Abstract
While endometrial cancer management remains challenging, a deeper understanding of the genetic diversity as well as the drivers of the various pathogenic states of this disease has led to development of divergent management approaches in an effort to improve therapeutic precision in this complex malignancy. This comprehensive review will provide an update on the epidemiology, pathophysiology, diagnosis and molecular classification, recent advancements in disease management, as well important patient quality of life considerations and emerging developments in the rapidly evolving therapeutic landscape of endometrial cancers.
Introduction
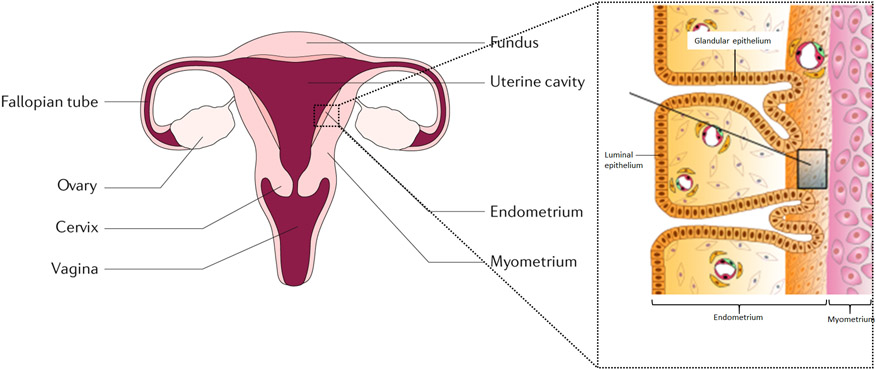
Endometrial cancer (EC) is a malignancy of the inner epithelial lining of the uterus (Fig. 1), with an increasing incidence and disease-associated mortality, worldwide.1 EC comprises distinct histological subtypes and molecular phenotypes. Historically, EC was categorized as Type I (association with unopposed estrogen stimulation, comprising low-grade cells that are more common and have a favorable prognosis) or Type II (not estrogen driven, comprising high-grade cells that are less common and have an unfavorable prognosis). Type I ECs are primarily composed of grade I or grade II endometrioid adenocarcinomas, whereas Type II ECs include grade III endometrioid adenocarcinomas, serous clear cell, undifferentiated and carcinosarcomas.
Figure 1. Uterine anatomy.
The endometrium is the inner lining of the uterus. Endometrial cancer arises from the endometrial glandular epithelium.
The incidence of EC in 2020 was 417,336, worldwide, and EC is the sixth most commonly occurring female cancer.2 Most cases occur between 65 and 75 years of age.3 Racial disparity and socioeconomic and geographical differences are important determinants of EC incidence and mortality. Although 67% of patients present with early-stage disease, which is associated with an 81% 5-year overall survival (OS), the 5-year OS for stage IVA and IVB EC are only 17% and 15%, respectively.4
As outlined in this Primer, although The Cancer Genome Atlas (TCGA) endeavor has substantially advanced our understanding of the biological heterogeneity of EC, optimal use of molecular classification relating to surgical staging, adjuvant therapy and surveillance scheduling has not been clearly defined. To comprehensively manage EC, dedicated efforts to more precisely delineate host factors, such as microbiome composition and the effect of body mass index (BMI), are imperative, as is a deeper understanding of the molecular and immunological drivers of response and resistance to emerging therapies, which is critically important for the optimal design of next-generation studies. Additionally, studies must consider not only efficacy and survival endpoints, but also quality of life (QOL) and economic implications of therapeutics.
Epidemiology
Incidence and mortality
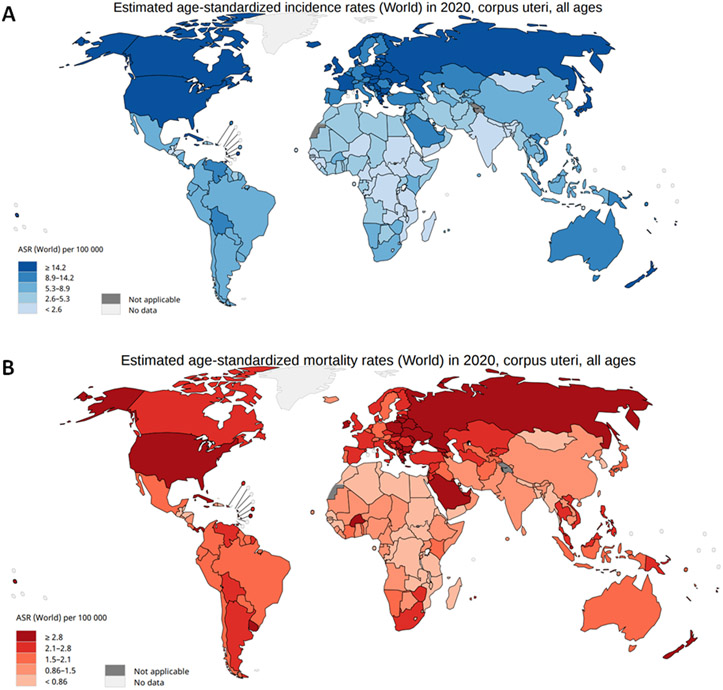
EC was diagnosed in 417,367 women in 2020, worldwide, with the highest disease burden in North America and Western Europe (Fig. 2). The incidence of EC is rapidly increasing.2 The high incidence rate in North America and Western Europe could be attributed to a high prevalence of lifestyle risk factors for EC, such as obesity, which is associated with ~50% of EC cases.5
Figure 2. Incidence and mortality of cancers of the corpus uteri.
A∣ Worldwide incidence of cancers of the corpus uteri. B∣ Worldwide mortality of cancers of the corpus uteri. Data from the Globocan Registry.
Based on a 2016 pooled analysis of epidemiological studies from 1971-2014, EC-associated mortality has increased by an average of 1.9% per year.6 In the United States, the number of women diagnosed with EC by 2030 will double to 122,000 cases per year if current trends continue.7 As of 2020, uterine cancer is the fourth most common female neoplasm in Europe, with an incidence of 12.9-20.2 per 100,000 women and a mortality of 2.0-3.7 per 100,000 women.8,9
Socioeconomic and racial disparities
Geographic, socioeconomic and racial disparities also affect EC incidence and mortality. EC is more prevalent in high-income countries compared with low-income and middle- income countries.2,10 Factors that contribute to geographical disparities in incidence and mortality may include access to high-quality healthcare, and oncologist density.11 In a Swedish study, variations in EC outcome were also influenced by socioeconomic status (SES), as assessed by, for example, income, social class, and educational attainment. Women of higher SES were less likely to present with advanced, type II EC and typically had more favorable outcomes due to enhanced access to healthcare compared with women of lower SES.12 Diagnosis at late cancer stages and reduced survival were more common in individuals of lower SES.12 Moreover, Black women from lower SES are twice as likely to receive delayed treatment and 2.5 times more likely to die from EC due to a lack of healthcare insurance, compared with white women from higher SES.13
Studies in the USA have shown that Black women are diagnosed with poorer prognostic histological subtypes and higher stage and grade of EC compared with white women, and Black women also have worse outcomes at every stage, grade and histological type of EC.14 Indeed, one study found worse OS in non-Hispanic black women with EC compared with non-Hispanic white women with EC, with survival disparities persisting despite adjustment for factors including age, tumor histology, grade, stage, and adjuvant treatment, suggesting that additional factors, such as molecular phenotypic differences, may contribute to differences in these outcomes.15 Black women are also less likely to receive chemotherapy and radiation therapy13 or undergo hysterectomy compared with white women.16,17 Socioeconomic factors such as lower median household income and access to healthcare coverage, and differences in medical comorbidities and genetic susceptibility to malignancy, are also believed to play a role.
Furthermore, compared with White women, Black women have experienced an increase in incidence of aggressive, high-grade, type II tumors compared with white.18,19 Tumor genomic differences are also important factors, and it is postulated that TP53 and PIK3R1 mutations in type II EC in Black women, along with higher HER2 expression, may be associated with a more unfavorable prognosis.16,20
Of note, a SEER data analysis from 1992-2001 found that Asian women presented with EC at a younger age (mean 58.4 years) compared with white women (mean age 65.1) and with more advanced-stage disease (21.5% for Asian women versus 15.4% for white women). Moreover, the 5-year OS for Asian women was 79.4% compared with 75.2% for white women, and 5-year OS for both early-stage (stage I or II) and late-stage (III or IV) EC were significantly higher in Asian women compared with white women (stages I/II 89.3% in Asian women versus 82.3% in white women, and for stages III or IV, 41.2% in Asian women and 34% in white women.21 US-born Asian women compared with immigrant Asian woman have type I EC at a significantly higher rate (65% vs. 56%, respectively; P < .01).22
Risk factors
Increased risk of EC is associated with increased age, certain ethnicities, higher BMI, endogenous or exogenous estrogen exposure, tamoxifen use, early menarche, late menopause, lower parity, metabolic syndrome, family history and genetic predisposition. By contrast, a lower risk of EC is associated with normal BMI, higher parity and oral contraception use.23
Health and lifestyle factors.
Prolonged unopposed estrogen exposure (such as with estrogen replacement therapy, chronic anovulation and tamoxifen treatment), and age ≥55 years are very well-known risk factors for EC.3,24 In developed countries, an increasing rate of obesity has been paralleled by an increasing incidence of EC.2,5,6 The association between obesity and EC is well established and is particularly pronounced for endometrioid EC, with approximate relative risks of 1.5 for those with overweight, 2.5 for those with class 1 obesity (BMI 30.0-34.9 kg/m2), 4.5 for those with class 2 obesity (BMI 35.0-39.9 kg/m2) and 7.1 for class 3 obesity (those with BMI ≥40.0 kg/m2).6,25 The main mechanism by which obesity promotes endometrial carcinogenesis is through increased estrogen production by conversion of androgens to estrogen by adipocytes, which in turn stimulates endometrial proliferation and, potentially, the development of hyperplasia and cancer.26 Moreover, obesity-associated hyperglycemia and insulin resistance can lead to abnormalities in IGF-1 signaling and activation of the Mammalian target of rapamycin (mTOR) pathway, resulting in increased cell proliferation. Associated inflammation and oxidative stress, as well as alterations in cytokines, steroid hormones and adipokine pathophysiology, and cellular and vascular perturbations, can also promote endometrial oncogenesis in women with obesity.27 Remedial actions such as intentional weight loss have provided encouraging data for this as an effective preventive measure. Indeed, after adjustment for baseline BMI, post-menopausal women who lost ≥5% of body weight had an ~30% reduction in EC risk, which rose to a 66% reduction for women with obesity.28
Meta-analyses have demonstrated an independent association between diabetes mellitus and increased risk of EC.29-31 Insulin resistance, hyperinsulinemias, hyperglycemia, inflammation and disturbances in the IGF-1 pathway may contribute to carcinogenesis in individuals with diabetes.32 In addition, a positive association between metabolic syndrome (that is, obesity, hypertension, insulin resistance and dyslipidemia) and risk of EC has been established33-35 and has been confirmed by the findings of a Women’s Health Initiative cohort study. Patients with metabolic syndrome have approximately a 2-fold increased risk of EC development.36 Of note, after excluding obesity from the definition of metabolic syndrome, the association remained positive although it no longer reached statistical significance, suggesting that the association between metabolic syndrome and EC risk is not solely explained by level of adiposity.36 Studies have yielded inconsistent results on the association of bisphosphonates and EC risk.37-39 Data from one meta-analysis of seven studies found that the risk of EC in postmenopausal women was decreased by 27% after bisphosphonate administration with a duration of use of up to 1 year or more.40 The anti-tumor effects of bisphosphonates are not fully understood and may involve complex underlying mechanisms including decreased cancer cell proliferation, disturbed mitosis, decreased angiogenesis, and an effect on immune cells/immune cell interactions.41-43
Genetic factors.
Some germline mutations increase the risk of EC, of which Lynch syndrome has the strongest association. This autosomal dominant syndrome is characterized by a germline mutation in one of the MMR genes: MLH1 (encoding MutL homologue 1), MSH2 (encoding MutS homologue2), MSH6 (encoding MutS homologue 6), or PMS2 (encoding postmeiotic segregation increased 2). Approximately 3% of ECs are due to Lynch syndrome,44 and the estimated lifetime risk of EC by age 70 years is approximately 46-54% for women with MLH1 mutations, 21-51% for women with MSH2 mutations, 16-49% for women with MSH6 mutations and 13-24% for women with PMS2 mutations.45-47
Somatic mutations in PTEN are common in sporadic EC, whereas germline PTEN mutations are rare and associated with Cowden syndrome.48 Cowden syndrome is characterized by an increased risk of breast cancer, thyroid cancer and EC, along with other diseases. The lifetime risk of EC in women with Cowden syndrome has been reported to be as high as 28%.49
The association between BRCA germline mutations and the risk of EC remains controversial, especially for serous carcinomas. A link between BRCA1 mutations and uterine serous cancer has been suggested in small, retrospective studies, whereas other studies have established no such risk.50,51 In addition, as many studies include only Ashkenazi Jewish populations or are confounded by tamoxifen exposure, results are challenging to interpret. One large study found an increased risk of serous and serous-like in BRCA1-positive patients undergoing risk-reducing salpingo-oophorectomy (RRSO) without hysterectomy52 in which 4 of 627 patients with BRCA1-mutations developed serous/serous-like EC (a 2.6-4.7% risk of developing these carcinomas by age 70). However, as these data are based on only 4 cases, making firm recommendations regarding the use of risk-reducing hysterectomy in BRCA mutation carriers is not feasible. In a study of 1,170 women with EC in which germline panel testing was performed, 4 women had BRCA1 mutations (including one serous carcinoma and one carcinosarcoma) and 3 had BRCA2 mutations (all endometrioid histology). These findings indicate a low incidence of germline BRCA mutations in this cohort of unselected patients with EC, and provides insufficient data to justify prophylactic hysterectomies in patients with BRCA mutations.53
Mechanisms/pathophysiology
Precursor lesions
EC is often a hormone-sensitive disease thought to commonly arise in the context of excessive estrogenic stimulation of the endometrial lining of the uterus. This estrogenic stimulation leads to mitogenic stimulation, and ultimately, malignant transformation of the endometrial glandular epithelium, and accounts for the development of the more common and lower grade endometrioid ECs. Risk factors for hyperestrogenism include obesity, hormone therapy (such as tamoxifen), ovarian cortical hyperplasia (hyperthecosis), polycystic ovarian syndrome, and hormone-producing tumors. Other histological subtypes of EC, including serous, clear cell, undifferentiated carcinoma and carcinosarcoma, are not as commonly associated with hyperestrogenism.
Endometrioid ECs develop through malignant transformation of the precursor lesions atypical endometrial hyperplasia (AEH) also known as endometrial intraepithelial neoplasia54,55. AEH often contains somatic PTEN mutations; loss of PTEN is necessary for the development of AEH but is insufficient for progression to invasive carcinoma.56 ARID1A has a critical role in the transition of precursor AEH lesions to invasive endometrioid carcinomas,57 and inactivation of TGFB also contributes to the progression of AEH to invasive carcinoma.58
The less common and more aggressive uterine serous carcinomas and the rarer uterine clear cell carcinomas are likely manifestations of increased genotoxic stress that is directly mediated through mutational and epigenetic activation of endometrial precursor cells. These EC subtypes often arise from precursor lesions such as serous endometrial intraepithelial carcinoma (SEIC). SEIC precursor lesions are thought to contain initiating TP53 mutations, as evidenced by abnormal immunohistochemistry staining of p53 and some reports of identifiable somatic TP53 mutations in SEIC precursor lesions and invasive disease.59
Uterine carcinosarcomas – characterized by carcinomatous and sarcomatous elements - are high-grade biphasic carcinomas with a rising incidence, and account for ~5% of ECs.60 Uterine carcinosarcomas are considered high-grade epithelial endometrial carcinomas with mutational profiles that overlap those of endometrioid and serous endometrial carcinomas.61-64
Molecular subgroups
Four molecular subgroup of EC defined by mutation burden and copy number alterations have been categorized in a study of 373 cases of EC by the TCGA61 (Fig. 3). Most ECs have near diploid tumors or focal copy number alterations. Moreover, the mutational burden of most ECs reflects that of most solid tumors, with ~2-3 somatic mutations per megabase sequenced.61
Figure 3.
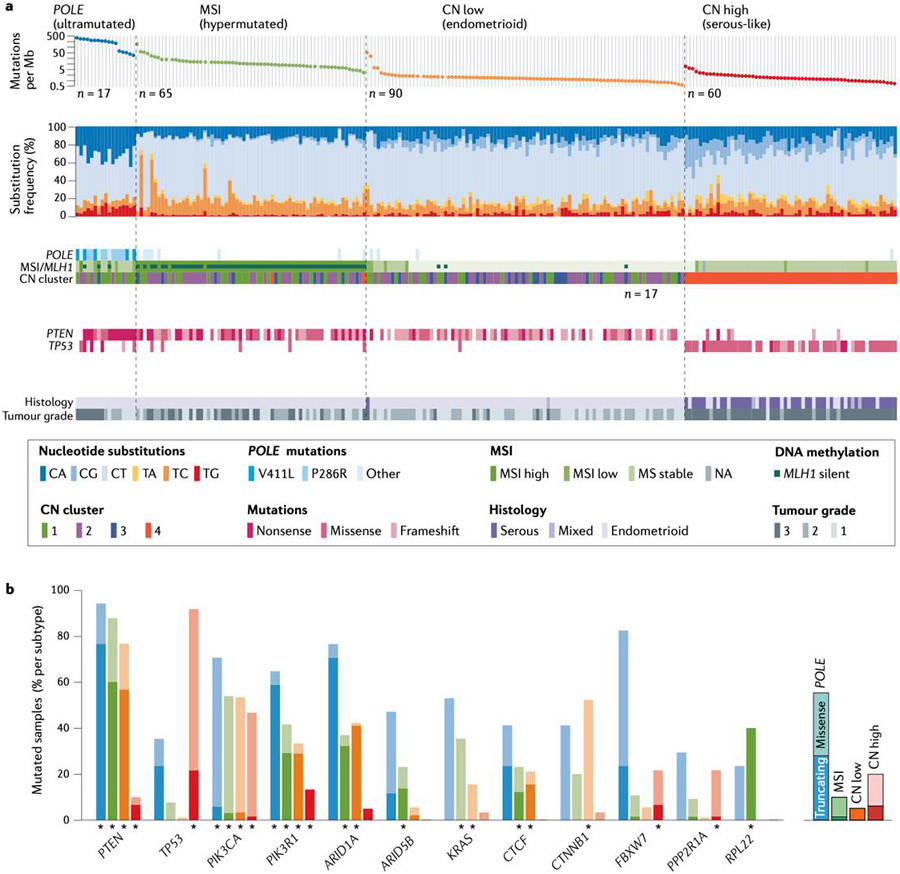
Molecular subgroup of EC. A. Genomic features of the four molecular subgroups of endometrial cancer (EC) identified by The Cancer Genome Atlas project. B. The frequency of mutations in specific EC-associated genes varies between molecular subgroups. MSI, microsatellite instability; Mb, megabase; CN, copy number. From The Cancer Genome Atlas Research Network; Nature 2013;497(7447):67-73. Springer Nature.
In this study, one subgroup was defined by widespread genomic alterations and extensive amplifications and deletions and was termed the copy number high (CNH) group. The CNH group contained most high-grade, aggressive cancers, and included all uterine serous carcinomas and ~25% of the high-grade endometrioid tumors. The clinical outcome of this subgroup was poor. Most of these tumors had pathogenetic mutations in TP53. These tumors also have frequent somatic mutations in PIK3CA, and mutations in FBXW7 and PPP2R1A, which are unique to CNH tumors. Moreover, these tumors have frequent amplification of CCNE1, which can lead to increased replication stress and likely chemoresistance.65 Some tumors also have amplification of ERBB2, which is a therapeutic target being evaluated in prospective clinical trials.66 L1CAM (a cell adhesion molecule that can affect cell motility) is commonly expressed in uterine serous carcinomas and has been associated with poor outcomes.67,68
Another subgroup of EC was tumors with microsatellite instability (MSI) (Fig. 3). These tumors have mismatch repair defects and a tumor mutational burden that is ~10-fold greater than that of a general mutational background. These tumors have mutations in many genes owing to their generally high mutation burden, therefore, it can be difficult to differentiate passenger from driver mutations. PTEN, ARID1A, PIK3CA, PIK3R1 and RPL22 are all commonly mutated in the MSI subgroup of EC. Moreover, mutations or epigenetic silencing of MLH1, MSH2, MSH6, PMS2, and less commonly EPCAM, are often responsible for MSI. These tumors often have unusual histological morphology that can make classification challenging, but they are uniformly endometrioid tumors often with cancer involvement of the lower part of the uterine body and with extensive tumor-infiltrating lymphocytes (TILs).
The third subgroup was identified owing to the discovery of recurrent mutations in POLE (encoding the exonuclease domain of polymerase-ε), which is present in ~7% of tumors. These tumors have a mutational burden ~100-fold greater than that of a general mutational background, and therefore, have somatic mutations in many frequently mutated cancer genes with difficult attribution to either passenger or driver mutations. These tumors also have a brisk lymphocytic infiltration, which may account for the better prognosis of this subgroup. Initially, tumors in this group were not thought to recur; however, there are some reports of recurrence, but at a lower rate than seen in any of the other molecular subgroups.69 Whether the favorable outcomes in this subgroup are due to tumor inherent biology or the interaction of adjuvant therapy with the tumor microenvironment is unclear. Mutations in ECs with POLE mutations are likely the consequence of both replication errors and increased polymerase activity, leading to amplification of these errors reflected in the genomic mutation rate.70
The fourth molecular subgroup consists of tumors with low amount of copy number alterations and no increased mutation burden, termed the copy number low (CNL) group (also known as the no specific molecular profile (NSMP) group by the TransPORTEC consortium).71 This group uniformly contains endometrioid tumors that are generally low grade. Unique to this subgroup are common CTNNB1 mutations that are associated with a worse outcome that would otherwise be expected from these tumors with favorable histopathologic features, presumably through activation of the canonical WNT pathway.72 This group also contains a subgroup of tumors with amplification of chromosome arm 1q, which seem to have a much worse outcome than would be expected.73 These two molecular features, CTNNB1 mutations and 1q amplifications, make this a fascinating group for future study and for stratified clinical trials.
The identification of the molecular subgroups has rapidly changed the way ECs are stratified and treated. Several groups have taken these initial findings from TCGA and extrapolated them for better application to clinical practice. One approach - known as ProMisE (proactive molecular risk classifier for EC) - uses IHC to identify mismatch repair proteins and p53, and sequences the POLE exonuclease domain.74 ProMisE has identified four molecular subtypes of EC that are analogous but not identical to the four genomic subtypes described in TCGA study: MMRd, DNA POLE (corresponding to the ultramutated (POLE mutated) subtype), p53abn (which demonstrates aberrant p53 immunohistochemical staining and corresponds to the CNH subtype) and p53wt (which corresponds to the CNL subtype). Cases lacking enough information to classify are designated NSMP.75,76
The National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) has completed an integrated proteogenomic analysis of EC specimens.77 This approach further defined a critical role linking Wnt signaling and histone acetylation for a subset of low-grade endometrial tumors and identified new regulatory pathways linking circRNAs to the epithelial-mesenchymal transition (EMT). Other important molecular features that may help to explain the response to immuno-oncology agents were also reported, and all data are in the public domain.
Uterine Carcinosarcomas
Endometrioid-like mutations in uterine carcinosarcoma include ARID1A (occurring in 10-25% of tumours), KRAS (10-15%) and PTEN (10-50%); and serous-like mutations include TP53 (60-90%), FBXW (10–40%), and PPP2R1A (15-30%),62,64,78-80 with TP53 and FBXW7 mutations occurring more frequently in uterine carcinosarcoma than in other EC subtypes.78 Additional work has found that the four TCGA molecular subgroups, previously described, can be reproduced in carcinosarcoma tumor samples.81 Further molecular analyses confirm that uterine carcinosarcoma differentiation develops through EMT. Evaluation of the immune microenvironment of uterine carcinosarcoma found a higher immune infiltration in tumors with increased mutation burden,82 with increased plasma cells and M2 macrophages in the sarcomatous component. The immune profile of uterine carcinosarcoma was associated with clinical outcome.82
Immune landscape and tumor microenvironment (TME)
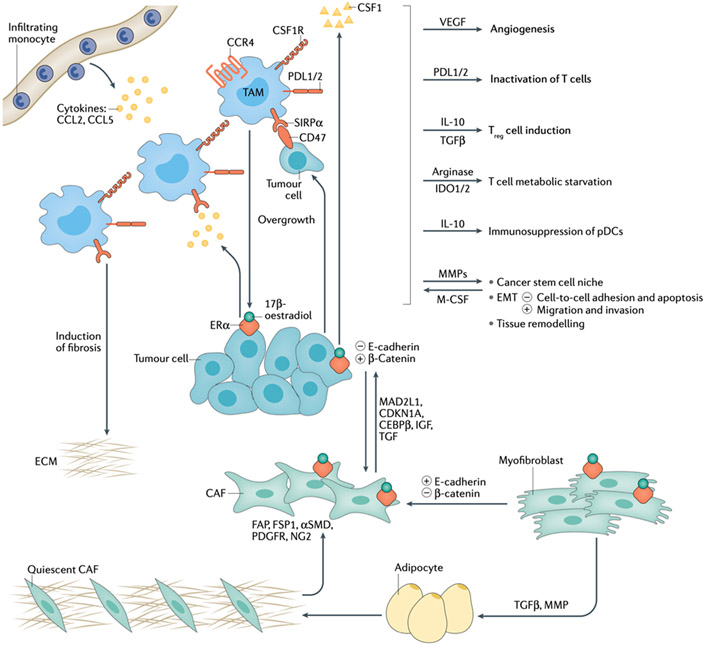
In normal endometrium, the immune system protects against pathogens and safeguards feto-maternal tolerance, whereas in carcinomatous endometrium, it exerts both pro-tumorigenic and anti-tumorigenic functions.83 The tumor microenvironment includes supporting cells of stromal cells, mainly composed of ER-α-positive fibroblasts and myofibroblasts, considered cancer-associated fibroblasts (CAFs), inflammatory cells and endothelial cells, which facilitate EMT induction and drive metastatic progression through interaction with cancer cells (Fig. 4).84 Leukocytes, especially tumor-associated macrophages (TAMs), as well as fibroblasts and myofibroblasts, have a crucial role malignant progression of hyperplasia to EC.85
Figure 4. Crosstalk endometrial cancer’s cell, TAMs and microenvironment.
Infiltrating monocytes are recruited to tumor foci via chemokines such as CCL2 and CCL5 which induced accumulation of tumour-associated macrophages (TAMs). Tumors produce CSF-1, which acts as both a chemoattractant and a mitogen for circulating monocytes which differentiate into TAMs. TAMs produce angiogenic factors such as VEGF to stimulate tumor-associated blood vessel growth. Through the expression of the immune checkpoint ligands PD-L1/2 and secretion of immunosuppressive factors like prostaglandins, TAMs create an immunosuppressive environment by acting on T cells. Reciprocally, TAMs remodel the extracellular matrix via matrix metalloproteases (MMPs) to create an environment that is a niche for cancer stem cells and conducive for the epithelial-mesenchymal transition (EMT), which leads to metastasis. Amino acid metabolism in TAMs causes metabolic starvation of T cells via IDO1/2 pathway. IL-10 and TGFβ secreted by TAMs promote regulatory T cells (Treg) activity, leading to immunosuppression. Fibroblasts and myofibroblasts, activated by the binding 17-β-estradiol and its receptor ERα, secret cell-cycle-related proteins (MAD2L1, CDKN1A and CEBPβ) and growth factors (IGF and TGF), leading to EMT, which reduces cell-cell adhesion and triggers the ability of cells to escape from apoptosis, migrate and invade. The contemporary loss of E-cadherin and the up-regulation of β-catenin drive the EMT process leading to the alteration of the endometrial architecture and the subsequent multistep process towards endometrial cancer (EC). Fibroblasts and myofibroblasts act as sentinel and amplifier of estrogens on the neighboring endometrium. They are characterized by an opposite expression of E-cadherin/β-catenin compared to the epithelium.
Stromal and immune cells.
The carcinogenic process involves fibroblast migration and proliferation at the neoplastic site, leading to increased collagen production and alpha-smooth muscle actin expression by fibroblasts - termed the desmoplastic response - which in the presence of the growth factor PDGF, is a hallmark of CAFs.86 And the reduced ER-α expression in this setting of CAFs, associated with a more myofibroblastic phenotype, is sufficient to promote endometrial hyperplasia and carcinogenesis.87 CAFs, through modulation of the PI3K/Akt and MAPK/ERK pathways and promote immune cell recruitment, can also stimulate EC proliferation.88 Moreover, APC controls Wnt/β-catenin signaling, which is involved in uterine cancer development. Preclinical studies in mice suggest APC decreases ERα and PR expression and induces a lack of response to estradiol-17β (E2) treatment in uterine stromal cells, in addition to decreasing stromal levels of TGFβ and BMP activity, and increasing levels of VEGF and stromal derived factor signaling components that can lead to the development of EC.
The initial step in EC formation is the recruitment of leukocytes from blood, which is regulated by several chemokines, including SDF-1α (also known as CXCL12) and its receptor, CXCR4.14,88 Monocytes attracted to tumors via CCL2, differentiate into TAMs,89 with higher tumor microenvironment macrophage density correlated with poor prognosis.90 Compared with benign endometrium, ECs have a higher CD68+ macrophage density in epithelial and stromal compartments,91 which is associated with higher risk of myometrial invasion, lymph-vascular space invasion (LVSI), lymph node metastasis, higher International Federation of Gynecology and Obstetrics (FIGO) stage, histological grade, as well as angiogenesis, higher micro-vessel density, and expression of Ki-67 and p53.92
TAMs have several functions, including stimulation of tumor-associated blood vessel growth via secretion of VEGF and creation of an immunosuppressive microenvironment through expression of PD-L1 and PD-L2 immune checkpoint ligands and secretion of immunosuppressive factors like prostaglandins. Additionally, matrix metalloproteases (MMPs) induce remodeling of the extracellular matrix via TAMs and metabolic starvation of T cells through arginase activity and production of immunosuppressive metabolites by IDO1/2. TAMs also promote the activity of regulatory T cells (Tregs) via secretion of IL-10 and TGFβ, creating an immune-suppressive milieu that is conducive for EMT and subsequent metastasis.93 TAM density is also inversely correlated with the expression of PR and enhanced estradiol sensitivity and the addiction of EC cells to estrogen and progesterone hormones via ER expression94 (Fig. 4).
In comparison to early well-differentiated EC, CD8+ and CD4+ T cells, Tregs, and Tregs/CD8+ or Tregs/CD4+ ratios are significantly higher in advanced poorly differentiated EC with LVSI.95 Additionally, high Treg counts and high Tregs/CD8+ ratios are associated with a worse prognosis.95 Although POLE-ultramutated and mismatch repair deficient (MMRd) subtypes are enriched for TIL-high tumors, 22% of these tumors have been shown to be TIL-low, and TIL-low tumors are also enriched for p53abn (abnormal) and p53wt (wild-type).96,97
In contrast to other subtypes of EC, POLE ultramutants have a robust cytotoxic T-cell response, denoted by increased CD8+ TILs and CD8A expression, augmented tumor-infiltrating T-cell gene signature, upregulation of T-cell cytotoxic differentiation, and overexpression of T-bet, Eomesodermin, IFN-γ, perforin and granzyme B effector markers.98,99 Additionally, T-cell exhaustion markers (including LAG3 TIM-3 and TIGIT) and T-cell inhibitors (PD-1 and CTLA-4) are expressed, suggesting prolonged antigenic exposure.100 Inhibition of dendritic cell function also seems to be involved in the development of EC. In support of a role, one study reported dendritic cell invasion in 49% of patients with endometrioid carcinoma, which was significantly higher than that for control normal endometrium.101
PD-1/PD-L1 axis.
One study found a high level of PD-L1expression in 92% of EC samples.102 By contrast, PD-L2 expression was very low, as also reported in ovarian and cervical cancers.83 POLE tumors, followed by MMRd tumors, have the highest epithelial PD-1+CD8− (putative CD4+) and PD-1+CD8+ TILs densities, whereas p53abn and p53wt tumors are associated with significantly lower levels of these cells.96 In the stromal compartment, a similar trend is noted, with the exception of the p53abn subtype, which ranks second for PD-1+CD8− TILs. Among POLE, MMRd, and p53abn tumors, the proportion of PD-1+CD8+ cells is similar (75-79%), with the percentage declining sharply to 33% in p53wt tumors.97 One study reported infrequent PD-L1 expression in tumor cells, which was common in intraepithelial immune cells.96 Greater PD-L1 expression in Lynch syndrome–associated ECs compared with MLH1 promoter hypermethylated EC was shown by another study.103 Similar to PD-L1, IDO-1 expression was significantly higher in TIL-high tumors across the four molecular subtypes. However, IDO-1 was expressed in only 21% of the examined EC samples.83 A higher prevalence of IDO-1 expression in MMRd tumors, particularly Lynch syndrome–associated EC, has previously been suggested.104
Endothelial cells, angiogenesis & EMT.
In response to cytokine and growth factor synthesis and hypoxia due to perfusion or diffusion dysregulation or anemia in the setting of malignancy, soluble proangiogenic factors are secreted by endothelial cells.105 These factors include VEGF, bFGF, TNFα, and IL-1β.14
Angiogenesis, marked by VEGF and MMP production, is essential to the normal functioning of the endometrium both during and after the menstrual cycle. However, MMP and VEGF overexpression in EC are associated with tumorigenesis and metastasis.106 In addition, hypoxia and EMT are key occurrences in tumor invasion and metastasis. HIF1 stabilization controls the expression of EMT regulators SNAIL, SIP and ZEB, and the HIF1A/TWIST/E-cadherin system appears to have a key role in the acquisition and progression of metastatic phenotype in endometrioid EC.107
Moreover, E-cadherin loss and upregulation of β-catenin promotes EMT in numerous malignancies, including EC.108 CTNNB1 mutations, which are frequently associated with endometrial hyperplasia and endometrioid EC, result in abnormal expression of β-catenin.109 This mesenchymal microenvironment homeostatic imbalance leads to a compromise of epithelial cell-to-cell adhesion and subsequent apoptotic escape, as well as the ability of malignant cells, in response to ER activation, to migrate and invade85 (Fig. 4).
Role of hormones in microenvironment
Development of endometrioid adenocarcinomas has been linked to increased circulating levels of E2.110 Both E2 and stromal cell-derived pathways activate ER. These pathways include SDF-1alpha/CXCR4 and HGF/c-Met activated downstream kinases, including MAPK and PI3K/AKT, which phosphorylate ER.111 Surrounding stromal cells also contribute directly to estrogen biosynthesis in which peritumoral stromal cells are primarily comprised of ERα+ fibroblasts and myofibroblasts that are activated by the binding of E2 and the ERα stromal receptor, leading to upregulation of cell-cycle-related proteins (CDKN1A) and certain growth factors (IGF-1, TGF and MAD2L1) that mediate E2 proliferative stimulation of adjacent endometrium, promoting the paracrine secretion of mesenchymal cells.112 Additionally, uterine epithelial differentiation and proliferation, as well as interactions between β-catenin and paracrine hormonal stimuli, are mediated by stromal cells. The higher β-catenin/E-cadherin expression in the post-menopausal state also suggests that this pathway is under the influence of the endometrial hormonal milieu108 (Fig. 4).
Involvement of the uterine microbiome
The microbiome is suspected to have an effect on carcinogenesis by stimulating proinflammatory cytokine or growth factor secretion,113 in addition to affecting the hormonal status of the host or serum levels of sex hormones. Numerous mechanisms, including preventing apoptosis, stimulating proliferation and driving genomic instability, which are hallmarks of cancer, can be driven by microbioma. Higher proportions of Firmicutes at the phylum level and Lactobacillus, Gardnerella, Bifidobacterium, Streptococcus, and Alteromonas were reported in samples of patients with chronic endometriosis, a known risk factor for EC development, compared with healthy individuals. High vaginal pH (an indicator of vaginal dysbiosis) was also significantly associated with EC in one study.114 Of note, evidence supporting a resident population of bacteria in the uterus is lacking. Accordingly, the uterine microbiota is likely comprised by non-resident bacteria.115
Diagnosis, screening, and prevention
Clinical presentation and diagnostic assessment
Among perimenopausal and postmenopausal women, postmenopausal bleeding (PMB) accounts for approximately two-thirds of all gynecological visits and is a common symptom of EC. Indeed, one meta-analysis found that PMB occurred in ~90% of patients with EC; however, it led to a diagnosis of EC in only 9% of cases.116 Although abnormal uterine bleeding is the most common symptom of EC, bleeding can be accompanied by vaginal discharge and pyometra (uterine infection) in some women. Patients diagnosed with advanced EC might also present with symptoms similar to those of advanced ovarian cancer, such as pain and abdominal distension along with either constipation or diarrhoea.
Diagnostic work-up is recommended to rule out EC in all women presenting with PMB. The standard work-up to investigate and determine the cause of PMB may comprise pelvic ultrasonography, endometrial biopsy or dilatation and curettage (D&C; involves cervical dilation with scraping of the endometrial lining) with or without hysteroscopy.117,118 Measurement of the endometrial thickness using transvaginal ultrasonography should be performed in the sagittal plane at the thickest point; the majority of authors consider 5 mm as the normal upper limit for endometrial thickness in postmenopausal women, and this cut-off value has a sensitivity of 96% and a specificity of 61% for EC in post-menopausal women with abnormal uterine bleeding.119,120 Pelvic ultrasonography can be omitted in patients who already have an endometrial sampling showing an invasive cancer. When histopathological findings from endometrial biopsy are insufficient to confirm diagnosis, a D&C should be carried out; of note, biopsy under hysteroscopy has a higher accuracy than ‘blind’ D&C and remains the gold standard for the diagnosis of EC when possible.121
Of note, biomarkers of EC, namely, CA-125 nor HE4, can be incorporated into routine diagnostic and follow-up practice for EC management owing to lack of evidence in support of their clinical impact.122-125
Pre-operative staging: imaging
Although EC is a surgically staged disease, preoperative staging using imaging may help establish those at risk of recurrence and inform surgical management, as imaging can identify myometrial or cervical invasion and lymph node metastasis (Fig. 5). Staging using imaging is indicated for patients who present with symptoms suggestive of extrapelvic disease and those with a poor performance status who are unable to independently perform activities of daily life for whom metastatic disease must be ruled out and in consequence surgery would not be an option.
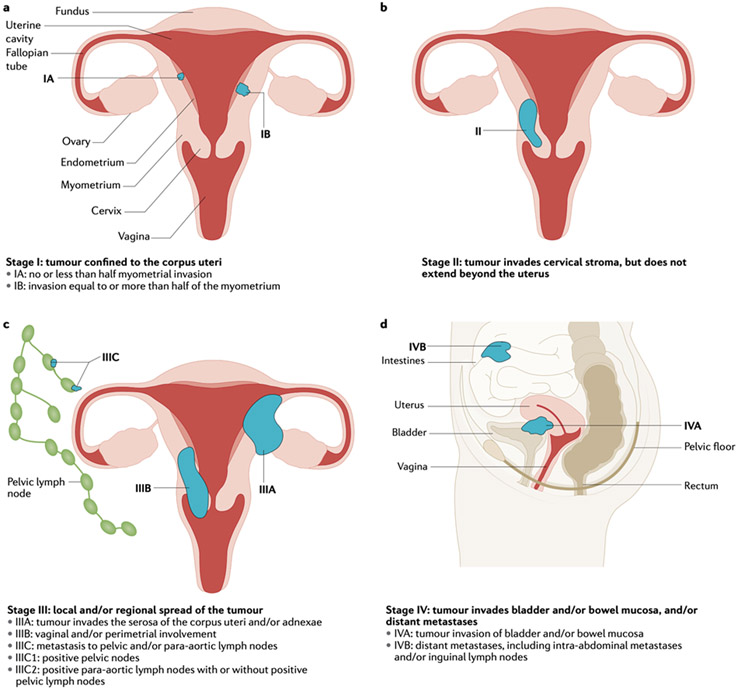
Figure 5. Staging of endometrial cancer.
Endometrial cancer is staged according to 2009 International Federation of Gynecology and Obstetrics (FIGO) criteria. This staging system comprises four stages (I-IV): tumour limited to the corpus uteri (Stage I; panel a), tumor invasion of cervical stroma but confined to the uterus (stage II; panel b), local and/or regions tumor spread (stage III; panel c) and tumour invasion of bladder and/or bowel mucosa and/or distant metastases (stage IV; panel d).
MRI is the most accurate imaging technique for preoperative staging of EC due to its excellent soft tissue contrast resolution. Depth of myometrial invasion and cervical stromal invasion are both important aspects of EC staging and can be determined using dynamic contrast-enhanced MRI (DCE-MRI) and T2-weighted imaging (T2WI).126 Diffusion-weighted imaging (DWI) can be carried out in patients who cannot receive intravenous gadolinium, which is typically used for DCE-MRI. In support of the use of DWI, one meta-analysis did not identify differences in the diagnostic performance of DWI compared with DCE-MRI, and combining T2WI and DWI was superior to DWI or DCE-MRI alone.127
CT has low sensitivity (83%) and specificity (42%) for myometrial and cervical stromal invasion and is generally not used for the initial diagnosis of EC.127 However, CT is useful in evaluating the extent of disease in women with more advanced disease with extra-uterine spread. For detection of lymph node metastases, both CT and MRI have a sensitivity of 27-66% and a specificity of 73-99%.127 Integrated PET and CT (PET-CT) is not an appropriate screening tool for detection of primary EC due to its limited spatial resolution and is only indicated for initial staging if extra-uterine involvement is suspected or is observed on a preoperative MRI. Moreover, when PET-CT is performed as initial staging, primary intratumoral heterogeneity of 18F-fluorodeoxyglucose (18F-FDG) uptake seems to be a negative prognostic sign, correlating with a greater likelihood of tumor recurrence128; these patients may benefit from more aggressive monitoring. In advanced disease, PET-CT may be particularly useful in detecting pelvic and paraaortic lymph node metastasis, with a sensitivity and specificity ranging from 51-69% and 90-100%, respectively. In addition, PET-CT and either CT or MRI has a higher sensitivity and specificity for detecting recurrence compared to CT and/or MRI alone.127 Nevertheless, no correlation was found between early detection of recurrence and overall prognosis. Therefore, integration of PET-CT in the diagnosis and follow-up of EC is not routinely indicated.129
Histological and molecular classification
Historically, EC is broadly classified into two subtypes — type I and type II — based on histological characteristics, grade and hormone receptor (ER and PR) expression.130 Type I EC is the most common subtype, and comprises low-grade, endometrioid, diploid, hormone-receptor–positive EC that have a good prognosis (5-year OS rate of 85%).2 Type II EC is non-endometrioid, high-grade, aneuploid, TP53-mutated, hormone-receptor-negative EC, and is associated with a higher risk of metastasis and a poorer prognosis (5-year OS rate of ~55%).131 These two types of EC have mutational profiles that differ substantially. Of note, it is increasingly clear that this traditional dualist classification of EC is suboptimal as a guide to risk classification, and consequently, as a tool to tailor therapy.
The histopathological classification for EC is a challenging task even among expert gynecopathologists, resulting in a frequent lack of consensus.132 As a result of this, EC risk assessment has been inaccurate and has possibly led to over- or undertreatment.133 In this context, the molecular classification of EC provides a reproducible and prognostically relevant classification system; therefore, its integration into EC diagnostic procedures should be requisite.
As previously described, the TCGA identified four molecular subtypes of EC based on genomic abnormalities (Figs. 3 and 6). Although the TCGA study is a milestone in EC classification, these methods require fresh-frozen material and costly and complex methodologies. To try and decrease costs and technical challenges associated with these methods, a simplified, pragmatic molecular classifier—ProMisE—has been developed using methods that can be easily adopted at most centers.74,134,135 As previously mentioned, this classification system identifies four molecular subtypes of EC: MMRd, DNA POLE, p53abn and p53wt. Cases lacking enough information to classify are designated NSMP.75,76 Molecular classification drives the treatment of EC in the adjuvant and recurrent metastatic setting.
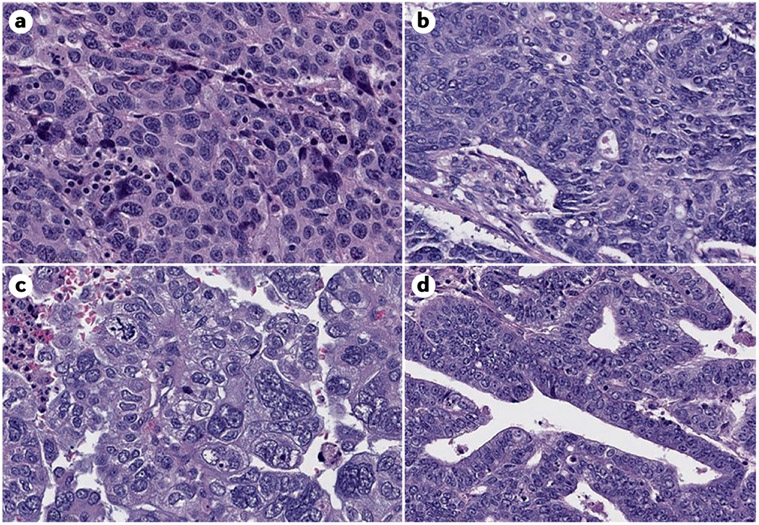
Figure 6. Histological features of endometrial cancer.
A. POLE ultramutated. B. Microsatellite Instability High. C. Copy number high. D. Copy number low. Image credit to Dr. Lora H. Ellenson, Chief Attending, GYN pathology.
Although determining MMR and p35 status using IHC is widely available, sequencing POLE cannot be widely carried out. IHC analyses as part of an EC diagnosis should be universally implemented to guide and personalize the treatment of patients with EC.
Prognosis by molecular subtype
The survival differences between the molecular subtypes of EC have been replicated in several studies incorporating the surrogate marker approach (Fig. 7).74,134-136 In these studies, the p53abn group consistently had the poorest prognosis, with a risk of death or progressive/recurrent disease 3-5–fold higher than that of the p53wt group, and 2-fold higher when adjusted for clinicopathological features. The MSI group was associated with a 1.5-2–fold increased risk of death compared with the p53wt group, which was non-significant upon adjusting for clinicopathological features. However, heterogeneity of the latter group might account for the prognostic overlap with the p53wt group, underscoring the need for further stratification of the p53wt group. The POLE-mutant group was least affected by clinicopathological features and was associated with the most favorable prognosis. The integration of biomarkers into prospective clinical trials is vitally important to more optimally delineate the relationships between molecular phenotypes and outcomes.
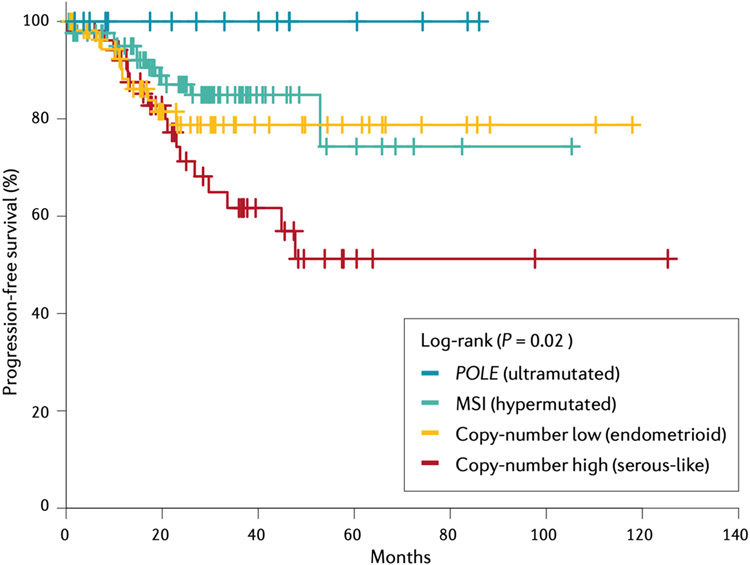
Figure 7. Progression-free survival of EC.
Survival varies according to the molecular subtype of endometrial cancer (EC). Copy-number high EC is associated with the poorest prognosis out of all subtypes, whereas the ultramutated subtype is associated with high survival. Adapted from From The Cancer Genome Atlas Research Network; Nature 2013;497(7447):67-73. Springer Nature.
Screening
The strongest risk association for the development of EC is with Lynch Syndrome. Although some clinicopathologic features, such as <50 years of age at diagnosis, a personal or family history of Lynch syndrome-associated cancers and the presence of lymphocytic infiltration in the tumor specimen, which may indicate the need for germline testing for Lynch Syndrome, none are sensitive enough.137 A more sensitive strategy is universal molecular tumor testing for all ECs diagnosed in women ≤60 years of age. The most cost-effective screening strategy is IHC for MLH1, MSH2, MSH6, and PMS2 expression in tumor samples, which can be performed in most pathology laboratories. However, most cases of MLH1 loss are due to MLH1 hypermethylation, with only 2-6% associated with germline mutations in MMR genes138,139; therefore, tumor samples with loss of MLH1 should subsequently be analyzed for MLH1 hypermethylation, followed by germline testing in those without MLH1 hypermethylation. Although universal screening in patients with EC who are <70 years of age is cost-effective owing to prevention of colorectal cancer in both patients with EC and their relatives,140 there are logistical barriers to such screening, for example, insufficient tissue for testing and patients declining tissue or germline testing.138
Management
Estimating the risk of disease recurrence has historically been challenging for EC given variability in surgical practice and the lack of reproducible pathological classification.141 Consequently, treatment of newly diagnosed EC is variable between regions and across treatment centres. For early-stage disease, the main treatment is surgery. Depending on stage of disease and other risk factors, adjuvant radiotherapy and/or chemotherapy can be used to reduce risk of recurrence.142 Studies investigating adjuvant endocrine therapy have been small and negative; therefore, endocrine therapy is not recommended in the adjuvant setting.143 Options for metastatic disease are limited, with both chemotherapy and endocrine therapy considered standard of care.144 More recently, immunotherapy alone or in combinations has become standard of care, although these therapies are not universally available across all jurisdictions.145
Surgery
Surgery is the primary treatment for women with localized EC. Surgical staging is used for prognostication and identification of women who might benefit from adjuvant treatment. Total hysterectomy with bilateral salpingo-oophorectomy (BSO) is standard of care and can be performed by an open or a minimally invasive approach. Minimally invasive techniques have similar oncological outcomes, shorter hospital stay and fewer complications compared with open laparotomy in early-stage EC.146-149 Intraoperative complications, such as bleeding and bowel-associated complications, occurred in ~8% of women who underwent laparotomy compared with 10% who underwent laparoscopy. Moreover, postoperative adverse events such as infection, thrombosis and fistula formation occur less often in those who undergo laparoscopy compared with laparotomy.149 The benefit of minimally invasive surgery is arguably greatest for women with morbid obesity and the frail elderly, in whom perioperative morbidity from laparotomy is highest.150 However, minimally invasive surgery should only be performed by a surgeon with appropriate training, and the uterus should be removed intact, not morcellated. Neoadjuvant chemotherapy can sometimes be considered but has not been universally adopted.151 Surgery for advanced or recurrent EC is more controversial. Most benefit has been described for those who undergo optimal (microscopic) cytoreduction.152
Lymphadenectomy is a controversial topic in the management of EC. GOG 33 found a role for lymphadenectomy in staging EC,153 and retrospective analysis found a survival benefit for pelvic and paraaortic lymphadenectomy in patients with higher risk histologies and for those with intermediate-risk or high-risk disease.154,155 The necessity of paraaortic lymphadenectomy (to detect metastatic deposits) is also controversial and can be particularly challenging in patients with obesity. The risk of paraaortic metastases if no metastatic deposits are identified in the pelvic lymph nodes is dependent on other factors, such as LVSI, tumor histology, and grade.156 Two randomized studies did not find a survival benefit with lymphadenectomy in early-stage EC, however, lymphadenectomy is important in staging.157,158 In addition, a Cochrane review did not find evidence that lymphadenectomy was associated with a decreased risk of death or EC recurrence compared with no lymphadenectomy.159
Practice differs across jurisdictions, ranging from no lymph node assessment, sentinel lymph node mapping, and complete pelvic and paraaortic lymphadenectomy as standard of care.157,160 At a minimum, lymph node assessment with removal of suspicious-appearing lymph nodes is recommended. Sentinel lymph node evaluation can be considered as a strategy for nodal assessment for low-risk or intermediate-risk EC and it can be omitted in patients without myometrial invasion (MMI). Lymphadenectomy should be considered for tumors exhibiting MMI >50% or grade 3 or non-endometrioid histology.142 Moreover, the National Comprehensive Cancer Network (NCCN) guidelines suggest evaluation of the pelvic nodes, with or without paraaortic nodal dissection (based on higher risk features such as deeply invasive lesions and high-grade histology), and suggests sentinel lymph node mapping may be considered.161 ESMO-ESGO-ESTRO guidelines do not recommend lymphadenectomy for low-grade, low-risk disease but suggest reserving it for patients with medium- or high-risk disease. Lymphadenectomy can be considered (with sentinel lymph node dissection as an option) for >50% MMI and is recommended for all stages of non-endometrioid histology and grade 3 tumors with >50% MMI. Gross extra-uterine disease should be biopsied and removed where possible.23 Removal of the omentum is considered in those with higher risk histology (such as serous and carcinosarcoma)162 owing to the high incidence of involvement even when EC is apparently confined to the uterus. The British Gynecological Cancer Society (BGCS) does not advise lymphadenectomy for grade 1 and 2 presumed FIGO stage I-II EC. Surgical staging, including pelvic and paraaortic lymphadenectomy and omental biopsy, may be appropriate for high-grade disease and non-endometrioid EC.163 Moving forward, the incorporation of molecular subgroups, in addition to traditional histopathologic parameters, may offer better predictive models to guide surgical decision making.141
Conservative management of EC
Fourteen percent of ECs are diagnosed in premenopausal women, and 5% occur in women aged <40 years,164 usually in those who are nulliparous, and often at an early stage. Women with low-grade endometrioid EC and no evidence of MMI on imaging (including MRI) who wish to preserve their fertility can be considered for a non-surgical approach. High-dose oral progestins and/or levonorgestrel-release intrauterine devices (LNG-IUDs) can be used with careful monitoring to ensure disease progression is not occurring.165,166 According to NCCN and ESGO guidelines,161,164 monitoring should encompass endometrial evaluation with a D&C or endometrial biopsy every 3-6 months. Surgery is recommended if EC is identified at 6-12 months, whereas conception with continued surveillance followed by completion surgery when childbearing is complete or progression occurs is advised in women with a complete response.
The rationale for progestin therapy is the role of unopposed estrogen in the development of precursor and low-grade EC development. Although the mechanisms of action of progestin therapy and resistance to this therapy are not fully known, progestin exposure downregulates ER and PR and reduces mitotic indices.167
Oral progestins can be used for EC treatment but are associated with some challenges, including weight gain and compliance. LNG-IUDs have been explored for early-stage cancers, as they result in constant low-dose progestin exposure within the uterus. In one meta-analysis of oral medroxyprogesterone acetate, a pooled regression rate of 76.2% (95% CI: 68-85.3%) and a live birth rate of 28% was reported, which were similar to response rates with LNG-IUDs.165 However, although response rates are high with these treatments, relapse rates are also high, therefore, these fertility-sparing options are temporizing measures.165
Treatment with LNG-IUD may also be appropriate in patients, in addition to those wishing to preserve fertility, such as those with significant comorbidities. Indeed, one study demonstrated a response rate of 75% in patients with complex atypical hyperplasia and early-grade endometrioid EC (grade 1, 67%; grade 2, 75%).168 Clinical trials to improve the efficacy of progesterone therapy in this population are warranted. Combining endocrine therapy with lifestyle interventions, particularly those that target obesity, is also an attractive approach.
Ovarian preservation can be considered in younger women with low-grade, early-stage EC to mitigate the menopausal symptoms associated with more extensive surgery. One study using SEER population data suggested that overall survival might be improved for women <50 with low-grade, early-stage disease, as risk of cardiovascular death is reduced.169 However, defining which low-risk women are candidates for this type of surgical approach is challenging, and careful expert discussion and counselling is required.
Adjuvant treatment
Low-risk EC.
Adjuvant treatment is not required for women with EC at low risk of recurrence, defined as low-grade (1 or 2) endometrioid histology, disease limited to the endometrium, or disease exhibiting <50% MMI. Local control rates are >95% with surgery alone.170,171
Intermediate-risk EC.
Intermediate-risk EC includes tumors of higher grade endometrioid histology that exhibit MMI (up to occult stage II disease). In addition, the GOG and PORTEC groups have defined a group of women at high-intermediate risk (HIR) based on age, tumor grade, MMI (outer one-third [GOG] and ≥50% [PORTEC]), and the presence of LVSI (GOG).172-174
Postoperative radiotherapy can significantly reduce the risk of local recurrence for women with intermediate-risk EC.172-174 However, the lack of an OS benefit coupled with variability in lymph node staging practices has resulted in a failure to establish a uniform adjuvant approach globally.175 Guidelines and treatment algorithms differ across jurisdictions, leading to variability in clinical practice.148 Whole pelvis external-beam radiation therapy (EBRT) and intracavity vaginal brachytherapy (VBT) can be used to deliver postoperative radiotherapy in patients with EC. Although no OS benefit was observed in GOG-99 and PORTEC-1 with adjuvant EBRT compared with observation in women with intermediate-risk EC, adjuvant EBRT decreased the recurrence rate by ~9%. For women with high-intermediate risk (HIR) features, the benefit was 13-20%, although for those at lower risk, the benefit was only 3-4%.172-174 Based on these studies, women with HIR disease should be offered radiotherapy with either EBRT or VBT. The presence of LVSI (focal vs diffuse) should guide decisions for other patients with intermediate-risk EC who do not fully meet HIR criteria.
For intermediate-risk EC in the absence of LVSI, VBT can be considered, but decisions need to be balanced against the risk of toxicity and the potential effect on QOL as part of the patient-physician decision-making process.175 The ESMO-ESGO-ESTRO guidelines suggest that in intermediate-risk patients no adjuvant therapy may be considered, especially for patients older than 60 years of age. The BGCS guidelines are also less in favor of radiotherapy. Guidelines differ across jurisdictions in women with intermediate-risk cancers. Consideration of tumor characteristics and discussion with the individual patient is essential.
The most common site of recurrence in the GOG-99 and PORTEC-1 studies was vaginal vault. In the PORTEC-2 trial, >96% of women remained free of vaginal vault recurrence with either VBT and EBRT.176 Moreover, 10-year survival data confirmed excellent similar rates of isolated pelvic recurrence, distant metastasis and OS. Accordingly, VBT alone is an option for some women with HIR EC based on careful evaluation of clinicopathologic risk factors.176 Combining EBRT and VBT can be considered on a case-by-case basis for women thought to be at high risk for both pelvic and vaginal recurrence. Factors such as deeply invasive grade 3 tumors, LVSI, or those with limited or no lymph node staging may be taken into account when considering EBRT and VBT. Although no randomized data are available, data from retrospective series support this practice.175
Of note, the addition of chemotherapy to VBT for patients with HIR or high-risk EC did not result in a superior outcome for those with early-stage disease in GOG-249 (Supplementary Table 1). The addition of 3 cycles of chemotherapy did not improve OS or recurrence endpoints compared to pelvic EBRT alone.177 However, chemotherapy was associated with increased toxicity. These results suggest pelvic radiation therapy as the standard treatment for HIR disease.
High-risk EC.
High-risk EC includes stage III-IVA optimally debulked disease and any stage of disease with high-risk histology (serous or clear cell). For these patients, chemotherapy or a combination of radiotherapy and chemotherapy should be considered. Two randomized trials have addressed the relative roles of chemotherapy and radiotherapy in this high-risk patient population (Supplementary Table 1).
In PORTEC-3, patients were randomized to receive CTRT or EBRT alone. Although there was no improvement in OS, an improvement in failure-free survival (FFS) was observed in the CTRT arm. The greatest benefit was observed in women with stage III disease and in women whose tumor exhibited serous histology.178 Interestingly, molecular subgroup analysis demonstrated an improvement in FFS with CTRT over EBRT in women with p53abn EC.71 Other molecular subgroups did not appear to derive benefit from the addition of chemotherapy, although there was a trend toward benefit in the NSMP subgroup. With long-term follow-up, no significant differences in grade ≥3 adverse events between women who received CTRT or those who received EBRT were reported. However, 25% of patients reported the persistence of sensory neurological symptoms.179
GOG-258 found no difference in recurrence-free survival or OS between women with stage III/IVA EC who received chemotherapy or CTRT. However, rates of vaginal recurrence and pelvic/para-aortic recurrence were higher in the chemotherapy arm compared with the CTRT arm. This was balanced by a higher rate of distant recurrence in the CTRT arm compared to chemotherapy arm.180 This has resulted in many centers in the US omitting radiotherapy for stage III disease.
Taking these studies and other smaller trials178,181-183 into consideration, the optimal approach for treating women with high-risk disease has yet to be defined. Planned or ongoing studies are looking at whether adjuvant strategies for higher stage EC should be informed by molecular subgroup. These include trials considering de-escalation of treatment for women with POLE cancers and the inclusion of immunotherapy for women with MMRd tumors. Furthermore, the optimal sequencing of chemotherapy and radiation therapy has yet to be determined and remains controversial, with various smaller studies providing evidence for sequential, sandwich, and concurrent CTRT.178,181-183
Recurrent and metastatic disease
Although EC generally carries a good prognosis, molecular subgroup data suggest that women with p53abn (serous or serous like) EC have the highest risk of metastatic spread. When considering high-risk cancers, 5-year relapse-free survival rates were 46.6% for p53abn ECs compared with 98% for POLE, and 77.1% and 74.4% for MMRd and NSMP ECs, respectively.71 Recurrence can occur locally within the vagina or in the pelvic lymph nodes or within paraaortic lymph nodes or peritoneum. Distant metastases most commonly occur in the lung and lymph nodes. Less common sites of metastasis include the bones, brain, and intra-abdominal organs.184 Treatment for local disease includes the consideration of surgery or radiotherapy, whereas systemic therapy is considered for more distant disease.
Chemotherapy.
Most women with metastatic EC, except those with low-grade, low-volume metastatic EC (confined to the pelvis, managed by radiation therapy), will receive chemotherapy. Frontline chemotherapy for metastatic EC is platinum-based; GOG-209 confirmed the non-inferiority of carboplatin/paclitaxel compared with the more toxic TAP regimen (cisplatin/doxorubicin/paclitaxel), with an OS of ~15 months.185 The benefit of second-line chemotherapy is debatable, with response rates <20% and PFS around 4 months.186 Retreatment with platinum-based chemotherapy is an option for women who demonstrate prior benefit, with retrospective data suggesting response rates of ~40% with a platinum-free interval <12 months and ≥60% with a platinum-free interval >12 months.187,188
HER2 overexpression occurs in 25% of serous or serous-like ECs.61 Based on a small, randomized phase II study, the combination of trastuzumab and carboplatin/paclitaxel improved PFS (12.9 versus 8 months) and OS (24.4 versus 29.6 months) compared with chemotherapy alone in HER2 overexpressing advanced or serous EC.66,189 This treatment regimen has become a standard of care for this subgroup of women in the United States.
Modest benefits for single-agent anti-angiogenics and the rapalog class of mTOR inhibitors, with PFS of around 3 months (some women experiencing durable response) resulted in their inclusion, post chemotherapy, as a treatment option. However, these agents are not universally available, have no identified predictive biomarkers (with the possible exception detailed below), and their use needs to be balanced against their toxicity in the context of modest gain.144 The combination of carboplatin/paclitaxel with either bevacizumab or temsirolimus did not demonstrate a benefit compared with chemotherapy alone.190 However, a subgroup analysis suggested that the presence of a TP53 mutation in the tumor may predict greater benefit to bevacizumab but not temsirolimus.191 This finding warrants further investigation.
Endocrine therapy.
A range of endocrine therapy agents are used in the clinic, including progestins, aromatase inhibitors, and tamoxifen.161 Despite the strong rationale for its use owing to ER and/or PR expression, one Cochrane review in 2010 could not conclude that endocrine therapy improved survival for women diagnosed with advanced EC. This result was due to a lack of randomized trials and too many small studies conducted across more than 6 decades.192 In a meta-analysis, endocrine therapy in an unselect EC population had a response rate of 21.6%, with an associated PFS of 2.8 months193 and a higher response in those with ER/PR EC (35.5%) and a response of 0% in ER/PR-negative tumors.193 There is no accepted standard for measuring or defining ER/PR positivity in EC. In addition, given the modest benefit, it is likely better if predictive signatures are required to optimize the use of this approach in the future. Planned studies incorporating endocrine therapy require high-quality correlative studies to answer fundamental questions and to incorporate patient-reported outcomes to better inform patients and clinicians of any potential benefit. Current indications for the use of endocrine therapy are based on clinicopathologic features; endocrine/hormonal agents can be considered for low-grade endometrioid histology with low-volume/indolent disease. Randomized comparative data on single-agent efficacy sequencing of endocrine therapy to inform subsequent use is lacking.194
Given that endocrine therapy is generally well tolerated they are attractive options for EC treatment. Furthermore, downstream interactions of the ER/PR pathways lend themselves to potential combinations with some of the newer targeted agents (Supplementary Table 2).187-192 The combination of everolimus (an oral mTOR inhibitor) and letrozole appears as a standard of care option within NCCN Guidelines.
Immunotherapy.
Approximately 30% of ECs belong to the MSI-H/MMRd subgroups.186 These tumors have higher mutational burden and are susceptible to immune checkpoint blockade.
The Keynote-158 study reported an objective response rate (ORR) of 57.1% with pembrolizumab treatment in 49 EC patients whose tumors were MSI-H/MMRd, with a complete response in 16% and partial response in 40%.201 In this study, median PFS was 25.7 months. Activity was also observed in women with tumor mutation burden-high (TMB-H) solid tumors, defined as ≥10 mut/Megabases, with an ORR of 29%.202 Accordingly, pembrolizumab is FDA approved for the treatment of previously treated MMRd/MSI-H and TMB-H EC.
The GARNET trial is a phase Ib study of the anti-PD1 agent dostarlimab, and has found ORRs of 44.7% for patients with MMRd EC and 13.4% for those with MMRp with dostarlimab treatment.202 Based on these results, dostarlimab has been granted approval by the FDA and conditional marketing authorization by the European Commission for women with MMRd or MSI-H EC that has progressed after prior therapy.
Several other studies have evaluated the activity of checkpoints inhibitors in EC. For example, the Phaedra Study203 evaluating durvalumab (anti–PD-L1 agent) reported an overall tumor response rate (OTRR) according to iRECIST of 43% for women with MMRd EC.203 In addition, a non-randomized, two-cohort, phase II study of avelumab (anti–PD-L1 agent) in women with advanced or recurrent EC after at least one previous therapy found an overall response rate of 26.7% in the MMRd cohort and 6.25% in the MMRp/non-POLE cohort. In the MMRd cohort, 40% of patients were progression free at 6 months.204
The KEYNOTE-146/Study 111 and the randomized phase III study KEYNOTE-775/Study309 evaluated the combination of pembrolizumab with lenvatinib, an oral multikinase inhibitor in women with previously treated EC. In KEYNOTE-146, with a primary endpoint of ORR at 24 weeks (ORRWk24) as evaluated by investigators per iRECIST, 87% of patients had MMRp/MSS tumours. The ORRWk24 was 38.0%, and response occurred regardless of MSI and PD-L1 status or histology. Median PFS was 7.4 months and median OS was 16.7 months, with long-term responders observed. As a result of these data, pembrolizumab with lenvatinib have been granted accelerated approval by the FDA, Australian Therapeutic Good Administration, and Health Canada for treatment of advanced non-MSI-H/ non-MMRd EC that has progressed following prior therapy.205 Adverse events were manageable but significant; 69% experienced grade 3/4 toxicities, of which hypertension was the most frequent (32.4%). Other adverse events of any grade were hypertension, diarrhea, decreased appetite, fatigue, and hypothyroidism. Dose interruption occurred in 72%, and lenvatinib dose reduction occurred in 65% of patients.
The KEYNOTE-775/Study 309 included patients with advanced or recurrent EC who had received at least one prior platinum-based regimen, and found a significant improvements in OS, PFS, and ORR with lenvatinib and pembrolizumab compared with treatment of physician’s choice, regardless of MMR status.206 For the entire population, the median OS was 18.3 vs. 11.4 months, the median PFS was 7.2 vs. 3.8 months, and the ORRs were 31.9% vs. 14.7% favoring lenvatinib and pembrolizumab. Based on these data, lenvatinib and pembrolizumab combination therapy was FDA approved for patients with advanced EC that is not MSI-H or MMRd, who have disease progression following prior systemic therapy in any setting, and by the European Medicines Agency for all EC following prior platinum-based therapy in any setting. Furthermore, large, randomized trials exploring this and a number of other novel treatment combinations are ongoing.
Quality of life
In general, several adverse effects are associated with EC treatment, including lymphedema, neurotoxicity, fatigue and bowel-bladder issues, and result in a decline of QOL. However, few studies focused exclusively on QOL in patients with EC have been carried out. Measurement of QOL has been generally conducted by patient-reported outcome (PRO) questionnaires, including the EORTC QLQ-C30, SF-36, FACT-G and FSFI, among others. These questionnaires are widely used to assess the QOL of patients with cancer, although not specifically for EC. Based on a systematic review of PROs in women who survived EC,207 obesity was associated with lower QOL and physical functioning. Moreover, surgical procedures, specifically laparoscopy over laparotomy was associated with better QOL outcomes, and vaginal brachytherapy was associated with better outcomes over external-beam radiation.
As most cases of EC are estrogen-linked, BSO is recommended in addition to total hysterectomy, in many patients (see Management, above even in young patients. In premenopausal patients, reduction of QOL associated with hypoestrogenism due to BSO needs to be managed. In general, climacteric and emotional symptoms caused by surgical menopause are more severe than those associated with spontaneous menopause.208 Performing BSO in those <50 years of age is associated with increased risk of all-cause mortality, coronary heart disease and stroke.209,210 Moreover, patients undergoing BSO for EC have a higher frequency of hypertriglyceridemia compared with those undergoing BSO for non-EC reasons, and serum low-density lipoprotein cholesterol levels are higher in those with a history of premenopausal BSO for EC.211 Cardiovascular disease is the most common cause of death in survivors of EC.212 Treatment of EC by BSO, radiation therapy and chemotherapy may increase the risk of reduction of bone mass and osteoporosis; however, some reports have shown that total adipose mass is correlated with a high bone mass,213 and that osteoporosis is less frequent in patients with EC with hypertriglyceridemia.214
Hormone replacement therapy is an effective treatment for symptoms caused by BSO, and was not associated with increased risk of EC recurrence in a meta-analysis and randomized controlled trial.211,215 However, an increased risk of recurrence was observed in Black patients who received estrogen replacement therapy in the randomized controlled trial.216 These findings suggest that conjugated estrogens or E2 can be used to alleviate hypoestrogenism-associated symptoms after EC surgery. Selective estrogen-receptor modulators (SERMs) may be another option, but no comparisons of long-term treatment with estrogen and SERMs have been carried out.217 As previously mentioned, no significant increase in risk of EC recurrence was observed in a meta-analysis of EC survivors using hormonal replacement therapy; however, in subgroup analysis, a protective effect on EC recurrence was seen with combined estrogen-progesterone hormone replacement therapy compared with estrogen-only therapy. Of note, these data must be interpreted with caution, as there was significant heterogeneity across estrogen-alone studies. Progesterone can increase the risk of breast cancer; therefore, it is important to provide accurate information about these hormones to patients. Patients who are considered poor candidates for hormone replacement therapy (such as smokers, those with a history of breast cancer or multiple strokes) may be considered for non-hormonal therapies, such as selective serotonin reuptake inhibitors (SSRIs), serotonin, norepinephrine uptake inhibitors (SNRIs), and anticonvulsants.218,219
Outlook
As previously discussed, EC is associated with many molecular aberrations that present myriad opportunities for targeted therapy and precision medicine.61,220,222 Although four major molecular subgroups have been identified, the majority of patients are treated in the same way, regardless of molecular differences in their tumors. However, recent publications and novel clinical trials are beginning to change this. Over the past year two new therapies have been approved by the FDA for advanced or recurrent EC: pembrolizumab for MSI-H/MMRd tumors222 and pembrolizumab plus lenvatinib for non-MMRd/non-MSI-H tumors.205
Based on TCGA findings, two groups of investigators developed pared down programmatic molecular classifiers that could be incorporated into clinical decision-making and counseling of patients with EC. The TransPORTEC initiative established a stratification model based on TP53 mutation, MSI, POLE mutation or NSMP status.223 These features were associated with the rate of distant metastases and recurrence-free survival.223 The TransPORTEC model has been further refined through the integration of clinical factors (LVSI) and other important molecular features (CTNNB1 mutation status and L1-CAM expression).224 The PORTEC-4a trial is prospectively testing this model to assign treatment to patients with HIR EC (Fig. 8).225 This exciting trial focuses on the use of radiation as a treatment strategy; thus, there still remains opportunity to incorporate targeted agents and immunotherapy into adjuvant therapy based on TransPORTEC classification.
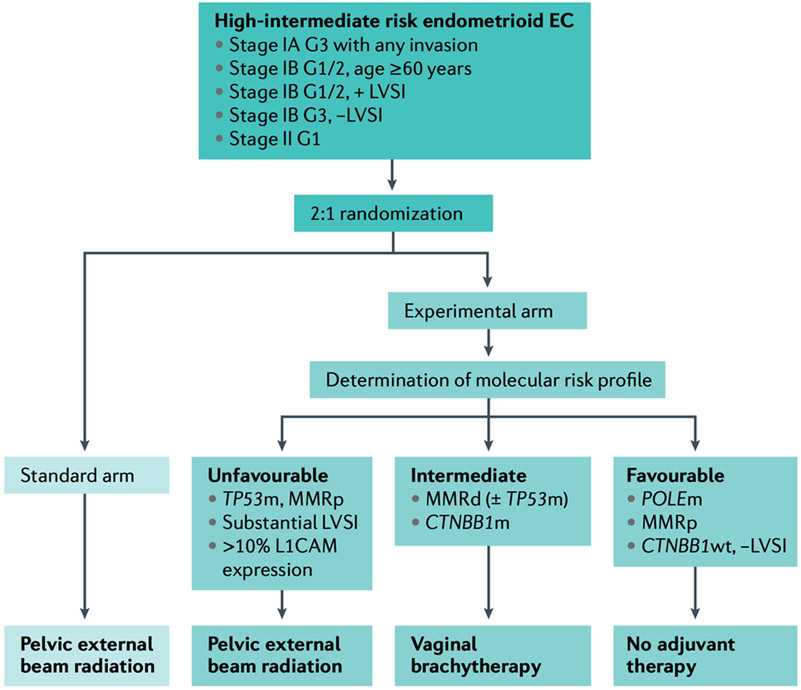
Figure 8. PORTEC-4a Trial Schema.
This was a multicenter randomized phase III trial in high-intermediate risk endometrial cancer investigating the role of an integrated clinicopathological and molecular risk profile to determine adjuvant management with options of no adjuvant therapy, vaginal brachytherapy, or external-beam radiotherapy-based profile compared to standard adjuvant vaginal brachytherapy. G1: Grade 1; G2: Grade 2; G3: Grade 3; LVSI: lymphovascular space invasion; POLEm: POLE mutant; MMRp: mismatch repair proficient; wt: wildtype; MMRd: mismatch repair deficient; CTNBB1m: CTNBB1 mutant; TP53m: TP53 mutant
ProMisE uses surrogate markers to create a stepwise path to stratify patients with EC analogous to TCGA molecular subtypes.134 Tumors are classified based on MMRd, presence of POLE mutation, presence of p53 loss or overexpression on immunohistochemistry, or no abnormality. Similar to the TransPORTEC model, these four subtypes were associated with disease-free survival and OS.134 More recently, another group71 confirmed the association of MMRd, P53 IHC, and POLE mutational status with prognosis, using samples collected as part of the PORTEC-3 clinical trial. Furthermore, the molecular subgroups may have predictive value. Women whose tumors belonged to the P53-aberrant group appeared to benefit from the addition of chemotherapy to radiation, whereas those with MMRd tumors did not appear to derive a benefit. In addition, women within the POLE-mutant group had an excellent prognosis regardless of treatment arm.71 These findings require further exploration and have important implications for the future design of adjuvant therapy trials.
Immune checkpoint inhibitors as monotherapy,201,222,226 as well as in combination with lenvatinib, have led to an increase in clinical trials designed to identify the ideal timeframe to employ immunotherapy in the treatment of EC. Response rates of checkpoint inhibition in MMRd/MSI-H EC ranges from 27-57% based on the agent assessed. These responses are durable and deep, but there are still many unanswered questions. For example, whether these treatments should be used for adjuvant treatment. The NRG Oncology group is looking to answer this question by randomizing patients with MMRd HIR EC to adjuvant radiotherapy with or without pembrolizumab (NCT04214067). In addition to survival outcomes, this study will explore important QOL endpoints to determine whether the addition of pembrolizumab to radiation, compared with radiation alone, is associated with decreased QOL at 6 and 24 weeks, as measured by the Functional Assessment of Cancer Therapy (FACT)-Endometrial (En) Trial Outcome Index (TOI); with increased gastrointestinal symptoms, as measured by the gastrointestinal subscale; and with increased fatigue as measured by the PROMIS-Fatigue scale.
Another key question relates to the maximization of the treatment of patients with advanced and recurrent EC. Preclinical evidence indicates that combining chemotherapy and immune checkpoint inhibition has the potential for synergy in solid tumors, including EC.227 Based on these data and the exciting single-agent activity of checkpoint inhibitors, there are a number of large randomized phase III trials exploring the addition of checkpoint inhibition to chemotherapy in women with advanced or recurrent EC (Supplementary Table 3). Importantly, these trials also incorporate assessment of the checkpoint inhibitor as a maintenance strategy. Moreover, there is interest in replacing chemotherapy entirely in the frontline setting. The LEAP-001 study is a randomized phase III trial comparing standard of care platinum-based chemotherapy to the lenvatinib and pembrolizumab combination in stage III, stage IV, and treatment-naïve EC (NCT 03884101). Beyond single-agent checkpoint inhibition, there are a number of interesting novel immunotherapy techniques that may warrant exploration in EC, including dual checkpoint inhibition and adoptive cell therapies. Furthermore, to better determine the relevance of the microbiome, future research incorporating studies of host–microbiota and host–metabolome interactions within the endometrial environment are needed.
Outside of immunotherapy, there are a number of novel targeted agents demonstrating promise in EC. One study found an OS benefit with the addition of trastuzumab to standard chemotherapy in women with HER2-overexpressing uterine serous cancers.66 This success led to a compendium listing and several planned trials assessing dual HER2 combinations in this population. Moreover, although p53 has long been considered an ‘undruggable’ target, the development of Wee1 inhibition to create a synthetic lethal situation in the setting of TP53 mutation has improved the prospects of action on this aberration. A phase II study of the Wee1 inhibitor adavosertib in uterine serous tumors led to durable and deep responses in ~30% of patients.228 Larger studies are moving forward to determine the ideal setting for this class of drugs. As noted above, TP53 mutations has also been explored as a potential predictor of benefit from the combination of chemotherapy and bevacizumab in the advanced/recurrent setting.1912 Additional studies will be necessary to confirm TP53 as a marker that can be used for decision-making. Finally, due to presence of homologous recombination deficiency in EC,229 PARP inhibitors are also under development, alone and in combination with other targeted therapies. Ultimately, the success of precision medicine in EC will depend on careful validation of biomarker candidates and thorough vetting of agents with innovative trial designs. Window of opportunity studies hold promise for rapid assessment of novel therapies with pre-therapy and post-therapy tissue assessments that can guide biomarker development and treatment selection.230,231
A key point is that none of these advances will yield maximum impact if health disparities persist in EC. Outcomes for women with EC are drastically different based on ethnicity. As previously discussed, Black women have significantly higher mortality rates compared with white women, even when controlling for known high-risk factors such as stage and non-endometrioid histology.232 The reasons for this disparity are not entirely clear but may include quality of care given to women of color, implicit racial bias and systemic racism.233 Further, the lack of inclusion of underrepresented minorities in clinical trials and molecular studies, such as TCGA, is a missed opportunity to improve outcomes for all women with EC.234,235 Therefore, there is a critical need for systematic investigation and research funding so these disparities can be scrutinized, addressed, and successfully overcome.
Supplementary Material
Acknowledgements
V.M.is supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Dr. Westin is supported in through the NIH/NCI Cancer Center Support Grant (NIH CA016672) and the NIH SPORE in Uterine Cancer (NIH 5P50CA098258-13).
Footnotes
Competing interests
Outside the submitted work, V.M. reports research (all funding to institution)/consultant/advisory board member support from Merck, Eisai, Karyopharm, AstraZeneca, Clovis, Moreo, Takeda, Zymeworks, and Genentech, GSK, Dicephera, Faeth. D.L. has held consulting/advisory role for Tesaro/GSK, Merck, receives research funding to his institution from Merck, Tesaro, Clovis Oncology, Regeneron, Agenus, Takeda, Immunogen, VBL Therapeutics, Genentech, Celsion, Ambry, and Splash Pharmaceuticals. D.L. is a founder of Nirova BioSense, Inc. D.L. is currently a full-time employee of Merck. I.R-C. reports honoraria (self) from Abbvie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; honoraria (institution) from GSK, MSD, Roche and BMS; advisory/consulting fees from Abbvie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; research grant/funding (self) from MSD, Roche and BMS; research grant/funding (institution) from MSD, Roche, BMS, Novartis, AstraZeneca and Merck Sereno; and travel support from Roche, AstraZeneca and GSK. A.O. has served on advisory boards for Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Inmunogen, Genmab, Mersana Therapeutic, GSK and Deciphera Pharmarceutial and has received support for travel or accommodation from Roche, AstraZeneca, and PharmaMar. H.M.K. has served on the advisory boards of Merck, Essai, GSK, and AstraZeneca. D.A. has research grant support from Aska Pharmaceutical, Takeda, and Tsumura; has served as consultant for AstraZeneca, Takeda, MSD, Myriad genetics, AbbVie, Chugai, and Ono; and has received honoraria from AbbVie, MSD, Aska Pharmaceutical, AstraZeneca, Takeda, Chugai, Ono, Taiho, and Bayer. S.N.W. reports research report from ArQule, AstraZeneca, Bayer, Bio-Path, Clovis Oncology, Cotinga Pharmaceuticals, GSK/Tesaro, Mereo, Novartis, Roche/Genentech, and Zentalis; and has served as consultant for Agenus, AstraZeneca, Circulogene, Clovis Oncology, Eisai, EQRX, GSK/Tesaro, Lilly, Merck, Novartis, Pfizer, Roche/Genentech, and Zentalis.
REFERENCES
- 1.Henley SJ et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 126, 2225–2249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer. J. Clin 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Morice P, Leary A, Creutzberg C, Abu-Rustum N & Darai E Endometrial cancer. Lancet. 387, 1094–1108 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD & Jemal A Cancer statistics, 2018. CA. Cancer. J. Clin 68, 7–30 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Calle EE, Rodriguez C, Walker-Thurmond K & Thun MJ Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med 348, 1625–1638 (2003). Prospective study showing that excess body weight was assocaited with increased death rates for endometerial cancer and all malignancies combined.
- 6.Lauby-Secretan B et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med 375, 794–798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahib L et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer. Res 74, 2913–2921 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer. J. Clin 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Zhang S et al. Global, regional, and national burden of endometrial cancer, 1990-2017. Results from the global burden of disease study, 2017. Front. Oncol 9, 1440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee S, Gupta D, Caputo TA & Holcomb K Disparities in Gynecological Malignancies. Front. Oncol 6, 36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svanvik T, Marcickiewicz J, Sundfeldt K, Holmberg E & Stromberg U Sociodemographic disparities in stage-specific incidences of endometrial cancer: a registry-based study in West Sweden, 1995-2016. Acta. Oncologica 58, 845–851 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Bain RP, Greenberg RS & Chung KC Racial differences in survival of women with endometrial cancer. Am. J. Obstet. Gynecol 157, 914–923 (1987). [DOI] [PubMed] [Google Scholar]
- 14.Felix AS et al. Factors associated with Type I and Type II endometrial cancer. Cancer. Causes. Control 21, 1851–1856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park AB et al. Racial disparities in survival among women with endometrial cancer in an equal access system. Gynecol. Oncol 163, 125–129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long B, Liu FW & Bristow RE Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol. Oncol 130, 652–659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madison T, Schottenfeld D, James SA, Schwartz AG & Gruber SB Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am. J. Public. Health 94, 2104–2111 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cote ML, Ruterbusch JJ, Olson SH, Lu K & Ali-Fehmi R The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer. Epidemiol. Biomarkers. Prev 24, 1407–1415 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Feinberg J et al. Ten-year comparison study of type 1 and 2 endometrial cancers: risk factors and outcomes. Gynecol. Obstet. Invest 84, 290–297 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Althubiti MA Mutation frequencies in endometrial cancer patients of different ethnicities and tumor grades: an analytical study. Saudi. J. Med. Sci 7, 16–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang MM et al. Improved survival of Asians with corpus cancer compared with whites: an analysis of underlying factors. Obstet. Gynecol 107, 329–335 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Simons E et al. Foreign- vs US-born Asians and the association of type I uterine cancer. Am. J. Obstet. Gynecol 212, 43.e41–46 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Colombo N et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann. Oncol 27, 16–41 (2016). [DOI] [PubMed] [Google Scholar]
- 24.SGO Clinical Practice Endometrial Cancer Working Group; Burke WM et al. Endometrial cancer: a review and current management strategies: part I. Gynecol. Oncol 134, 385–392 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Setiawan VW et al. Type I and II endometrial cancers: have they different risk factors? J. Clin. Oncol 31, 2607–2618 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renehan AG, Zwahlen M & Egger M Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer 15, 484–498 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Hopkins BD, Goncalves MD & Cantley LC Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol 34, 4277–4283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J et al. Intentional Weight Loss and Endometrial Cancer Risk. J. Clin. Oncol 35, 1189–1193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao C, Zhang D, Mungo C, Tompkins DA & Zeidan AM Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol. Oncol 135, 163–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saed L et al. The effect of diabetes on the risk of endometrial Cancer: an updated a systematic review and meta-analysis. BMC. Cancer 19, 527 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang ZH, Su PY, Hao JH & Sun YH The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: a meta-analysis of prospective cohort studies. Int. J. Gynecol. Cancer 23, 294–303 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Shikata K, Ninomiya T & Kiyohara Y Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer. Sci 104, 9–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito K et al. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 45, 28–36 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Rosato V et al. Metabolic syndrome and endometrial cancer risk. Ann. Oncol 22, 884–889 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Trabert B et al. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer. Epidemiol. Biomarkers. Prev 24, 261–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arthur RS et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: a prospective study. Cancer. Causes. Control 30, 355–363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alford SH, Rattan R, Buekers TE & Munkarah AR Protective effect of bisphosphonates on endometrial cancer incidence in data from the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Cancer. 121, 441–447 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Newcomb PA et al. Oral bisphosphonate use and risk of postmenopausal endometrial cancer. J. Clin. Oncol 33, 1186–1190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rennert G, Rennert HS, Pinchev M & Lavie O The effect of bisphosphonates on the risk of endometrial and ovarian malignancies. Gynecol. Oncol 133, 309–313 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Zhang XS et al. Risk reduction of endometrial and ovarian cancer after bisphosphonates use: A meta-analysis. Gynecol. Oncol 150, 509–514 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Clezardin P Bisphosphonates' antitumor activity: an unravelled side of a multifaceted drug class. Bone. 48, 71–79 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Coleman R et al. Effects of bone-targeted agents on cancer progression and mortality. J. Natl. Cancer. Inst 104, 1059–1067 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Gnant M, Clézardin P Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer. Treat. Rev 38, 407–415 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Ryan NAJ et al. The proportion of endometrial cancers associated with Lynch syndrome: a system review of the literature and meta-analysis. Genet. Med 21, 2167–2180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonadona V et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 305, 2304–2310 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Moller P et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 67, 1306–1316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ten Broeke SW et al. Cancer Risks for PMS2-Associated Lynch Syndrome. J. Clin. Oncol 36, 2961–2968 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ring KL et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod. Pathol 29, 1381–1389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan MH et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer. Res 18, 400–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goshen R et al. Is uterine papillary serous adenocarcinoma a manifestation of the hereditary breast-ovarian cancer syndrome? Gynecol. Oncol 79, 477–481 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Lavie O et al. BRCA germline mutations in Jewish women with uterine serous papillary carcinoma. Gynecol. Oncol 92, 521–524 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Shu CA et al. Uterine Cancer After Risk-Reducing Salpingo-oophorectomy Without Hysterectomy in Women With BRCA Mutations. JAMA. Oncol 2, 1434–1440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long B et al. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol. Oncol 152, 20–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lacey JV Jr. et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J. Clin. Oncol 28, 788–792 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baak JP et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 103, 2304–2312 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi A & Ellenson LH PI3K/PTEN/AKT Genetic Mouse Models of Endometrial Carcinoma. Adv. Exp. Med. Biol 943, 261–273 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Rahmanto YS et al. Inactivation of Arid1a in the endometrium is associated with endometrioid tumorigenesis through transcriptional reprogramming. Nat. Commun 11, 2717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Y, Lin P, Lydon JP & Li Q Conditional abrogation of transforming growth factor-β receptor 1 in PTEN-inactivated endometrium promotes endometrial cancer progression in mice. J. Pathol 243, 89–99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarboe EA et al. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod. Pathol 22, 345–350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantrell LA, Blank SV & Duska LR Uterine carcinosarcoma: a review of the literature. Gynecol. Oncol 137, 581–588 (2015). [DOI] [PubMed] [Google Scholar]
- 61. Cancer Genome Atlas Research Network; Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 497, 67–73 (2013). Performed genomic, epigenomic, transcriptomic, and proteomic characterizations of endometrial carcinomas and uterine carcinomas, delineating unique molecular phenotypic features and subtypes of these malignancies.
- 62. Cherniack AD et al. Integrated molecular characterization of uterine carcinosarcoma. Cancer. Cell 31, 411–423 (2017). Performed genomic, epigenomic, transcriptomic, and proteomic characterizations of endometrial carcinomas and uterine carcinomas, delineating unique molecular phenotypic features and subtypes of these malignancies.
- 63.Crane E et al. Molecular variations in uterine carcinosarcomas identify therapeutic opportunities. Int. J. Gynecol. Cancer 30, 480–484 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Urick ME & Bell DW Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 19, 510–521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuhn E, Bahadirli-Talbott A & Shih Ie M Frequent CCNE1 amplification in endometrial intraepithelial carcinoma and uterine serous carcinoma. Mod. Pathol 27, 1014–1019 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Fader AN et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer. Res 26, 3928–3935 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bosse T et al. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled PORTEC trial results. Eur. J. Cancer 50, 2602–2610 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Van Gool IC et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod. Pathol 29, 174–181 (2016). [DOI] [PubMed] [Google Scholar]
- 69.McConechy MK et al. Endometrial Carcinomas with POLE Exonuclease Domain Mutations Have a Favorable Prognosis. Clin. Cancer. Res 22, 2865–2873 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Xing X et al. A recurrent cancer-associated substitution in DNA polymerase ε produces a hyperactive enzyme. Nat. Commun 10, 374 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leon-Castillo A et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol 38, 3388–3397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurnit KC et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 30, 1032–1041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Depreeuw J et al. Amplification of 1q32.1 Refines the Molecular Classification of Endometrial Carcinoma. Clin. Cancer. Res 23, 7232–7241 (2017). [DOI] [PubMed] [Google Scholar]
- 74. Talhouk A et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer. 123, 802–813 (2017). A simplified, pragmatic molecular classifier enabling the identification of four prognostically distinct molecular subtypes to diagnostic specimens, faciliating earlier informed decision-making.
- 75.Sari A et al. Interobserver Agreement for Mismatch Repair Protein Immunohistochemistry in Endometrial and Nonserous, Nonmucinous Ovarian Carcinomas. Am. J. Surg. Pathol 43, 591–600 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Vermij L, Smit V, Nout R & Bosse T Incorporation of molecular characteristics into endometrial cancer management. Histopathology. 76, 52–63 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dou Y et al. Proteogenomic characterization of endometrial carcinoma. Cell. 180, 729–748 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cuevas IC et al. Fbxw7 is a driver of uterine carcinosarcoma by promoting epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 116, 25880–25890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leskela S et al. Molecular basis of tumor heterogeneity in endometrial carcinosarcoma. Cancers. (Basel) 11, 964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao S et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 113, 12238–12243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gotoh O et al. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat. Commun 10, 4965 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gotoh O et al. Immunogenomic landscape of gynecologic carcinosaroma. Gynecol. Oncol 160, 547–556 (2021). [DOI] [PubMed] [Google Scholar]
- 83.Vanderstraeten A, Tuyaerts S & Amant F The immune system in the normal endometrium and implications for endometrial cancer development. J. Reprod. Immunol 109, 7–16 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Vong S & Kalluri R The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes. Cancer 2, 1139–1145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Nola R et al. The Crowded Crosstalk between Cancer Cells and Stromal Microenvironment in Gynecological Malignancies: Biological Pathways and Therapeutic Implication. Int. J. Mol. Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tlsty TD Stromal cells can contribute oncogenic signals. Semin. Cancer. Biol 11, 97–104 (2001). [DOI] [PubMed] [Google Scholar]
- 87.Tanwar PS, Zhang L, Roberts DJ & Teixeira JM Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer. Res 71, 1584–1596 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coussens LM & Werb Z Inflammation and cancer. Nature. 420, 860–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pollard JW Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Peña CG et al. LKB1 loss promotes endometrial cancer progression via CCL2-dependent macrophage recruitment. J. Clin. Invest 125, 4063–4076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dun EC et al. Infiltration of tumor-associated macrophages is increased in the epithelial and stromal compartments of endometrial carcinomas. Int. J. Gynecol. Pathol 32, 576–584 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Pegram MD et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol 16, 2659–2671 (1998). [DOI] [PubMed] [Google Scholar]
- 93.Krishnan V, Schaar B, Tallapragada S & Dorigo O Tumor associated macrophages in gynecologic cancers. Gynecol. Oncol 149, 205–213 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Ning C et al. Infiltrating Macrophages Induce ERα Expression through an IL17A-mediated Epigenetic Mechanism to Sensitize Endometrial Cancer Cells to Estrogen. Cancer. Res 76, 1354–1366 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Yamagami W et al. Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int. J. Gynecol. Cancer 21, 1628–1634 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Howitt BE et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA. Oncol 1, 1319–1323 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Talhouk A et al. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer. Res 25, 2537–2548 (2019). [DOI] [PubMed] [Google Scholar]
- 98.van Gool IC et al. POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer. Clin. Cancer. Res 21, 3347–3355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jumaah AS, Salim MM, Al-Haddad HS, McAllister KA & Yaseen AA The frequency of POLE-mutation in endometrial carcinoma and prognostic implications: a systemic review and meta-analysis. J. Pathol. Transl. Med 54, 471–479 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anderson AC, Joller N & Kuchroo VK Lag-3, Tim-3, and TIGIT co-inhibitory receptors with specialized functions in immune regulation. Immunity. 44, 989–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lijun Z et al. Tumor-infiltrating dendritic cells may be used as clinicopathologic prognostic factors in endometrial carcinoma. Int. J. Gynecol. Cancer 22, 836–841 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Sungu N Expression of immunomodulatory molecules PD-1, PD-L1, and PD-L2, and their relationship with clinicopathologic characteristics in endometrial cancer. Int. J. Gynecol. Pathol 38, 404–413 (2019). [DOI] [PubMed] [Google Scholar]
- 103.Willis BC, Sloan EA, Atkins KA, Stoler MH & Mills AM Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod. Pathol 30, 1622–1632 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Mills A et al. Indoleamine 2,3-dioxygenase in endometrial cancer: a targetable mechanism of immune resistance in mismatch repair-deficient and intact endometrial carcinomas. Mod. Pathol 31, 1282–1290 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Challapalli A, Carroll L, & Aboagye EO Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 5, 225–253 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahecha AM & Wang H The influence of vascular endothelial growth factor-A and matrix metalloproteinase-2 and −9 in angiogenesis, metastasis, and prognosis of endometrial cancer. Onco. Targets. Ther 10, 4617–4624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Franceschi T et al. Role of epithelial-mesenchymal transition factors in the histogenesis of uterine carcinomas. Virchows. Arch 475, 85–94 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Senol S et al. Stromal Clues in Endometrial Carcinoma: Loss of Expression of β-Catenin, Epithelial-Mesenchymal Transition Regulators, and Estrogen-Progesterone Receptor. Int. J. Gynecol. Pathol 35, 238–248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim G et al. Nuclear β-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Mod. Pathol 31, 1553–1559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hecht JL & Mutter GL Molecular and pathologic aspects of endometrial carcinogenesis. J. Clin. Oncol 24, 4783–4791 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Teng F et al. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J. Hematol. Oncol 9, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Winuthayanon W et al. Juxtacrine Activity of Estrogen Receptor α in Uterine Stromal Cells is Necessary for Estrogen-Induced Epithelial Cell Proliferation. Sci. Rep 7, 8377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwabe R & Jobin C The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walther-Antonio MRS et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome. Med 8, 122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baker JM, Chase DM & Herbst-Kralovetz MM Uterine microbiota: residents, tourists, or invaders? Front. Immunol 9, 208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clarke MA et al. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA. Intern. Med 178, 1210–1222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet. Gynecol 131, e124–e129 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Breijer MC, Timmermans A, van Doorn HC, Mol BW & Opmeer BC Diagnostic strategies for postmenopausal bleeding. Obstet. Gynecol. Int 2010, 850812 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gull B, Karlsson B, Milsom I, Wikland M & Granberg S Transvaginal sonography of the endometrium in a representative sample of postmenopausal women. Ultrasound. Obstet. Gynecol 7, 322–327 (1996). [DOI] [PubMed] [Google Scholar]
- 120.Smith-Bindman R et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 280, 1510–1517 (1998). [DOI] [PubMed] [Google Scholar]
- 121.Lee DO, Jung MH & Kim HY Prospective comparison of biopsy results from curettage and hysteroscopy in postmenopausal uterine bleeding. J. Obstet. Gynaecol. Res 37, 1423–1426 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Abbink K et al. HE4 is superior to CA125 in the detection of recurrent disease in high-risk endometrial cancer patients. Tumour. Biol 40, 1010428318757103 (2018). [DOI] [PubMed] [Google Scholar]
- 123.Bignotti E et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br. J. Cancer 104, 1418–1425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brennan DJ et al. Serum HE4 as a prognostic marker in endometrial cancer--a population based study. Gynecol. Oncol 132, 159–165 (2014). [DOI] [PubMed] [Google Scholar]
- 125.Saarelainen SK et al. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma. Am. J. Obstet. Gynecol 209, 142 e141–146 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Peungjesada S, Bhosale PR, Balachandran A & Iyer RB Magnetic resonance imaging of endometrial carcinoma. J. Comput. Assist. Tomogr 33, 601–608 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Faria SC, Devine CE, Rao B, Sagebiel T & Bhosale P Imaging and Staging of Endometrial Cancer. Semin. Ultrasound. CT. MR 40, 287–294 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Kang SY et al. Prediction of Recurrence by Preoperative Intratumoral FDG Uptake Heterogeneity in Endometrioid Endometrial Cancer. Transl. Oncol 10, 178–183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Akin EA, Kuhl ES & Zeman RK The role of FDG-PET/CT in gynecologic imaging: an updated guide to interpretation and challenges. Abdom. Radiol. (NY) 43, 2474–2486 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Bokhman JV Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol 15, 10–17 (1983). [DOI] [PubMed] [Google Scholar]
- 131.Brinton LA et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol. Oncol 129, 277–284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gilks CB, Oliva E & Soslow RA Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol 37, 874–881 (2013). [DOI] [PubMed] [Google Scholar]
- 133.de Boer SM et al. Clinical consequences of upfront pathology review in the randomised PORTEC-3 trial for high-risk endometrial cancer. Ann. Oncol 29, 424–430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol 29, 1180–1188 (2018). [DOI] [PubMed] [Google Scholar]
- 135.Talhouk A et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 113, 299–310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raffone A et al. TCGA molecular groups of endometrial cancer: Pooled data about prognosis. Gynecol. Oncol. 155, 374–383 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Lin DI & Hecht JL Targeted Screening with Combined Age- and Morphology-Based Criteria Enriches Detection of Lynch Syndrome in Endometrial Cancer. Int. J. Surg. Pathol 24, 297–305 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Bruegl AS et al. Clinical Challenges Associated with Universal Screening for Lynch Syndrome-Associated Endometrial Cancer. Cancer. Prev. Res. (Phila) 10, 108–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Watkins JC et al. Universal Screening for Mismatch-Repair Deficiency in Endometrial Cancers to Identify Patients With Lynch Syndrome and Lynch-like Syndrome. Int. J. Gynecol. Pathol 36, 115–127 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Goverde A et al. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70 years of age. Gynecol. Oncol 143, 453–459 (2016). [DOI] [PubMed] [Google Scholar]
- 141.McAlpine JN, Temkin SM & Mackay HJ Endometrial cancer: Not your grandmother's cancer. Cancer. 122, 2787–2798 (2016). [DOI] [PubMed] [Google Scholar]
- 142.Brooks RA et al. Current recommendations and recent progress in endometrial cancer. CA. Cancer. J. Clin 69, 258–279 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Gien L, Kwon J, Oliver TK & Fung-Kee-Fung M Adjuvant hormonal therapy for stage I endometrial cancer. Curr. Oncol 15, 126–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.MacKay HJ, Freixinos VR & Fleming GF Therapeutic Targets and Opportunities in Endometrial Cancer: Update on Endocrine Therapy and Nonimmunotherapy Targeted Options. Am. Soc. Clin. Oncol. Educ. Book 40, 1–11 (2020). [DOI] [PubMed] [Google Scholar]
- 145.Green AK, Feinberg J & Makker V A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer. Am. Soc. Clin. Oncol. Educ. Book 40, 1–7 (2020). [DOI] [PubMed] [Google Scholar]
- 146.Gaia G et al. Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches: a systematic review. Obstet. Gynecol 116, 1422–1431 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Galaal K, Donkers H, Bryant A & Lopes AD Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane. Database. Syst. Rev 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janda M et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet. Oncol 11, 772–780 (2010). [DOI] [PubMed] [Google Scholar]
- 149.Walker JL et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol 27, 5331–5336 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Malley DM, Smith B & Fowler JM The role of robotic surgery in endometrial cancer. J. Surg. Oncol 112, 761–768 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Rabinovich A Neo-adjuvant chemotherapy for advanced stage endometrial carcinoma: a glimmer of hope in select patients. Arch. Gynecol. Obstet 293, 47–53 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Barlin JN, Ueda SM & Bristow RE Cytoreductive surgery for advanced and recurrent endometrial cancer: a review of the literature. Womens. Health. (Lond) 5, 403–411 (2009). [DOI] [PubMed] [Google Scholar]
- 153.Creasman WT et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 60, 2035–2041 (1987). [DOI] [PubMed] [Google Scholar]
- 154.Lutman CV et al. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol. Oncol 102, 92–97 (2006). [DOI] [PubMed] [Google Scholar]
- 155.Todo Y et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 375, 1165–1172 (2010). [DOI] [PubMed] [Google Scholar]
- 156.Abu-Rustum NR et al. What is the incidence of isolated paraaortic nodal recurrence in grade 1 endometrial carcinoma? Gynecol. Oncol 111, 46–48 (2008). [DOI] [PubMed] [Google Scholar]
- 157.group A. s. et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 373, 125–136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Benedetti Panici P et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J. Natl. Cancer. Inst 100, 1707–1716 (2008). [DOI] [PubMed] [Google Scholar]
- 159.Frost JA, Webster KE, Bryant A & Morrison J Lymphadenectomy for the management of endometrial cancer. Cochrane. Database. Syst. Rev 10, CD007585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Benedetti Panici P et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J. Natl. Cancer. Inst 100, 1707–1716 (2008). [DOI] [PubMed] [Google Scholar]
- 161.NCCN Guidelines. Version1 2021. <nccn.org>.
- 162.Metindir J & Dilek GB The role of omentectomy during the surgical staging in patients with clinical stage I endometrioid adenocarcinoma. J. Cancer. Res. Clin. Oncol 134, 1067–1070 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.155 Sundar S et al. BGCS Uterine Cancer Guidelines: Recommendations for Practice, https://www.bgcs.org.uk/wp-content/uploads/2019/05/BGCS-Endometrial-Guidelines-2017.pdf (2019). [DOI] [PubMed] [Google Scholar]
- 164.Rodolakis A et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: Clinical Recommendations for Fertility-Sparing Management in Young Endometrial Cancer Patients. Int. J. Gynecol. Cancer 25, 1258–1265 (2015). [DOI] [PubMed] [Google Scholar]
- 165.Gallos ID et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am. J. Obstet. Gynecol 207, 266 e261–212 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Hawkes AL et al. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device +/− metformin +/− weight loss in endometrial cancer (feMME) trial. Contemp. Clin. Trials 39, 14–21 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Zaino RJ et al. Histologic effects of medroxyprogesterone acetate on endometrioid endometrial adenocarcinoma: a Gynecologic Oncology Group study. Int. J. Gynecol. Pathol 33, 543–553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pal N et al. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obstet. Gynecol 131, 109–116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matsuo K et al. Ovarian Conservation and Overall Survival in Young Women With Early-Stage Cervical Cancer. Obstet. Gynecol 129, 139–151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Johnson N & Cornes P Survival and recurrent disease after postoperative radiotherapy for early endometrial cancer: systematic review and meta-analysis. BJOG. 114, 1313–1320 (2007). [DOI] [PubMed] [Google Scholar]
- 171.Sorbe B et al. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: a controlled randomized study. Int. J. Gynecol. Cancer 19, 873–878 (2009). [DOI] [PubMed] [Google Scholar]
- 172.Creutzberg CL et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys 81, e631–638 (2011). [DOI] [PubMed] [Google Scholar]
- 173. Creutzberg CL et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 355, 1404–1411 (2000). Phase III study of postoperative radiotherapy versus surgery alone in stage 1 endometrial carcinoma that showed that postoperative radiotherapy reduces locoregional recurrence but has no impact on overall survival.
- 174.Keys HM et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol 92, 744–751 (2004). [DOI] [PubMed] [Google Scholar]
- 175.Harkenrider MM et al. American Brachytherapy Task Group Report: Adjuvant vaginal brachytherapy for early-stage endometrial cancer: A comprehensive review. Brachytherapy. 16, 95–108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wortman BG et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br. J. Cancer 119, 1067–1074 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Randall ME et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol 37, 1810–1818 (2019). This phase III trial of adjuvant pelvic radiation versus vaginal brachytherapy (VCB) plus paclitaxel and carboplatin chemotherapy in high-intermediate risk and high-risk early-stage endometrial cancer failed to show superiority of VCB plus chemotherapy compared with pelvic radiation.
- 178. de Boer SM et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet. Oncol 19, 295–309 (2018). Phase III trial showing that adjuvant chemotherapy administered during and after radaiion therapy in high-risk endometrial cancer did not improve overall survival.
- 179.De Boer SM et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 17, 1114–1126 (2016). [DOI] [PubMed] [Google Scholar]
- 180. Matei D et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med 380, 2317–2326 (2019). Phase III trial showing that chemotherapy plus radiation did not prolong relapse-free survival compared to chemotherapy alone in stage III or IVA endometrial cancer.
- 181.Geller MA et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a "Sandwich" method for stage III, IV, and recurrent endometrial cancer. Gynecol. Oncol 121, 112–117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hogberg T et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies. Eur. J. Cancer 46, 2422–2431 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Randall ME et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J. Clin. Oncol 24, 36–44 (2006). [DOI] [PubMed] [Google Scholar]
- 184.Kurra V et al. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer. Imaging 13, 113–122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Miller DS et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J. Clin. Oncol 38, 3841–3850 (2020). This phase III trial showed that paclitaxel and carbopaltin chemotherapy was noninferior to paclitaxel-doxorubicin-cisplatin chemotherapy as first-line therapy for advanced endometrial cancer.
- 186.Fleming GF Second-Line Therapy for Endometrial Cancer: The Need for Better Options. J. Clin. Oncol 33, 3535–3540 (2015). [DOI] [PubMed] [Google Scholar]
- 187.Nagao S et al. What is an appropriate second-line regimen for recurrent endometrial cancer? Ancillary analysis of the SGSG012/GOTIC004/Intergroup study. Cancer. Chemother. Pharmacol 76, 335–342 (2015). [DOI] [PubMed] [Google Scholar]
- 188.Rubinstein M et al. Retreatment with carboplatin and paclitaxel for recurrent endometrial cancer: A retrospective study of the Memorial Sloan Kettering Cancer Center experience. Gynecol. Oncol. Rep 28, 120–123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fader AN et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol 36, 2044–2051 (2018). [DOI] [PubMed] [Google Scholar]
- 190.Aghajanian C et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol 150, 274–281 (2018).29804638 [Google Scholar]
- 191.Leslie KK et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: An NRG Oncology study. Gynecol. Oncol 161, 113–121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kokka F, Brockbank E, Oram D, Gallagher C & Bryant A Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane. Database. Syst. Rev CD007926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ethier JL, Desautels DN, Amir E & MacKay H Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol. Oncol 147, 158–166 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Jerzak KJ, Duska L & MacKay HJ Endocrine therapy in endometrial cancer: An old dog with new tricks. Gynecol. Oncol 153, 175–183 (2019). [DOI] [PubMed] [Google Scholar]
- 195.Fiorica JV et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol 92, 10–14 (2004). [DOI] [PubMed] [Google Scholar]
- 196.Fleming GF et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study. Gynecol. Oncol 132, 585–592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mirza MR et al. LBA28 A randomised double-blind placebo-controlled phase II trial of palbociclib combined with letrozole (L) in patients (pts) with oestrogen receptor-positive (ER+) advanced/recurrent endometrial cancer (EC): NSGO-PALEO / ENGOT-EN3 trial. Ann. Oncol 31, S1160 (2020). [Google Scholar]
- 198.Slomovitz BM et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol 33, 930–936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Soliman PT et al. Everolimus, Letrozole, and Metformin in Women with Advanced or Recurrent Endometrioid Endometrial Cancer: A Multi-Center, Single Arm, Phase II Study. Clin. Cancer. Res 26, 581–587 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Heudel P et al. Victoria: A multicentric, randomized, open-label, phase I/II of mTOR inhibitor (VISTUSERTIB) combined with anastrozole in patients with hormone receptor-positive advanced/metastatic endometrial cancer—A CLIPP program INCA in collaboration with GINECO group. J. Clin. Oncol 39 (2021). [Google Scholar]
- 201. Marabelle A et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol 38, 1–10 (2020). Phase II trial that established monotherapy activity of the PD-1 inhibitor pembrolizumab in MSI-H/MMRd solid tumors.
- 202.Marabelle A et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020). [DOI] [PubMed] [Google Scholar]
- 203. Oaknin A et al. 935PD - Preliminary safety, efficacy, and PK/PD characterization from GARNET, a phase I clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-H endometrial cancer. Ann. Oncol 29, viii334 (2018). Phase I study that established monotherapy activity of the PD-1 inhibitor dostarlimab in endometrial cancer.
- 204.Konstantinopoulos PA et al. Phase II Study of Avelumab in Patients With Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol 37, 2786–2794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Antill YC, Kok PS & Robledo K Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J. Clin. Oncol 37, 5501 (2019). [Google Scholar]
- 206.Makker V et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J. Clin. Oncol 38, 2981–2992 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Makker V et al. A multicenter, open-label, randomized, phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer: Study 309/KEYNOTE-775. 2021 Society of Gynecologic Oncology Annual Meeting. March 19-21, 2021. Virtual. Abstract. Phase III trial of lenvatinib and pembrolizumab in advanced endometrial cancer that led to the FDA approval (non MSI-H/MMRd) and EMA approval (all endometrial cancer) of this combination therapy in patients previously treted with platinum-based chemotherapy in any setting.
- 208.Shisler R et al. Life after endometrial cancer: A systematic review of patient-reported outcomes. Gynecol. Oncol 148, 403–413 (2018). [DOI] [PubMed] [Google Scholar]
- 209.Singh P & Oehler MK Hormone replacement after gynaecological cancer. Maturitas. 65, 190–197 (2010). [DOI] [PubMed] [Google Scholar]
- 210.Parker WH et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet. Gynecol 113, 1027–1037 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hirasawa A et al. Osteoporosis is less frequent in endometrial cancer survivors with hypertriglyceridemia. Jpn. J. Clin. Oncol 45, 127–131 (2015). [DOI] [PubMed] [Google Scholar]
- 212.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD & Melton LJ 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet. Oncol 7, 821–828 (2006). [DOI] [PubMed] [Google Scholar]
- 213.Ward KK et al. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol. Oncol 126, 176–179 (2012). [DOI] [PubMed] [Google Scholar]
- 214.Douchi T et al. Bone mineral density in postmenopausal women with endometrial cancer. Maturitas. 31, 165–170 (1999). [DOI] [PubMed] [Google Scholar]
- 215.Barakat RR, Bundy BN, Spirtos NM, Bell J & Mannel RS Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol 24, 587–592 (2006). [DOI] [PubMed] [Google Scholar]
- 216.Shim SH, Lee SJ & Kim SN Effects of hormone replacement therapy on the rate of recurrence in endometrial cancer survivors: a meta-analysis. Eur. J. Cancer 50, 1628–1637 (2014). [DOI] [PubMed] [Google Scholar]
- 217.Maxwell GL, Tian C, Risinger JI, Hamilton CA & Barakat RR Racial disparities in recurrence among patients with early-stage endometrial cancer: is recurrence increased in black patients who receive estrogen replacement therapy? Cancer. 113, 1431–1437 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pinkerton JV, Utian WH, Constantine GD, Olivier S & Pickar JH Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 16, 1116–1124 (2009). [DOI] [PubMed] [Google Scholar]
- 219.Loprinzi CL, Barton DL & Qin R Nonestrogenic management of hot flashes. J. Clin. Oncol 29, 3842–3846 (2011). [DOI] [PubMed] [Google Scholar]
- 220.Sideras K & Loprinzi CL Nonhormonal management of hot flashes for women on risk reduction therapy. J. Natl. Compr. Canc. Netw 8, 1171–1179 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cheung LW et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer. Discov 1, 170–185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liang H et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome. Res 22, 2120–2129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Le DT et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med 372, 2509–2520 (2015). This study showed for the first time that MMR status predicted for clinical benefit to pembrolizumab.
- 224.Stelloo E et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol 28, 836–844 (2015). [DOI] [PubMed] [Google Scholar]
- 225.Stelloo E et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer. Res 22, 4215–4224 (2016). [DOI] [PubMed] [Google Scholar]
- 226.van den Heerik A et al. PORTEC-4a: international randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 30, 2002–2007(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Galluzzi L, Buque A, Keep O, Zitvogel L & Kroemer G Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer. Cell 28, 690–714 (2015). [DOI] [PubMed] [Google Scholar]
- 228.Liu JF et al. A phase II trial of the Wee1 inhibitor adavosertib (AZD1775) in recurrent uterine serous carcinoma. J. Clin. Oncol 38, 6009 (2020). [DOI] [PubMed] [Google Scholar]
- 229.de Jonge MM et al. Frequent Homologous Recombination Deficiency in High-grade Endometrial Carcinomas. Clin. Cancer. Res 25, 1087–1097 (2019). [DOI] [PubMed] [Google Scholar]
- 230.Duska LR et al. A Surgical Window Trial Evaluating Medroxyprogesterone Acetate with or without Entinostat in Patients with Endometrial Cancer and Validation of Biomarkers of Cellular Response. Clin. Cancer. Res 27, 2734–2741 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Soliman PT et al. Prospective evaluation of the molecular effects of metformin on the endometrium in women with newly diagnosed endometrial cancer: A window of opportunity study. Gynecol. Oncol 143, 466–471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cote ML, Ruterbusch JJ, Olson SH, Lu K & Ali-Fehmi R The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer. Epidemiol. Biomarkers. Prev 24, 1407–1415 (2015). [DOI] [PubMed] [Google Scholar]
- 233.Doll KM, Snyder CR & Ford CL Endometrial cancer disparities: a race-conscious critique of the literature. Am. J. Obstet. Gynecol 218, 474–482.e472 (2018). [DOI] [PubMed] [Google Scholar]
- 234.Spratt DE et al. Racial/ethnic disparities in genomic sequencing. JAMA. Oncol 2, 1070–1074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Awad E et al. Minority participation in phase 1 gynecologic oncology clinical trials: Three decades of inequity. Gynecol. Oncol 157, 729–732 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.