Abstract
Background
Helium plasma dermal resurfacing (HPDR) is an emerging off-label use for an existing FDA-approved device.
Objectives
Retrospective evaluation of patient satisfaction and adverse events (AEs) following facial skin resurfacing with HPDR technology.
Methods
Single-site, retrospective review of 301 patient charts following HPDR treatment of the face. Patient satisfaction data were collected during review of medical records. AE data were analyzed to determine the effects of demographic, procedural, and posttreatment variables on the presence or absence of AEs.
Results
HPDR was performed concurrently with other facial/non-facial surgical procedures in 193 of 301 patients (64.1%) including over undermined facial skin in 58 patients (19.3%) during rhytidectomy. No serious AEs were observed. Nonserious AEs were noted, however, in 20 patients (7.3%) and included erythema/prolonged erythema, hyperpigmentation, milia, slow healing, and upper lip hypertrophic scar. Among the 288 patients returning for follow-up (mean 2 months postprocedure), satisfaction with HPDR treatment results was documented in 275 patients (95.5%); the remaining 13 patients’ charts did not reference satisfaction or dissatisfaction, and no AEs were recorded for this patient subgroup.
Conclusions
This retrospective study supports the use and safety of HPDR technology for facial skin rejuvenation; no serious AEs and relatively few nonserious AEs were observed following either sole modality HPDR or HPDR with concurrent treatment of undermined skin tissue during rhytidectomy procedures. Patient satisfaction and observed results are comparable to full-field laser skin resurfacing treatments.
Level of Evidence: 3

Contemporary options for wrinkle reduction and reversal of photograph damage and dyschromia include ablative lasers (10,600-nm CO2, 2940-nm Er:YAG, or 2790-nm Er:YSGG),1-6 chemical peels (phenol based),7 and plasma energy (nitrogen plasma skin regeneration).8-11 Lack of predictability, potential for complications (eg, prolonged erythema and hypopigmentation), time requirement for recovery, and variable applicability to diverse skin types have led to continuing innovation in the area of skin rejuvenation. However, treatments that are fractional, non-ablative, and/or more superficial in nature are generally less effective for rhytid reduction and reversal of photograph damage and dyschromia and may require multiple treatments.7
A novel plasma-based device that uses helium gas to deliver radio-frequency (RF) energy to the tissue is an emerging option for skin rejuvenation. The Renuvion Cosmetic Technology (Apyx Medical Corporation, Clearwater, FL) helium-based plasma system is a second-generation device that is FDA cleared for cutting, coagulation, and ablation of soft tissue; the original device was approved in 2009 (FDA 191542). The system consists of an electrosurgical generator unit, a handpiece, and a supply of helium gas. RF energy is delivered to the handpiece by the generator and used to energize an electrode. When helium gas is passed over the energized electrode, helium plasma is produced that enables propagation and delivery of RF energy to the tissue in a non-contact and non-chromophore-dependent manner.
Proof of concept for off-label use of helium plasma energy for skin rejuvenation (JDB) and preclinical study (JDH), both using the first-generation device, led to updates to the electrosurgical generator and subsequent preclinical and clinical studies with the updated second-generation device. Preclinical evaluation in a porcine animal model highlighted the unique bimodal energy transfer of the helium plasma device wherein both topdown thermal conduction and RF-based Joule tissue heating enhance tissue tightening while limiting the depth of effect.12 A low-energy, single-pass multi-center study showed significant improvement in rhytidosis with high patient satisfaction and few non-anticipated adverse events (AEs).13 Further analysis of VISIA-CR data from this initial clinical study showed statistically significant improvements in wrinkle depth, dyschromia (number of brown spots), and pore size.14 A high-energy, double-pass multi-center study evaluating efficacy for wrinkle reduction and safety showed high patient satisfaction and marked improvement in Facial Wrinkle Scores (mean Independent Photographic Reviewer reduction of −3.6) along with mostly mild to moderate and mostly anticipated AEs that resolved in a reasonable time frame.15 Several hypertrophic scarring AEs were clustered at a single study site and were thought to be related to secondary wounding or to a variance in technique during energy delivery.15
The objectives of this study include retrospective evaluation of patient satisfaction and safety profile using the first-generation helium plasma dermal resurfacing (HPDR) technology for facial skin rejuvenation as a sole treatment modality and concurrent with aesthetic surgery of the face and neck.
METHODS
Study Design
The authors conducted a single-site, retrospective review of 301 patient charts following HPDR treatment of the face completed over an 8-year period (2012–2020) wherein patients underwent treatment of up to 6 facial zones (forehead, periorbital, nose, cheeks, perioral, and mandibular border). The number of patients treated varied for each zone is as follows: forehead (n = 153), periorbital (n = 176), nose (n = 150), cheeks (n = 250), perioral (n = 217), and mandibular border (n = 250). This was an IRB-approved (Sterling IRB, Atlanta, GA) study where data on AEs, patient satisfaction, and both demographic and treatment variables were collected and analyzed. The IRB approved a waiver of consent as patients were not contacted, and Health Information Portability and Accountability Act (HIPAA)-protected data were not collected.
Patients
Patients comprised 290 females (96.3%) and 11 males (3.7%) aged 34 to 82 years (64.0 ± 12.0, median ± interquartile range [IQR]) with Fitzpatrick skin types I (18.6%), II (50.8%), III (26.2%), and IV (4.3%). IQR, a nonparametric counterpart of the standard deviation, is the difference between the 75th and 25th percentile. Eligible patients were greater than 21 years of age, had facial wrinkles, and were treated with the helium plasma device. Patients were excluded if they had undergone concurrent therapy or surgery that would interfere with the evaluation of the safety and patient satisfaction of the study treatment.
Procedure
All patients were pretreated with Retin-A cream 0.1% (topically to the face once daily at bedtime) for 2 weeks before treatment with the helium-based device. Each patient underwent HPDR treatment of one or more of 6 facial zones (Figure 1). Most patients underwent 3 passes of the treatment areas (1-4) at 35% power (25%-48%) with continuous energy delivery (no pulsing) and helium gas flow rate of 4 liters per minute. Desiccated superficial skin tissue layers were gently wiped away between the first and second pass using saline moistened gauze. Posttreatment skin care consisted of one or more silicone gels, Avene Cicalfate + Restorative Protective Cream (Pierre Fabre, Castres, Midi-Pyrenees, France), Stratamed Advanced Film-Forming Wound Dressing (Stratapharma, Basel, Switzerland), and Xeroform Petrolatum Gauze (Covidien/Medtronic Minneapolis, MN). Two hundred eighty-eight patients had satisfaction documented at a follow-up visit with the majority of patient satisfaction assessments completed between days 31 and 90 after treatment. While safety assessments tracked concurrent with satisfaction survey time points, patients were encouraged to report any subsequent concerns to ensure that all significant AEs would be captured.
Figure 1.
Helium plasma dermal resurfacing (HPDR) facial treatment zones. Diagram representing the 6 regional facial treatment zones used in HPDR treatments include the forehead, periorbital, nose, cheeks, perioral, and chin/mandibular transition area.
Concurrent Treatment
Face and neck aesthetic procedures completed concurrently with HPDR included upper blepharoplasty, cervical rhytidectomy, and cervicofacial rhytidectomy. HPDR was not performed over infrabrow and upper eyelid skin concurrent with upper blepharoplasty. HPDR was carried across the lower mandibular border for blending in all cases but was not performed more inferiorly. During cervicofacial rhytidectomy, undermined facial skin was treated with typical treatment power (eg, 35%) with 2 passes and with desiccated superficial skin tissue layers gently wiped away between the first and second pass using saline moistened gauze. Management of the facial skin flap during cervicofacial rhytidectomy included maximizing flap thickness in the preauricular area with the development of a thicker fat-up, fat-down cutaneous flap with undermining forward to the vertical plane of the lateral canthus whereupon the dissection was transitioned through the superficial muscular aponeurotic system (SMAS) to a deeper sub-SMAS dissection plane. Additionally, the risk of distal flap complications was minimized by avoiding excess tension and stopping HPDR treatment 2 cm before the end of the flap.
Data Analysis
Results were analyzed by nonparametric statistics because data were either small whole numbers, not continuous, or not normally distributed as shown by the Shapiro-Wilk test. Comparisons among groups were done by the Wilcoxon-Mann-Whitney test (2 groups) or the Kruskal-Wallis test (more than 2 groups). Proportions among groups were compared using Pearson’s Chi-Square test. The cutoff value for significance was P < 0.05. Statistical analysis was performed by Fred Wilson (Wilwrite Writing and Editing, Syracuse, NY) using Analyze-It for Microsoft Excel version 5.81 (Redmond, WA).
RESULTS
Patient Satisfaction
Among patients who returned for follow-up (n = 288), 275 commented on their reaction to the treatment: 80 were satisfied, 172 were pleased, and 21 were happy. One patient said, “looks great,” and another said, “huge difference.” The remaining 13 patients did not respond to this question. The majority (83%) of documented satisfaction queries occurred between days 31 and 90 posttreatment; the remaining charts (n = 13) did not reference satisfaction or dissatisfaction, and no AEs were observed in this patient subgroup. The average satisfaction score completion time point was 60 days (range, 4-510 days; median 46 days).
Zones of Treatment
For both the AE and no AE group, the median number of passes along the jawline/mandibular border was 2. For all other zones, the median number of passes was 3 for both the AE and no AE groups. Fewer passes were used along the jawline/mandibular border to minimize the line of demarcation between the treated tissue of the face and the untreated tissue of the neck. Fewer passes were also employed over undermined facial skin during cervicofacial rhytidectomy to limit the potential for flap tissue compromise.
Concurrent Treatment
HPDR was performed concurrently with other facial/non-facial procedures in 193 of 301 patients (64.1%), whereas 108 of 301 patients (35.9%) underwent HPDR as a sole modality (Table 1). Concurrent procedures included upper blepharoplasty in 49 patients (16.3%), cervical rhytidectomy in 86 patients (28.6%), and cervicofacial rhytidectomy in 58 patients (19.3%) (Table 1).
Table 1.
HPDR and Face and Neck Aesthetic Surgical Procedures
| Concurrent procedure | No. of patients (%) |
|---|---|
| None (HDPR only) | 108 (35.9) |
| Cervical rhytidectomy | 86 (28.6) |
| Cervicofacial rhytidectomy | 58 (19.3) |
| Upper blepharoplasty | 49 (16.3) |
HPDR sole modality and concurrent HPDR with face and neck aesthetic surgical procedures, number of patients, and percent of total HDPR treatments performed. HPDR, helium plasma dermal resurfacing.
Adverse Events
Safety assessments were tracked concurrently with satisfaction survey time points. No serious AEs were observed with or without concurrent face and neck aesthetic surgical procedures, including over undermined facial skin in 58 patients (116 flaps) during cervicofacial rhytidectomy. Nonserious AEs were noted in 20 patients (7.3%) and included redness/prolonged redness (n = 14), hyperpigmentation (n = 3), milia (n = 1), slow healing (n = 1), and upper lip hypertrophic scar (n = 1). All AEs resolved. The upper lip scar resolved after 3 injections of triamcinolone 40 mg/mL spaced 6 weeks apart. The presence or absence of AEs was not significantly affected by patient demographics (age, gender, skin type) or treatment variables (number of passes in each treatment zone, posttreatment skin care type). Statistical analysis reveals no correlation between demographic, procedural, or posttreatment skin care and the presence or absence of AEs (Table 2).
Table 2:
Correlation of Adverse Events (AEs) With Demographic, Procedural, and Post-treatment Variables
| Variable | Adverse events | No adverse events | P value |
|---|---|---|---|
| Demographic | |||
| Age (med, IQR) | 63.5 (6.6) | 63.0 (12.0) | 0.2961 |
| Gender (n [%]) | |||
| Female | 19 (7.2) | 245 (92.8) | 0.8127 |
| Male | 1 (9.1) | 10 (90.9) | |
| Skin type (n) | |||
| I | 6 | 49 | |
| II | 9 | 130 | 0.6787 |
| III | 4 | 66 | |
| IV | 1 | 10 | |
| Procedural | |||
| Number of passes | |||
| Median (range) | |||
| Perioral | 3 (2-3) | 3 (2-4) | 0.4650 |
| Periorbital | 3 (2-3) | 3 (2-3) | 0.3824 |
| Forehead | 3 (3-3) | 3 (2-3) | 0.4550 |
| Nose | 3 (3-3) | 3 (2-3) | 0.4784 |
| Cheeks | 3 (2-3) | 3 (2-3) | 0.8089 |
| Mandibular border | 2 (2-3) | 2 (1-3) | 0.8672 |
| Posttreatment (n) | 0.8695 | ||
| Cicalfate | 0 | 1 | |
| Silicone | 1 | 19 | |
| Silicone-Cicalfate | 7 | 109 | |
| Stratamed | 0 | 4 | |
| Stratamed-Cicalfate | 8 | 84 | |
| Xeroform | 0 | 1 | |
| Xeroform-Stratamed-Cicalfate | 0 | 5 | |
| Xeroform-Stratamed-Cicalfate | 2 | 10 |
Results of statistical analysis attempting to identify any correlations with AEs. n = number of patients. IQR = interquartile range, the difference between the 75th and 25th percentile, a measure of dispersion. Range = maximum minus minimum. Of note, no statistically significant correlation between any AE and any demographic, procedure or post-treatment variables was identified.
Examples of Favorable Outcomes
Before and after photographs were selected from several patients from the group to demonstrate favorable outcomes relatively early on after treatment (Figures 2, 3; both 6 weeks posttreatment) and at much longer intervals (Figure 4; including 3 and 8 years posttreatment).
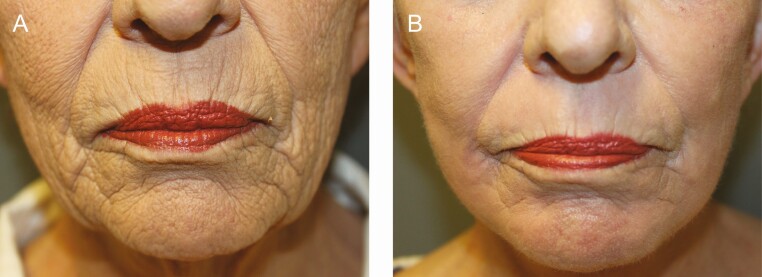
Figure 2.
Before and after photographs of perioral helium plasma dermal resurfacing (HPDR) treatment: (A) before and (B) 6 week after photographs of perioral HPDR treatment (3 passes, 40% power) in a 59-year-old female with Fitzpatrick II skin type demonstrating significant improvement of vertical lip lines and perioral folds as well as skin tone and superficial skin texture.
Figure 3.
Before and after photographs of perioral helium plasma dermal resurfacing (HPDR): (A) before and (B) 6 week after photographs of perioral HPDR treatment (3 passes, 40% power) in a 65-year-old female with Fitzpatrick II skin type demonstrating significant improvement of vertical lip lines and perioral folds as well as skin tone and superficial skin texture. Both upper and lower blepharoplasty and cervicofacial rhytidectomy were completed as concurrent procedures.
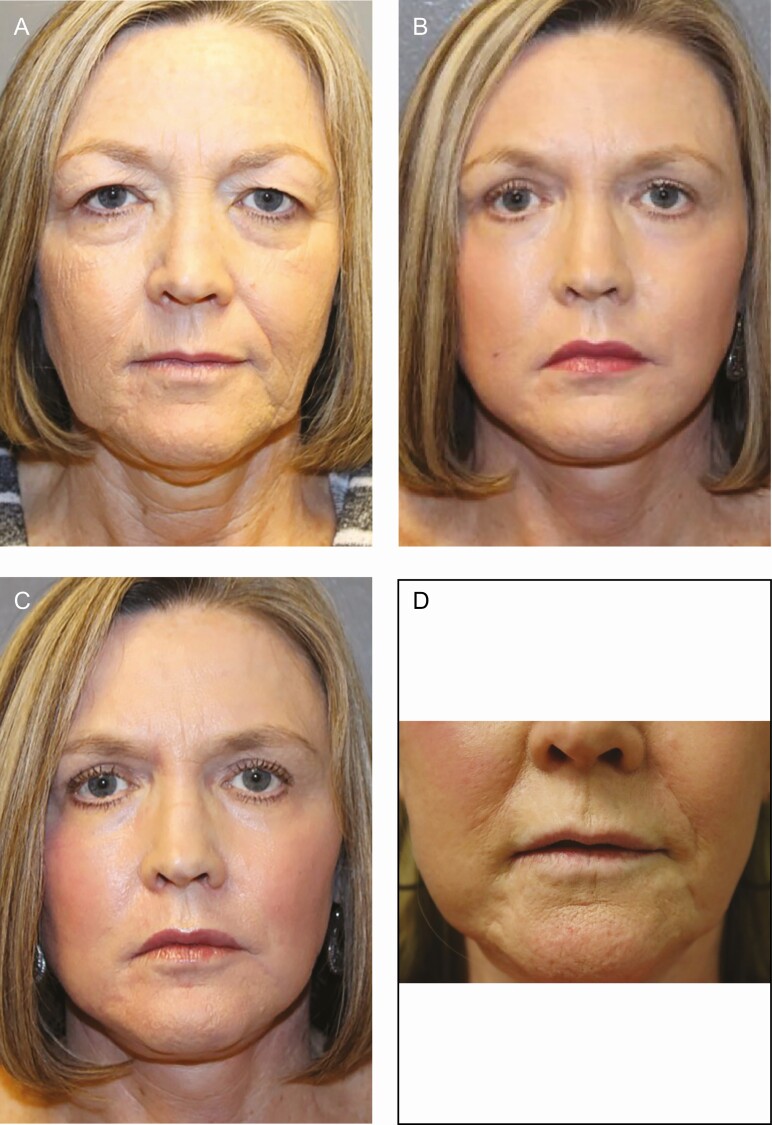
Figure 4.
Before and after photographs of full-face helium plasma dermal resurfacing (HPDR) treatment: (A) before, (B) 6 week, (C) 3 year, and (D) 8 year after photographs of full-face HPDR treatment (3 passes, 40% power) in a 58-year-old female with Fitzpatrick II skin type demonstrating (B) very early and (C) enduring significant improvement of facial rhytidosis as well as substantial skin tightening and improvement in skin tone and superficial skin texture. Both upper blepharoplasty and surgical neck lift were completed as concurrent procedures.
DISCUSSION
The author’s (JBD) experience with off-label HPDR was not restricted to sole modality HPDR treatment—HPDR was performed concurrently with face and neck aesthetic surgery procedures in 193 of 301 patients (64.1%). The author’s (JBD) overall experience also includes another 153 cases that did not meet photograph-documentation or follow-up criteria for inclusion herein but bring his total HPDR case number to 454 from 2012 through 2020. Each of these procedures used the original helium plasma technology with disposable generators located within bulky handpieces. The author’s success with off-label HPDR treatment led to efforts to characterize helium plasma skin tissue interaction and to upgrade the device with a new non-disposable generator with an updated, proprietary energy delivery algorithm and new ergonomic much more user-friendly handpieces. Apart from this study, others’ efforts to further develop helium plasma energy skin rejuvenation technology have also led to the recognition of the potential of this new therapy, to development of consensus guidelines for safe and effective treatment, and, ultimately (as of May 26, 2022), to 510(k) clearance from the FDA for the use of the Renuvion Dermal Handpiece (Apyx Medical, Clearwater, FL) for specific (low energy) dermal resurfacing procedures.16-19
While this is a retrospective review of patients treated with the original helium plasma device, the findings are certainly clinically relevant for this still-emerging technology. Although the RF waveform for the original helium plasma device was modified in the second-generation device to improve the uniformity of energy delivery, the energy delivered to the tissue remained equivalent between devices. That no serious AEs were observed among the 301 patients treated with the original more powerful helium plasma device suggests that the therapeutic window should be similar or even more favorable for the second-generation device. Twenty patients (7.3%) experienced nonserious AEs including redness/prolonged redness, hyperpigmentation, milia, slow healing, and upper lip hypertrophic scar. All AEs resolved in a reasonable timeframe, and intervention was required only for the hypertrophic scar (serial triamcinolone injections). No new AEs unique to this technology were identified—all AE types recorded in this study have also been observed with other full-field facial skin resurfacing treatments including nitrogen plasma skin regeneration and laser skin resurfacing (CO2 and Erbium YAG).20-22 Similar AEs were observed in a recent clinical study using the updated helium plasma device wherein only a single pass at a lower power setting (20%) was performed13; more severe AEs were observed in a recent clinical study using the Erbium YAG laser for deep resurfacing23 (see comparison of AEs; Table 3).
Table 3.
Comparison of AEs Among 3 Skin Resurfacing Studies
| AE type | AEs this study (n = 301) HPDR 3 passes, 35% power |
AEs, Holcomb et al13 (n = 55) HPDR 1 pass, 20% power |
AEs, Weniger et al23 (n = 472) Erbium-YAG laser deep resurfacing |
|---|---|---|---|
| Acne/milia | 1 | 5+ | Unknown |
| Hyperpigmentation | 3 | 8 | Unknown |
| Hypertrophic scarring | 1 | 2 | 7a |
| Hypopigmentation | 0 | 0 | 6a |
| Prolonged erythema | 10 | 0 | 70a |
| Slow healing | 1 | 4 | Unknown |
AEs observed in this study compared with similar AEs in two recent facial skin resurfacing studies including HPDR (lower energy, single pass treatment; Holcomb et al) and dual mode erbium YAG laser skin resurfacing (Weniger et al23).
aNumber of patients determined based on percentage observed and total patients treated. AE, adverse event; HPDR, helium plasma dermal resurfacing.
Nonserious AEs that were observed in the initial single-pass, low-energy HPDR study but not observed or recorded as AEs per se in this study included pruritis, pain, transient bleeding, skin hypersensitivity (one or more of edema, erythema, induration, urticaria), sensitivity to topical care, and several others. Since the 2 HPDR studies used different generation devices and handpieces, energies, and treatment protocols, it is interesting that the relative incidences of acne/milia, hyperpigmentation, and slow healing are greater with HPDR using only a single pass at lower power, but it is not unexpected that prolonged erythema, defined as erythema lasting longer than 3 months posttreatment, was more prevalent in this study with HPDR using multiple passes and higher power. On average, patients in this retrospective review experienced erythema for 6 to 8 weeks postprocedure. The greater incidences of acne/milia and slow healing in the HPDR study using only a single pass and lower power may be explained by differences in the pretreatment and posttreatment skin care regimen whereas that for hyperpigmentation may be related to patient skin type, environmental, and other factors.
Comparison of AEs between HPDR with 3 passes (mean) at 35% power (mean) with erbium YAG deep laser resurfacing reveals a much higher incidence of prolonged erythema for the latter (approximately 15% vs approximately 3%) as well as higher incidences of hypopigmentation and hypertrophic scarring with the laser treatment (Table 3). Acne/milia, hyperpigmentation, and slow healing were either not observed or not recorded as AEs by Weniger et al.23 Additional AEs that were observed by Weniger et al with erbium YAG laser deep resurfacing but not with HPDR include bacterial infection, cold sores, and yeast infection.23
The greater incidence of prolonged erythema with erbium YAG deep laser resurfacing may be in part related to treatment parameters including the use of “coagulation” with its pseudo-long-pulse energy delivery to increase energy density, depth of effect, tissue heating, and regenerative healing response. Despite mean treatment parameters of 3 passes and 35% power, other aspects of the treatment, the method of energy delivery with rapid movement of the handpiece over the tissue minimizing energy density, may have contributed to a lower incidence of prolonged erythema (greater than 3 months posttreatment) with the HPDR treatment in this study. Certainly, patient, posttreatment skin care, and environmental factors may also play a role in the development and duration of prolonged erythema. Of note, prolonged erythema has been linked to the development of permanent hypopigmentation following erbium YAG laser skin resurfacing.24,25 Despite this association, the precise etiology of permanent hypopigmentation following erbium YAG laser skin resurfacing remains ill-defined.
The greater incidence of hypertrophic scarring observed with the erbium YAG laser deep resurfacing study may be explained in part by energy density upon treatment, depth of tissue ablation/coagulation potentially reaching the reticular dermis, posttreatment healing, or patient factors. During HPDR treatment, the initial pass results in coagulation of the superficial layers of tissue that may remain intact as a natural biological dressing if only a single pass is performed and at the discretion of the treating physician. If a second pass is performed, the superficially coagulated tissue is gently removed via wiping with moistened gauze.19 Second and subsequent passes do not result in enough superficial tissue desiccation to warrant further tissue removal between successive passes; therefore, the overall depth of tissue ablation with multi-pass HPDR treatment as performed in this study is likely to be significantly less than that with the deep erbium YAG laser resurfacing treatments described by Weniger et al.23 A more superficial depth of tissue injury with HDPR treatment could account for the differences in AEs and is consistent with findings of the preclinical animal study comparing depth of tissue injury with the predicate nitrogen plasma skin regeneration device.12
Numerous studies have previously evaluated the benefits and safety of concurrent facial skin resurfacing and aesthetic facial surgery, including CO2 laser skin resurfacing,26-30 erbium YAG laser skin resurfacing,31,32 and nitrogen plasma skin regeneration.33 Benefits of concurrent treatment include avoiding the need for extended downtime for a secondary procedure and more homogenous skin tone and improved skin texture with full-face resurfacing vs regional resurfacing and avoidance of treatment of undermined skin flaps. Patients receiving concurrent treatment with HPDR and cervicofacial rhytidectomy have responded well to treatment and have experienced additional efficacy in terms of perioral and periorbital wrinkle reduction and improvement in dyschromia. However, the decision to perform HPDR on undermined tissue should be assessed on each individual patient. Special consideration should be paid to patient variables such as smoking history, poor nutrition, previous radiation therapy, decreased vascularity, autoimmune disorders, and any other factor that may impact the healing of the flap.
While the potential for tissue compromise impacting flap survival is greater in smokers with or without concurrent resurfacing of undermined tissue, the premise that full-face facial skin resurfacing may be safely performed concurrently with cervicofacial rhytidectomy in nonsmokers is generally accepted. Safety of concurrent resurfacing of undermined facial skin during cervicofacial rhytidectomy may be improved by technical choices that include increased flap thickness (eg, composite flap) and/or limiting energy density during treatment of undermined tissue (eg, fewer passes, lower power, and specific to HPDR optimization of treatment speed). In this current study, a somewhat thicker flap (fat-up, fat-down tissue plane before transitioning to a deeper tissue plane) was utilized with a novel resurfacing technology that has a limited depth of tissue injury, and while 2 passes were performed over the undermined facial skin flaps, treatment was stopped approximately 2 cm from the flap edge and treatment speed was optimized to avoid inadvertent increases in energy density and desiccated skin tissue was carefully debrided between the first and second passes to ensure homogenous energy delivery.
Patient satisfaction was consistently high among patients who commented on their reaction to treatment (n = 275 of 288 available for follow-up or 95.5%). No patients reported dissatisfaction with the HPDR procedure, and 2 patient comments were “huge difference” and “looks great.” Figure 4 shows before (Figure 4A) and after (Figure 4B-D) photographs from a “satisfied” patient where very significant improvement in skin texture, wrinkle reduction, and modest skin tightening at 6 weeks remain evident as long as 8 years after treatment. Limitations of this study include the subjective nature of the efficacy assessments, relatively short duration of follow-up, lack of formal objective efficacy evaluation, lack of stratification of outcomes by age and skin type, nonuniform photography, and the limitations inherent with a retrospective study.
CONCLUSIONS
Skin rejuvenation with HPDR when performed under the conditions of the present study appears to be a safe treatment with favorable patient satisfaction and the potential for very dramatic positive outcomes. The infrequent, nonserious nature of AEs in this study suggests that this nascent helium plasma-based technology is suitable for deep dermal resurfacing in appropriate skin types. While HPDR may be safely performed concurrently with face and neck aesthetic surgical procedures, technical aspects of surgical management of undermined tissues and of energy delivery that are specific to this new technology should be carefully optimized. Additional studies are needed that objectively evaluate long-term treatment efficacy.
Disclosures
Dr DeLozier is a clinical trial investigator for Apyx Medical (Clearwater, FL), and he owns privately purchased stock and stock options of Apyx Medical, the sponsor of the study. Dr Holcomb is a consultant and clinical trial investigator for Apyx Medical (no purchased stock or stock options).
Funding
Manuscript preparation was funded by Apyx Medical Corporation (Clearwater, FL).
REFERENCES
- 1. Ross EV, Miller C, Meehan K, et al. One-pass CO2 versus multiple-pass Er:YAG laser resurfacing in the treatment of rhytides: a comparison side-by-side study of pulsed CO2 and Er:YAG lasers. Dermatol Surg. 2001;27(8):709-715. doi: 10.1046/j.1524-4725.2001.01015.x [DOI] [PubMed] [Google Scholar]
- 2. Rostan EE, Fitzpatrick RE, Goldman MP. Laser resurfacing with a long pulse erbium:YAG laser compared to the 950 ms pulsed CO(2) laser. Lasers Surg Med. 2001;29(2):136-141. doi: 10.1002/lsm.1099 [DOI] [PubMed] [Google Scholar]
- 3. Khatri KA, Ross V, Grevelink JM, et al. Comparison of erbium:YAG and carbon dioxide lasers in resurfacing of facial rhytides. Arch Dermatol. 1999;135(4):391-397. doi: 10.1001/archderm.135.4.391 [DOI] [PubMed] [Google Scholar]
- 4. Rostan EE, Fitzpatrick RE, Goldman MP. Laser resurfacing with a long pulse erbium:YAG laser compared to the 950 ms pulsed CO(2) laser. Lasers Surg Med. 2001;29(2):136-141. doi: 10.1002/lsm.1099 [DOI] [PubMed] [Google Scholar]
- 5. Newman JB, Lord JL, Ash K, et al. Variable pulse erbium:YAG laser skin resurfacing of perioral rhytides and side-by-side comparison with carbon dioxide laser. Lasers Surg Med. 2000;26(2):208-214. doi: [DOI] [PubMed] [Google Scholar]
- 6. Rhie JW, Shim JS, Choi WS. A pilot study of skin resurfacing using the 2,790-nm erbium:YSGG laser system. Arch Plast Surg. 2015;42(1):52-58. doi: 10.5999/aps.2015.42.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wambier CG, Lee KC, Soon L, et al. Advanced chemical peels: phenol croton oil peel. Am Acad Dermatol. 2019;81(2):327-336. doi: 10.1016/j.jaad.2018.11.060 [DOI] [PubMed] [Google Scholar]
- 8. Bogle MA, Arndt KA, Dover JS. Evaluation of plasma skin regeneration technology in low-energy full-facial rejuvenation. Arch Dermatol. 2007;143(2):168-174. doi: 10.1001/archderm.143.2.168 [DOI] [PubMed] [Google Scholar]
- 9. Kilmer S, Semchyshyn N, Shah G, et al. A pilot study on the use of a plasma skin regeneration device (PortraitPSR3) in full facial rejuvenation procedures. Lasers Med Sci. 2007;22(2):101-109. doi: 10.1007/s10103-006-0431-9 [DOI] [PubMed] [Google Scholar]
- 10. Fitzpatrick R, Bernstein E, Iyer S, Brown D, Andrews P, Penny K. A histopathologic evaluation of the Plasma Skin Regeneration System (PSR) versus a standard carbon dioxide resurfacing laser in an animal model. Lasers Surg Med. 2008;40(2):93-99. doi: 10.1002/lsm.20547 [DOI] [PubMed] [Google Scholar]
- 11. Foster KW, Moy RL, Fincher EF. Advances in plasma skin regeneration. J Cosmet Dermatol. 2008;7(3):169-179. doi: 10.1111/j.1473-2165.2008.00385.x [DOI] [PubMed] [Google Scholar]
- 12. Holcomb JD, Schucker A. Helium plasma skin regeneration: evaluation of skin tissue effects in a porcine model and comparison to nitrogen plasma skin regeneration. Lasers Surg Med. 2020;52(1):23-32. doi: 10.1002/lsm.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holcomb JD, Kelly M, Hamilton TK, DeLozier JB, 3rd. A prospective study evaluating the use of helium plasma for dermal resurfacing. Lasers Surg Med. 2020;52(10):940-951. doi: 10.1002/lsm.23257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holcomb JD. Helium plasma dermal resurfacing: VISIA CR assessment of facial spots, pores, and wrinkles – preliminary findings. J Cosmet Dermatol. 2021;20(6):1668-1678. doi: 10.1111/jocd.14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holcomb JD, Doolabh V, Lin M, Zimmerman E. High energy, double pass helium plasma dermal resurfacing: a prospective, multicenter, single-arm clinical study. Lasers Surg Med. 2022. doi: 10.1002/lsm.23524 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gentile RD. Plasma energy skin rejuvenation. Adv Cosmet Surg. 2020;3(1):39-50. doi: 10.1016/j.yacs.2020.01.017 [DOI] [Google Scholar]
- 17. Gentile RD. Renuvion RF-helium plasma for subdermal skin tightening, facial contouring and skin rejuvenation of the face and neck. Facial Plast Surg Aesthet Med. 2020;22(4):304-306. doi: 10.1089/fpsam.2020.0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holcomb JD. Plasma energy skin rejuvenation. Facial Plast Surg Clin North Am. 2020;28(1):67-74. doi: 10.1016/j.fsc.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 19. Holcomb JD, Duncan D, Lin M, et al. Helium plasma dermal resurfacing: consensus guidelines. Dermatol Rev. 2020;1:97-107. doi: 10.1002/der2.22 [DOI] [Google Scholar]
- 20. Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. An evaluation of 500 patients. Dermatol Surg. 1998;24(3):315-320. doi: 10.1111/j.1524-4725.1998.tb04161.x [DOI] [PubMed] [Google Scholar]
- 21. Alster TS, Lupton JR. Treatment of complications of laser skin resurfacing. Arch Facial Plast Surg. 2000;2(4):279-284. doi: 10.1001/archfaci.2.4.279 [DOI] [PubMed] [Google Scholar]
- 22. Hamilton MM, Kao R. Recognizing and managing complications in laser resurfacing, chemical peels, and dermabrasion. Facial Plast Surg Clin North Am. 2020;28(4): 493-501. doi: 10.1016/j.fsc.2020.06.008 [DOI] [PubMed] [Google Scholar]
- 23. Weniger FG, Weidman AA, Barrero Castedo CE. Full-field Erbium:YAG laser resurfacing: complications and suggested safety parameters. Aesthet Surg J. 2020;40(6):NP374-NP385. doi: 10.1093/asj/sjz319 [DOI] [PubMed] [Google Scholar]
- 24. Kim YJ, Lee HS, Son SW, et al. Analysis of hyperpigmentation and hypopigmentation after Er:YAG laser skin resurfacing. Lasers Surg Med. 2005;36(1):47-51. doi: 10.1002/lsm.20120 [DOI] [PubMed] [Google Scholar]
- 25. Ko NY, Ahn HH, Kim SN, et al. Analysis of erythema after Er:YAG laser skin resurfacing. Dermatol Surg. 2007;33(11):1322-1327. doi: 10.1111/j.1524-4725.2007.33283.x [DOI] [PubMed] [Google Scholar]
- 26. Mayl N, Felder DS. CO(2) laser resurfacing over facial flaps. Aesthet Surg J. 1997;17:285-292. doi: 10.1016/s1090-820x(97)80017-9 [DOI] [PubMed] [Google Scholar]
- 27. Fulton JE. Simultaneous face lifting and skin resurfacing. Plast Reconstr Surg. 1998;102:2480-2489. doi: 10.1097/00006534-199812000-00035 [DOI] [PubMed] [Google Scholar]
- 28. Graf RM, Bernardes A, Auerswald A, Noronha L. Full-face laser resurfacing and rhytidectomy. Aesthetic Plast Surg. 1999;23:101-106. doi: 10.1007/s002669900250 [DOI] [PubMed] [Google Scholar]
- 29. Roberts TL III, Pozner JN, Ritter E. The RSVP facelift: a highly vascular flap permitting safe, simultaneous, comprehensive facial rejuvenation in one operative setting. Aesthetic Plast Surg. 2000;24:313-322. doi: 10.1007/s002660010054 [DOI] [PubMed] [Google Scholar]
- 30. Koch BB, Perkins SW. Simultaneous rhytidectomy and full-face carbon dioxide laser resurfacing: a case series and meta-analysis. Arch Facial Plast Surg. 2002;4:227-233. doi: 10.1001/archfaci.4.4.227 [DOI] [PubMed] [Google Scholar]
- 31. Weinstein C, Pozner J, Scheflan M, Achauer BM. Combined erbium:YAG laser resurfacing and face lifting. Plast Reconstr Surg. 2001;107:593-594. doi: 10.1097/00006534-200102000-00045 [DOI] [PubMed] [Google Scholar]
- 32. Scheuer JF 3rd, Costa CR, Dauwe PB, et al. Laser resurfacing at the time of rhytidectomy. Plast Reconstr Surg. 2015;136(1):27-38. doi: 10.1097/PRS.0000000000001397 [DOI] [PubMed] [Google Scholar]
- 33. Holcomb JD, Rousso DR, Kent KJ. Nitrogen plasma skin regeneration and aesthetic facial surgery: multicenter evaluation of concurrent treatment. Archiv Facial Plast Surg. 2009;11(3):184-193. doi: 10.1001/archfacial.2009.29 [DOI] [PubMed] [Google Scholar]