Abstract
Background
Abnormal activation of immune system is an important pathogenesis of Parkinson’s disease, but the relationship between peripheral inflammation, central microglia activation and dopaminergic degeneration remains unclear.
Objectives
To evaluate the brain regional microglia activation and its relationship with clinical severity, dopaminergic presynaptic function, and peripheral inflammatory biomarkers related to adaptive immunity.
Methods
In this case–control study, we recruited 23 healthy participants and 24 participants with early-stage Parkinson’s disease. 18F-PBR06 PET/MR for microglia activation, 18F-FP-DTBZ for dopaminergic denervation, total account of T cells and subpopulations of T helper (Th1/Th2/Th17) cells, and the levels of serum inflammatory cytokines were assessed. Sanger sequencing was used to exclude the mix-affinity binders of 18F-PBR06-PET.
Results
Compared to healthy controls, patients with Parkinson’s disease had an increased 18F-PBR06-PET standardized uptake value ratio (SUVR) in the putamen, particularly in the ipsilateral side of the motor onset. 18F-PBR06-PET SUVR was positively associated with 18F-FP-DTBZ-PET SUVR in the brainstem and not associated with disease severity measured by Hoehn and Yahr stage, MDS-UPDRS III scores. Patients with Parkinson’s disease had elevated frequencies of Th1 cells and serum levels of IL10 and IL17A as compared to healthy controls. No significant association between peripheral inflammation markers and microglia activation in the brain of PD was observed.
Conclusion
Parkinson’s disease is associated with early putaminal microglial activation and peripheral phenotypic Th1 bias. Peripheral adaptive immunity might be involved in microglia activation in the process of neurodegeneration in PD indirectly, which may be a potential biomarker for the early detection and the target for immunomodulating therapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-022-02574-z.
Keywords: Parkinson’s disease, Microglia, Cytokine, Th cell, Lymphocyte, Positron emission tomograph
Introduction
The pathophysiology of Parkinson’s disease (PD) results from a complex interplay among α-synuclein aggregation, neuroinflammation, and dysfunction of mitochondria, lysosomes and synaptic transport [1]. The presence of activated microglial cells was observed in the substantia nigra and putamen in postmortem studies of PD, supporting the role of central neuroinflammation [2, 3]. In addition, aberration in peripheral inflammatory cytokine levels and immune cell counts were demonstrated in blood and cerebrospinal fluid from PD patients [4, 5], suggesting the involvement of peripheral innate and adaptive immunity in the degenerative process of PD. However, associations between peripheral inflammation, central microglia activation and dopaminergic degeneration have not been clearly described in PD.
Recent studies have showed the infiltration of T lymphocytes (CD4+ and CD8+) but not B lymphocytes in the affected brain regions in PD [6], and T cells from patients with PD could recognize alpha-synuclein peptides [7]. Adoptive transfer of both T helper 1 (Th1) and Th17 cells significantly potentiated the degree of neurodegeneration in mice model, and the IL-17-producing Th17 cells displayed the highest potency [8]. Moreover, Th17 cells isolated from PD patients could provoke midbrain neurons derived from induced pluripotent stem cells to undergo cell death [9], indicating a key role of T lymphocytes in PD pathogenesis.
The 18-kDa translocator protein (TSPO), is known to be expressed in glia cells including microglia and astrocytes, and can be quantified using positron emission tomography (PET) and thus, can be considered a putative biomarker of microglia activation in vivo [10, 11]. Several studies have observed an increase of TSPO binding in the midbrain, striatum or cortex of PD mainly using the first-generation TSPO tracer 11C-(R)-PK11195 [12–16] or the second-generation tracers 11C-DPA713/18F-DPA714 [17, 18], while some other studies showed a lack of binding differences between PD and healthy controls using the second-generation TSPO tracers 18F-FEPPA or 11C-PBR28 [19–21]. Studies also evaluated the associations between microglia activation and dopaminergic denervation measured by either tracers of dopaminergic transporter or Fdopa, which reported inconsistent results [12, 15, 18, 21].
Taking advantage of lower impact of the rs6971 polymorphism of TSPO gene in the Chinese population, the current study has evaluated (i) the central microglia activation in PD using the second-generation TSPO radioligand 18F-PBR06; (ii) the relationship between central microglia activation, disease severity and dopaminergic denervation; (iii) the associations between the central microglia activation and peripheral inflammatory.
Materials and methods
Participants and clinical assessments
Participants were recruited between September 2019 to January 2021 in Xuanwu Hospital, Capital Medical University. PD participants were diagnosed by movement disorder specialists according to the MDS clinical diagnostic criteria for PD [22]. Patients with dementia, neuropsychiatric disease or receiving anti-inflammation drugs, i.e., aspirin, were excluded. The genotyping of rs6971 polymorphism was carried out with sanger sequencing [23]. Participants carrying the TSPO rs6971 A/G were mixed-affinity binders, while those with G/G, were defined as high-affinity binders. According to the 1000 Genomes Project Phase 3, the proportion of high-affinity binder is around 95% for the Chinese population; thus, only high-affinity binders were included in the finial statistical analysis. The study was approved by the Clinical Research Ethics Board of Xuanwu Hospital and all participants provided written informed consent.
Neurological assessments including Hoehn and Yahr stage, MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Geriatric Depression Scale (GDS), REM Sleep Behavior Disorder Questionnaire-Hong Kong (RBDQ-HK) were performed, the Hoehn and Yahr stage and MDS-UPDRS were preformed in OFF state in PD patients (off medication for at least 12 h). Venous blood samples were obtained from all participants on the same day before 18F-PBR06 PET scan.
Measurement of cytokines in the plasma
Venous blood was centrifuged at 2500×g for 10 min at 4 °C within 30 min of blood collection. The extracted plasma sample was aliquoted and stored at − 80 °C until analysis. V-PLEX Custom Human Biomarkers Kits (K151A9H) were used to measure 11 cytokines simultaneously, according to the manufacturer’s instructions (Meso Scale Discovery, Rockville, MD, USA). Discovery Workbench software (version 4.0.12; Meso Scale Diagnostics, LLC) was used to establish calibration curves and determine cytokine levels as reported previously [24].
Flow cytometric analysis of CD4+ T helper cells in blood
Peripheral blood mononuclear cells were separated using Ficoll density gradient centrifugation within 6 h, and stored in cell cryopreservation box at 4 °C for 30 min and − 80 °C overnight, then transferred to liquid nitrogen for storage. Peripheral blood mononuclear cells were resuscitated in a 37 °C water bath and washed with PBS, Leukocyte Activation Cocktail was added for stimulation and FVS510 to identify dead cells. All the samples were incubated with a cocktail of anti-human CD3, CD4, CD8 antibodies at room temperature for 20 min in the dark, and washed twice with PBS. After washing, BD cytofix/cytoperm fixation/permeabilization solution kit was used to break the membrane and fix the cells for 20 min according to the instructions. IL-4-APC, IFN-γ-FITC and IL-17A-PE were used for intracellular staining, incubated in the dark at room temperature for 20 min. The cells were resuspended in 1% paraformaldehyde. After staining, all samples were collected on the BD Canto II flow cytometer, and analyzed with Flowjo 7.6.1 software. Th cells were delineated by the membranous CD3 and CD4 expression. Th cell subpopulations were separated based on the staining results of IFN-γ, IL-4, and IL-17A: Th1 cells (CD4+ IFN-γ+), Th2 cells (CD4+ IL-4+), and Th17 cells (CD4+ IL-17A+).
Production of radiopharmaceuticals
Two PET imaging tracers were used in the study: 18F-FP-DTBZ for vesicular monoamine transporter 2 (dopaminergic denervation) and 18F-PBR06 for TSPO (microglia activation). Both were prepared from the corresponding tosyl precursors through a one-step nucleophilic substitution fluorination reaction with no-carrier-added 18F, followed by purification with semi-preparative high-performance liquid chromatography [25]. The preparation processes were performed on Neptis perform synthesizer with disposable cassettes. Each tracer was formulated in a mixture of ethanol (< 12%) and 0.5% sodium ascorbate saline solution before final sterilization by 0.22 μm filtration (Millex GV filter, Millipore).
PET/MR scanning
PET/MR scans were performed on a hybrid 3.0-T PET/MR scanner (uPMR790, UIH, Shanghai, China) with a voxel size of 1.82 × 1.82 × 2.78 mm [26]. A single frame was acquired between 60 and 90 min after an intravenous bolus injection of 370 MBq 18F-PBR06. Similarly, a single frame was acquired between 90 and 105 min after an intravenous bolus injection of 222 MBq 18F-FP-DTBZ. The 18F-FP-DTBZ PET/MR scans were performed in OFF state in PD patients. The 18F-FP-DTBZ and 18F-PBR06 scans were performed within a week for all participants. The subject’s head was stabilized with foam pads to reduce motion. During PET data acquisition, two anatomical brain MRI scans were performed. T2-weighted imaging was used for clinical evaluation and exclusion of pathology. 3D T1-weighted imaging was used for co-registration with the PET images and spatial normalization [27].
Image analysis
The PET/MR frames were spatially segmented, co-registered and normalized for each subject using the PNEURO utilities suite. A standardized set of regions of interest was automatically placed on bilateral caudate, putamen, substantia nigra and brainstem using Hammer’s atlas. The putamen was further divided into the anterior dorsal part, anterior ventral part, the posterior dorsal part, and the posterior ventral part. Regional standardized uptake value ratio (SUVR) for 18F-PBR06-PET was calculated for each participant based on normalization of the individual region’s SUV to the global brain SUV [28, 29]. To assess the appropriateness of using global SUV as a reference for normalization, global SUV was compared between the PD and HC groups and showed no significant difference (1.93 ± 0.54 vs. 2.00 ± 0.77, p = 0.751). Regional SUVR for 18F-FP-DTBZ-PET were calculated for each participant using the occipital cortex as reference. Due to the asymmetric feature of neurodegeneration in PD, regional analysis was applied in which ipsilateral and contralateral sides were referred to the hemisphere associated with first onset of clinical symptoms.
Statistical analyses
Independent two-tailed T-test was used for comparisons of age, MDS-UPDRS III scores, MMSE, and MoCA between PD and control, and a binomial variable with Fisher’s exact test was used for gender. GDS and RBDQ-HK were compared using Mann–Whitney test. The SUVR of 18F-PBR06-PET was analyzed using independent two-tailed t test and covariance was adjusted for age and gender. We compared the level or percentages of the peripheral markers using T-test or Mann–Whitney test. Linear regressions were applied for correlation between 18F-PBR06-PET SUVR and disease duration, MDS-UPDRS and 18F-FP-DTBZ-PET SUVR. Pearson’s or Spearman correlation was applied between 18F-PBR06-PET SUVR and peripheral inflammatory makers depending on the distributions. P values less than 0.05 were set as significantly different. The statistical analysis was done with SPSS for Windows, version 19.0 (SPSS, Chicago, IL, USA). Multiple comparisons were corrected by controlling the false discovery rate using the Benjamini–Hochberg procedure.
Results
Clinical characteristics
Among 24 PD participants and 23 healthy controls recruited, only 2 PD and 1 healthy control were mix-affinity binders for the rs6971 polymorphism of TSPO gene and were excluded for analysis. The demographic and clinical characteristics are shown in Table 1. No differences in age and gender were noted between PD patients and controls, and no adverse events occurred during the PET scans. The PD patients were in the early disease stage with an average disease duration 2.5 years (30.18 ± 16.59 months) after any self-reported motor symptoms and a Hoehn and Yahr stage of two (1.82 ± 0.57). No between-group differences were found in GDS, MMSE, MoCA or RBDQ-HK (Table 1).
Table 1.
Demographic and clinical features
| Healthy control | Parkinson’s disease | P | Healthy control | Parkinson’s disease | |
|---|---|---|---|---|---|
| High-affinity binders | Mix-affinity binders | ||||
| No. | 22 | 22 | – | 1 | 2 |
| Age | 58.91 ± 9.78 | 59.27 ± 11.96 | 0.913 | 59 | 60, 66 |
| Gender (M/F) | 10/12 | 15/7 | 0.128 | 0/1 | 1/1 |
| MDS-UPDRS III scores | 0.41 ± 1.33 | 21.23 ± 10.14 | < 0.0001 | 0 | 11, 5 |
| GDS | 6.09 ± 6.29 | 6.27 ± 5.44 | 0.680a | 2 | 7, 16 |
| MMSE | 28.45 ± 1.79 | 26.95 ± 6.35 | 0.236 | 30 | 28, 30 |
| RBDQ-HK | 6.95 ± 8.28 | 12.73 ± 14.71 | 0.163a | 5 | 4, 9 |
| LEDD | – | 508.33 ± 239.7 | – | – | 0, 0 |
| Dose (18F-FP-DTBZ) | 283.82 ± 60.34 | 298.00 ± 67.01 | 0.469 | 346 | 267, 253 |
| Dose (18F-PRB06) | 348.14 ± 19.61 | 353.35 ± 21.54 | 0.430 | 360 | 373, 332 |
GDS Geriatric Depression Scale, MDS-UPDRS III MDS-Unified Parkinson’s Disease Rating Scale motor scores, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, P p-value, RBDQ-HK REM Sleep Behavior Disorder Questionnaire-Hong Kong, SUVR standardized uptake value ratio
aCalculated with Mann–Whitney test
Increase of microglia activation in the basal ganglia of PD
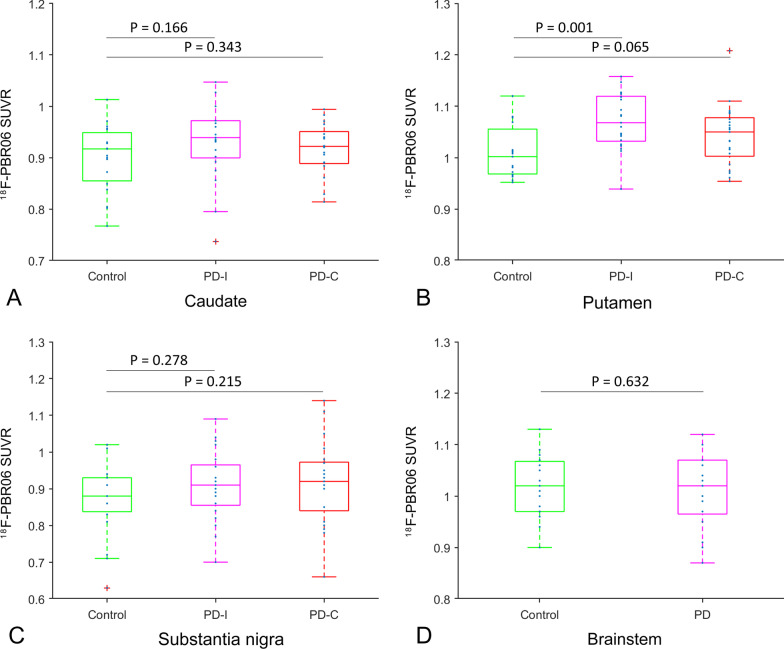
Comparing to healthy controls, the 18F-PBR06-PET SUVR was higher in the putamen, but statistically only on the ipsilateral to the side first observed motor symptoms of PD (Fig. 1). The increase of 18F-PBR06 SUVR remained significant after controlling for age and gender and survived the correction for multiple comparisons (Additional file 1: Table S1). No differences were observed in the caudate nucleus or substantia nigra (Fig. 1). Further post hoc analyses on the putaminal subregions showed significant increase of the 18F-PBR06-PET SUVR in all subregions of putamen on the ipsilateral side but only in the posterior ventral putamen on the contralateral side (Additional file 1: Table S2).
Fig. 1.
Neuroinflammation in striatum subregions, substantia nigra and brainstem. The SUVR of 18F-PBR06 between patients with Parkinson’s disease and healthy controls in the A caudate, B putamen, C substantial nigra, D brainstem. CContralateral side to the onset side of motor symptoms. I Ipsilateral side to the onset side of motor symptoms. PD Parkinson’s disease, SUVR standardized uptake value ratio
Association between brain microglia activation, disease severity and dopaminergic presynaptic disruption
No significant correlations were found between 18F-PBR06-PET SUVR, disease severity as measured by Hoehn and Yahr stage and MDS-UPDRS III scores in all subregions of striatum and substantia nigra (Table 2). However, the 18F-PBR06-PET SUVR of brainstem was negatively associated with MDS-UPDRS III score (R = − 0.452, P = 0.040), and positively associated with dopaminergic function measured by 18F-FP-DTBZ SUVR.
Table 2.
Linear regressions between microglia activation, disease duration, UPDRS III, and dopaminergic disruption in PD
| VOI | Side | Duration | Hoehn and Yahr stages | MDS-UPDRS III scores | 18F-FP-DTBZ SUVR | |
|---|---|---|---|---|---|---|
| Caudate | Ipsilateral | R | − 0.163 | 0.003 | − 0.288 | − 0.042 |
| P | 0.480 | 0.990 | 0.206 | 0.862 | ||
| Putamen | R | − 0.184 | 0.398 | 0.412 | 0.204 | |
| P | 0.424 | 0.074 | 0.063 | 0.389 | ||
| Substantia nigra | R | 0.007 | − 0.206 | − 0.403 | 0.148 | |
| P | 0.975 | 0.370 | 0.070 | 0.533 | ||
| Caudate | Contralateral | R | − 0.019 | − 0.023 | − 0.366 | − 0.141 |
| P | 0.935 | 0.920 | 0.103 | 0.553 | ||
| Putamen | R | − 0.138 | 0.421 | 0.349 | 0.124 | |
| P | 0.551 | 0.058 | 0.121 | 0.603 | ||
| Substantia nigra | R | − 0.194 | 0.017 | − 0.400 | 0.187 | |
| P | 0.399 | 0.940 | 0.072 | 0.430 | ||
| Brainstem | R | 0.269 | − 0.140 | − 0.452 | 0.688 | |
| P | 0.238 | 0.544 | 0.040* | 0.001* | ||
R correlation coefficient, P p-value, MDS-UPDRS III MDS-Unified Parkinson’s Disease Rating Scale motor scores, SUVR standardized uptake value ratio
*P < 0.05
Peripheral inflammation in PD
The count of total T cells, and frequencies of Th2 or Th17 cells among T cells in blood did not differ between PD patients and healthy participants, while the frequencies of Th1 cells among total T cells was increased in PD comparing to controls (12.85 ± 3.19% vs. 9.81 ± 2.42%, P = 0.008), Th2 cells slightly decreased and Th17 cells increased, respectively (Table 3). For the cytokines, levels of cytokines IL17A (0.577 ± 0.352 vs. 0.395 ± 0.204, P = 0.045) and IL10 (0.202 ± 0.195 vs. 0.083 ± 0.052, P = 0.002) were significantly higher in patients with PD than that of healthy controls, and the increase of IL10 level survived the correction for multiple comparisons.
Table 3.
Peripheral inflammation in the blood of PD patients and healthy controls
| Control n = 22 |
PD n = 22 |
P | |
|---|---|---|---|
| Total T cells/lymphocytes | 73.24% ± 8.78% | 72.95% ± 7.47% | 0.976 |
| Th1/total T cells | 9.81% ± 2.42% | 12.85% ± 3.19% | 0.008* |
| Th2/total T cells | 3.25% ± 2.51% | 3.15% ± 1.46% | 0.842 |
| Th17/total T cells | 1.65% ± 0.79% | 2.10% ± 1.53% | 0.743 |
| IFNγ | 6.30 ± 2.63 | 5.62 ± 2.35 | 0.375 |
| TNFα | 1.88 ± 0.38 | 1.92 ± 0.42 | 0.707 |
| IL6 | 0.746 ± 0.462 | 0.659 ± 0.432 | 0.343a |
| IL8 | 7.02 ± 2.86 | 7.89 ± 2.82 | 0.320 |
| IL10 | 0.083 ± 0.052 | 0.202 ± 0.195 | 0.002*a |
| IL12p70 | 0.078 ± 0.117 | 0.070 ± 0.049 | 0.762 |
| IL22 | 0.605 ± 0.652 | 0.440 ± 0.354 | 0.921a |
| IL17A | 0.395 ± 0.204 | 0.577 ± 0.352 | 0.045* |
P p-value, PD Parkinson’s disease, Th T helper cells
*P < 0.05
aCalculated with Mann–Whitney test
Associations between peripheral inflammation and brain microglia activation in PD
In general, there was no significant association between levels of microglia activation in the brain and peripheral inflammation markers. Associations were noted within the 18F-PBR06 SUVR of regions in the central nervous system, and within the levels of peripheral markers, but not in-between them (Additional file 1: Fig. S1).
Discussion
Using 18F-PBR06-PET SUVR, a marker of TSPO mainly representing brain microglia activation, the current study found increased regional microglia activation in the putamen and relatively increased blood subpopulation of Th1 lymphocytes and levels of IL10 and IL17A cytokines in patients with early PD. The increase of activated microglia was more prominent in the ipsilateral side of the motor onset, and not associated with disease severity measured by Hoehn and Yahr stage, MDS-UPDRS III scores, and dopaminergic terminal density. No significant association between peripheral inflammation markers and microglia activation in the brain of PD was observed. Our data support the notion that adaptive immunity is involved in the early phase of neurodegenerative processes.
TSPO-PET has been deemed a surrogate marker of neuroinflammation levels since the level of TSPO significantly increase in microglia upon activation. Two generations of TSPO tracers were developed to date. The first-generation TSPO tracer 11C-PK111195 was the most widely used so far, but suffered from non-specific bindings and low brain uptake. Several second-generation tracers (11C-PBR28, 18F-PBR06, 11C-DPA713, 18F-DPA714, 18F-FEPPA) was developed later with better binding potential to TSPO, but the uptake of the tracers was soon found to be significantly influenced by the rs6971 polymorphism of TSPO [23]. Interestingly, studies with the application of the first-generation TSPO tracer 11C-PK111195 showed an increase of microglia activation in the brain of PD patients uniformly [12–16], while recent findings of studies with the second-generation TSPO tracers were less consistent [17–21]. One possible explanation is that approximately 40–45% of the Caucasian population are high-affinity binders and 40–45% are median-affinity binders, which significantly dilute the power of statics analysis. Taking advantage that the difference on rs6971 polymorphism is rare in Asian populations, we have observed an increase of microglia activation in the putamen of PD patients in a genetically homogeneous group of participants for TSPO polymorphism in this study. Our results further support the involvement of microglia activation in early PD using the second-generation TSPO tracer.
Microglia activation was found selectively increased in the putamen of early-stage PD patients, particularly in the potentially mild lesion side ipsilateral to the motor onset. Moreover, the increased microglia activation in the putamen was not associated with disease severity demonstrated by neither clinical symptoms nor loss of dopaminergic terminals. Recent multimodal PET studies showed that while microglial activation was increased in brain regions such as midbrain, frontal cortex, and putamen, the regional activation levels did not correlate with the severity of motor symptoms, disease duration nor dopaminergic uptake [15, 18, 21], inconsistently, one study showed parallel changes in microglial activation and corresponding dopaminergic terminal loss [12]. It appears to suggest that the regional level of microglia activation may be an early event during neurodegeneration rather than a marker of disease progression. The initiation of microglia activation can be pre-manifesting, since evidence suggests the presence of microglia activation in the prodromal phase of PD. Stokholm et al. has reported the elevation of microglia activation in the substantial nigra of individuals with high risk for development of PD, including REM sleep behavior disorders and non-manifesting carriers with LRRK2 G2019S mutation [30, 31]. Similarly, Mullin et al. reported increased microglia activation in the substantial nigra of carriers with GBA mutations [32]. Consistently, we found 18F-PBR06 SUVR in the brainstem was positively correlated with local 18F-FP-DTBZ SUVR (Table 2). However, whether the microglia activation is neuroprotective or detrimental can not be answered due to the inability to distinguish the proinflammatory M1 phenotype of microglia and the anti-inflammatory M2 microglia with TSPO-PET, as discussed by Stokholm et al. and Mullin et al. [30, 32].
The proinflammatory phenotypes, such as Th1 and Th17 cells, promote inflammation by cytokine production, while Th2 and T regulatory cells help B lymphocytes can alleviate inflammation [33]. A number of studies have demonstrated a decreased number of CD3+ and CD4+ Th lymphocytes and increased serum levels of IL-6, TNF-α, IL-1β, IL-2, and IL-10 in PD [4, 5]. Consistent with our results, a previous comprehensive study on the phenotypic and functional profile of CD4+ T cell subsets in peripheral blood of early PD found a reduced circulating Th2, Th17, Th1/17, and Treg leading to a relative increase of Th1 cells, a complex Th1-biased adaptive immune response [34]. This alteration independent from PD progression and severity suggests a systematic proinflammatory status and the contribution of Th1-related mechanisms to neuroinflammation and neurodegeneration in PD. We also found an elevation of frequencies of Th17 cells and serum level of proinflammatory IL17A and significant increase in serum level of anti-inflammatory IL-10, in agreement with the findings of increased serum levels of IL-6, TNF-α, IL-1β, IL-2, and IL-10 in PD patients from a meta-analysis of 25 studies and 2654 participants [5]. Our results, also confirmed the involvement of IL17A cytokine in the pathogenesis of PD which was reported in MPTP-injected mice model and midbrain neuron model derived from induced pluripotent stem cells [8, 9]. However, while the increase of both central microglia activation and peripheral inflammation markers was noted, no significant correlation was found between central and peripheral inflammation markers in the study. A trend of positive association was noted between the level of proinflammatory markers (IFNγ, TNFα, IL6, IL8 and IL17A) and putaminal microglia activation (Additional file 1: Fig. S1), but none of the association could survive the corrections for multiple comparisons. The lack of association was inconsistent with previous studies [35–37], possibly due to the limited number of PD participants enrolled and global normalization.
There are several limitations of the current study. Most TSPO tracers are well described with a two-tissue compartmental model using an arterial input function [38]. However, acquisition of blood samples required for a plasma input function modeling approach which could not be done in our center, thus, a clinically feasible PET quantitation measure SUVR with the average global brain SUV as a reference was adopted [29]. This method was supported by evidence that the distribution volume of 18F-PBR06 remained stable during 60–120 min [39], and the test–retest reliability of the SUVR approach was high [28, 29, 39, 40]. Furthermore, the global SUV was generally used to compared between patient and control which was not significant different in our study. We agree that caution should be exercised when interpreting the meaning of the TSPO SUVR in a relative regional activation of microglia compared to the entire brain. However, PD is a disease with relatively focal degeneration on the nigra-striatum regions. Another limitation was that the VOI of substantia nigra was segmented automatically with an atlas build in the MNI space. The procedure was challenging due to the small size of the anatomical structure of substantia nigra, which could introduce potential noise to the SUVR. However, the normalization of PET/MR data was closely monitored to ensure the location of substantia nigra of each individual in the MNI space, and the bias from researchers could be reduced with an atlas-based automatic VOIs comparing to VOIs drawn manually. A third limitation was that while TSPO-PET imaging is widely applied as a non-invasive marker of neuroinflammation in vivo, there are questions about specificity of elevated TSPO to activated microglia. The protein is known to be expressed by multiple cell types such as astrocytes [41]. Future development of tracers specifically targeting microglia activation is required to enable pivotal studies elucidating the role of neuroinflammation in disease onset and progression.
Conclusions
Our results suggest microglial activation in the putamen and peripheral phenotypic Th1 bias is a characteristic of early PD. Adaptive immunity might be involved in microglia activation in the process of neurodegeneration in PD, which may be a potential biomarker for the early detection and the target for immunomodulating therapy.
Supplementary Information
Additional file 1: Table S1. The 18F-PBR06 SUVR between PD and healthy controls. Table S2. Microglia activation in the subregions of putamen in PD and control. Figure S1. Associations between peripheral inflammation and brain microglia activation.
Acknowledgements
We would like to thank all study participants and Fang-Wei Qiao, Cai-Yun Qi for clinical data collection in the study. We are also grateful for the help of Qiu-Xia Wang in the process of PBMC.
Abbreviations
- GDS
Geriatric Depression Scale
- MDS-UPDRS III
MDS-Unified Parkinson’s Disease Rating Scale motor scores
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson’s disease
- RBDQ-HK
REM Sleep Behavior Disorder Questionnaire-Hong Kong
- SUVR
Standardized uptake value ratio
- Th
T helper
- TSPO
18-kDa translocator protein
Author contributions
SYL, HWQ and PC: study concept and design. SYL and XLL: clinical data collection. YXY, CSZ and YNC: blood sample preprocessing and cytokines analysis. CSZ: sequencing and analysis of gene results. SLX: flow cytometric analysis. HWQ, TBS and GT: imaging data collection. SYL and OB: imaging data analysis. SYL: manuscript writing. VS, JL and PC: manuscript revision for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81901285, 81701726) and the Ministry of Science and Technology of China (2021YFC2501200; 2018YFC1312001).
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
All participants provided informed consent and the study was approved by the Clinical Research Ethics Board of Xuanwu Hospital.
Consent for publication
The paper has not been published previously and is not under consideration for publication elsewhere. All coauthors have read and approved the submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shu-Ying Liu and Hong-Wen Qiao contributed equally to this work
Contributor Information
Shu-Ying Liu, Email: liu_shuy@xwhosp.org.
Piu Chan, Email: pbchan@hotmail.com.
References
- 1.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 2.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/WNL.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 3.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S, Gao H, Luo Q, Wang P, Yang X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: a meta-analysis. Neurol Sci. 2017;38(8):1373–1380. doi: 10.1007/s10072-017-2988-4. [DOI] [PubMed] [Google Scholar]
- 5.Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 2016;73(11):1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 6.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. I Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Investig. 2009;119(1):182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature. 2017;546(7660):656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184(5):2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer A, Maxreiter F, Krach F, Fadler T, Grosch J, Maroni M, et al. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell. 2018;23(1):123–31.e6. doi: 10.1016/j.stem.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhang PF, Gao F. Neuroinflammation in Parkinson’s disease: a meta-analysis of PET imaging studies. J Neurol. 2022;269(5):2304–2314. doi: 10.1007/s00415-021-10877-z. [DOI] [PubMed] [Google Scholar]
- 11.Vivash L, O'Brien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57(2):165–168. doi: 10.2967/jnumed.114.141713. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57(2):168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 13.Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, et al. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):47–52. doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, et al. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology. 2013;38(6):938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21(2):404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Kang Y, Mozley PD, Verma A, Schlyer D, Henchcliffe C, Gauthier SA, et al. Noninvasive PK11195-PET image analysis techniques can detect abnormal cerebral microglial activation in Parkinson’s disease. J Neuroimaging. 2018;28(5):496–505. doi: 10.1111/jon.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terada T, Yokokura M, Yoshikawa E, Futatsubashi M, Kono S, Konishi T, et al. Extrastriatal spreading of microglial activation in Parkinson’s disease: a positron emission tomography study. Ann Nucl Med. 2016;30(8):579–587. doi: 10.1007/s12149-016-1099-2. [DOI] [PubMed] [Google Scholar]
- 18.Lavisse S, Goutal S, Wimberley C, Tonietto M, Bottlaender M, Gervais P, et al. Increased microglial activation in patients with Parkinson disease using [(18)F]-DPA714 TSPO PET imaging. Parkinsonism Relat Disord. 2021;82:29–36. doi: 10.1016/j.parkreldis.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Ghadery C, Koshimori Y, Coakeley S, Harris M, Rusjan P, Kim J, et al. Microglial activation in Parkinson’s disease using [(18)F]-FEPPA. J Neuroinflamm. 2017;14(1):8. doi: 10.1186/s12974-016-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshimori Y, Ko JH, Mizrahi R, Rusjan P, Mabrouk R, Jacobs MF, et al. Imaging striatal microglial activation in patients with Parkinson’s disease. PLoS ONE. 2015;10(9):e0138721. doi: 10.1371/journal.pone.0138721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varnäs K, Cselényi Z, Jucaite A, Halldin C, Svenningsson P, Farde L, et al. PET imaging of [(11)C]PBR28 in Parkinson's disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding. Eur J Nucl Med Mol Imaging. 2019;46(2):367–375. doi: 10.1007/s00259-018-4161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 23.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32(1):1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Wang T, Li Y, Mao W, Hao S, Huang Z, et al. Plasma immune markers in an idiopathic REM sleep behavior disorder cohort. Parkinsonism Relat Disord. 2020;78:145–501. doi: 10.1016/j.parkreldis.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Gao M, Miller KD, Zheng QH. Synthesis of [11C]PBR06 and [18F]PBR06 as agents for positron emission tomographic (PET) imaging of the translocator protein (TSPO) Steroids. 2011;76(12):1331–1340. doi: 10.1016/j.steroids.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Shan Y, Wang Z, Song S, Xue Q, Ge Q, Yang H, et al. Integrated positron emission tomography/magnetic resonance imaging for resting-state functional and metabolic imaging in human brain: what is correlated and what is impacted. Front Neurosci. 2022;16:824152. doi: 10.3389/fnins.2022.824152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XL, Liu SY, Barret O, Tamagnan GD, Qiao HW, Song TB, et al. Diagnostic value of striatal 18F-FP-DTBZ PET in Parkinson’s disease. Front Aging Neurosci. 2022;14:931015. doi: 10.3389/fnagi.2022.931015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair A, Veronese M, Xu X, Curtis C, Turkheimer F, Howard R, et al. Test–retest analysis of a non-invasive method of quantifying [11C]-PBR28 binding in Alzheimer’s disease. EJNMMI Res. 2016;6(1):72. doi: 10.1186/s13550-016-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singhal T, O'Connor K, Dubey S, Belanger AP, Hurwitz S, Chu R, et al. 18F-PBR06 versus 11C-PBR28 PET for assessing white matter translocator protein binding in multiple sclerosis. Clin Nucl Med. 2018;43(9):e289–e295. doi: 10.1097/RLU.0000000000002179. [DOI] [PubMed] [Google Scholar]
- 30.Stokholm MG, Iranzo A, Ostergaard K, Serradell M, Otto M, Svendsen KB, et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case–control study. Lancet Neurol. 2017;16(10):789–796. doi: 10.1016/S1474-4422(17)30173-4. [DOI] [PubMed] [Google Scholar]
- 31.Stokholm MG, Garrido A, Tolosa E, Serradell M, Iranzo A, Østergaard K, et al. Imaging dopamine function and microglia in asymptomatic LRRK2 mutation carriers. J Neurol. 2020;267(8):2296–2300. doi: 10.1007/s00415-020-09830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullin S, Stokholm MG, Hughes D, Mehta A, Parbo P, Hinz R, et al. Brain microglial activation increased in glucocerebrosidase (GBA) mutation carriers without Parkinson’s disease. Mov Disord. 2021;36(3):774–779. doi: 10.1002/mds.28375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Punt J, Stranford SA, Jones PP, Owen JA. Kuby immunology. 8. New York: W. H. Freeman and Company; 2018. [Google Scholar]
- 34.Kustrimovic N, Comi C, Magistrelli L, Rasini E, Legnaro M, Bombelli R, et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflam. 2018;15(1):205. doi: 10.1186/s12974-018-1248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farmen K, Nissen SK, Stokholm MG, Iranzo A, Østergaard K, Serradell M, et al. Monocyte markers correlate with immune and neuronal brain changes in REM sleep behavior disorder. Proc Natl Acad Sci USA. 2021;118(10):e2020858118. doi: 10.1073/pnas.2020858118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanegawa N, Collste K, Forsberg A, Schain M, Arakawa R, Jucaite A, et al. In vivo evidence of a functional association between immune cells in blood and brain in healthy human subjects. Brain Behav Immun. 2016;54:149–157. doi: 10.1016/j.bbi.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Surendranathan A, Su L, Mak E, Passamonti L, Hong YT, Arnold R, et al. Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain. 2018;141(12):3415–3427. doi: 10.1093/brain/awy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura Y, Zoghbi SS, Simeon FG, Taku A, Pike VW, Innis RB, et al. Quantification of translocator protein (18 kDa) in the human brain with PET and a novel radioligand, (18)F-PBR06. J Nucl Med. 2009;50(7):1047–1053. doi: 10.2967/jnumed.108.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herranz E, Louapre C, Treaba CA, Govindarajan ST, Ouellette R, Mangeat G, et al. Profiles of cortical inflammation in multiple sclerosis by (11)C-PBR28 MR-PET and 7 Tesla imaging. Mult Scler. 2020;26(12):1497–1509. doi: 10.1177/1352458519867320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavisse S, Guillermier M, Hérard A-S, Petit F, Delahaye M, Camp NV, et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. 2012;32(32):10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The 18F-PBR06 SUVR between PD and healthy controls. Table S2. Microglia activation in the subregions of putamen in PD and control. Figure S1. Associations between peripheral inflammation and brain microglia activation.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.