consumption, respectively, for 24 h. The cell proliferation was evaluated by WST-1 assays. The expressions of osteogenic and fibrotic proteins, PI3K/AKT signaling, insulin receptor substrate 1 and mitochondrial dynamic proteins were detected by Western blot analyses. The mitochondrial oxidative phosphorylation (OXPHOS) was examined by Seahorse analyzer.
Results
hVICs proliferation was significantly suppressed by 20 mM fructose. The expressions of alkaline phosphatase (ALP) and osteocalcin were enhanced concurrent with the upregulated PI3K p85, AKT, phospho(p)S473-AKT, and pS636-insulin receptor substrate 1 (p-IRS-1) by high fructose. Moreover, ATP production capacity and maximal respiratory capacity were enhanced in the high fructose groups. Synchronically, the expressions of mitochondrial fission 1 and optic atrophy type 1 were increased.
Conclusions
These results suggested that high fructose stimulated the osteogenic differentiation of hVICs via the activation of PI3K/AKT/mitochondria signaling at the early stage. These results implied that high fructose at physiological level might have a direct, hazard effect on the progression of AS.
Keywords: Fructose, Aortic valve stenosis, Valve interstitial cells, PI3K/AKT signaling, Oxidative phosphorylation, Mitochondrial dynamics
At a glance of commentary
Scientific background on this subject
Aortic stenosis (AS) is a common, lethal cardiovascular disease. There is no cure except the valve replacement at the last stage. Metabolic syndrome (MetS) is one of the significant risk factors of AS whereas fructose overconsuming is a major risk factor of MetS. However, whether fructose under physiological level induces AS is currently unknown.
What this study adds to this field?
In this study, we demonstrated that high fructose promotes the osteogenic differentiation of human valve interstitial cells (hVICs) via activating the PI3K/AKT signaling. By increasing the expressions of FIS1 and OPA1, the activated PI3K/AKT signaling enhanced mitochondrial respiration leading to the osteogenic differentiation of hVICs.
Aortic valve stenosis (AS), a status of aortic valve disease, is a common, lethal cardiovascular disease in the elderly. The development of AS is often subtle at first and progresses over time until months or years after the beginning. Once the progression of AS initiated, it is considered as irreversible. Fibrosis and calcification are the two hallmarks of aortic stenosis whereas valve interstitial cells (VICs) differentiated myofibroblasts and osteoblasts are considered as their origin, respectively [1]. The limited knowledge makes AS currently unable to be earlier diagnosis for intervention. Therefore, a precise delineation of the mechanism of the AS progression is imperative.
Several risk factors are involved in the progression of AS including age, hypertension and dyslipidemia [[2], [3], [4]] which are the major criteria of metabolic syndrome [5]. Under physiological status, insulin binds to membrane-bonded insulin receptor to phosphorylate insulin receptor substrate (IRS)-1 for the activation of the phosphoinositide 3-kinase (PI3K)/phosphoinositide-dependent kinase 1 (PDK-1)/Akt pathway for glucose uptake [6] in response to the carbohydrate supply. Any disturbance in this cellular pathway, for instance, the upregulation of IRS-1 and PI3K p85, could initiate the progression of metabolic syndrome [7]. Notably, the PI3K/AKT signaling promotes osteogenic differentiation of mesenchymal stem cells [8,9] though the downstream signaling is inconclusive.
AKT signaling regulate mitochondrial oxidative phosphorylation [10,11]. Mitochondrion is the major source of cellular energy support by oxidative phosphorylation (OXPHOS). Intriguingly, the activation of OXPHOS drives calcium accumulation in mitochondria (Reviewed by Carafoli 2010 [12]). Recently, accumulating evidence indicated that active mitochondria positively regulate osteogenic differentiation of mesenchymal stem cells [13,14]. It is conceivable that the AKT-activated mitochondrial OXPHOS may play roles in the osteogenic differentiation in hVICs.
High activation results in large-scale impairment of mitochondria. To maintain the quality, mitochondria are highly dynamics and are strictly regulated by mitochondrial fission/fusion, and mitochondrial autophagy (mitophagy) in response to environmental nutrition [15,16]. Mitochondrial fission is controlled by dynamin-related protein 1 (DRP1), fission protein 1 (FIS1) and mitochondrial fission factor (Mff). Overnutrition may result in mitochondrial fission and the increased number of damaged mitochondria. The impaired mitochondria are divided by fission, engulfed by mitophagy [17,18]. In mammals, the best-studied proteins involved in mitochondrial fusion are the optic atrophy type 1 (OPA1), mitofusin 1 (Mfn1) and Mfn2. Mitophagy is responsible for the clearance of damaged mitochondria. The best-studied proteins involved in mitophagy are the PTEN-induced kinase 1 (PINK1) and Parkin [19]. Under physiological status, PINK1 is cleaved by presenilin-associated rhomboid-like serine protease (PARL) in mitochondria [20] while full length PINK1 recruits Parkin to initiate mitophagy [19]. However, rare study has been focused on the role of valve mitochondrial fission/fusion and mitophagy in response for the nutrient supply.
Fructose is a common sweetener in nature fruits and in our daily desserts. Overconsumption of fructose is a pressing worldwide health issue. A large body of evidence from both human and animals suggest that high fructose intake induces metabolic syndrome (MetS) [[21], [22], [23], [24], [25]] while metabolic syndrome is one of the major risk factor of valve stenosis [5]. It is conceivable that excessive fructose may contribute to the progression of valve stenosis. Moreover, high fructose diet-altered mitochondrial function has been documented in MetS [26]. In this study, we conducted the in vitro model to investigate the initiation of valve stenosis at different time points by using the human valve interstitial cells (hVICs) co-incubated with various concentrations of fructose. The levels of cell proliferation, cell fibrotic markers (e.g. α-smooth muscle actin, and collagen III), cell osteogenic markers (e.g. alkaline phosphatase, and osteocalcin), the PI3K/AKT signaling, mitochondrial OXPHOS, and mitochondrial dynamic proteins were detected by Western blot analysis. The level of calcium deposition was evaluated by Alizarin Red staining.
Materials and methods
Human valve interstitial cell culture
The human valve interstitial culture cells (hVICs) were purchased from Innoprot (Bizkaia Spain) and the hVICs specific medium (Fibroblast Medium II) were used. The cells from passage five were seeded into 6-cm culture dish coated with Poly-l-Lysine at a density of 1.0 × 105 cells/mL and grow for 4 h in the serum-free medium with FGF for further study. Cells were incubated in a humidified incubator at 37 °C in 5% CO2. After dripping fructose (0, 0.2, 2, or 20 mM) into cells, the cells were incubated at 37 °C in a CO2 incubator for 24, 48, 72, or 96 hours (h) prior test.
WST-1 cell proliferation assay
WST-1 cell proliferation assay kit (Takara Bio Inc., Shiga, Japan) was used to evaluate the cell proliferation following the guideline of the kit. 1 × 104 hVICs were seeded into a 96-well flat-bottomed plate for 24 h at 37 °C with 5% CO2 then subjected to various fructose (0, 0.2, 2 or 20 mM). At 24, 48, 72 and 96 h incubation, the cells were washed with PBS and replaced with 100 μL fresh medium. After washed by PBS, 100 μL of WST-1 Reagent were added to each well, and the plate was incubated for 2 h on an orbital shaker at room temperature. The Luminescence was detected by spectrophotometer. The luminescence of wells with no reagent were measured as Blank control. The value of Blank control were deducted from the values of the experimental wells. Values of proliferation of the treated-cells were expressed as a percentage of that from corresponding control cells. All experiments were repeated in triplicates.
Mitochondrial respiratory rate detection by the XF analyzer
XF24 Extracellular Flux Analyzer were used to perform all XF assays (Seahorse Bioscience; MA, USA). The sensor cartridge contains four reagent delivery chambers per well for injecting compounds, including inhibitors of mitochondrial respiratory complex I (rotenone), III (antinomycin A, AMA), and V (oligomycin) as well as an uncoupling agent that disrupts the proton gradient (FCCP), into the wells during an assay to evaluate the rates of O2 consumption rate (OCR). 1 × 105 hVICs were seeded into 24-well XF24 plates for overnight attachment (except for background correction wells). After 24 h co-incubation with fructose (e.g. 0, 2, 20 mM), culture medium was washed out by PBS and substituted by 1X MAS buffer (Seahorse Bioscience) with substrate. The plate was then transferred to the XF24 instrument to initiate the measurement.
Total protein isolation
For Western blotting analysis, hVICs from each treatment were harvested after 3 times wash with PBS. Samples were homogenized with a Dounce grinder with a tight pestle in ice-cold lysis buffer (15 mM HEPES, pH 7.2, 60 mM KCl, 10 mM NaCl, 15 mM MgCl2, 250 mM Sucrose, 1 mM EGTA, 5 mM EDTA, 1 mM PMSF, 2 mM NaF, 4 mM Na3VO4). A protease inhibitor cocktail (Sigma–Aldrich) was included in the isolation buffer to prevent protein degradation. The lysate was stored at −80 °C for later use. The concentration of the total protein extracted was estimated by Micro Bicinchoninic acid (BCA) Protein Assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Western blotting
Samples from each group contain equivalent total protein concentration. Total proteins were separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (SDS-PAGE), using 8, 10 or 12% gels and a running buffer of 24 mM Tris–HCl, 0.19 M glycine, 0.5% SDS, pH 8.3. The electrophoretical proteins were transferred onto polyvinylidene difluoride (PVDF) membrane (Immobilon-P membrane; Millipore; Bedford, MA, USA) and probed with specific antibodies against α-SMA (1:1000, Abcam, Cambridge, UK), MMP9 (1:1000, Abcam), ALP (1:1000, Abcam), osterocalcin (1:1000, Abcam), IRβ (1:1000, Abcam), phospho-IRS1 (S312) (1:1000, Abcam), PI3K p85 (1:1000, Abcam), AKT (1:1000, Abcam), phospho-AKT (T308) (1:1000, Abcam) and phospho-AKT (S473) (1:1000, Abcam). Membranes were then incubated with appropriate horseradish peroxidase–conjugated secondary antibody. Specific antibody–antigen complex was detected using an enhanced chemiluminescence Western Blot detection system (Thermofisher Bioscience). The amounts of detected proteins were quantified by ImageJ software (NIH, MD, USA), and were normalized by β-actin protein.
The alizarin red staining
Human valve interstitial cells from different treatments were stained by alizarin red. The cells were fixed with 10% formaldehyde for 1 h. The cells was washed with PBS (pH 7.4) for 3 times followed by 3 times wash with PBS (pH 4.1). After incubation with alizarin red solution for 1 h, excessive dye was removed by washing with PBS (pH 4.1) then PBS (pH 7.4). The calcified nodules were observed by Olympus light microscope (IX51, Tokyo, Japan).
Statistical analysis
Data are expressed as means ± SEM. Nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method was used for comparisons between groups. The differences were considered statistically significant when p < 0.05. Calculations were performed by GraphPad Prism (version 5) software (GraphPad Software, San Diego, CA).
Results
Fructose suppressed proliferation of human valve interstitial cells at high concentration
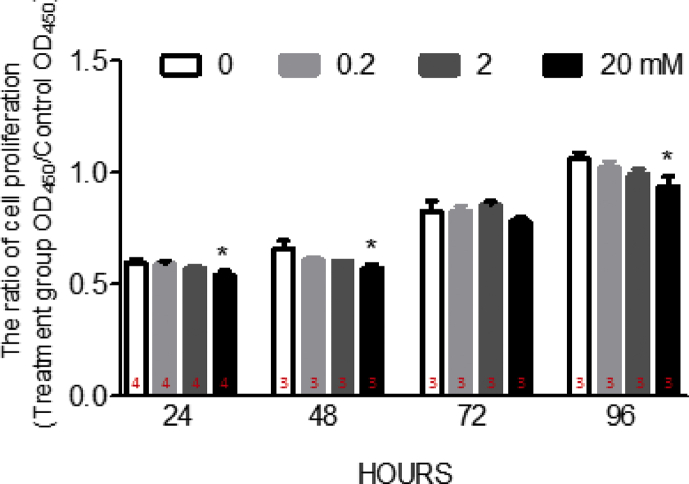
Fructose overconsuming is one of the major risk factor of metabolic syndrome [27,28], which plays important role in the progression of aortic valve stenosis [3,29,30]. The valve interstitial cell (VIC) is the predominant cell type in the aortic valve [31,32]. As a fibroblast-like cell type, VICs is responsible for the valvular calcification [33,34]. However, whether high fructose provides direct effect to drive the human valve interstitial cells (hVICs) to calcification is unknown. According to previous study, the fasting serum fructose concentration is 1.9 ± 0.4 mM, and the peak of postprandial serum fructose concentration is 17.2 ± 1.1 mM in healthy volunteers [35].Therefore, the fructose concentration used for in vitro study were ranged between 0 and 20 mM. To evaluate whether fructose impairs the cell proliferation of hVICs, the cells were co-incubated with various fructose concentration (e.g. 0, 0.2, 2, 20 mM) for 24, 48, 72 or 96 h for proliferation assays. The results indicated that the hVICs proliferation was significantly suppressed in these groups with extra added 20 mM fructose at 24, 48 and 96 h [Fig. 1]. At lower fructose dosage (e.g. 0.2 or 2 mM), hVICs proliferation showed the trends of decrement without statistical significant. Based on these results, the time point of 24-h was selected to further reveal the underlying mechanism of fructose (0, 2, 20 mM)-induced impairment.
Fig. 1.
The cell proliferation of human valve interstitial cells was suppressed by fructose at high concentration. The cell proliferation of human valve interstitial cells (hVICs) after 24, 48, 72 and 96 h incubated with 0, 0.2, 2, or 20 mM fructose. Values are mean ± SEM, n = 3–4 in each experimental group. The sample size of each group was noted on the bar. ∗p < 0.05 versus 0 mM fructose, time-matched group using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method.
Fructose induced the expressions of alkaline phosphatase and osteocalcin in human valve interstitial cells at high concentration
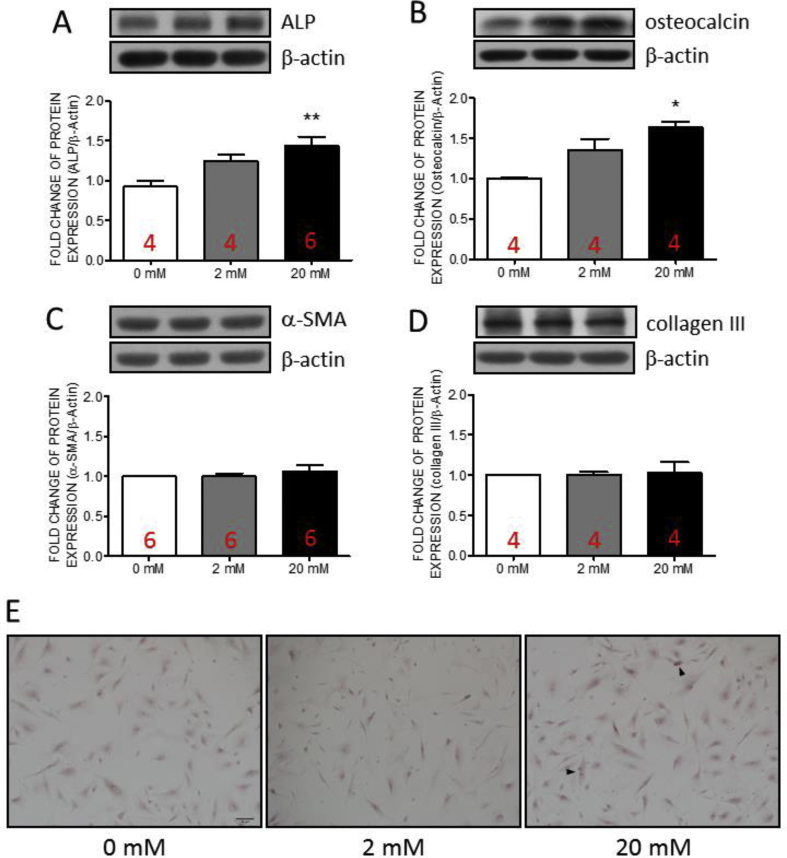
The VICs can be differentiated as osteoblast or myofibroblasts. Valvular calcification is the primary cause of AS. Alkaline phosphatase (ALP) is an early calcification marker, and osteocalcin is an indicator of later-stage calcification [31,32]. We, therefore, examined the expressions of ALP and osteocalcin in hVICs co-incubated with various fructose concentration (0, 2, and 20 mM) at 24-h. The results indicated that the expressions of ALP [Fig. 2A] and osteocalcin [Fig. 2B] were significantly increased at high fructose (20 mM) group. At lower fructose group (2 mM), the expressions of ALP or osteocalcin showed the trends of increment without statistically significant. On the contrary, the expressions of fibrotic markers, α-smooth muscle actin (α-SMA, Fig. 2C) and collagen III [Fig. 2D], showed no significant difference between groups. These results suggested that high fructose stimulation maybe dominate the progression of calcification in hVICs rather than fibrosis. Further, the results of the Alizarin-Red staining indicated that the mineral deposition was slightly increased in the high fructose-dose (20 mM) group when compared with the control (0 mM) and the low fructose-dose (2 mM) groups [Fig. 2E].
Fig. 2.
The expressions of alkaline phosphatase and osteocalcin as well as mineral deposition in human valve interstitial cells were increased by fructose at high concentration. Representative gels and densitometric analyses of (A) alkaline phosphatase (ALP) (B) osteocalcin (C) α-smooth muscle actin (α-SMA) and (D) collagen III detected at 24 h incubated with 0, 2, or 20 mM fructose (E) Mineral deposition detected by Alizarin Red staining. Values are mean ± SEM, n = 4–6 in each experimental group. The sample size of each group was noted on the bar. ∗p < 0.05 versus 0 mM fructose group using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method. Arrow head: mineral deposition. Scale: 100 μm.
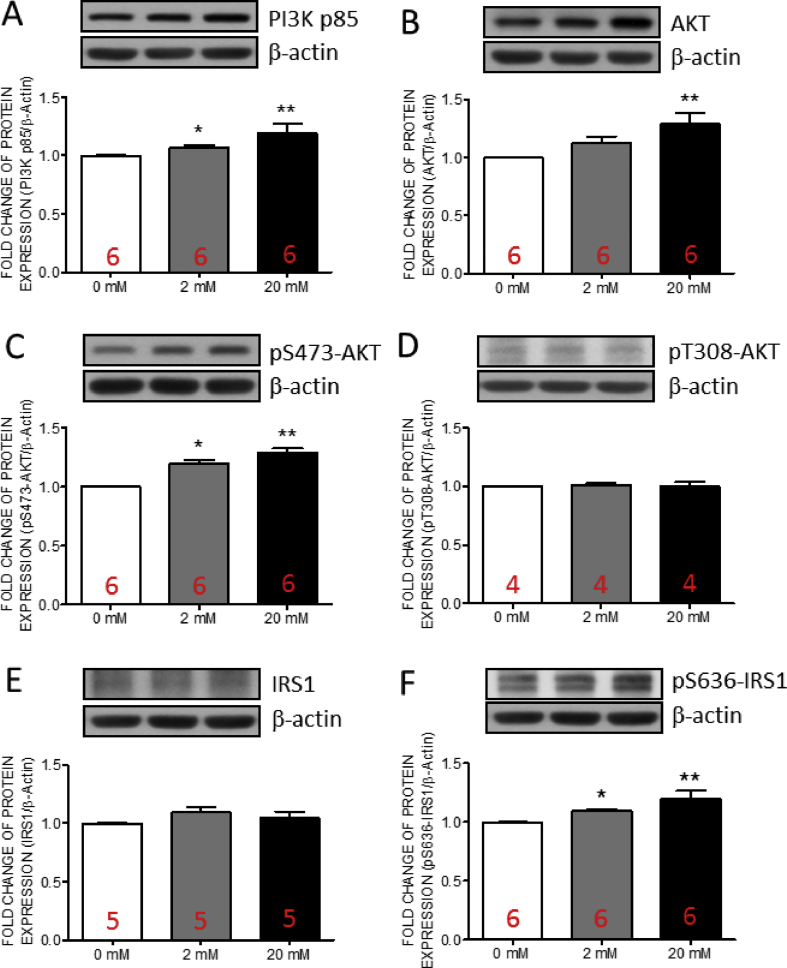
Fructose induced the expression of PI3K/AKT signaling and serine phosphorylation of insulin receptor substrate-1 in human valve interstitial cells at high concentration
The activation of PI3K/AKT signaling contributes to the osteogenic differentiation [8,9]. In addition, the increase of p85 subunit of phosphatidylinositol 3-kinase (PI3K) involves in the progression of MetS [7]. We detected the expressions of p85 subunit of PI3K, AKT and phospho(p)-AKT in hVICs by Western blotting. The results indicated that the expressions of PI3K p85 [Fig. 3A], AKT [Fig. 3B] and pS473-AKT [Fig. 3C] were significantly increased at high fructose group while no significant change was detected in the expression of pT308-AKT [Fig. 3D]. At lower fructose group, only the expressions of pS473-AKT [Fig. 3C] was significantly increased whereas PI3K p85 [Fig. 3A] and AKT [Fig. 3B] showed the trends of increment without statistically significant. These results suggested that PI3K/AKT cascade may mediate the high fructose-triggered progression of osteogenesis in hVICs at early stage.
Fig. 3.
The expressions of insulin receptor β and phospho-S636 insulin receptor substrate 1 in human valve interstitial cells were increased by fructose at high concentration. Representative gels and densitometric analyses of (A) PI3K p85 (B) total AKT (C) phospho(p)-S473-AKT (D) pT308-AKT (E) insulin receptor substrate 1 (IRS1), and (F) p-S636 IRS1 detected at 24 h incubated with 0, 2, or 20 mM fructose. Values are mean ± SEM, n = 4–6 in each experimental group. The sample size of each group was noted on the bar. ∗p < 0.05, ∗∗p < 0.01 versus 0 mM fructose group using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method.
The activated AKT exhibits the capability to phosphorylate insulin receptor substrate-1 (IRS-1) leading to the attenuation of T308-AKT phosphorylation [36]. Therefore, we examined the expressions of IRS-1 and p-IRS1 in hVICs by Western blotting. The expression of pS636-IRS1 [Fig. 3F] were significantly increased at high fructose group while no significant change was detected in the expression of IRS1 [Fig. 3E]. At lower fructose group, the expression of pS636-IRS1 [Fig. 3F] showed the trends of increment without statistically significant. These results provided the possibility that high fructose-increased pS473-AKT may in turn activate IRS-1 to suppress the phosphorylation of T308-AKT in hVICs.
Fructose enhanced mitochondrial oxygen consumption rate in human valve interstitial cells at high concentration
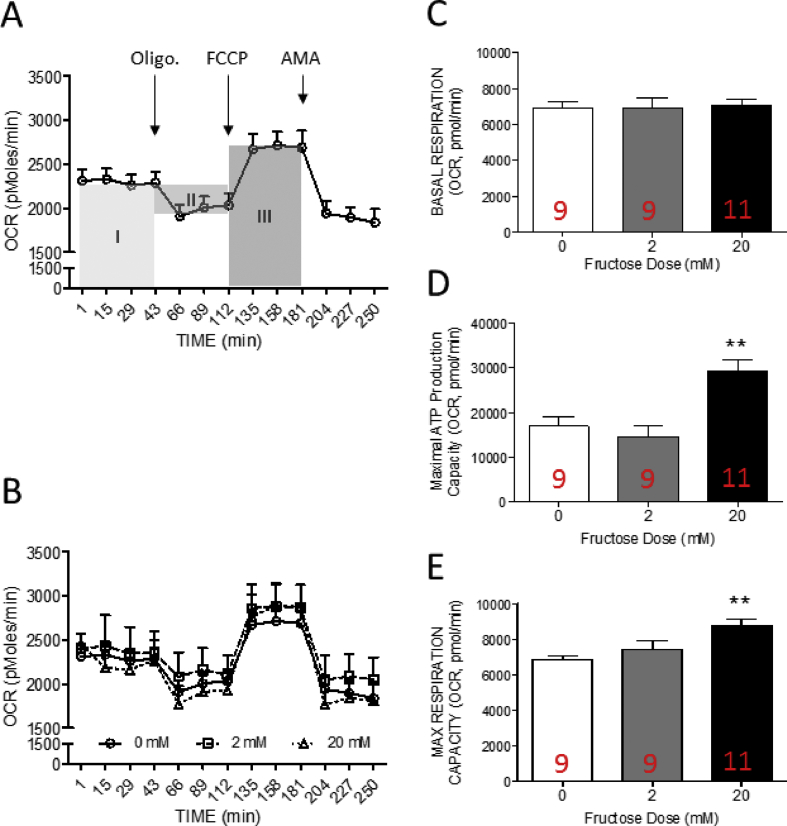
The activation of AKT signaling promotes mitochondrial oxidative phosphorylation [10,11]. Intriguingly, the activation of mitochondrial respiration enhance calcium accumulation [12]. Oxygen consumption rate is an important index of mitochondrial aerobic respiration. To examine whether the mitochondrial respiration was altered by high fructose incubation, the oxygen consumption rate (OCR) of each group was measured by the XF24 Extracellular Flux Analyzer (Seahorse). The maximal respiratory capacity, basal respiratory capacity, and ATP production capacity of hVICs were further dissected from OCR curve.
The results indicated that OCR of hVICs was reduced to ∼78% of baseline rates after oligomycin A (a mitochondrial ATP synthase inhibitor) in control group (common medium with 0 mM fructose) indicating that ∼22% of oxygen consumption was related to ATP production. Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) is a mitochondrial protonophoric uncoupler. FCCP application eliminated the proton gradient across mitochondrial inner membrane and enhanced OCR to the maximal of baseline rate (∼117.15%). These results suggested that the maximal respiratory capacity of hVICs is about 1.2 times higher than the basal respiration [Fig. 3A and B]. In the high fructose (20 mM) group, the FCCP-treatment significantly decreased the OCR values when compared with the control group [Fig. 3B]. These results indicated that the capacity of maximal (∼33.69%) oxygen consumption were enhanced by high fructose co-incubation [Fig. 3B].
To further evaluate the detail alteration in mitochondrial OXPHOS, the elements of oxygen consumption capacity were dissected from the area under curve of OCR as previous study described [[37], [38], [39]]. The area under curve before oligomycin A (Oligo.) treatment (area I) was defined as the basal respiration. The values between basal state and oligomycin A treatment of OCR curve and the time-period between oligomycin and FCCP treatment (area II) is regarded as the ATP production capacity of the cells. The area under curve between the oligomycin A and antinomycin A (AMA) treatment (area III) was defined as the maximal respiration capacity. The results indicated that ATP production capacity [Fig. 3D] and maximal respiratory capacity [Fig. 3E] were significantly increased in the high fructose group while the basal respiration [Fig. 3C] showed no significant difference between groups. These results suggested that high fructose treatment induced oxidative phosphorylation in the hVICs. Fig. 4.
Fig. 4.
The mitochondrial oxygen consumption rate in human valve interstitial cells were enhanced by fructose at high concentration (A) Schematic illustration of the elements of basal respiration, ATP production capacity and maximal respiratory capacity calculated from the curve of oxygen consumption rate (OCR) (B) The profile of oxygen consumption rates (OCR) of hVICs (C) basal respiration (area under curve of OCR before oligo. Injection; area I) (D) ATP production capacity (area under curve of OCR between oligo. and FCCP injection; area II), and (E) maximal respiratory capacity (area under curve of OCR between FCCP and AMA injection; area III) of hVICs detected at 24 h incubated with 0, 2, or 20 mM fructose. OCR was measured under basal conditions followed by the sequential addition of oligomycin (oligo.; 0.25 μM), FCCP (1 μM), and antimycin A (AMA 1 μM; as arrow indicated). Each data point represents an OCR measurement. Values are mean ± SEM of analyses (n = 9–11 independent experiments). The sample size of each group was noted on the bar. ∗p < 0.05 versus the common medium with 0 mM fructose using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method.
Fructose induced the expression of mitochondrial fission 1 protein in human valve interstitial cells at high concentration
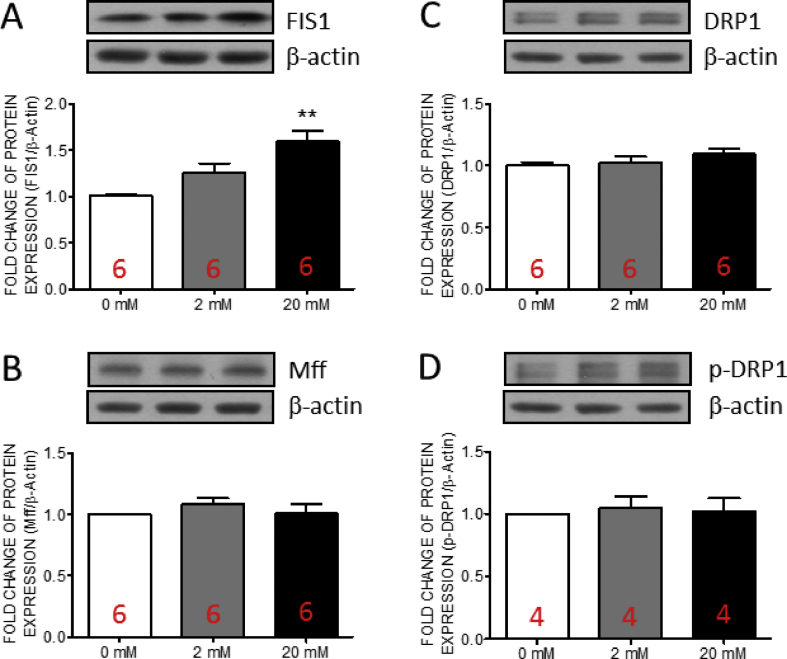
Mitochondria produce reactive oxygen species during the processes of ATP production [[40], [41], [42]]. Accumulated oxidative stress damages mitochondria in turn. Mitochondrial fission works to maintain the mitochondrial function by eliminating the damaged fraction of mitochondria [43]. Therefore, we further examined the expressions of three key factors of mitochondrial fission, including FIS1, Mff, DRP-1 and p-DRP-1 in the hVICs by Western blotting. The results indicated that the expressions of FIS1 [Fig. 5A] was significantly increased at high fructose group while no significant change was detected in the expressions of Mff [Fig. 5B], DRP1 [Fig. 5C] or p-DRP1 [Fig. 5D]. At lower fructose group, FIS1 [Fig. 5A] only showed the trends of increment without statistical significant. These results suggested that high fructose may trigger the FIS1-associated mitochondrial fission in hVICs.
Fig. 5.
The expressions of FIS1 in human valve interstitial cells was increased by fructose at high concentration. Representative gels and densitometric analyses of (A) mitochondrial fission 1 protein (FIS1) (B) mitochondrial fission factor (Mff) (C) dynamin-related protein 1 (DRP1) and (D) p-DRP1 detected at 24 h incubated with 0, 2, or 20 mM fructose. Values are mean ± SEM, n = 4–6 in each experimental group. The sample size of each group was noted on the bar. ∗∗∗p < 0.001 versus 0 mM fructose group using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method were used for comparisons between groups.
Fructose induced the expression of optic atrophy type 1 in human valve interstitial cells at high concentration
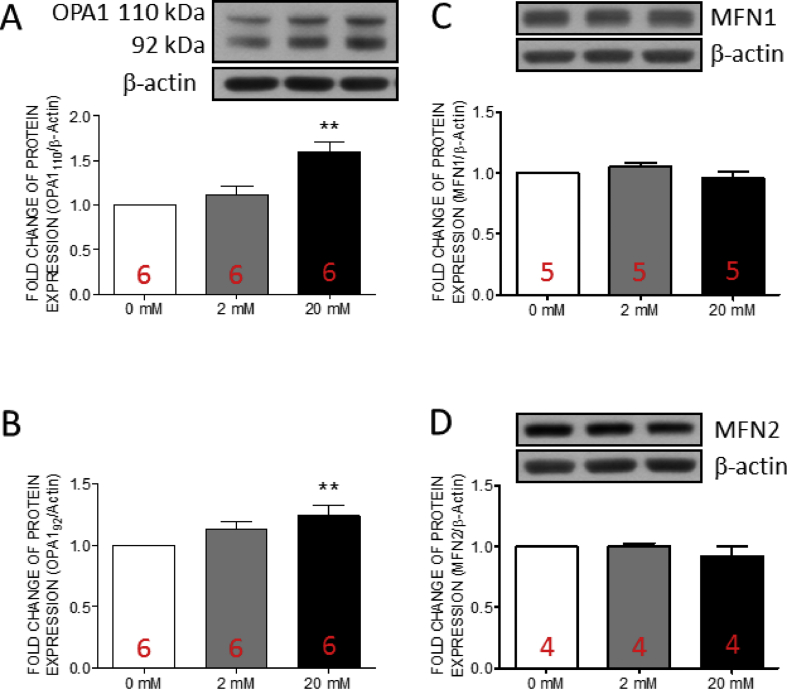
Mitochondrial fusion works to maintain the mitochondrial function by fusing the health and functional mitochondria [43]. Recently, optic atrophy type 1 (OPA1)-mediated mitochondrial fusion has been demonstrated to protects cells from calcium deposition [44]. Therefore, we examined the expressions of three key proteins of mitochondrial fusion, including OPA1, mitofusin (MFN1), and MFN2 in hVICs by Western blotting. The results indicated that the expressions of full length OPA1 and cleavage OPA1 [Fig. 6A and B] were significantly increased at high fructose group while no significant change was detected in the expressions of MFN1 [Fig. 6C], or MFN2 [Fig. 6D]. These results suggested the OPA1-associated mitochondrial fusion in hVICs with high fructose.
Fig. 6.
The expressions of OPA1 in human valve interstitial cells was increased by fructose at high concentration. Representative gels and densitometric analyses of (A) full length optic atrophy 1 (OPA1) (B)cleaved OPA1 (C) mitofusin 1 (MFN1) and (D) MFN2 detected at 24 h incubated with 0, 2, or 20 mM fructose. Values are mean ± SEM, n = 4–6 in each experimental group. The sample size of each group was noted on the bar. ∗p < 0.05, ∗∗p < 0.01 versus 0 mM fructose group using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method.
Fructose induced the expression of optic atrophy type 1 in human valve interstitial cells at high concentration
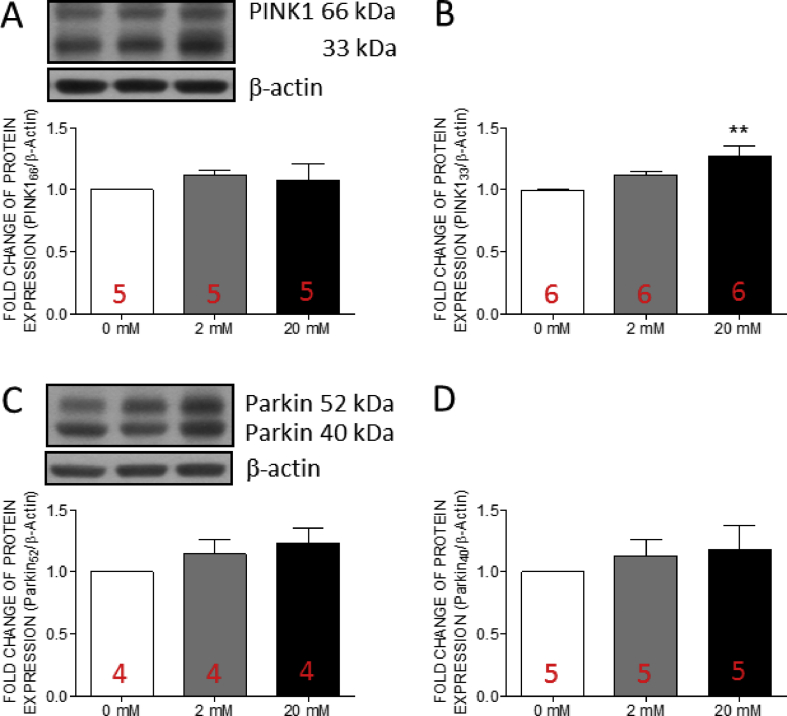
Mitochondrial autophagy is a protective mechanism for mitochondrial quality control [45]. Under physiological status, the process of mitophagy is strictly regulated. In this study, we examined the expressions of the key proteins of mitophagy, including phosphatase and tensin homologue-induced kinase 1 (PINK1), and Parkin in hVICs by Western blotting. The results indicated that the expression of cleaved PINK1 [Fig. 7B] was significantly increased at high fructose group while no significant change was detected in the expressions of full length PINK1 [Fig. 7A], full length Parkin [Fig. 7C], or short form Parkin [Fig. 7D]. These results suggested that the mitophagy function was maintained in hVICs with high fructose co-incubation.
Fig. 7.
The expressions of cleaved PINK1 in human valve interstitial cells was increased by fructose at high concentration. Representative gels and densitometric analyses of (A) full length phosphatase and tensin homolog (PTEN)-induced putative kinase protein 1 (PINK1) (B) cleaved PINK1 (C) full length Parkin, and (D) cleaved Parkin detected at 24 h incubated with 0, 2, or 20 mM fructose. Values are mean ± SEM, n = 4–6 in each experimental group. The sample size of each group was noted on the bar. ∗∗p < 0.01 versus 0 mM fructose group using the nonparametric, Kruskal–Wallis test followed by the Dunn post hoc method.
Discussion
This study provided novel evidence to demonstrate that high fructose has direct effect on upregulating the calcification-related genes, ALP and osteocalcin, of hVICs after 24 h incubation. The underlying mechanisms involve the activation of PI3K/AKT signaling, the enhancement of mitochondrial OXPHOS, and the increase of mitochondrial fission/fusion concurrent with the limited proliferation ability. This finding suggested that the high fructose initiated an early onset of hVICs calcification via the activation of PI3K/AKT/mitochondrial OXPHOS pathway. This study provides new insights into the progression of valve stenosis accelerated by fructose overconsuming.
Fructose is a common sweetener in our daily diet and has been linked to the progression of metabolic disorder, including cardiovascular diseases. In the human study [35], the mean serum fructose concentration in fasting healthy volunteers was 1.9 ± 0.4 mM and after ingestion of fructose and glucose-containing drink dose to 16.3 ± 1.2 mM at 15 min and peaked at 30 min when serum fructose was 17.2 ± 1.1 mM. Valve interstitial cells are critical for the development of calcific aortic valve disease. Therefore, we examined the adverse effect of fructose stimulation on the hVICs calcification at the levels of 2 and 20 mM to mimic the serum fructose levels of fasting (e.g. 2 mM) and of post-fructose consuming (20 mM) in human. The results indicated that high fructose triggered the progression of calcification in the hVICs by upregulation of ALP and osteocalcin as well as the mineral deposition. These data further implied that the repeated high fructose stimulation during daily life may initiate the development of calcified aortic valve disease.
Aortic valve stenosis is a common valvular heart disease. According to the conventional wisdom, the progression of valve stenosis is thought to be initiated from the valve fibrosis and gradually transformed into calcification. The progression of AS is strongly bounded to the differentiation of VICs to osteoblast [46]. In human study, high dietary fructose increased the concentration of serum ALP [47]. In line with previous evidence [47], our data further indicated that as early after 24 h incubation, high fructose increased the expressions of ALP and osteocalcin, the initial and finalized markers of calcification, respectively. These results suggested that high fructose could induce an early onset of valve stenosis through the calcification of VICs. Consist with previous study [48], these results indicated that hVICs do not need to progress through a fibrotic stage before reaching an osteogenic stage. In other cell types, high fructose-induced cell proliferation and the fibrosis have been reported [49,50]. Different from those cell types, reduced proliferation of hVICs by high fructose at a series of time points was constantly detected in this study. The hVICs is a cell type prone to differentiate to osteoblasts or myofibroblasts in response for the metabolic stress. Our data further demonstrated the upregulation of ALP and osteocalcin, which are essential for the differentiation of osteoblast. Follow this line, we reason that the high fructose incubation might promote the switch from proliferation to osteogenic differentiation.
hVICs from healthy and calcified valves have various sensitivities when encounter the osteogenic stimuli. Cultured with common medium, hVICs from calcified valves appeared positive calcium deposits with Alizarin Red staining, but not significantly different from healthy hVICs. Stimulating with osteogenic medium, hVICs from calcified valves increased calcification [51]. The evidence suggests that hVICs may be more vulnerable for calcification under environmental stimulation. Therefore, we used a commercial primary hVICs to avoid the bias from the selected subject. The pathogenesis of aortic valve stenosis (AS) is a life-long progression. Repeated stimulation could be a risk factor to initiate AS. Fructose is a common sugar in beverages, desserts and even fresh fruits. Overconsumption of fructose has been demonstrated to induce metabolic syndrome which is a top risk factor of AS [[2], [3], [4], [5]]. Valve interstitial cells (VICs) play important role in the development of calcific aortic valve disease. In this study, we investigated that whether high fructose (∼20 mM, the postprandial level of serum fructose [35]) might be a risk factor to initiate the progression of fibrosis and calcification of valve interstitial cells. In this study, the results indicated that high fructose triggered the expressions of ALP and osteocalcin, the downstream candidates of the calcification-related genes, instead of the fibrosis-related genes. These results further imply that as a common sweetener in our daily life, fructose could be a silent inducer of valve calcification. Although the increments of ALP and osteocalcin were not drastic, the stable accumulation of these calcification-related proteins in the hVICs could lead to calcification in the long run by repeated high fructose stimulation.
The upregulation of p85 subunit of PI3K involves in the progression of metabolic disorders [7]. In addition, the activation of PI3K/AKT signaling has been linked to the osteogenic differentiation of mesenchymal stem cells [8,9]. The involvement of PI3K/AKT signaling in the progression of calcification in various cells has been reported recently [52,53]. ALP and osteocalcin have been suggested to be the downstream candidates of the calcification-related genes. Accordingly, it is reasonable that the high fructose-increased PI3K p85 concurrent with the upregulation of ALP and osteocalcin. Recently, the role of pS473-AKT in positive regulation of osteogenesis has been reported [54]. Consistently, our results demonstrated that the high fructose-induce AKT expression and phosphorylation at S473 accompanied with the increment of ALP and osteocalcin. These results implied the linkage between the activation of PI3K/pS473-AKT signaling and osteogenic differentiation in the high fructose-stimulated hVICs. On the other hand, accumulating evidence suggests that the increased pS473-AKT in turn activates the MTORC1 cascade to phosphorylate insulin receptor substrate-1 (IRS-1) leading to the attenuation of T308-AKT phosphorylation [36]. Our results further indicated that the p-IRS-1 was increased by high fructose. Follow these lines, we reasoned that the unchanged pT308-AKT could be a result of the increased pS473-AKT.
Valve is a thin, passive tissue with rare blood vessels support. These characteristics might make the hVICs exhibit different features of energy support from other energy-dependent cells. According to the seahorse data, mitochondria in hVICs only responded for ∼22% of ATP production. The data implied the characteristic of the energy support in hVICs as the glycolysis dominant. Whereas high fructose enhanced the mitochondrial OXPHOS. These results suggested that increased dietary fructose could boost the energy production of mitochondria in hVICs. Previous study indicated that the metabolic activity of VICs is enhanced when the cells are exposed to diabetic conditions [55] which may lead the VICs towards differentiation and sequel pathogenesis. Similarly, our data demonstrated that the maximal respiratory capacity and ATP production capacity were enhanced in hVICs concurrent with the markers of osteogenesis at the fructose level of post-fructose consumption. These lines of evidence support that the enhancement of mitochondrial activity in hVICs could be a landmark of valve stenosis at early stage.
The activity of mitochondrial oxidative phosphorylation could be enhanced by the activation of AKT signaling [10,11]. In this study, we further indicated that the impairment of mitochondrial bioenergetics correlate to the PI3K/AKT signal in the high fructose-induced progression of hVICs calcification. Under physiological status, mitochondria uptake large amounts of calcium driven by respiratory energy [56]. Intriguingly, as one of the major storage organelles of Ca2+, the activation of mitochondrial respiration enhance Ca2+ accumulation [12]. However, the role of mitochondrial respiration in the development of valvular calcification has not been carefully evaluated. In this study, we assayed the fructose-associated change of mitochondrial respiration of hVICs. The results indicated that high fructose enhanced the maximal capacity and ATP production capacity of mitochondrial oxidative phosphorylation. Most importantly, the increment of mitochondrial aerobic respiration were consistent with the upregulation of ALP and osteocalcin in a fructose dose-dependent manner. These lines of evidence implies that high fructose may initiate a vicious circle of valvular calcification via high fructose-boosted activity of mitochondrial respiration in a long run.
Mitochondrial fission and fusion are critical regulators for maintaining the function of mitochondria in response to the environmental nutrient support [16]. Overnutrition may enhance mitochondrial activity to accelerate mitochondrial oxidative damage [41,42,57]. Mitochondrial fission is essential for the elimination of damaged mitochondria. Although rare study has been conducted in the role of mitochondrial fission in the valve calcification, our results indicated that the high fructose-induced FIS1 was concurrent with the upregulation of ALP and osteocalcin implying the involvement of FIS1-mediated mitochondrial fission in the development of valvular calcification. Consist with this research, our results further indicated that the upregulation of OPA1 in hVICs may be a compromise results in response to the increased mitochondrial OXPHOS in the first place. Furthermore, OPA1 has been linked to the progression of calcification [44]. It is conceivable that the high fructose-increased mitochondrial dynamic signals could initiate a vicious cycle to develop valve stenosis. We, therefore, linked the increment of mitochondrial aerobic respiration and dynamics as the mediator of PI3K/AKT-induced ALP and osteocalcin upregulation. Follow these lines, it is possible that the activation of PI3K/AKT/mitochondria axis could be the key mechanisms to shift hVICs from healthy to the calcification-prone status.
Mitophagy strictly controls the mitochondrial quality [58]. In healthy mitochondria, PINK1, which translocates from cytosol to mitochondria is rapidly cleaved by PARL [20] while uncleaved PINK1 recruits Parkin to initiate mitophagy [19]. Previous study indicated that the suppression of mitophagy contributes to vascular calcification [59]. In this study, the PINK1 cleavage was increased in response to the increase fructose concentration. These results suggested that the mitochondria function properly under high fructose at 24 h incubation. These results implied that the mitophagy mechanism may not be altered by high fructose at the early stage.
Conclusions
By using a cell model to mimic the fasting and post fructose-consuming for the study of the progression of AS, we demonstrated that the proliferation of hVICs was reduced by high fructose concurrent with the activation of PI3K/AKT signaling, the increased capacity of mitochondrial respiration, and the enhancement of mitochondrial dynamics to initiate the calcification even at the physiological level of post-fructose consuming. Together, these results imply that PI3K/AKT/mitochondrial respiration of hVICs might be a feasible therapeutic target of aortic valve stenosis as early at the initiation of AS progression.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by grants from the Taiwan Association for Integration of Cardiology and Surgery (TAICS-107-4-Y1 to CLC) and the Chang Gung Medical Foundation (CMRPG8J0721, CMRPG8J0722, CMRPG8J0723 to KLHW).
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Ching-Li Cheng, Email: clcheng@mail.ntin.edu.tw, NTIN.Jenny@gmail.com.
Kay L.H. Wu, Email: klhwu@adm.cgmh.org.tw, wlh0701@yahoo.com.tw.
References
- 1.Rutkovskiy A., Malashicheva A., Sullivan G., Bogdanova M., Kostareva A., Stenslokken K.O., et al. Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briand M., Lemieux I., Dumesnil J.G., Mathieu P., Cartier A., Despres J.P., et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–2236. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 3.Katz R., Budoff M.J., Takasu J., Shavelle D.M., Bertoni A., Blumenthal R.S., et al. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA) [published correction appears in Diabetes. 2009;58:1937] Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz R., Wong N.D., Kronmal R., Takasu J., Shavelle D.M., Probstfield J.L., et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill S., O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 6.van der Heide L.P., Ramakers G.M., Smidt M.P. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes. 2006;55:2392–2397. doi: 10.2337/db06-0391. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang N., Zhang P., Fu R., Shen G., Jiang L., Fang B. Mechanical strain promotes osteogenic differentiation of bone mesenchymal stem cells from ovariectomized rats via the phosphoinositide 3kinase/Akt signaling pathway. Mol Med Rep. 2018;17:1855–1862. doi: 10.3892/mmr.2017.8030. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee A., Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci. 2009;122:716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haileselassie B., Mukherjee R., Joshi A.U., Napier B.A., Massis L.M., Ostberg N.P., et al. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J Mol Cell Cardiol. 2019;130:160–169. doi: 10.1016/j.yjmcc.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C., Li Y., He L., Agarwal A.R., Zeng N., Cadenas E., et al. PI3K/AKT signaling regulates bioenergetics in immortalized hepatocytes. Free Radic Biol Med. 2013;60:29–40. doi: 10.1016/j.freeradbiomed.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carafoli E. The fateful encounter of mitochondria with calcium: how did it happen? Biochim Biophys Acta. 2010;1797:595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Gao Z., Chen Y., Guan M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell. 2017;8:439–445. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shares B.H., Busch M., White N., Shum L., Eliseev R.A. Active mitochondria support osteogenic differentiation by stimulating beta-catenin acetylation. J Biol Chem. 2018;293:16019–16027. doi: 10.1074/jbc.RA118.004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo A.Y., Joseph A.M., Dutta D., Hwang J.C., Aris J.P., Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesa M., Palacin M., Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 17.Scarpulla R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventura-Clapier R., Garnier A., Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 19.Pickrell A.M., Youle R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin S.M., Lazarou M., Wang C., Kane L.A., Narendra D.P., Youle R.J. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bantle J.P. Dietary fructose and metabolic syndrome and diabetes. J Nutr. 2009;139:1263S–1268S. doi: 10.3945/jn.108.098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khitan Z., Kim D.H. Fructose: a key factor in the development of metabolic syndrome and hypertension. J Nutr Metab. 2013;2013:682673. doi: 10.1155/2013/682673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A., Adeli K. Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol. 2008;24:204–209. doi: 10.1097/MOG.0b013e3282f3f4c4. [DOI] [PubMed] [Google Scholar]
- 24.Wu K.L., Chao Y.M., Tsay S.J., Chen C.H., Chan S.H., Dovinova I., et al. Role of nitric oxide synthase uncoupling at rostral ventrolateral medulla in redox-sensitive hypertension associated with metabolic syndrome. Hypertension. 2014;64:815–824. doi: 10.1161/HYPERTENSIONAHA.114.03777. [DOI] [PubMed] [Google Scholar]
- 25.Lin I.C., Wu C.W., Tain Y.L., Chen I.C., Hung C.Y., Wu K.L.H. High fructose diet induces early mortality via autophagy factors accumulation in the rostral ventrolateral medulla as ameliorated by pioglitazone. J Nutr Biochem. 2019;69:87–97. doi: 10.1016/j.jnutbio.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Cioffi F., Senese R., Lasala P., Ziello A., Mazzoli A., Crescenzo R., et al. Fructose-rich diet affects mitochondrial DNA damage and repair in rats. Nutrients. 2017;9:323. doi: 10.3390/nu9040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basciano H., Federico L., Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Pozo S.E., Schold J., Nakagawa T., Sanchez-Lozada L.G., Johnson R.J., Lillo J.L. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 29.Go J.L., Prem K., Al-Hijji M.A., Qin Q., Noble C., Young M.D., et al. Experimental metabolic syndrome model associated with mechanical and structural degenerative changes of the aortic valve. Sci Rep. 2018;8:17835. doi: 10.1038/s41598-018-36388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Testuz A., Nguyen V., Mathieu T., Kerneis C., Arangalage D., Kubota N., et al. Influence of metabolic syndrome and diabetes on progression of calcific aortic valve stenosis. Int J Cardiol. 2017;244:248–253. doi: 10.1016/j.ijcard.2017.06.104. [DOI] [PubMed] [Google Scholar]
- 31.Liu A.C., Joag V.R., Gotlieb A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor P.M., Batten P., Brand N.J., Thomas P.S., Yacoub M.H. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35:113–118. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 33.Mohler 3rd., E.R. Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. doi: 10.1016/j.amjcard.2004.08.013. A6. [DOI] [PubMed] [Google Scholar]
- 34.Schoen F.J., Levy R.J. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Hui H., Huang D., McArthur D., Nissen N., Boros L.G., Heaney A.P. Direct spectrophotometric determination of serum fructose in pancreatic cancer patients. Pancreas. 2009;38:706–712. doi: 10.1097/MPA.0b013e3181a7c6e5. [DOI] [PubMed] [Google Scholar]
- 36.Harrington L.S., Findlay G.M., Lamb R.F. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Divakaruni A.S., Paradyse A., Ferrick D.A., Murphy A.N., Jastroch M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol. 2014;547:309–354. doi: 10.1016/B978-0-12-801415-8.00016-3. [DOI] [PubMed] [Google Scholar]
- 38.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C.W., Hung C.Y., Hirase H., Tain Y.L., Lee W.C., Chan J.Y.H., et al. Pioglitazone reversed the fructose-programmed astrocytic glycolysis and oxidative phosphorylation of female rat offspring. Am J Physiol Endocrinol Metab. 2019;316:E622–E634. doi: 10.1152/ajpendo.00408.2018. [DOI] [PubMed] [Google Scholar]
- 40.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxidants Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva Ramos E., Larsson N.G., Mourier A. Bioenergetic roles of mitochondrial fusion. Biochim Biophys Acta. 2016;1857:1277–1283. doi: 10.1016/j.bbabio.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen W.R., Zhou Y.J., Yang J.Q., Liu F., Wu X.P., Sha Y. Melatonin attenuates calcium deposition from vascular smooth muscle cells by activating mitochondrial fusion and mitophagy via an AMPK/OPA1 signaling pathway. Oxid Med Cell Longev. 2020;2020:5298483. doi: 10.1155/2020/5298483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin S.M., Youle R.J. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajamannan N.M. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milne D.B., Nielsen F.H. The interaction between dietary fructose and magnesium adversely affects macromineral homeostasis in men. J Am Coll Nutr. 2000;19:31–37. doi: 10.1080/07315724.2000.10718911. [DOI] [PubMed] [Google Scholar]
- 48.Monzack E.L., Masters K.S. Can valvular interstitial cells become true osteoblasts? A side-by-side comparison. J Heart Valve Dis. 2011;20:449–463. [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama T., Kosugi T., Gersch M., Connor T., Sanchez-Lozada L.G., Lanaspa M.A., et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Ren Physiol. 2010;298:F712–F720. doi: 10.1152/ajprenal.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfrom C., Kadhom N., Raulin J., Raynaud N., Gautier M. Fructose-induced enhanced mitogenicity of diploid human cells: possible relationship with cell differentiation. In Vitro Cell Dev Biol Anim. 1994;30A:263–268. doi: 10.1007/BF02632049. [DOI] [PubMed] [Google Scholar]
- 51.Bogdanova M., Zabirnyk A., Malashicheva A., Enayati K.Z., Karlsen T.A., Kaljusto M.L., et al. Interstitial cells in calcified aortic valves have reduced differentiation potential and stem cell-like properties. Sci Rep. 2019;9:12934. doi: 10.1038/s41598-019-49016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He X., Jiang H., Gao F., Liang S., Wei M., Chen L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-kappaB signaling pathway. Microsc Res Tech. 2019;82:2000–2006. doi: 10.1002/jemt.23369. [DOI] [PubMed] [Google Scholar]
- 53.Pan J.M., Wu L.G., Cai J.W., Wu L.T., Liang M. Dexamethasone suppresses osteogenesis of osteoblast via the PI3K/Akt signaling pathway in vitro and in vivo. J Recept Signal Transduct Res. 2019;39:80–86. doi: 10.1080/10799893.2019.1625061. [DOI] [PubMed] [Google Scholar]
- 54.Yan D.Y., Tang J., Chen L., Wang B., Weng S., Xie Z., et al. Imperatorin promotes osteogenesis and suppresses osteoclast by activating AKT/GSK3 beta/beta-catenin pathways. J Cell Mol Med. 2020;24:2330–2341. doi: 10.1111/jcmm.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selig J.I., Ouwens D.M., Raschke S., Thoresen G.H., Fischer J.W., Lichtenberg A., et al. Impact of hyperinsulinemia and hyperglycemia on valvular interstitial cells - a link between aortic heart valve degeneration and type 2 diabetes. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2526–2537. doi: 10.1016/j.bbadis.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 56.Vasington F.D., Murphy J.V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- 57.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998;1366:53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 58.Truban D., Hou X., Caulfield T.R., Fiesel F.C., Springer W. PINK1, parkin, and mitochondrial quality control: what can we learn about Parkinson's disease pathobiology? J Parkinsons Dis. 2017;7:13–29. doi: 10.3233/JPD-160989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y., Ji J.J., Yang R., Han X.Q., Sun X.J., Ma W.Q., et al. Lactate accelerates calcification in VSMCs through suppression of BNIP3-mediated mitophagy. Cell Signal. 2019;58:53–64. doi: 10.1016/j.cellsig.2019.03.006. [DOI] [PubMed] [Google Scholar]