Abstract
Background
Although targeted agents have been gradually applied in the treatment of HER2-mutated non-small cell lung cancer (NSCLC) in recent years, patients’ therapeutic demands are far from being met. PATHER2 was the first phase 2 trial to explore the efficacy and safety of the HER2-targeted tyrosine kinase inhibitor (TKI) pyrotinib plus the antiangiogenic agent apatinib in previously treated HER2-altered metastatic NSCLC patients.
Methods
HER2-mutated or HER2-amplified metastatic NSCLC patients who had failed at least first-line chemotherapy or HER2-targeted TKIs received oral pyrotinib 400 mg plus apatinib 250 mg once daily until disease progression, intolerable toxicity, or death. The primary endpoint was the investigator-assessed objective response rate (ORR).
Results
Between March 2019 and December 2020, 33 patients were enrolled; 13 (39.4%) presented brain metastases, and 16 (48.5%) had received at least two lines of prior chemotherapy or HER2-targeted TKIs. As of September 20, 2021, the median follow-up duration was 11.3 (range, 3.5–26.0) months. The investigator-assessed ORR was 51.5% (17/33; 95% CI, 33.5 to 69.2%), and the disease control rate was 93.9% (31/33; 95% CI, 79.8 to 99.3%). The median duration of response, progression-free survival, and overall survival were 6.0 (95% CI, 4.4 to 8.6) months, 6.9 (95% CI, 5.8 to 8.5) months, and 14.8 (95% CI, 10.4 to 23.8) months, respectively. The most frequent grade ≥ 3 treatment-related adverse events included diarrhea (3.0%) and hypertension (9.1%). No treatment-related deaths were reported.
Conclusions
Pyrotinib plus apatinib demonstrated promising antitumor activity and a manageable safety profile in HER2-mutated or HER2-amplified metastatic NSCLC patients.
Trial registration
Chinese Clinical Trial Registry Identifier: ChiCTR1900021684.
Keywords: Pyrotinib, Apatinib, HER2 mutation, HER2 amplification, Non-small cell lung cancer
Background
Non-small cell lung cancer (NSCLC) is a heterogeneous malignant disease with a high degree of genomic diversity. During the past decade, the development of targeted therapies against specific molecular aberrations (e.g., epidermal growth factor receptor [EGFR] mutations, anaplastic lymphoma kinase [ALK] and ROS1 fusions) has contributed to the improvement of patient prognosis, and targeted therapies have emerged as the standard of care for oncogene-driven NSCLC [1, 2]. In the era of genetic testing, more oncogenic driver genes, such as human epidermal growth factor receptor 2 (HER2 or ERBB2), have been identified. HER2 alterations, mainly manifested as protein overexpression, gene amplification, or gene mutation, occur in 2–4% of NSCLC patients [3, 4]. HER2 alterations are commonly observed in female, never-smokers and lung adenocarcinoma patients, who have a higher probability of developing brain metastases than those without HER2 alterations or other molecular mutations [3, 5, 6].
Unlike NSCLC patients with other common molecular aberrations, who have a remarkable response to tyrosine kinase inhibitors (TKIs), HER2-altered patients are relatively insensitive to HER2-targeted TKIs such as afatinib, neratinib, poziotinib, and dacomitinib, with an objective response rate (ORR) of less than 30% [7–10]. The standard of care for HER2-altered population is still chemotherapy or immunotherapy, also with limited benefit [11–13]. Currently, only two anti-HER2 antibody–drug conjugates (ADCs), ado-trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (DS-8201), have shown encouraging results in HER2-mutated NSCLC, with an ORR of 44–55% [14, 15]. However, pulmonary toxicities may limit their application. Thus, more anti-HER2 therapeutic approaches need to be explored.
Tumor angiogenesis is a critical feature of cancer pathogenesis, not only providing oxygen to the tumor but also providing an important pathway for the metastatic spread of cancer cells [16]. Antiangiogenic agents targeting the dominant angiogenic mediators vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) could normalize pathologic tumor vasculature, modulate the tumor microenvironment, and suppress neovascularization [16, 17]. Previous evidence has shown that EGFR-TKIs could diminish VEGF expression and inhibit tumor angiogenesis; when combined with antiangiogenic agents, they facilitate substantial survival benefits in advanced NSCLC patients [18–20]. VEGF expression is also modulated by HER2 signaling [21], and additional suppression of VEGF/VEGFR activity might potentiate the antitumor effects of HER2 inhibitors.
Pyrotinib is an irreversible oral pan-ErbB TKI targeting EGFR/HER1, HER2, and HER4. In a phase II study, pyrotinib monotherapy as a second- or above-line treatment in HER2-mutant NSCLC patients showed an acceptable safety profile and promising antitumor activity, with an ORR of 30% [22]. Apatinib, an oral small-molecule TKI that selectively targets VEGFR-2, has displayed clinical benefit in patients with advanced NSCLC when combined with chemotherapy or EGFR-TKIs [19, 23]. In addition, apatinib exhibited synergistic antitumor effects with pyrotinib in vivo [24]. Herein, we conducted a single-arm phase 2 clinical study to explore the efficacy and safety of pyrotinib combined with apatinib in metastatic NSCLC patients harboring HER2 mutations or amplification.
Methods
Study design and participants
PATHER2 is an open-label, single-arm, phase 2 clinical study (ChiCTR1900021684) conducted at National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China). Eligible patients were aged 18–70 years; had histologically or cytologically confirmed HER2-altered stage IV NSCLC (AJCC 8th edition); had at least one measurable lesion per the Response Evaluation Criteria in Solid Tumours, version 1.1 (RECIST 1.1); had received at least one kind of prior chemotherapy or anti-HER2 TKIs for metastatic disease; had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1; and had adequate organ function. HER2 alterations included HER2 exon 20 insertion mutations, HER2 missense mutations in the tyrosine kinase domain (TKD), and HER2 amplification. Asymptomatic brain metastasis was permitted, except for meningeal metastasis. The key exclusion criteria included uncontrolled hypertension, significant gastrointestinal function abnormalities, a history of bleeding or the presence of clinically meaningful bleeding symptoms or definite bleeding tendency, an active and severe infection, and prior exposure to pyrotinib and/or apatinib.
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices and was approved by the Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences. Written informed consent was obtained from each participant before study initiation.
Procedures
All eligible patients were administered oral pyrotinib 400 mg and apatinib 250 mg once daily in a continuous 28-day cycle until disease progression, intolerable toxicity, or death. Dose reduction of pyrotinib was allowed according to adverse events, with a gradual reduction from 400 to 320 mg to 240 mg. In terms of apatinib, dose modification was not permitted, but dose interruption or discontinuation was allowed.
The initial tumor response was evaluated 4 weeks after the initiation of pyrotinib with apatinib and then every 8 weeks thereafter as per RECIST 1.1. Patients achieving a complete response (CR) or partial response (PR) had to be confirmed at least 4 weeks following the initial response. Computed tomography (CT) scans of the neck, chest, and abdomen were performed at baseline and then every 8 weeks until disease progression. Brain magnetic resonance imaging (MRI) and SPECT whole body bone scans were also performed at baseline and were not routinely taken unless metastasis of the central nervous system (CNS) or bone was clinically indicated. Adverse events (AEs) were collected from the onset of study treatment until 30 days after the last administration and were graded according to the National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 5.0.
HER2 alterations were documented by next generation sequencing (NGS) or amplification refractory mutation system polymerase chain reaction (ARMS-PCR) assay. DNA samples extracted from tumor tissues or plasma was used for HER2 testing. Circulating tumor DNA from plasma was used only in cases of the inadequacy of tumor tissues. NGS testing was performed based on the Illumina sequencing system.
Outcomes
The primary endpoint was investigator-assessed ORR, defined as the proportion of patients with a CR or PR as per RECIST 1.1. The secondary endpoints included progression-free survival (PFS, defined as the time from the initiation of study treatment to the first evidence of disease progression or death of any cause), disease control rate (DCR, defined as the proportion of patients with a CR, PR or stable disease [SD] as per RECIST 1.1), duration of response (DoR, defined as the time from the first documented response to the first evidence of disease progression or death of any cause), overall survival (OS, defined as the time from the initiation of study treatment to death of any cause), and safety.
Statistical analysis
The sample size of this trial was calculated in accordance with Simon's optimal two-stage design, with a one-sided α error of 5% and a power of 80%. Ten evaluable patients were required in the first stage; if two or more of them achieved CR or PR, 19 additional evaluable patients were recruited in the second stage. An ORR of 10% was deemed unacceptable, whereas a 30% ORR was considered to be clinically promising and would merit further investigation. To account for a 10% dropout rate for unevaluable patients, a total of 33 patients were required.
Efficacy and safety were evaluated in patients undergoing at least one dose of study treatment. Patient characteristics and treatment-related adverse events (TRAEs) were summarized by descriptive statistics. The 95% confidence intervals (CI) for the ORR and DCR was calculated using the Clopper-Pearson method. The Kaplan–Meier method was performed to estimate PFS, DoR, and OS, and the 95% CI for median survival time and survival rate were calculated using the Brookmeyer-Crowley and complementary log–log method, respectively. Post hoc subgroup analyses for ORR and PFS were conducted on the basis of patient characteristics. Statistical analyses were performed by the SAS software, version 9.4 (SAS Institute). Forest plots were generated with the R software, version 4.3.1.
Results
Baseline characteristics
A total of 33 eligible patients with lung adenocarcinoma were enrolled from March 2019 to December 2020 in this study and received scheduled treatment with pyrotinib combined with apatinib. HER2 status was determined by local or institutional laboratory tests in 32 patients (29 patients with tissues, 3 patients with plasma) by NGS and one patient with tissue by PCR in the institutional laboratory. The median age of the enrolled patients was 54 (range, 35–70) years. Twenty-four (72.7%) patients had an ECOG PS of 0, 13 (39.4%) presented brain metastases, and 16 (48.5%) had received at least two lines of prior chemotherapy or HER2-targeted TKIs. Of the 33 patients, 28 (84.8%) harbored HER2 exon 20 insertions, with A775_G776insYVMA being the predominant insertion variant (60.6%). Three patients carried HER2 TKD missense mutations, with one each of the G776V, R811L with Q820K and G727A mutations, and the remaining two patients harbored primary HER2 amplification. The detailed baseline characteristics are listed in Table 1. As of September 20, 2021, the median treatment duration was 6.9 months (range, 0.9 to 17.3), with three patients (9.1%) still on treatment. The median follow-up duration was 11.3 (range, 3.5–26.0) months.
Table 1.
Baseline characteristics of patients
| Characteristic | N = 33 |
|---|---|
| Median age, years (range) | 54 (35–70) |
| ≤ 60, n (%) | 25 (75.8) |
| > 60, n (%) | 8 (24.2) |
| Gender, n (%) | |
| Male | 17 (51.5) |
| Female | 16 (48.5) |
| Smoking history, n (%) | |
| Former/current | 12 (36.4) |
| Never | 21 (63.6) |
| ECOG PS, n (%) | |
| 0 | 24 (72.7) |
| 1 | 9 (27.3) |
| Brain metastases, n (%) | |
| Presence | 13 (39.4) |
| Absence | 20 (60.6) |
| No. of lines of prior systemic treatment, n (%) | |
| 1 | 17 (51.5) |
| 2 | 9 (27.3) |
| ≥ 3 | 7 (21.2) |
| Median (range) | 1 (1–5) |
| Prior Chemotherapy, n (%) | |
| Yes | 24 (72.7) |
| No | 9 (27.3) |
| Prior anti-HER2 TKI therapy, n (%) | |
| Yes | 17 (51.5) |
| No | 16 (48.5) |
| HER2 alterations, n (%) | |
| Exon 20 insertion mutations | 28 (84.8) |
|
A775_G776insYVMA Non-YVMA insertions* |
20 (60.6) 8 (24.2) |
| TKD missense mutations** | 3 (9.1) |
| Amplification | 2 (6.1) |
*Non-YVMA insertions included P780_Y781insGSP (n = 6), G776delinsVC (n = 1), and G776_V777delinsCVC (n = 1)
**TKD missense mutations included G776V (n = 1) and R811L with Q820K (n = 1) at exon 20, and G727A (n = 1) at exon 18
ECOG PS Eastern Cooperative Oncology Group Performance Status, TKD tyrosine kinase domain
Clinical efficacy
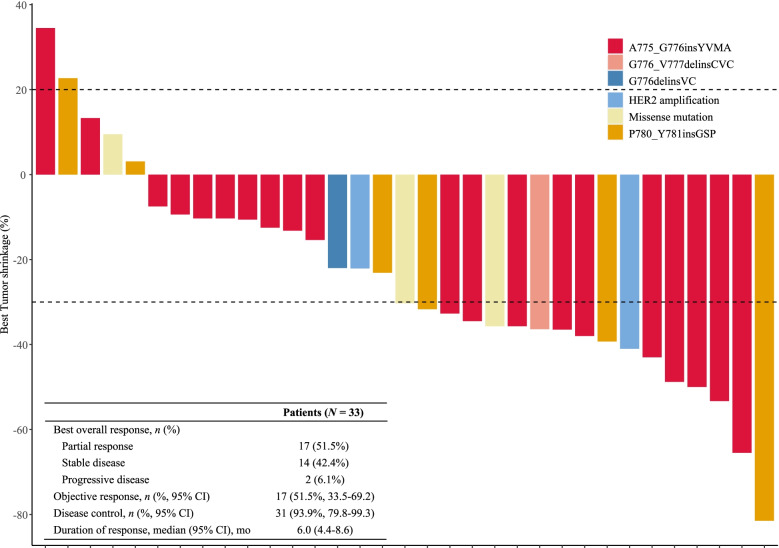
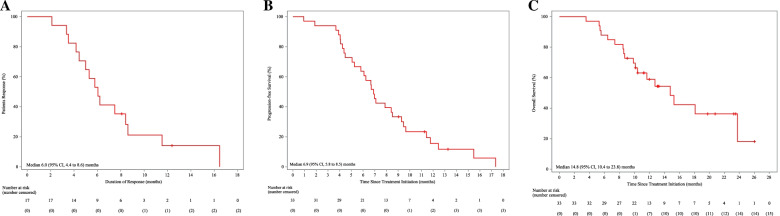
Among the 33 evaluable patients, 17 (51.5%) achieved a PR, and 14 (42.4%) showed SD, with a confirmed investigator-assessed ORR of 51.5% (17/33; 95% CI, 33.5% to 69.2%) and a DCR of 93.9% (31/33; 95% CI, 79.8% to 99.3%). The independent review committee-assessed ORR was 48.5% (16/33, 95% CI 30.8% to 66.5%). In total, 84.8% (28/33) of patients experienced a decrease in target lesion size from baseline (Fig. 1). The median DoR was 6.0 (95% CI, 4.4 to 8.6) months, and the median PFS was 6.9 (95% CI, 5.8 to 8.5) months (Fig. 2A, B). At the time of data cut-off, 18 (54.5%) of 33 patients had died, with a median OS of 14.8 (95% CI, 10.4 to 23.8) months (Fig. 2C) and a 1-year overall survival rate of 58.9% (95% CI, 39.6–73.9%).
Fig. 1.
Waterfall plot of best response
Fig. 2.
Kaplan–Meier estimates of the duration of response (A), progression-free survival (B), and overall survival (C)
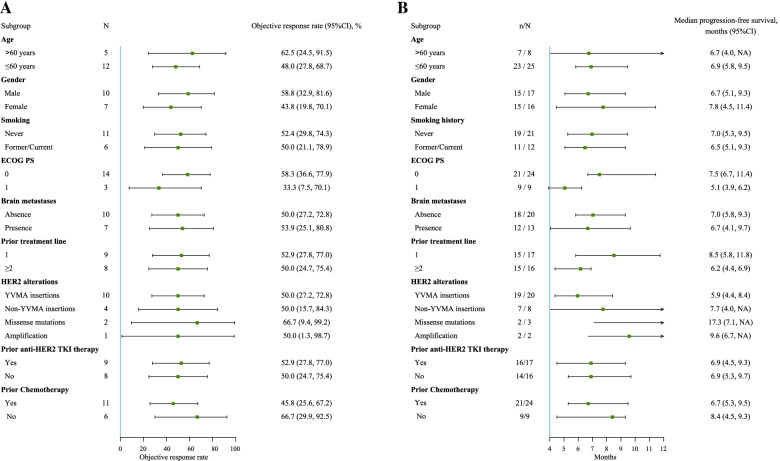
In the post hoc subgroup analyses, benefits in terms of ORR and PFS were observed across all subgroups stratified by patient characteristics (Fig. 3). Similar ORRs were observed when pyrotinib combined with apatinib was used in the second-line (52.9%, 9/17; 95% CI, 27.8 to 77.0%) or third- or above-line settings (50.0%, 8/16; 95% CI, 24.7 to 75.4%), and the median PFS times in the second-line therapy group and in the later-line therapy group were 8.5 months (95% CI, 5.8 to 11.8) and 6.2 months (95% CI, 4.4 to 6.9), respectively. In the absence or presence of baseline brain metastases, the ORR was 50.0% (10/20; 95% CI, 27.2 to 72.8%) versus 53.8% (7/13; 95% CI, 25.1 to 80.8%), and the median PFS times were 7.0 (95% CI, 5.8 to 9.3) months and 6.7 (95% CI, 4.1 to 9.7) months, respectively. Among patients with different HER2 exon 20 insertions, the ORR was 50.0% (95% CI, 27.2 to 72.8%) for those with YVMA insertions and 50.0% (95% CI, 15.7 to 84.3%) for those with non-YVMA insertions; the median PFS times were 5.9 (95% CI, 4.4 to 8.4) months and 7.7 (95% CI, 4.0 to NA) months, respectively.
Fig. 3.
Subgroup analyses of objective response rate (A) and progression-free survival (B)
Safety
All patients experienced any grade of TRAEs, of whom 12.1% had at least one grade 3 TRAE. As summarized in Table 2, the vast majority of TRAEs were grade 1 or 2, with the most common being diarrhea (90.9%), hypertension (72.7%), anorexia (54.5%), nausea (51.5%), and oral mucositis (45.5%). Grade 3 TRAEs were diarrhea (3.0%) and hypertension (9.1%). No grade 4 or 5 TRAEs were reported. One patient (3.0%) discontinued study treatment because of intolerable vomiting and nausea, and 10 patients (30.3%) experienced pyrotinib dose reduction (320 mg once daily) or dose interruption related to TRAEs, including seven with grade 2 diarrhea and one each with grade 3 diarrhea, grade 2 vomiting, and grade 2 nausea and vomiting. Five patients (15.2%) discontinued apatinib treatment owing to grade 3 hypertension, grade 2 hand-foot syndrome, and grade 2 nausea and vomiting.
Table 2.
Treatment-related adverse events by pyrotinib combined with apatinib
| Event | Patients (N = 33) | |
|---|---|---|
| Any grade, n (%) | Grade 3, n (%) | |
| Diarrhea | 30 (90.9) | 1 (3.0) |
| Hypertension | 24 (72.7) | 3 (9.1) |
| Anorexia | 18 (54.5) | 0 (0.0) |
| Nausea | 17 (51.5) | 0 (0.0) |
| Oral mucositis | 15 (45.5) | 0 (0.0) |
| Erythra | 13 (39.4) | 0 (0.0) |
| Vomiting | 10 (30.3) | 0 (0.0) |
| Hand-foot syndrome | 9 (27.3) | 0 (0.0) |
| Abdominal pain | 7 (21.2) | 0 (0.0) |
| Blood creatinine increased | 6 (18.2) | 0 (0.0) |
| Thrombocytopenia | 4 (12.1) | 0 (0.0) |
| Anemia | 3 (9.1) | 0 (0.0) |
| ALT increased | 3 (9.1) | 0 (0.0) |
| AST increased | 3 (9.1) | 0 (0.0) |
| Paronychia | 3 (9.1) | 0 (0.0) |
| Leukopenia | 2 (6.1) | 0 (0.0) |
| Proteinuria | 2 (6.1) | 0 (0.0) |
ALT Alanine aminotransferase, AST Aspartate aminotransferase
Discussion
To our knowledge, this is the first trial to report the efficacy and safety of combination therapy of a HER2-targeted TKI and an antiangiogenic agent in patients with HER2-altered metastatic NSCLC who have failed at least first-line treatment. This phase 2 trial met its primary endpoint, with pyrotinib combined with apatinib yielding a confirmed ORR of 51.5%. In addition, the DCR was 93.9%, median DoR was 6.0 months, median PFS was 6.9 months and median OS was 14.8 months.
As per previous reports, poor antitumor activity was observed with single-agent afatinib, dacomitinib, and neratinib in advanced NSCLC with HER2 mutations, with an ORR of only 3.8%-12.0% and a median PFS of 3–5.5 months [7, 9, 10]. Recently, Elamin and colleagues reported that poziotinib resulted in an ORR of 27%, median PFS of 5.5 months and median OS of 15 months in 30 patients with HER2 exon 20 mutated NSCLC [8]. In patients with advanced HER2-mutated lung adenocarcinoma who had received platinum-based chemotherapy, pyrotinib monotherapy showed an ORR of 30.0%, median PFS of 6.9 months and median OS of 14.4 months [22]. Although cross-trial comparisons should be interpreted with caution, our findings suggest that the combination of pyrotinib and apatinib produced a meaningful improvement in ORR.
In addition to HER2-targeted TKIs, other promising strategies have been explored in HER2-mutated NSCLC. In the MyPathway study, patients with HER2-mutated tumors received trastuzumab plus pertuzumab and had an ORR of 21% [25]. In the IFCT-1703 R2D2 trial, triplet trastuzumab, pertuzumab, and docetaxel yielded an ORR of 29% and a median PFS of 6.8 months in patients with advanced NSCLC harboring HER2 mutations [26]. Additionally, T-DM1 presented an ORR of 44% and a median PFS of 5 months in HER2-mutant lung cancers [14]. DS-8201 produced a 55% ORR and median PFS of 8.2 months in patients with pretreated HER2-mutated NSCLC [15]. It should be noted that 26% of patients treated with DS-8201 developed adjudicated drug-related interstitial lung disease, and two patients died [15]. Unlike the above targeted agents (trastuzumab, T-DM1 and DS-8201), which are administered intravenously, both pyrotinib and apatinib are orally administered, implying a potential improvement in patient compliance.
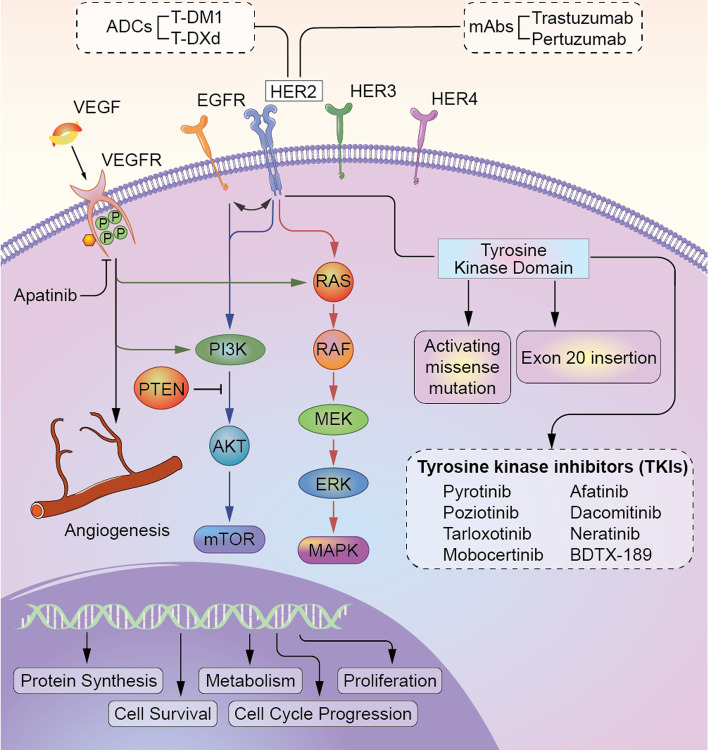
Subgroup analysis showed that comparable responses were observed in patients receiving pyrotinib plus apatinib either as second-line or as third- or above-line therapy, while patients treated with this regimen as second-line therapy had better PFS than those treated with this regimen as third- or above-line therapy (9.8 vs. 6.1 months), which might indicate pyrotinib plus apatinib might be more efficacious in earlier lines for HER2-altered NSCLC patients; however, more studies on this topic are needed. The HER2 receptor is a member of the ErbB family of transmembrane tyrosine kinase receptors, and its dimerization catalytically activates its downstream signaling cascades through the MEK/ERK/MAPK and PI3K/AKT/mTOR pathways and mediates tumor angiogenesis by activating the VEGF signaling pathway (Fig. 4). This might offer a mechanistic basis for the good efficacy in HER2-altered NSCLC patients.
Fig. 4.
Landscape of HER2 signaling pathways and mechanistic basis for angiogenesis inhibitor and HER2-targeted agents in HER2-altered NSCLC
The safety profile of pyrotinib plus apatinib was consistent with that of previous reports for each single agent, without any emerging safety signals identified [22, 27, 28]. The most common TRAEs included diarrhea, hypertension, anorexia, nausea, and oral mucositis, with grades 1–2 being dominant. Four patients experienced grade 3 TRAEs: three experienced hypertension, and one reported diarrhea. All TRAEs were tolerable and well manageable with dose reduction/interruption and symptom-based treatment. Additionally, the low incidence of any-grade proteinuria (6.1%) might be attributed to the low-dose administration of apatinib at 250 mg, which has been proven to be as appropriate and effective as 500 mg and has been applied in numerous clinical studies in NSCLC and other solid tumors [29–31].
Several limitations should be noted in our trial. First, this was a single-center, single-arm, exploratory phase 2 study with a small sample size; thus, selection bias seems unavoidable. Second, only a few patients with HER2 missense mutations or HER2 amplification were included, with no confirmatory information on the clinical efficacy of pyrotinib combined with apatinib in this setting.
Conclusions
Pyrotinib combined with apatinib showed encouraging antitumor activity and an acceptable safety profile in metastatic NSCLC patients with heterogeneous HER2 mutations or amplification, indicating that it might be a potential effective strategy for HER2-mutant or HER2-amplified NSCLC.
Acknowledgements
We thank the patients and the families of the patients who participated in this study. We also thank Li Zhang (a former medical manager) and Yanhua Xu (a medical writer) for medical writing assistance and Lifeng Yin and Xiaowei Ji (two statisticians) for statistical checks from Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Abbreviations
- ADCs
Antibody-drug conjugates
- AEs
Adverse events
- ALK
Anaplastic lymphoma kinase
- CI
Confidence interval
- CNS
Central nervous system
- CR
Complete response
- CT
Computed tomography
- DCR
Disease control rate
- DoR
Duration of response
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EGFR
Epidermal growth factor receptor
- HER2
Human epidermal growth factor receptor 2
- MRI
Magnetic resonance imaging
- NCI-CTCAE
National Cancer Institute-Common Toxicity Criteria for Adverse Events
- NGS
Next-generation sequencing
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PCR
Polymerase chain reaction
- PFS
Progression-free survival
- PR
Partial response
- RECIST 1.1
Response Evaluation Criteria in Solid Tumours, version 1.1
- SD
Stable disease
- TKD
Tyrosine kinase domain
- TKIs
Tyrosine kinase inhibitors
- TRAEs
Treatment-related adverse events
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Authors’ contributions
GY and HX contributed equally to this study as co-first authors. YW and JW has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: GY, HX, and YW. Data acquisition and analysis: GY, HX, YY, SZ, FX, XH1, JL, PX, XH2, YL, LW, LL, ZW, JD, and JW. Data interpretation: GY, HX, and YY. Manuscript drafting: GY and HX. Critical revision of the manuscript: JW and YW. Statistical analysis: GY, HX, YY, and SZ. All authors read and approved the final manuscript.
Funding
This study was supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd, the inventor and producer of pyrotinib and apatinib, and funded by the Beijing Health Promotion Association (Grant No. 2021–053-ZZ).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol was approved by the Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (No.19–062/1847) in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from each participant before the onset of any trial-related treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guangjian Yang and Haiyan Xu contributed equally to this work.
Contributor Information
Jie Wang, Email: zlhuxi@163.com.
Yan Wang, Email: wangyanyifu@163.com.
References
- 1.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–66. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, Campo MJ, Dahlberg SE, Doebele RC, Garon E, Gerber DE, et al. Molecularly targeted therapies in non-small-cell lung cancer annual update 2014. J Thorac Oncol. 2015;10(1 Suppl 1):S1–63. doi: 10.1097/JTO.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910–4918. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 5.Bu S, Wang R, Pan Y, Yu S, Shen X, Li Y, et al. Clinicopathologic characteristics of patients with HER2 insertions in non-small cell lung cancer. Ann Surg Oncol. 2017;24(1):291–297. doi: 10.1245/s10434-015-5044-8. [DOI] [PubMed] [Google Scholar]
- 6.Offin M, Feldman D, Ni A, Myers ML, Lai WV, Pentsova E, et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer. 2019;125(24):4380–4387. doi: 10.1002/cncr.32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dziadziuszko R, Smit EF, Dafni U, Wolf J, Wasąg B, Biernat W, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European Thoracic Oncology Platform (ETOP) J Thorac Oncol. 2019;14(6):1086–1094. doi: 10.1016/j.jtho.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Elamin YY, Robichaux JP, Carter BW, Altan M, Gibbons DL, Fossella FV, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. 2022;40(7):702–709. doi: 10.1200/JCO.21.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554(7691):189–194. doi: 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O'Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26(7):1421–1427. doi: 10.1093/annonc/mdv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna NH, Robinson AG, Temin S, Baker S, Jr, Brahmer JR, Ellis PM, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39(9):1040–1091. doi: 10.1200/JCO.20.03570. [DOI] [PubMed] [Google Scholar]
- 12.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Ding N, Xu X, Zhang Y, Ye M, Li C, et al. Clinical outcomes of patients with HER2-mutant advanced lung cancer: chemotherapies versus HER2-directed therapies. Ther Adv Med Oncol. 2020;12:1758835920936090. doi: 10.1177/1758835920936090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36(24):2532–2537. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 17.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 18.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, et al. Apatinib plus gefitinib as first-line treatment in advanced EGFR-mutant NSCLC: the phase III ACTIVE study (CTONG1706) J Thorac Oncol. 2021;16(9):1533–1546. doi: 10.1016/j.jtho.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther. 2011;131(1):80–90. doi: 10.1016/j.pharmthera.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2(10):727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J Clin Oncol. 2020;38(24):2753–2761. doi: 10.1200/JCO.20.00297. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Xiong Y, Xia Q, Wu F, Liu L, Zhou Y, et al. Efficacy and safety of apatinib plus vinorelbine in patients with wild-type advanced non-small cell lung cancer after second-line treatment failure: a nonrandomized clinical Trial. JAMA Netw Open. 2020;3(3):e201226. doi: 10.1001/jamanetworkopen.2020.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su B, Huang T, Jin Y, Yin H, Qiu H, Yuan X. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021;24(2):352–367. doi: 10.1007/s10120-020-01126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6):536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 26.Mazieres J, Lafitte C, Ricordel C, Greillier L, Pujol JL, Zalcman G, et al. Combination of trastuzumab, pertuzumab and docetaxel in patients with advanced non-small cell lung cancer (NSCLC) harboring HER2 mutation: Final results from the IFCT-1703 R2D2 trial. J Clin Oncol. 2021;39(15_suppl):9015–9015. doi: 10.1200/JCO.2021.39.15_suppl.9015. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P, et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin Cancer Res. 2019;25(17):5212–5220. doi: 10.1158/1078-0432.CCR-18-4173. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 29.Yang G, Xu H, Yang L, Xu F, Zhang S, Yang Y, et al. Apatinib in combination with pemetrexed-platinum chemotherapy for chemo-naive non-squamous non-small cell lung cancer: a phase II clinical study. Lung Cancer. 2020;147:229–236. doi: 10.1016/j.lungcan.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Zhang R, Du N, Yang M, Zang A, Liu L, et al. An open label, multicenter, noninterventional study of apatinib in advanced gastric cancer patients (AHEAD-G202) Ther Adv Med Oncol. 2020;12:1758835920905424. doi: 10.1177/1758835920905424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort A report in a multicenter phase Ib/II trial. J Immunother Cancer. 2021;9(3):e002191. doi: 10.1136/jitc-2020-002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.