Abstract
Background
Fecal microbiota transplantation (FMT) has been shown to highly effective in the treatment of recurrent or refractory Clostridioides difficile infection (rCDI) in many countries of the world. Not until 2018, Ministry of Health and Welfare, Taiwan approved the application of FMT for rCDI under a special law. The study reported the first implementation of the technology in the medical center in Taiwan and the preliminary outcome.
Methods
FMT was used to treat patients with rCDI in Chang Gung Memorial Hospital. FMT was delivered by gastroenterologists using colonoscope. Strict donor screening was performed according to the guidelines. We followed up the clinical course of patients after FMT. 16S rRNA sequencing of fecal samples for donor, and also recipient before and after FMT was carried out.
Results
From September 2018 to June 2020, 39 patients with rCDI received FMT, with a successful rate of 89.7%. Two patients died due to causes unrelated to FMT, and two other cases showed no clinical improvement after the procedure. High school and college students showed the best pass rate during donor screening. The presence of multi-drug resistant pathogen was the most common cause for screening failure. We demonstrated in a case the use of rRNA sequencing as a biomarker indicating for the improvement of dysbiosis in a patient after FMT.
Conclusions
FMT was successfully implemented in a medical center in Taiwan and showed a comparable successful rate in treating rCDI, compared to other countries. Safety remains the most important issue when applying FMT in the clinical setting.
Keywords: Fecal microbiota transplantation, Clostridioides difficile infection, Donor screening, 16S rRNA sequencing, Outcome
At a glance commentary
Scientific background on the subject
Fecal microbiota transplantation (FMT) has been shown to be highly effective in the treatment of recurrent or refractory Clostridioides difficile infection (rCDI) in many countries of the world. Not until 2018, Ministry of Health and Welfare, Taiwan approved the application of FMT for rCDI under a special law.
What this study adds to the field
FMT was successfully implemented in a medical center and showed a comparable successful rate in treating rCDI. Safety remains the most important issue when applying FMT in the clinical setting.
Fecal microbiota transplantation (FMT) is to deliver microbiota from feces of healthy donors to patients to treat gut dysbiosis related diseases. FMT has been used to treat human diseases for over a thousand years [1,2]. Since FMT was recommended as the optional treatment for recurrent or refractory C. difficile infection (rCDI) in 2013 in the United States, a series of guidelines and consensuses focusing on FMT have been published to move FMT forward in the treatment of adults and children [[3], [4], [5], [6], [7], [8], [9], [10]]. Increasing use of FMT in various indications have been reported, though the clinical findings from different reports based on different FMT-related methods vary a lot among those indications beyond CDI, including ulcerative colitis [[11], [12], [13], [14], [15]], Crohn's disease [[16], [17], [18]], hepatic encephalopathy [19,20] and other diseases [[21], [22], [23], [24]]. In 2018, the Ministry of Health and Welfare announced the “Administrative Measures on the Implementation or Use of Specific Medical Technology Inspection and Medical Instruments” that allowed the application of FMT to treat rCDI in Taiwan. The treatment technique is to implant the intestinal flora of healthy donors into the patient's intestinal system by enema, endoscopy or oral capsules to restore the balance of intestinal colonies and achieve the therapeutic effect. The current open administration is applicable to patients with C. difficile infection that is repetitive or ineffective with conventional treatment. Medical centers or regional hospitals with teaching hospital qualifications, qualified operating personnel and facilities, and qualified equipment can implement FMT after applying for approval and registration with the municipal, county (city) competent authority. The study reported the implementation of FMT in Chang Gung Memorial Hospital (CGMH) and the preliminary outcome of 35 patients, as of August 2020.
Material and methods
FMT indication
FMT was used to treat CDI in either of the following circumstances: 1) Recurrent or relapsing CDI, defined as recurrent CDI within eight weeks of completion of therapy; and 2) refractory or complicated CDI, defined by at least one of the following: not responding to standard therapy (metronidazole, vancomycin), admission to the ICU, hypotension with or without the use of vasopressors, ileus or significant abdominal distention/toxic megacolon, mental status changes, WBC ≥ 35,000 cells/mm3, or < 2 cells/mm3, serum lactate >2.2 mmol/L or any evidence of end-organ failure.
Donor selection
Donor screening was performed according to the protocols approved by our local institutional review board and the Food and Drug Administration (FDA). Except for pediatric donors, majority of the donors were young adults 18–50 years of age with a body-mass index (the weight in kilograms divided by the square of the height in meters) of 18.5–25.0. Medical history, physical examination findings, and laboratory test results for each donor must have met the standards outlined in the protocols [16]. All volunteers underwent comprehensive laboratory testing and once they passed the screening, they were asked to donate the stool at least three times in 1–2 weeks. Donors were supplied a toilet hat and clean, sealable containers for collection and transport of stool. Containers were labeled with the name, date of birth and date/time of stool collection. Collected stool were immediately refrigerated, transferred to the laboratory facility for processing on ice within a biohazard bag (but not frozen) and used within 6 h collection.
Detection of antimicrobial resistance genes
US FDA issued an alert in 2019 regarding two patients with Escherichia coli sepsis who received FMT by capsules but developed bacteremia 8 and 17 days, respectively, following FMT. To reduce donor-derived risk of FMT because of the inadequate donor screening by the current criteria and to setup a domestic safer stool bank for FMT, efforts to optimize the efficiency of donor screening were applied. All the fecal donations were tested for the common resistance genes in Gram-negative bacteria in Taiwan by PCR. We also tested the presence of Pseudomonas aeruginosa, a common human pathogen, in fecal donations from the donors. All the fecal transplants must be proved negative for these genes or P. aeruginosa before they were used in the clinical setting. The targets and primer sequences are shown in [Table 1]. The specific primers used for the detection of common antimicrobial resistance genes, including blaNDM (GenBank: FN396876.1), blaKPC (GenBank: AF297554.1), and blaVIM (GenBank: Y18050.2), in this study were designed by the internet-accessible software Primer3 [33].
Table 1.
Common human pathogen targets and primer sequences.
| Pathogens | Genes | Enzymes | Primer sequences | PCR product | References |
|---|---|---|---|---|---|
| Enterobacteriaceae (K. pneumoniae/E. coli/Salmonella) | blaCMY | AmpC β-lactamase | 5′-CTGCTGCTGACAGCCTCTTT-3′ | 1109 bp | [25] |
| 5′-TTTTCAAGAATGCGCCAGGC-3′ | |||||
| blaDHA | AmpC β-lactamase | 5′-CGTCTGACCATAATCCACCTGT-3′ | 1300 bp | [26] | |
| 5′-CCAGTGCACTCAAAATAGCCT-3′ | |||||
| blaCTX-M-3 | ESBL | 5′-CCAGAATAAGGAATCCCAT-3′ | 911 bp | [27] | |
| 5′-CCCATTCCGTTTCCGCTA-3′ | |||||
| blaCTX-M-14 | ESBL | 5′-AACACGGATTGACCGTCTTG-3′ | 906 bp | [28] | |
| 5′-TTACAGCCCTTCGGCGAT-3′ | |||||
| blaSHV | ESBL | 5′-GGGTTATTCTTATTTGTCGC-3′ | 928 bp | [29] | |
| 5′-TTAGCGTTGCCAGTGCTC-3′ | |||||
| mcr-1 | phosphoethanolamine transferases | 5ʹ-CGGTCAGTCCGTTTGTTC-3ʹ | 308 bp | [30] | |
| 5ʹ-CTTGGTCGGTCTGTAGGG-3ʹ | |||||
| blaNDM | Class B metallo-β-lactamase | 5′-ACGGTTTGGCGATCTGGTTT-3′ | 663 bp | GenBank: FN396876.1 | |
| 5′-ATGCGGGCCGTATGAGTGATT-3′ | |||||
| blaKPC | Class A metallo-β-lactamase | 5′-CGCCGTCTAGTTCTGCTGTCTTGT-3′ | 879 bp | GenBank: AF297554.1 | |
| 5′-TTCAGAGCCTTACTGCCCGTTGAC-3′ | |||||
| P. aeruginosa | blaVIM | Class B metallo-β-lactamase | 5′-GATGGTGTTTGGTYGCATA-3′ | 390 bp | GenBank: Y18050.2 |
| 5′-CGAATGCGCAGCACCRG-3′ | |||||
| algD | GDP mannose dehydrogenase gene | 5′-TTCCCTCGCAGAGAAAACATC-3′ | 520 bp | [31] | |
| 5′-CCTGGTTGATCAGGTCGATCT-3′ | |||||
| exoT | Exotoxin T | 5′-AATCGCCGTCCAACTGCATGCG-3′ | 152 bp | [32] | |
| 5′-TGTTCGCCGAGGTACTGCTC-3′ |
Transplant material preparation
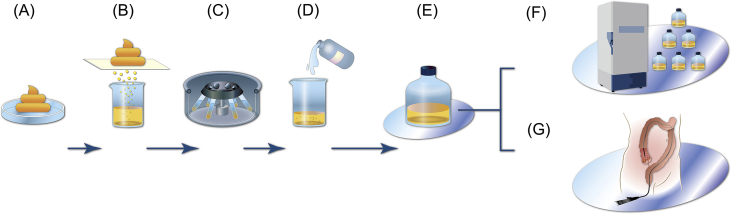
The outline of FMT material prepare procedure was shown in [Fig. 1]. Available quantity of stool (typically 50 ± 10 g) were added to sterile normal saline (250–300 ml) in sterile container [Fig. 1A]. The mixture can be homogenized by shaking or using a blender. This solution was then filtered to remove larger articulate matter repeat 3 times [Fig. 1B–D]. Finally, the fecal suspension was ready to use [Fig. 1E]. Either, the fecal suspension was divided into 50 ml sterile tube and frozen at −80 °C with 10% v/v glycerol [Fig. 1F] or drawn into aliquots of 50 ml slip (catheter) tip syringes was delivered to procedure area (usually endoscopic room) for administration [Fig. 1G] within 6 h.
Fig. 1.
The outline of FMT transplant preparation. Schematic diagram of the preparation process is from (A) 50 ± 10 g stool homogenized with 250 ml sterile normal saline; (B) filtration by sterile mesh; (C) centrifugation 1500×g/5mins to remove the suspend; (D) resuspending with fresh sterile normal saline; (E) ready-to-use FMT material; (F) adding 10% v/v glycerol and storage at −80 °C; or (G) administration of FMT material by colonoscopy.
FMT delivery
For endoscopic administration of FMT, the subjects were prepped with standard bowel purge administered the day before the procedure. Procedure will be performed within GI/endoscopy units of the centers. The method used to deliver the FMT depends on the individual characteristics of the patient. It is at the discretion of the treating physician, but in this report, all patients received colonoscopic delivery. This method allows full endoscopic examination of the colon and exclusion of co-morbid conditions, such as inflammatory bowel disease, microscopic colitis, malignancy, and diverticulosis, which may have an impact on patient's treatment or response to FMT.
16S rRNA sequencing and analysis
Fecal specimens were collected from patients before and after FMT for 16S rRNA sequencing. Application of the technique is for research purpose and approved by the Institutional Review Board of CGMH (approval number:201801263B0A3). All sequencing and analysis were done at Genomic Medicine core laboratory, Chang Gung Memorial Hospital, Linkou. DNA was first extracted from stool specimens using the QIAamp PowerFecal® DNA Kit (Qiagen, USA) according to manufacturer's protocol. PCR and sequencing will be performed using a modified version of the protocol [34]. Briefly, the 16S rRNA gene was amplified with region-specific primers that included the Illumina adaptor overhang nucleotide sequences (forward, 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′, and reverse, 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′). Subsequently, the product was subject to index PCR with Illumina® Nextera™ DNA UD Indexes. The final amplicon libraries were approximately 630 bp in length and were validated using the Agilent 2100 Bioanalyzer with Agilent HS DNA Kit. The sequencing of the multiplexed pooled libraries was carried out on the MiSeq System with MiSeq Reagent Kit v3 (600-cycle) (Illumina, USA).
The sequencing reads were initially de-multiplexed using MiSeq Reporter v2.6, according to sample barcodes. Processing of raw reads was performed mainly using USEARCH v11 (https://drive5.com/). Briefly, to join the pair reads and quality filtering by expected errors with maximum threshold = 1.0., only samples with merged reads ≥60,000 retained for subsequent analysis. The Cutadapt v2.3 package [35] used to remove forward and reverse sequencing primers from the merged reads of each dataset. Only sequence tags with length ≧ 400bp retained for subsequent analysis. The de-replication reads from effective reads were denoising attempts to identify all correct biological sequences using the UNOISE2 [36] into a zero-radius operational taxonomic unit (zOTU). Furthermore, the RDP training set (v16) database [37] was used as a reference set and final taxonomic assignments performed using the SINTAX classifier [38] with the α-diversity (e.g., Chao1 index and Shannon index) and β-diversity measurements calculated. Finally, we performed normalization using DEseq2 package [39] and graphic using ggplot2 package under R software (version 3.6.3).
Results
Donor screening
For the 39 recipients, donors were 87.2% from healthy volunteers and 12.8% from relatives. Students, including middle school, college, and graduate student, showed a successful pass rate of 66.7%, suggesting the student group may be a better donor source. The most common cause for screening failure was the presence of multidrug-resistant organisms (MDRO) (23.5%) [Table 2]. The second was a positive ANA (17.6%) and presence of Helicobacter pylori (17.6%). Notably, a long-term donor with previously multiple donations was removed from the donor list due to fatty liver.
Table 2.
Causes for donor screening failure.
| Causes for failure | Cases |
|---|---|
| Multiple drug resistance genes | 4 |
| ANA | 3 |
| Helicobacter pylori | 3 |
| High IgE | 1 |
| Vancomycin-resistant Enterococcus | 1 |
| Salmonella enterica serogroup B | 1 |
| High IgE & Clostridioides difficile | 1 |
| Helicobacter pylori & Clostridioides difficile | 1 |
| Plesiomonas shigelloides | 1 |
| Liver dysfunction (fatty Liver) |
1 |
| Total | 17 |
Preliminary outcome of FMT
From September 2018 to June 2020, the FMT was applied in 39 cases with rCDI in CGMH. Base on the negative results of C. difficile toxin testing and C. difficile culture examined during the 3-month follow-up, the success rate of FMT treatment for rCDI was 89.7%. Two cases showed no clinical improvement after FMT, but C. difficile toxin turned negative. Two cases expired within 6 months after FMT-unrelated serious adverse events (SAEs). One case was a 56-year-old female with acral lentiginous melanoma and multiple metastasis including brain and lung. She died 11 days post colonoscope-delivered FMT due to cancer-related aspiration pneumonia. Another case was a 63-year-old female with liver cirrhosis, end-stage renal disease under hemodialysis, and cerebral atherosclerosis disease. She died 84 days post colonoscope-delivered FMT due to ventricular tachycardia and severe sepsis during another admission.
FMT procedure and follow-up
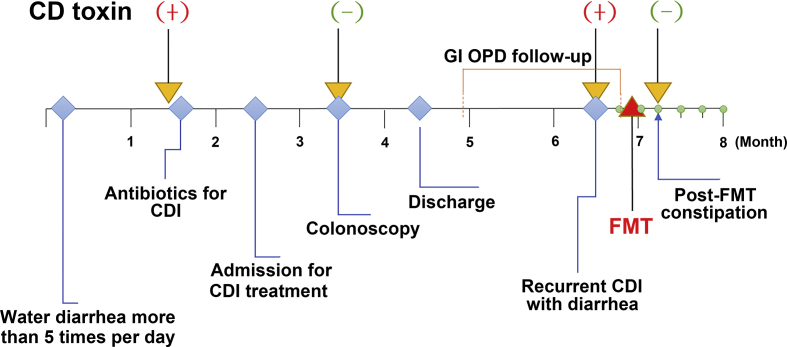
The FMT procedure and a follow-up timeline of a patient were summarized in [Fig. 2]. The patient is a 74-years-old male with chronic diarrhea over one month under C. difficile infection. But, persistent diarrhea and C. difficile infection were still noted after being treated with Metronidazole and Vancomycin over 7 days. Review of the patient's history, approximately four years ago, the patient received an operation for right buccal cancer and right lateral tongue cancer. Then, he needed long-term nasogastric tube feeding after tracheotomy and partial facial reconstruction in early May, 2019. After nasogastric tube feeding, the patient experienced the loss of appetite, severe abdominal distension, abdominal cramping pain, and persistent diarrhea caused by mucolytic fluid, combined with weight loss. Fecal examination showed positive for C. difficile toxins. After treatment with antibiotics such as vancomycin and metronidazole, the patient still had severe diarrhea over 5 times/day. He received FMT for the rCDI. After FMT, the patient's diarrhea improved after 2 days, and he later experienced a short period of post-FMT constipation. There was no recurrence of CDI during a 2-year period of follow-up according to negative C. difficile toxin gene screening and anaerobic C. difficile culture.
Fig. 2.
A timeline for fecal microbiota transplantation in a patient. Abbreviations: CD: C. difficile; CDI: C. difficile infection; GI: Gastroenterology; OPD: outpatient clinic; FMT: fecal microbiota transplantation. (+) indicates a positive testing result, and (−) indicates a negative result.
Diversity and relative abundances of microbiota
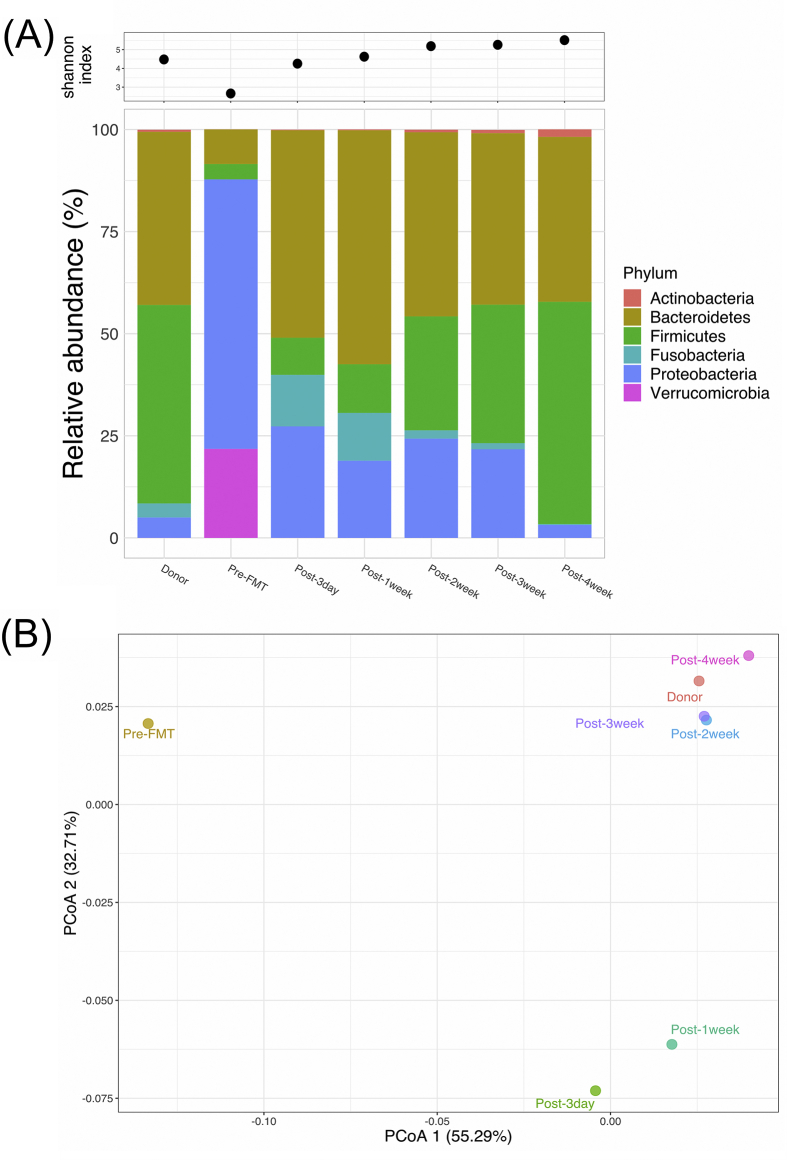
To compare the bacterial composition of gut microbiota in donor feces and in the recipient before and after FMT, 16S rRNA gene sequencing was carried out. The mean number of raw sequencing reads was 3.3 ± 0.9 × 105 per fecal sample. With quality filtration and PCR noise exclusion, the mean number of effective reads per fecal sample reduced to 1.1 ± 0.3 × 105. A total of 929 sequence variants were identified between the fecal samples of donor and FMT recipient. The relative abundance rates of bacteria were analyzed in the levels of phyla, genus and species over time. The most common phyla in donor were Firmicutes (48.6%) and Bacteroidetes (42.4%) [Fig. 3A]. However, the relatively abundant phyla in the patient were Proteobacteria (66.0%) and Verrucomicrobia (21.8%). The patient with gut dysbiosis symptom was confirmed by carrying pathogens of the family Enterobacteriaceae, including Klebsiella and Escherichia, but patient's microbiota became similar to that of the donor at the phyla level a week after FMT and during the follow-up [Fig. 3A]. Moreover, at the species level, the amounts of Bacteroidetes (Bacteroides) and Firmicutes (Ruminococcus, Faecalibacterium, and Eubacterium) increased but Proteobacteria (Klebsiella and Escherichia) decreased. In terms of Shannon index, lower level of microbiota diversity was exhibited in the fecal sample of the patient before FMT, compared to those in the case after FMT and in the donor [Fig. 3A upper]. In addition, with 16S rRNA sequencing at different time points after FMT, the relative abundance rate of C. difficile slightly increased on day 3 after FMT but became purged on day 7. In the comparison of fecal microbiota of the FMT-treated patient at different time points to that of donor according to the Principal Coordinates Analysis (PCoA) of the weighted Unifrac metric, a significant difference in overall community composition was observed among the three groups of gut microbiomes: (i) before FMT, (ii) within a week after FMT, and (iii) a week after FMT [Fig. 3B]. The PCoA results indicated that the FMT-treated rCDI patient successfully converted from a dysbiosis microbiota to a healthy gut environment.
Fig. 3.
Gut microbiota analysis before and after FMT. (A) Upper: richness of microbiota analysis was by Shannon index (alpha diversity); Lower: relative abundance at phyla level. (B) PCoA of weighted UniFrac for beta diversity.
Discussion
The Microbiota Therapy Center in CGMH, specifically established for donor screening and transplant preparation was a Biosafety Level 2 Laboratory according to the requirement from “The Regulation Governing the Application of Specific Medical Examination Technique and Medical Device” [40]. Donor screening and safe fecal banking reduced the donor-derived complications and optimized the entire FMT procedure. Previously, we have described main concerns on the safety of FMT [41,42]. Regarding the concerns on donor fecal screening, E. coli-associated sepsis reported in a previous study were found to develop 8 and 17 days in 2 patients after FMT by capsules, suggesting that delivery of FMT by colonoscopy in the vast majority of patients may be safer in terms of FMT delivery than by capsules [43,44]. In terms of the global incidence rate of FMT-related AEs, there was an overall 19.0% AEs, including diarrhea and abdominal discomfort/pain/cramping, and 1.4% SAEs, including infections and deaths, reported among 5688 FMT courses. Moreover, delivery of FMT by colonoscopy showed less AEs than by capsules (15.35% vs. 28.97%) [45]. In CGMH, all FMT were administered via colonoscopy; however, the patients who are not qualified for colonoscopic administration of FMT must then be considered to receive FMT by other methods. Notably, COVID-19, a globally ongoing pandemic since 2019, should be considered as a new target in donor screening because the SARS-CoV-2 can be detected in stool of patients with COVID-19 and theoretically, the virus could be transmittable via FMT delivery [42,46]. Given the important findings related to donor fecal screening in the present study, the detection of antimicrobial-resistant genes in bacterial pathogens, including Klebsiella pneumoniae, E. coli, Salmonella, Acinetobacter baumannii, and P. aeruginosa, as well as the emerging SARS-CoV-2 should be incorporated in fecal screening system not only in our hospital but also in other centers that are applying FMT in the clinical setting.
We noticed a low pass rate of donor screening reported from OpenBiome likely due to wide age range among fecal donors [47]. With intentional selection of young donors such as college students and young adults in our hospital, efficacy of donor screening was significantly improved to reach as high as 66.7% that was much higher than that of board-age range donor screening.
Based on our results, we also suggested that detection of P. aeruginosa should be incorporated into the current donor screening protocol [48]. Although a common protocol has been available for donor screening [49], certain bacterial species should be specifically screened according to local epidemiology in different countries. In addition, routine medical examinations can include microbiota analysis to evaluate the dynamic change of gut microbiota before and after FMT treatment. Interestingly, we observed that C. difficile was still present and relatively increased on day 3 in a patient after FMT, but the patient's diarrheal condition has been improved with the reversion of gut dysbiosis to homeostasis after FMT. The elimination of C. difficile was verified by C. difficile toxin test on day 7 after FMT, whereas the dynamic change of fecal microbiota profile may be easily evaluated by analysis of the alpha diversity in microbial richness according to the Shannon index [50].
Finally, new emerging resistance mechanisms are commonly observed [44], and therefore the donor screening protocol should be reevaluated and updated over time. The screening for antimicrobial-resistant organisms should better be carried out using molecular detection methods, because many organisms are usually unculturable, their resistance genes would not be easily detected using traditional culturing system. In addition, the gene function could be possibly predicted using molecular detection methods, such as next generation sequencing (NGS) [50]. The challenge of using NGS platform, however, remains high because of its time- and capital-consuming features. Recently, a novel sequencing platform, named MinION sequencer (Oxford Nanopore Technologies, UK), has been innovated for real-time sequencing with long reads and at an inexpensive price [51]. Moreover, the time for sequencing and identification of pathogenic bacteria and their antimicrobial resistance genes can be shortened to 1 h in the composition of MinION sequencer and NanoOK RT software package [52]. MinION platform may also allow us to boost up the speed and safety of donor screening as well as for fecal banking procedures.
This is the first study from Taiwan that reported the successful implementation of FMT in a medical center to treat rCDI. Our experience in donor screening, delivering of fecal transplant and follow-up for patients is useful to local health care workers who plan to set up and provide FMT service in their hospitals.
Conflicts of interest
The authors have no financial or ethical conflicts of interest to report.
Acknowledgments
This study was financially supported by grants from the Chang Gung Medical Foundation (CIRPG3H0041-2, CMRPG3J0571 and CMRPG3F1931). We thank Tzu-Chun Chuang, Kun-Jei Chen and Hung-Ju Chang, for their excellent technical assistance. The authors acknowledge the Genomic Medicine Core Laboratory for big data analytics.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Cheng-Tang Chiu, Email: ctchiu@cgmh.org.tw.
Cheng-Hsun Chiu, Email: chchiu@cgmh.org.tw.
References
- 1.Zhang F., Luo W., Shi Y., Fan Z., Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755–1756. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 2.Zhang F., Cui B., He X., Nie Y., Wu K., Fan D., et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9:462–473. doi: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debast S.B., Bauer M.P., Kuijper E.J., European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20 Suppl 2:1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 4.Sokol H., Galperine T., Kapel N., Bourlioux P., Seksik P., Barbut F., et al. Faecal microbiota transplantation in recurrent Clostridium difficile infection: recommendations from the French Group of Faecal microbiota Transplantation. Dig Liver Dis. 2016;48:242–247. doi: 10.1016/j.dld.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Konig J., Siebenhaar A., Hogenauer C., Arkkila P., Nieuwdorp M., Noren T., et al. Consensus report: faecal microbiota transfer - clinical applications and procedures. Aliment Pharmacol Ther. 2017;45:222–239. doi: 10.1111/apt.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cammarota G., Ianiro G., Tilg H., Rajilic-Stojanovic M., Kump P., Satokari R., et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cammarota G., Ianiro G., Kelly C.R., Mullish B.H., Allegretti J.R., Kassam Z., et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson M.R., Mitchell P.D., Alexander E., Ballal S., Bartlett M., Becker P., et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin Gastroenterol Hepatol. 2020;18:612–619. doi: 10.1016/j.cgh.2019.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng S.C., Kamm M.A., Yeoh Y.K., Chan P.K.S., Zuo T., Tang W., et al. Scientific frontiers in faecal microbiota transplantation: joint document of asia-pacific association of Gastroenterology (APAGE) and asia-pacific society for digestive endoscopy (APSDE) Gut. 2020;69:83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., Coffin S.E., et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. e6. [DOI] [PubMed] [Google Scholar]
- 12.Paramsothy S., Kamm M.A., Kaakoush N.O., Walsh A.J., van den Bogaerde J., Samuel D., et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 13.Costello S.P., Hughes P.A., Waters O., Bryant R.V., Vincent A.D., Blatchford P., et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sood A., Singh A., Mahajan R., Midha V., Mehta V., Gupta Y.K., et al. Acceptability, tolerability, and safety of fecal microbiota transplantation in patients with active ulcerative colitis (AT&S Study) J Gastroenterol Hepatol. 2020;35:418–424. doi: 10.1111/jgh.14829. [DOI] [PubMed] [Google Scholar]
- 15.Ding X., Li Q., Li P., Zhang T., Cui B., Ji G., et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019;42:869–880. doi: 10.1007/s40264-019-00809-2. [DOI] [PubMed] [Google Scholar]
- 16.Cui B., Feng Q., Wang H., Wang M., Peng Z., Li P., et al. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51–58. doi: 10.1111/jgh.12727. [DOI] [PubMed] [Google Scholar]
- 17.Vaughn B.P., Vatanen T., Allegretti J.R., Bai A., Xavier R.J., Korzenik J., et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn's disease. Inflamm Bowel Dis. 2016;22:2182–2190. doi: 10.1097/MIB.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Cui B., Li Q., Ding X., Li P., Zhang T., et al. The safety of fecal microbiota transplantation for Crohn's disease: findings from a long-term study. Adv Ther. 2018;35:1935–1944. doi: 10.1007/s12325-018-0800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajaj J.S., Kassam Z., Fagan A., Gavis E.A., Liu E., Cox I.J., et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj J.S., Salzman N.H., Acharya C., Sterling R.K., White M.B., Gavis E.A., et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. 2019;70:1690–1703. doi: 10.1002/hep.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrieze A., Van Nood E., Holleman F., Salojarvi J., Kootte R.S., Bartelsman J.F., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Wiesnoski D.H., Helmink B.A., Gopalakrishnan V., Choi K., DuPont H.L., et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakihana K., Fujioka Y., Suda W., Najima Y., Kuwata G., Sasajima S., et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnsen P.H., Hilpusch F., Cavanagh J.P., Leikanger I.S., Kolstad C., Valle P.C., et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]
- 25.Yan J.J., Ko W.C., Tsai S.H., Wu H.M., Jin Y.T., Wu J.J. Dissemination of CTX-M-3 and CMY-2 beta-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J Clin Microbiol. 2000;38:4320–4325. doi: 10.1128/jcm.38.12.4320-4325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su L.H., Chen H.L., Chia J.H., Liu S.Y., Chu C., Wu T.L., et al. Distribution of a transposon-like element carrying bla(CMY-2) among Salmonella and other Enterobacteriaceae. J Antimicrob Chemother. 2006;57:424–429. doi: 10.1093/jac/dki478. [DOI] [PubMed] [Google Scholar]
- 27.Chia J.H., Chu C., Su L.H., Chiu C.H., Kuo A.J., Sun C.F., et al. Development of a multiplex PCR and SHV melting-curve mutation detection system for detection of some SHV and CTX-M beta-lactamases of Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae in Taiwan. J Clin Microbiol. 2005;43:4486–4491. doi: 10.1128/JCM.43.9.4486-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Agamy M.H., Shibl A.M., Tawfik A.F. Prevalence and molecular characterization of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in Riyadh, Saudi Arabia. Ann Saudi Med. 2009;29:253–257. doi: 10.4103/0256-4947.55306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasheed J.K., Jay C., Metchock B., Berkowitz F., Weigel L., Crellin J., et al. Evolution of extended-spectrum beta-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41:647–653. doi: 10.1128/aac.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 31.DA Silva Filho L.V.F., Levi J.E., Bento C.N.O., DA Silva Ramos S.R.T., Rozov T. PCR identification of Pseudomonas aeruginosa and direct detection in clinical samples from cystic fibrosis patients. J Med Microbiol. 1999;48:357–361. doi: 10.1099/00222615-48-4-357. [DOI] [PubMed] [Google Scholar]
- 32.Ajayi T., Allmond L.R., Sawa T., Wiener-Kronish J.P. Single-nucleotide-polymorphism mapping of the Pseudomonas aeruginosa type III secretion toxins for development of a diagnostic multiplex PCR system. J Clin Microbiol. 2003;41:3526–3531. doi: 10.1128/JCM.41.8.3526-3531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 34.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J., et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetJ. 2011;17:10–12. [Internet], https://journal.embnet.org/index.php/embnetjournal/article/view/200. [cited 2021 Jan 15] [Google Scholar]
- 36.Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 37.Cole J.R., Wang Q., Fish J.A., Chai B., McGarrell D.M., Sun Y., et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar R.C. bioRxiv:74161 [Preprint]; 2016. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv 74161 [cited 2016 Sep 09]. Available from: https://www.biorxiv.org/content/10.1101/074161v1. [Google Scholar]
- 39.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ministry of Health and Welfare. Republic of China (Taiwan), The regulation governing the application of specific medical examination technique and medical device, https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0030054/;2021 [accessed 15 January 2021].
- 41.Chiu C.H., Chiu C.T. Drug-resistant bacteremia after fecal microbiota transplant. N Engl J Med. 2020;382:1960–1961. doi: 10.1056/NEJMc2002496. [DOI] [PubMed] [Google Scholar]
- 42.Chiu C.H., Tsai M.C., Cheng H.T., Le P.H., Kuo C.J., Chiu C.T. Fecal microbiota transplantation and donor screening for Clostridioides difficile infection during COVID-19 pandemic. J Formos Med Assoc. 2021;120:791–793. doi: 10.1016/j.jfma.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFilipp Z., Bloom P.P., Torres Soto M., Mansour M.K., Sater M.R.A., Huntley M.H., et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 44.Blaser M.J. Fecal microbiota transplantation for dysbiosis - predictable risks. N Engl J Med. 2019;381:2064–2066. doi: 10.1056/NEJMe1913807. [DOI] [PubMed] [Google Scholar]
- 45.Marcella C., Cui B., Kelly C.R., Ianiro G., Cammarota G., Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53:33–42. doi: 10.1111/apt.16148. [DOI] [PubMed] [Google Scholar]
- 46.Khanna S., Pardi D. Fecal microbiota transplantation for recurrent Clostridioides difficile infection: the COVID-19 era. Am J Gastroenterol. 2020;115:971–974. doi: 10.14309/ajg.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kassam Z., Dubois N., Ramakrishna B., Ling K., Qazi T., Smith M., et al. Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381:2070–2072. doi: 10.1056/NEJMc1913670. [DOI] [PubMed] [Google Scholar]
- 48.Chuang C.H., Janapatla R.P., Wang Y.H., Chang H.J., Huang Y.C., Lin T.Y., et al. Pseudomonas aeruginosa-associated diarrheal diseases in children. Pediatr Infect Dis J. 2017;36:1119–1123. doi: 10.1097/INF.0000000000001567. [DOI] [PubMed] [Google Scholar]
- 49.Konopiński M.K. Shannon diversity index: a call to replace the original Shannon's formula with unbiased estimator in the population genetics studies. PeerJ. 2020;8 doi: 10.7717/peerj.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu W., Deng X., Lee M., Sucu Y.D., Arevalo S., Stryke D., et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27:115–124. doi: 10.1038/s41591-020-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leggett R.M., Clark M.D. A world of opportunities with nanopore sequencing. J Exp Bot. 2017;68:5419–5429. doi: 10.1093/jxb/erx289. [DOI] [PubMed] [Google Scholar]
- 52.Leggett R.M., Alcon-Giner C., Heavens D., Caim S., Brook T.C., Kujawska M., et al. Rapid MinION profiling of preterm microbiota and antimicrobial-resistant pathogens. Nat Microbiol. 2020;5:430–442. doi: 10.1038/s41564-019-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]