Abstract
Background
Periventricular nodular heterotopia (PVNH) is caused by abnormal neuronal migration, resulting in the neurons accumulate as nodules along the surface of the lateral ventricles. PVNH often cause epilepsy, psychomotor development or cognition problem. Mutations in FLNA (Filamin A) is the most common underlying genetic etiology. Our purpose is to delineate the clinical and imaging spectrum that differentiates FLNA-positive and FLNA-negative PVNH patients.
Methods
We included 21 patients with confirmed PVNH. The detailed clinical information, electroencephalography, and other clinical findings were recorded. Detailed brain MR imaging was assessed. Mutation analysis of the FLNA gene was used Sanger sequencing or a next generation sequencing based assay.
Results
FLNA mutations were identified in 9 patients (7 females and 2 males), including two nonsense, two splice site, three frameshift, and two missense mutations. In FLNA-positive group, 8 patients had anterior predominant bilateral symmetric presentation and only one had asymmetrical distribution and dilated ventricles. Extra-cerebral features were more often observed in FLNA-positive group than FLNA-negative group.
Conclusion
Genetics of PVNH is heterogenous, and mutations in FLNA gene account for less than half of the patients in our cohort. Our finding between FLNA-positive and FLNA-negative patients could guide the clinicians to select relevant genetic testing.
Keywords: Periventricular heterotopia, MRI, Epilepsy, Brain malformation, FLNA
At a glance commentary.
Scientific background on the subject
Periventricular nodular heterotopia (PVNH) is a developmental brain anomaly caused by impaired neuronal migration. Pathogenic variants in FLNA (Filamin A) are the most common underlying genetic etiology of PVNH, which accounts for only 20–30% of PVNH cases in Western countries. The role of FLNA in Asian PVNH population is unexplored.
What this study adds to the field
The current study reported the percentage of FLNA mutations in Asian PVNH patients. It also delineated the clinical and imaging spectrum that differentiates the FLNA-positive and FLNA-negative PVNH patients, which could guide the clinicians to select relevant genetic testing.
Nodular heterotopia (NH) is one of the most common malformations of cortical development (MCD) related to epilepsy [1]. Periventricular nodular heterotopia (PVNH) is the most common type of NH [2]. The neuronal migration abnormality results in the neurons accumulate as nodules along the surface of the lateral ventricles. Patients with PVNH often had epilepsy with multiple epileptogenic zone and variable seizure severity, psychomotor development and/or cognition problem. PVNH is predominantly observed in women, due to an X-linked dominant mutation caused by FLNA (Filamin A) [2]. PVNH is often accompanied by other cerebral malformations such as cerebellar anomaly, ventricular abnormalities, mega cisterna magna and hypoplasia or agenesis of corpus callosum [2,3]. There are also several associated extra-cerebral findings, including cardiac valves disease [4], patent duct arteriosus [3,4], joint hyperextensibility [4], chronic constipation [3], chronic obstructive lung disease [3,5], or coagulopathy. Hitherto, mutations in FLNA genes account for only 20–30% of PVNH cases in Western countries [2,6]. Here, we report the percentage of FLNA mutations in Asian PVNH patients as well as to delineate the clinical and imaging spectrum that differentiates the FLNA-positive and FLNA-negative PVNH patients. By early detecting the FLNA mutations, we could provide genetic counseling to the family and offer medical surveillance for other organ systems to avoid complications.
Materials and methods
Subjects & clinico-imaging phenotyping
The study included 21 patients with radiologically confirmed PVNH, followed up at or referred by other neurologists to the Department of Neurology of Kaohsiung Chang Gung Memorial Hospital, Taiwan and Department of Neurology of Malaya Medical Center, University of Malaya. Most of the patients are Chinese ethnicity, except one Malay and one Indian. The detailed clinical information, electroencephalography (EEG), neurological examination and other associated manifestations were sourced from medical records. For those who were using antiseizure drugs (ASMs), types of ASMs and response to ASMs were detailed recorded. The study was approved by the local human research ethics committees and written consents were obtained from all subjects. In minors and those with intellectual disabilities, consents were obtained from their legal guardian.
Original brain MR imaging was sourced. The morphology, location, and symmetry of PVNH were analyzed. We classified periventricular heterotopia according to the location of heterotopic nodules as previously described with modification [2]. Patients were classified into four groups: 1. The heterotopia located bilaterally symmetric along the frontal and the body of the lateral ventricles, (anterior predominant type); 2. The heterotopia located in bilateral temporo-occipital and trigones of the lateral ventricles (Inferior type); 3. Bilateral asymmetric periventricular nodules with or without subcortical heterotopia; 4. Unilateral focal periventricular nodule (presence of isolated or multiple nodule heterotopia in a restricted area adjacent to the ventricle) with or without subcortical heterotopias [2,[7], [8], [9]]. We also reviewed the presence of associated abnormalities, including corpus callosum malformation, mega cisterna magna, white matter lesion, abnormal cortical gyration/cortical thinning, posterior fossa abnormality (defined as posterior fossa cysts, brain stem or cerebellar malformation), dilated ventricle, or intracranial aneurysms.
Molecular analysis of FLNA
Genomic DNA was extracted from patients’ peripheral blood leukocytes using QIAGEN DNA extraction kits (Qiagen, Germany). We designed an amplicon-based targeted resequencing technique covering all 48 coding exons of FLNA gene and their flanking (at least 10 base pairs) intronic regions. Multiplex polymerase chain reactions were used to amplify FLNA gene. The amplified libraries were sequenced using Illumina MiSeq platform. Standardized bioinformatics pipeline was used as previously described [10]. Four prediction programs, including SIFT (v1.03) [11], PolyPhen-2 (v2.2.2 build r394) [12], MutationTaster 2 [13], and Combined Annotation Dependent Depletion (CADD v1.2) [14] were used to prioritize variants. The cutoff value of CADD was set at 20. All identified pathogenic or likely pathogenic variants were confirmed by Sanger sequencing. The pathogenicity of the variants was classified according to the ACMG/AMP guideline [15].
Statistical analysis
Fisher exact or Chi-Squared test was used to compare the clinical and genetic features between the FLNA positive and negative groups.
Results
Patients
Among 21 patients with PVNH, FLNA mutations were identified in 9 (9/21, 42.9%) patients, including two nonsense mutations (case 1 & 2), two splice site mutations (case 3 & 4), three frameshift mutations (case 5, 6, & 7), and two missense mutations (case 8 & 9). The identified FLNA mutations were detailed in Table 1. Among the FLNA positive patients, seven were females and two were male. As for the remaining 12 patients without FLNA mutations, 6 were females and 6 were males. There was no gender difference between the two groups (p = 0.367).
Table 1.
Summary of the genetic and clinical data of FLNA positive patients.
Abbreviations: BTCS: bilateral tonic-clonic seizures; CBZ: Carbamazepine; EEG: electroencephalography; FAS: focal aware seizures; FIAS: focal impaired awareness seizures; LEV: Levetiracetam; LTG: Lamotrigine; N/A: not applicable; OXC: oxcarbazepine; PVNH: periventricular nodular heterotopia; VPA: Valproic acid.
The most common FLNA mutations were loss-of-function mutations (7/9, 77.8%), such as nonsense, frameshift and splicing, which were predictive to reduce the expression level of filamin A. Additionally, there were two missense mutations: p. Thr608Met and p.Glu1661Lys, which is located in the fourth and 15th repeat of Rod 1 domain, respectively. Both missense variants were predicted to deleterious by multiple in silico prediction algorithms. All of the variants were not presented in ExAc or gnomAD database and classified as pathogenic or likely pathogenic according to ACMG guideline. Interestingly, both missense mutations were identified in male patients in hemizygous status. In patient 8, the mutation was passed on to an affected daughter (heterozygous status) who has epilepsy but normal brain MRI.
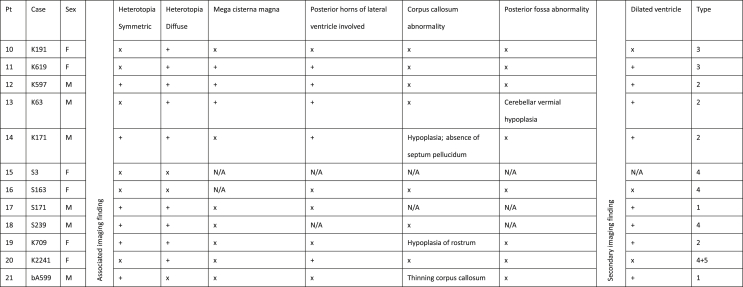
The clinical spectrum, epileptic features and neuroimaging findings were summarized in Table 2 (FLNA-positive) and Table 3 (FLNA-negative). All patients in both groups had epilepsy. Among patients with FLNA positive, most (8/9, 88.9%) patients had anterior predominant bilateral PVNH (type 1) on MRI except one had bilateral asymmetric PVNH with adjacent subcortical heterotopia (type 3). None of FLNA positive had inferior type or unilateral PVNH (type 2 and 4). In the FLNA negative group, there were two patients had anterior predominant type (type 1), 4 patients had inferior PVNH (type 2), and 2 patients had type 3 (bilateral asymmetric). Four patients had type 4, including three with unilateral focal nodular PVNH without subcortical heterotopia and one patient had unilateral focal nodule PVNH combined with subcortical heterotopia.
Table 2.
Clinical and brain MRI features of FLNA positive patients.
Abbreviations: IVS: interventricular septum; LA: left atrial; LV: left ventricle; MR: mitral regurgitation; N: normal; N/A: not applicable; PVNH: periventricular nodular heterotopia; TR: tricuspid regurgitation; +: present; -: absent.
Table 3.
Summary of imaging finding of FLNA negative patients.
Abbreviations: N/A: not applicable; +: present; x: absent.
We then compared the associated intracerebral malformation between FLNA positive and negative group. With regard to intracerebral malformations, corpus callosum abnormalities were seen in 3/9 (33.3%) FLNA positive versus 3/12 (25%) negative cases (p = 1); mega cisterna magna in 3/9 (33.3%) positive versus 3/12 (25%) negative cases (p = 1). Besides, posterior fossa abnormality was seen once in both groups (1/9, 11.1% versus 1/12, 8%). Dilated lateral ventricles tend to be more frequent in FLNA negative group (8/12, 66.7% versus 1/9, 11.1%) compared to FLNA positive group (p = 0.0244).
As for the systemic manifestations, the FLNA positive group frequently have variable systemic findings and connective tissue manifestations (7/9, 77.8%), including dysmorphic features, cardiovascular disease, skin and joint abnormality and intestinal dysfunction. On the contrary, there was no systemic, internal organ or connective tissue manifestations observed in FLNA negative group (0/12, p = 0.0003).
In terms of seizure outcome, the FLNA positive group had five (5/9) patients with medical refractory epilepsies, while the FLNA negative group had six (6/12) medical refractory patients (p = 0.8) [16].
Discussion
In our PVNH cohort, pathogenic variants in FLNA gene account for 43% of all cases. Most FLNA positive cases were female with loss-of-function variants; the neuroimaging showed anterior predominant bilateral PVNH. Patients with pathogenic FLNA variants were also more likely to have systemic manifestations, such as dysmorphism, cardiovascular disease, skin and joint abnormality, and intestinal dysfunction.
Among FLNA positive cases, there was an obvious female predominance (female-to-male ratio: 7:2), and loss of function variants. Female predominance was reported to be 93–100% in previous series [2,3,17], and only a few male patients were identified. In this study, both patients with missense variants were male, which is probably due to individuals with loss of function hemizygous FLNA variants are not viable. Previous reported male patients were all missense or distal truncating variants that have milder deleterious effect on Filamin A protein [[18], [19], [20], [21]]. Interestingly, there was suggestion that male FLNA patients have higher incidence (69% compared to 33.3% in female and 50% in all FLNA mutations) of cardiac or aortic abnormality and may not presented with intellectual disability or epilepsy [3,17,21]. One of our male patients also had cardiac valve insufficiency. The reason for the prevalence of cardiac involvement in male patients remains uncertain. Both of our missense male patients still had seizures and mild intellectual disability.
Intriguingly, the missense variant in case 8 was inherited from the proband to his daughter, who does not have PVNH but had a few self-limited seizures without the need of antiepileptic drug. A previous study also reported a father-daughter pair with missense FLNA variant and milder phenotype [2]. For missense variant, the survival of male patients and mild phenotype in female patients is probably due to the presence of a normal allele as well as residual function of missense Filamin A compared to loss of function variants [18,20,21].
All FLNA positive patients in our cohort had anterior predominant PVNH except one who had subcortical heterotopia on the same side of PVNH (the father of hemizygous missense variant). A few patients with FLNA variants without anterior predominant PVNH have been reported [2,6,21]. On the contrary, there were also two (2/10, 20%) of all anterior predominant PVNH patients were negative for FLNA. Previous studies also reported that 51–74% of anterior predominant PVNH were negative for FLNA variants [2,17,22].
As for other associated features, we found that FLNA positive cases are likely to have more systemic manifestations (∼78%) while none of the FLNA negative patients had associated internal organ abnormality or cardiovascular abnormality [[23], [24], [25]]. The most common extracerebral features are cardiac abnormalities followed by gut dysfunction and joint hypermobility. FLNA encodes for Filamin A protein, which is highly expressed in the arteries, gastrointestinal (esophagus and colon) and urogenital system (uterus and bladder) based on GTEx data. FLNA is an actin-binding protein that links actins to membrane glycoproteins, which plays an important role in the remodeling the cytoskeleton and cell-cell adhesions. Therefore, it is possible that the systemic manifestations are due to the non-CNS expression and function of FLNA. Whereas the genetic cause of FLNA negative PVNH cases remain unknown, it is possible that the causative genes have a more limited expression and function in the brain. On the contrary, the intracerebral malformation was not significantly different in the two groups, except for the enlarged ventricle which is more prominent in FLNA negative group. This is informative in the clinics where bilateral anterior predominant PVNH associated with systemic features is more likely to be positive for FLNA gene screening.
There was no difference of seizure outcomes between the two groups, and nearly half patients had refractory seizure using multiple antiseizure medications (ASMs). This is in accordance with previous studies where near a third patients with FLNA mutations were unable to reach seizure free despite multiple ASMs [3].
Our FLNA mutation positive rate is higher than previous reports in Western countries ranged from 21 to 33% [2,6]. This is probably because the referral bias. More than half cases were unsolved and may have hitherto unidentified genetic causes, which indicates the genetic heterogeneity of PVNH. Several genes, such as MAP1B, TMTC3, MEN1, NEDD4L, ACTG1, and ARFGEF2 have been recently associated with FLNA negative PVNH [[26], [27], [28], [29], [30], [31]]. Our study has some limitations: first, the patient number is limited due to the rare occurrence of PVNH. Due to small number in each group, the statistics may not have the power to show minor differences. Lastly, we only captured and sequenced the FLNA gene, deletion or copy number variations of FLNA gene may be missed. Further studies using advanced techniques, such as multiplex ligation-dependent probe amplification (MLPA) or whole genome/whole exome sequencing (WGS/WES), may be required to identify the underlying genetic cause of unsolved cases.
Conflicts of interest
There is no conflict of interest regarding the publication of this study.
Acknowledgments
We thank the patients and their families for participating in this study. This research was funded by CMRPG8G0252 to MHT and CMRPG8J0781 to YTL from Kaohsiung Chang Gung Memorial Hospital, Taiwan.
The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from: the GTEx Portal on 2021/03/31.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Gonzalez G., Vedolin L., Barry B., Poduri A., Walsh C., Barkovich A.J. Location of periventricular nodular heterotopia is related to the malformation phenotype on MRI. AJNR Am J Neuroradiol. 2013;34:877–883. doi: 10.3174/ajnr.A3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parrini E., Ramazzotti A., Dobyns W.B., Mei D., Moro F., Veggiotti P., et al. Periventricular heterotopia: phenotypic heterogeneity and correlation with Filamin A mutations. Brain. 2006;129:1892–1906. doi: 10.1093/brain/awl125. [DOI] [PubMed] [Google Scholar]
- 3.Lange M., Kasper B., Bohring A., Rutsch F., Kluger G., Hoffjan S., et al. 47 patients with FLNA associated periventricular nodular heterotopia. Orphanet J Rare Dis. 2015;10:134. doi: 10.1186/s13023-015-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinstein E., Frentz S., Morgan T., Garcia-Minaur S., Leventer R.J., McGillivray G., et al. Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in Filamin A. Eur J Hum Genet. 2013;21:494–502. doi: 10.1038/ejhg.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltahir S., Ahmad K.S., Al-Balawi M.M., Bukhamsien H., Al-Mobaireek K., Alotaibi W., et al. Lung disease associated with filamin A gene mutation: a case report. J Med Case Rep. 2016;10:97. doi: 10.1186/s13256-016-0871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Moron D., Vishnopolska S., Consalvo D., Medina N., Marti M., Cordoba M., et al. Germline and somatic mutations in cortical malformations: molecular defects in Argentinean patients with neuronal migration disorders. PloS One. 2017;12 doi: 10.1371/journal.pone.0185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srour M., Rioux M.F., Varga C., Lortie A., Major P., Robitaille Y., et al. The clinical spectrum of nodular heterotopias in children: report of 31 patients. Epilepsia. 2011;52:728–737. doi: 10.1111/j.1528-1167.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdel Razek A.A., Kandell A.Y., Elsorogy L.G., Elmongy A., Basett A.A. Disorders of cortical formation: MR imaging features. AJNR Am J Neuroradiol. 2009;30:4–11. doi: 10.3174/ajnr.A1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkovich A.J., Guerrini R., Kuzniecky R.I., Jackson G.D., Dobyns W.B. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai M.H., Chan C.K., Chang Y.C., Yu Y.T., Chuang S.T., Fan W.L., et al. DEPDC5 mutations in familial and sporadic focal epilepsy. Clin Genet. 2017;92:397–404. doi: 10.1111/cge.12992. [DOI] [PubMed] [Google Scholar]
- 11.Ng P.C., Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 14.Kircher M., Witten D.M., Jain P., O'Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg A.T., Vickrey B.G., Testa F.M., Levy S.R., Shinnar S., DiMario F., et al. How long does it take for epilepsy to become intractable? A prospective investigation. Ann Neurol. 2006;60:73–79. doi: 10.1002/ana.20852. [DOI] [PubMed] [Google Scholar]
- 17.Sole G., Coupry I., Rooryck C., Guerineau E., Martins F., Deves S., et al. Bilateral periventricular nodular heterotopia in France: frequency of mutations in FLNA, phenotypic heterogeneity and spectrum of mutations. J Neurol Neurosurg Psychiatr. 2009;80:1394–1398. doi: 10.1136/jnnp.2008.162263. [DOI] [PubMed] [Google Scholar]
- 18.Sheen V.L., Dixon P.H., Fox J.W., Hong S.E., Kinton L., Sisodiya S.M., et al. Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet. 2001;10:1775–1783. doi: 10.1093/hmg/10.17.1775. [DOI] [PubMed] [Google Scholar]
- 19.Moro F., Carrozzo R., Veggiotti P., Tortorella G., Toniolo D., Volzone A., et al. Familial periventricular heterotopia: missense and distal truncating mutations of the FLN1 gene. Neurology. 2002;58:916–921. doi: 10.1212/wnl.58.6.916. [DOI] [PubMed] [Google Scholar]
- 20.Guerrini R., Mei D., Sisodiya S., Sicca F., Harding B., Takahashi Y., et al. Germline and mosaic mutations of FLN1 in men with periventricular heterotopia. Neurology. 2004;63:51–56. doi: 10.1212/01.wnl.0000132818.84827.4d. [DOI] [PubMed] [Google Scholar]
- 21.Fergelot P., Coupry I., Rooryck C., Deforges J., Maurat E., Sole G., et al. Atypical male and female presentations of FLNA-related periventricular nodular heterotopia. Eur J Med Genet. 2012;55:313–318. doi: 10.1016/j.ejmg.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Fox J.W., Lamperti E.D., Eksioglu Y.Z., Hong S.E., Feng Y., Graham D.A., et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 23.Pisano T., Barkovich A.J., Leventer R.J., Squier W., Scheffer I.E., Parrini E., et al. Peritrigonal and temporo-occipital heterotopia with corpus callosum and cerebellar dysgenesis. Neurology. 2012;79:1244–1251. doi: 10.1212/WNL.0b013e31826aac88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandelstam S.A., Leventer R.J., Sandow A., McGillivray G., van Kogelenberg M., Guerrini R., et al. Bilateral posterior periventricular nodular heterotopia: a recognizable cortical malformation with a spectrum of associated brain abnormalities. AJNR Am J Neuroradiol. 2013;34:432–438. doi: 10.3174/ajnr.A3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallil Z., Pardoe H., Bachman R., Cunningham B., Parulkar I., Shain C., et al. Phenotypic and imaging features of FLNA-negative patients with bilateral periventricular nodular heterotopia and epilepsy. Epilepsy Behav : E&B. 2015;51:321–327. doi: 10.1016/j.yebeh.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheen V.L., Ganesh V.S., Topcu M., Sebire G., Bodell A., Hill R.S., et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36:69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- 27.Broix L., Jagline H., Ivanova E., Schmucker S., Drouot N., Clayton-Smith J., et al. Mutations in the HECT domain of NEDD4L lead to AKT-mTOR pathway deregulation and cause periventricular nodular heterotopia. Nat Genet. 2016;48:1349–1358. doi: 10.1038/ng.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhan S.M.K., Nixon K.C.J., Everest M., Edwards T.N., Long S., Segal D., et al. Identification of a novel synaptic protein, TMTC3, involved in periventricular nodular heterotopia with intellectual disability and epilepsy. Hum Mol Genet. 2017;26:4278–4289. doi: 10.1093/hmg/ddx316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinzen E.L., O'Neill A.C., Zhu X., Allen A.S., Bahlo M., Chelly J., et al. De novo and inherited private variants in MAP1B in periventricular nodular heterotopia. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montier L., Haneef Z., Gavvala J., Yoshor D., North R., Verla T., et al. A somatic mutation in MEN1 gene detected in periventricular nodular heterotopia tissue obtained from depth electrodes. Epilepsia. 2019;60:e104–e109. doi: 10.1111/epi.16328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vontell R., Supramaniam V.G., Davidson A., Thornton C., Marnerides A., Holder-Espinasse M., et al. Post-mortem characterisation of a case with an ACTG1 variant, agenesis of the corpus callosum and neuronal heterotopia. Front Physiol. 2019;10:623. doi: 10.3389/fphys.2019.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]