Abstract
Introduction
Ureteral stone is a worldwide disease and accounts for 20% of all urolithiasis. There is a widespread discussion on the preferred initial treatment method, whether medical or surgical, and each has its pros and cons. In this study, we aimed to assess the role of both ureteral wall thickness around the stone and inflammatory markers in guiding the decision-making process.
Methods
In this prospective study, 161 patients who presented with ureteric colic and were diagnosed with ureteral stone with NCCT were included. UWT around the stone was measured, and the NLR and PLR were calculated. The patients were given a single daily dose of tamsulosin 0.4 mg for 4 weeks with weekly follow-up to determine SSP or failure.
Results
Of the 161 patients with a mean age 40.12 ± 12.36 SD, 55.9% had a spontaneous stone passage. Receiver operating characteristics showed a cut off value of 2.45 mm UWT of non SSP patients with an 83% sensitivity and 86% specificity. Moreover, there was a significant correlation between higher NLR, PLR and increased UWT (Pearson correlation of 0.314 and 0.426 respectively). The combined higher NLR, PLR and increased UWT were associated with failure of SSP (p-value <0.001).
Conclusion
Many factors play a role in decision making for management of ureteral stones. Our study concludes that patients with high NLR, PLR, and UWT around the stone have lesser chance of SSP using MET. Their rise can be used as predictors to decide early intervention.
Keywords: Medical expulsive therapy, Urolithiasis, Ureteral stone passage, Inflammatory serum markers, Ureteral wall thickness
Highlights
-
•
Ureteral stone is a worldwide disease and accounts for 20% of all urolithiasis.
-
•
Impacted ureteral stones occupy the majority of emergency department visits due to urolithiasis.
-
•
The role of inflammatory serum markers and UWT around the stone on spontaneous passage are controversial.
1. Introduction
Urolithiasis is a worldwide common disease with an incidence as high as 20%. The prevalence of ureteral stones has increased over the past years, and it constitutes 20% of all urolithiasis [1,2]. Impacted ureteral stones occupy the majority of emergency department visits due to urolithiasis and causes a high economic burden to the health system [3]. This burden together with the rising incidence of ureteral stones, encourages a vigilant treatment plan, including medical expulsive therapy and timing of intervention [4]. Surgical intervention for ureteral stones could be regarded as overtreatment and adds extra costs because guidelines state that patients with uncomplicated ureteral stones smaller than 10 mm can be offered conservative management, including watchful waiting or medical expulsive therapy for 4 weeks. On the other hand, watchful waiting could have undesirable outcomes like urosepsis and renal impairment [[5], [6], [7]]. However, it is controversial which patient would likely benefit from conservative management or immediate intervention. For that reason, it is imperative to identify the factors predicting spontaneous ureteral stone passage or stone-related complications during the period of conservative treatment [8]. Distally located small-sized stones in the ureter have been found in most studies as a predictor of spontaneous stone passage [9]. Identifying ureteral wall thickness around the stone using CT scan and raised inflammatory serum marker, which could reflect the inflammation and impaction of the stone in the ureter, have also been investigated as parameters to predict SSP. However, their role is controversial [[10], [11], [12], [13], [14], [15]]. Some authors suggested that high leucocyte count is an indicator for stone passage, hypothesizing that stone passage causes ureteral wall inflammation in comparison to the static one [12]. On the contrary, other investigators found a high leucocyte count in correspondence to a low rate of spontaneous ureteral stone passages or even early intervention during MET [13,14]. Furthermore, a more recent study did not show a significant association between spontaneous ureteral stone passage and WBC count [15.16]. In the present study, we aimed to assess the role of both ureteral wall thickness around the stone and inflammatory serum markers in predicting SSP of <10 mm ureteral stones.
2. Methods
2.1. Registration
The current study was registered in accordance with the Helsinki declaration – “Every research study involving human subjects must be registered in a publicly accessible database before recruitment of the first subject”. The study was registered in the Research Registry with a registration number of (8072). The link ishttps://www.researchregistry.com/register-now#home/registrationdetails/62c2c16dda82dc001e5989dd/
2.2. Setting and study design
A prospective analysis was performed for 176 patients from January 2021 to December 2021 who presented with acute ureteric colic to the emergency unit. It was written in line with STROCSS 2021 guidelines [17].
2.3. Ethical considerations
The study was performed following the approval of the Ethical Committee from the Kurdistan Board for medical specialties (No 1135). Consent was taken from all participants.
2.4. Inclusion and exclusion criteria
Patients with a radiological diagnosis of a ureteric stone of ≤10 mm on a non–contrast-enhanced Computerized Tomography (NCCT), willing to receive medical expulsive therapy and further follow up, were included in the study. However, patients with multiple ureteral stones, concurrent renal stones, solitary kidneys, associated chronic inflammatory conditions, taking, positive urine culture, high fever on presentation, renal impairment, and patients who were lost in the follow-up were excluded from the study. Based on that, 15 participants were excluded, and the data of only 161 patients were analyzed.
2.5. Data collection
Demographic data such as age, gender, and body mass index (BMI) were collected. A medical history of diabetes mellitus and hypertension, as well as a history of previous ureteral stone passages were obtained.
2.6. Diagnostic assessment
During the acute phase, urine and blood samples were collected, and their results with radiographic examinations were recorded. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were then calculated as inflammatory markers.
The NCCT provided site (proximal, mid and distal), size (was defined by the stone's largest diameter), density (Hounsfield unit), degree of hydronephrosis (no, mild, moderate and severe using Onen classification [18]) and ureteral wall thickness (UWT) around the stone.
Axial NCCT images of 5 mm thickness slices with soft-tissue radiodensity (a window width of 360, a pitch of 1.5, a tube voltage of 120 kV and a tube current of 70–90 mAs were the setting parameters) were used to evaluate the stone and its surrounding tissue. The UWT was calculated by locating the point with the greatest soft-tissue thickness (ureteral wall ± peri-ureteral edema) around the circumference of the stone.
2.7. Management procedure
The patients were put on a daily single dose of tamsulosin 0.4 mg for four weeks with a regular weekly interval visit to check for spontaneous ureteral stone passage or stone-related complications. Failure of passage was defined as the presence of the stone on NCCT at the end of the 4 weeks or urgent intervention by drainage, shockwave lithotripsy, or URS due to stone-related complications within the period.
2.8. Statistical analysis
Data were analyzed using the Statistical Package for the social sciences, version 21.0 (IBM corporation, Armonk, NY). The p-value of <0.05 was regarded as significant. The clinical variables were compared using Chi-square or an independent t-test. Univariate and multivariate analysis was performed and the Odds ratio was calculated to assess the association of variables with the stone passage.
Receiver operator curve (ROC) analysis for the area under the curve (AUC) values was done to derive the cut-off values for UWT in non-spontaneous stone passage patients. Pearson correlation was performed for the association of UWT with both PLR and NLR. Manova test was used for the relation between NLR, PLR, and ureteral wall thickness for ureteral stone passage.
3. Results
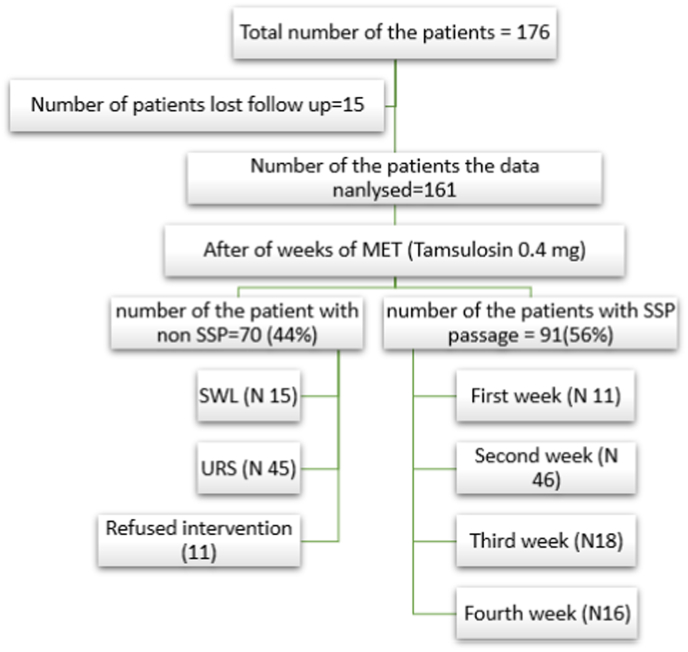
Of the 176 cases that fulfilled the inclusion criteria, 15 patients were excluded due to loss of follow-up. The mean age of the patients was 40.12 ± 12.36 SD, with the range being (17–78). Eighty-seven patients (54%) were female, and 74 patients (46%) were males. Fig. 1 shows the follow-up flow chart of the patients. The comparison of demographic data, patients, and stones characteristics between the SSP and non-SSP are mentioned in Table 1. Neutrophil, platelet, NLR and PLR were significantly higher in the non SSP patients with a p-value of 0.01, <0.001, 0.01, and <0,001 respectively. The stone size was also significantly larger in non-SSP patients (p value < 0.0001). Moreover, the distally located stones were more likely to spontaneously pass in comparison to proximal stones with a p-value of 0.019.
Fig. 1.
Follow up flow chart of the participants.
Table 1.
Comparison of demographic, laboratory, and radiological factors as predictors of spontaneous stone passage.
| Variable | Total | No SSP | SSP | p.value |
|---|---|---|---|---|
| Age(Year±SD) | 40.12 ± 12.36 | 42.27 ± 13.87 | 38.43 ± 10.81 | 0.050* |
| Gender | ||||
| Male (n,%) | 86(53.5) | 41(57.7) | 45(50) | 0.328** |
| Female (n,%) | 75(46.5) | 30(42.3) | 45(50) | |
| BMI(Kg/m2) | 22.47 ± 2.4 | 22.6 ± 2.35 | 22.29 ± 2.59 | 0.309* |
| Diabetic | ||||
| Yes (n,%) | 19(11.8) | 4(5.6) | 11(12.7) | 0.153** |
| No (n,%) | 142(88.2) | 67(9.4) | 79(87.7) | |
| Hypertension | ||||
| Yes (n,%) | 19(11.8) | 8(11.3) | 11(12.3) | 0.852** |
| No (n,%) | 142(88.2) | 63(88.7) | 79(87.8) | |
| WBC(mean ± SD) | 9462 ± 1861 | 9710 ± 1650 | 9267 ± 2001 | 0.136* |
| PLT mean ± SD) | 272.33 ± 48.6 | 293.9 ± 40.13 | 255.3 ± 48.1 | <0.001* |
| Neutrophil (%) | 63.59 ± 9.21 | 66.3 ± 7.7 | 61.4 ± 9.7 | 0.01* |
| PLR (mean ± SD) | 10.09 ± 3.8 | 11.47 ± 4.86 | 9.00 ± 2.30 | <0.001* |
| NLR (mean ± SD) | 2.39 ± 1.05 | 2.63 ± 1.35 | 2.20 ± 0.69 | 0.010* |
| Serum creatinine | 0.76 ± 0.16 | 0.74 ± 0.15 | 0.78 ± 0.17 | 0.109* |
| Stone size(mean ± SD) | 7.19 ± 1.68 | 7.76 ± 1.49 | 6.43 ± 1.60 | <0.001* |
| Stone location | ||||
| Upper(n,%) | 8(5) | 6(8.5) | 2(2.2) | |
| Mid(n,%) | 24(15) | 15(21) | 9(10) | 0.019** |
| Lower (n,%) | 129(80) | 50(70.4) | 79(87.8) | |
| HU | 710 | 726 ± 218 | 697 ± 215 | 0.403* |
| UWT | 2.42 ± 0.73 | 2.98 ± 0.67 | 1.98 ± 0.42 | <0.001* |
| HN | ||||
| No mild | 109(67.7) | 40(36.6) | 69(63.4) | 0.006** |
| Moderate-severe | 52(32.3) | 31(59.6) | 21(40.4) | |
BMI body mass index, WBC white blood cells, PLT platelet, PLR platelet lymphocyte ratio, NLR neutrophil lymphocyte ratio, UWT ureteral wall thickness, HU Hounsfield unit, SSP spontaneous stone passage, * independent t-test, ** chi-square test.
Our results showed that the younger patients were more likely to pass the stones than the older ages.
Concerning the grade of hydronephrosis, there was a significantly higher rate of stone passage in patients with no or mild hydronephrosis compared to moderate to severe hydronephrosis (p-value = 0.006). However, on multivariate analysis the association was not significant (p-value 0.051).
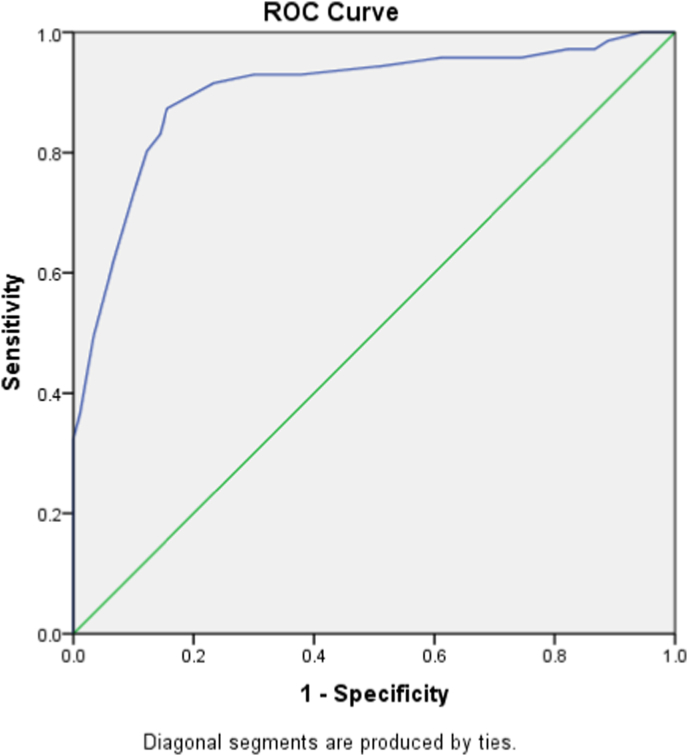
Ureteral wall thickness around the stone was another factor that significantly affected ureteral stone passage. Our result showed that lower ureteral wall thickness was associated with a higher chance of stone passage, and the correlation was highly significant (p-value < 0.0001). Receiver operating characteristic curves showed an area of 0.901 (95% CI 0.849–0.952) with a cut-off value of 2.45 mm of UWT with an 83% sensitivity and 86% specificity (Fig. 2). However, several other factors like sex, medical comorbidities, HU of the stone, creatinine level, and hematuria did not affect the outcome.
Fig. 2.
Receiver operating characteristic curve for ureteral wall thickness and non-spontaneous stone passage. Area 0.901 (95% CI 0.849–0.952) with a cut-off value of 2.45 mm of UWT with an 83% sensitivity and 86% specificity.
Univariate and multi multivariate analysis of the factors related to the failure of SSP revealed a significant correlation between higher UWT, NLR, PLR, and stone size with failure of SSP with an OR of 11.45, 3.24, 7.54, and 3.24, respectively (Table 2).
Table 2.
Univariate and multivariate analysis of the factors associated with failure of spontaneous ureteral stone passage.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | OR | 95% CI | P |
| UWT | 6.56 | 5.25–18.24 | <0.001 | 11.45 | 3.24–25.76 | 0.002 |
| NLR | 1.686 | 1.112–2.557 | 0.014 | 3.24 | 1.65–6.23 | <0.001 |
| PLR | 4.02 | 1.324–10.43 | 0.002 | 7.54 | 4.23–12.54 | 0.004 |
| Age | 1.026 | 1.00–1.053 | 0.053 | |||
| Gender | 1.367 | 0.730–2.557 | 0.328 | |||
| BMI | 1.060 | 0.934–1.203 | 0.368 | |||
| Hydronephrosis | 2.546 | 1.294–5.012 | 0.007 | 4.143 | 1.765–9.054 | 0.051 |
| Stone size | 1.690 | 1.357–2.104 | <0.001 | 3.25 | 2.11–8.25 | <0.001 |
| Previous history of ureteral stone | 0.43 | 0.017–0.564 | 0.03 | 1.09 | 0.71–4.98 | 0.354 |
BMI body mass index, WBC white blood cells, PLT platelet, PLR platelet lymphocyte ratio, NLR neutrophil lymphocyte ratio, UWT ureteral wall thickness, HU Hounsfield unit, SSP spontaneous stone passage.
Our results showed a correlation between higher NLR, PLR and UWT (Pearson correlation of 0.314 and 0.426 respectively with a p-value of both <0.001).
Manova test for the relation between PLR, and NLR with UWT for SSP showed a significant chance of SSP toward the lower PLR, and NLR with lower UWT (Table 3).
Table 3.
Manova test for relationship between NLR and ureteral wall thickness at sone site with Spontaneous stone passage.
| Variable | No SSP | SSP | p-value |
|---|---|---|---|
| NLR | 2.63 ± 1.35 | 2.20 ± 0.69 | <0.001 |
| UWT | 2.98 ± 0.67 | 1.98 ± 0.42 | |
| PLR | 11.47 ± 4.86 | 9.00 ± 2.30 | <0.001 |
| UWT | 2.98 ± 0.67 | 1.98 ± 0.42 |
PLR platelet lymphocyte ratio, NLR neutrophil lymphocyte ratio, UWT ureteral wall thickness, SSP spontaneous stone passage.
4. Discussion
A variety of management strategies exist to treat ureteral stones, ranging from conservative treatment (medical expulsive therapy) to shock wave lithotripsy to ureteroscopy [19]. Medical treatment is considered non-invasive and cheap; however, it may have undesirable consequences like renal impairment, urinary tract infection, recurrent colic, and patient discomfort. On the other hand, shockwave lithotripsy and endoscopic stone removal are safer and yield better stone-free rates than medical treatment, yet they are costly, and procedure-related complications such as urinary infection, hematoma formation, and urinary extravasation should be kept in mind [20]. Likewise, postponing surgical intervention until failure of medical therapy is stressful to the patient and adds to the cost of treatment when compared with immediate surgical interference [21]. This vagueness in clinical decision-making has prompted many investigators to explore markers to guide clinicians in triaging patients for optimal treatment plans [22].
Different pharmacological agents such as alpha-blockers, calcium channel blockers, phosphodiesterase inhibitors, and corticosteroids are studied to facilitate spontaneous ureteral stone expulsion. However, recent guidelines concluded the superiority of using alpha-blocker monotherapy in this regard [23]. In the current study, our patients received a single dose of 0.4 mg of tamsulosin daily for 4 weeks.
4.1. Stone size and location
Stone size and location are among the important factors to predict spontaneous stone passage. Stones < 5 mm in any part of the ureter have a 75% chance of spontaneous passage. As the size increases, the rates of spontaneous passage decline (60% for 5–7 mm, 48% for 7–9 mm, and 25% for > 9 mm). Based on the stone location, the SSP will change ranging from 79%, 60%, and 48% for distal, mid, and proximal ureteral stones respectively. The EAU/AUA panel studied SSP based on a meta-analysis that showed 68% for <5 mm stones and 48% for 5–10 mm stones [24]. The current study showed a 55.9% SSP rate, and the stone size was significantly smaller in comparison with non SSP patients (6.43 ± 1.60 versus 7.76 ± 1.49) with a p-value < 0.001, while the rate of SSP concerning the stone location was 25%, 37,5%, and 61.2% for the proximal, mid and lower ureteral stones respectively which is relatively lower than the previously mentioned studies. The overall lower SSP in our study, is likely related to the higher mean size of the stones in our patients (7.19 ± 1.68 mm).
4.2. Inflammatory serum markers and SSP
NLR is regarded as a parameter to evaluate the inflammatory status of a patient. Its prognostic efficacy has been proved in various kinds of cancers, postoperative complications, major cardiac events, and various infectious states, yet its role in predicting SSP is controversial [[25], [26], [27], [28], [29]]. Sfoungaristos et al. found that elevated WBC and neutrophile in patients with spontaneous ureteral passage. They hypothesized that stone passage causes ureteral wall inflammation, and this process does not occur in statis stones [12]. By contrast Nassib et al., in their analysis of 619 patients with ureteric stone <10 mm, observed an elevated NLR and PLR in SSP failure patients with an odds ratio of 2.96 (95% CI 1.80–5.49) for NLR between 2.87 and 4.87, and an OR of 3.28 (1.79–6.19) for PLR between 10.42 and 15.25 [22]. Another study by Kwang Suk Lee revealed a significant correlation between SSP and low NLR, with an odds ratio of 9.3 for NLR of <2.3 [30]. Our results are in line with the mentioned studies. We found a higher NLR and PLR among patients with failure of SSP, with an OR of 3.2 (95% CI 1.65–6.23), P-value <0.001 and an OR of 7.53 (95% CI 4.3–12.54), p-value 0.004 respectively. Nonetheless, Ahmed et al. and Senel C et al. did not find a relation between WBC count, NLR, and PLR with SSP [15,16].
4.3. UWT around the stone and SSP
Impacted ureteral stone causes inflammatory reactions that may result in ureteral wall edema, hypertrophy, and fibrosis which ultimately increases the ureteral wall thickness around the stone [31]. Several studies have evaluated the value of ureteral wall thickness in the treatment of ureteral stones. Ozbir et al., Elibol et al., and. Sarica et al. found in their studies that the UWT can be regarded as a predictor of stone impaction [[32], [33], [34]]. Moreover, Yoshida et al. concluded its use as a dependable factor to predict stone impaction and surgical outcome post ureteroscopy [35]. However, its value for predicting spontaneous ureteral stone passage and the outcome of MET is questionable [10,36]. Different cut-off values of UWT have been reported to predict SSP or outcome MET in the literature. Yoshida et al. [10] reported 2.71 mm as a cut-off level predicting SSP in 4 weeks, while Mohamed Samir et al. [11] reported a cut-off level of ≥3.75 mm predicting stone passage failure. The current study showed a cut-off value of 2.45 mm with an 83% sensitivity and 86% specificity, which is comparable with the literature. Additionally, our study revealed a significant positive correlation between NLR and PLR with ureteral wall thickness with a p-value of <0.001 on one hand, and on the other hand, the higher ureteral wall thickness with elevated NLR and PLR was associated with failure of SSP, the relation was statistically significant (p-value < 0.001). To the best of our knowledge, our study is the first of a kind to use both factors at the same time to predict SSP.
There are limitations to our study, starting with the small sample size and the single-center data. Another limitation is missing some important data like C reactive protein and ESR, which are important inflammatory markers that can be used to predict SSP. However, due to the economic burden, we could not send such investigations to all the patients. Another limitation is using a single sample during the patients' presentation rather than repeating it to observe the trend of inflammatory markers is regarded as another drawback to our study.
5. Conclusions
Many factors play a role in the decision making of ureteral stone, and it is a complex process. Ureteral stone passage is dependent on the stone size and location. Our findings propose that higher NLR and PLR, together with the higher ureteral wall thickness at the stone site, suggest failure of spontaneous stone passage during MET. However due to vast controversies in the literature, further prospective and comprehensive studies are recommended to confirm our results.
Ethical approval
The manuscript approved by ethical committee of the Kurdistan Board for medical specialties (No 1135).
Sources of funding
No source to be stated.
Author contribution
Ismaeel Aghaways: supervisor the project, final approval of the manuscript.
Rawa Bapir: literature review, writing the first draft of the manuscript, and final approval of the manuscript.
Karzan M. Salih: wrote and an amended the second draft, final approval of the manuscript.
Berwn A. Abdulla, Rawezh Q. Salih,: literature review, final approval of the manuscript.
Rebaz Ibrahim: data collection, follow up of the patient, final approval of the manuscript.
Registration of research studies
The study was registered in the Research Registry with a registration number of (8072). The link is https://www.researchregistry.com/register-now#home/registrationdetails/62c2c16dda82dc001e5989dd/
Guarantor
Rawa Bapir is Guarantor of this submission.
Consent
Consent has been taken from the patients and the family of the patients.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
There is no conflict to be declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104198.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Parmar M.S. Kidney stones. BMJ. 2004;328:1420–1424. doi: 10.1136/bmj.328.7453.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed A.F., Al-Sayed A.Y. Tamsulosin versus alfuzosin in the treatment of patients with distal ureteral stones Prospective, randomized, comparative study. Kor. J. Urol. 2010;51:193–197. doi: 10.4111/kju.2010.51.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearle M.S., Calhoun E.A., Curhan G.C. Urologic diseases in America project: urolithiasis. J. Urol. 2005;173(3):848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 4.Lotan Y. Economics and cost of care of stone disease. Adv. Chron. Kidney Dis. 2009;16(1):5–10. doi: 10.1053/j.ackd.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Garcia Morua A., Gutierrez Garcia J.D., Martinez Montelongo R., Gomez Guerra L.S. Use of alfuzosin for expulsion of stones in the distal third of ureter. Actas Urol. Esp. 2009;33(9):1005–1010. doi: 10.1016/s0210-4806(09)72901-8. [DOI] [PubMed] [Google Scholar]
- 6.Bensalah K., Pearle M., Lotan Y. Cost-effectiveness of medical expulsive therapy using alpha-blockers for the treatment of distal ureteral stones. Eur. Urol. 2008;53(2):411–418. doi: 10.1016/j.eururo.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Assimos D., Krambeck A., Miller N.L., et al. Surgical management of stones: American urological association/endourological society guideline, PART II. J. Urol. 2016;196:1161–1169. doi: 10.1016/j.juro.2016.05.091. [DOI] [PubMed] [Google Scholar]
- 8.Türk C., Petřk A., Sarica K., et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur. Urol. 2016;69:468–474. doi: 10.1016/j.eururo.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Coll D.M., Varanelli M.J., Smith R.C. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am. J. Roentgenol. 2002;178:101–103. doi: 10.2214/ajr.178.1.1780101. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T., Inoue T., Taguchi M., Omura N., Kinoshita H., Matsuda T. Ureteral wall thickness as a significant factor in predicting spontaneous passage of ureteral stones of≤ 10 mm: a preliminary report. World J. Urol. 2019 May;37(5):913–919. doi: 10.1007/s00345-018-2461-x. [DOI] [PubMed] [Google Scholar]
- 11.Samir M., Elawady H., Hamid E., Tawfick A. Can ureteral wall thickness (UWT) be used as a potential parameter for decision-making in uncomplicated distal ureteral stones 5–10 mm in size? A prospective study. World J. Urol. 2021 Sep;39(9):3555–3561. doi: 10.1007/s00345-021-03608-6. [DOI] [PubMed] [Google Scholar]
- 12.Sfoungaristos S., Kavouras A., Katafigiotis I., Perimenis P. Role of white blood cell and neutrophil counts in predicting spontaneous stone passage in patients with renal colic. BJU Int. 2012;110:E339–E345. doi: 10.1111/j.1464-410X.2012.11014.x. [DOI] [PubMed] [Google Scholar]
- 13.Park C.H., Ha J.Y., Park C.H., Kim C.I., Kim K.S., Kim B.H. Relationship between spontaneous passage rates of ureteral stones less than 8 mm and serum C-reactive protein levels and neutrophil percentages. Kor. J. Urol. 2013;54:615–618. doi: 10.4111/kju.2013.54.9.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abushamma F., Ktaifan M., Abdallah A., Alkarajeh M., Maree M., Awadghanem A., et al. Clinical and Radiological predictors of early intervention in acute ureteral colic. Int. J. Gen. Med. 2021;14:4051–4059. doi: 10.2147/IJGM.S322170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A.F., Gabr A.H., Emara A.A., Ali M., Abdel-Aziz A.S. Alshahrani S Factors predicting the spontaneous passage of a ureteric calculus of ≤10 mm. Arab J. Urol. 2015;13:84–90. doi: 10.1016/j.aju.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senel C., Aykanat I.C., Asfuroglu A., Keten T., Balci M., Aslan Y., Tuncel A. What is the role of inflammatory markers in predicting spontaneous ureteral stone passage? Aktuelle Urol. 2022 Jan 10 doi: 10.1055/a-1703-3099. [DOI] [PubMed] [Google Scholar]
- 17.Mathew G., Agha R., for the STROCSS Group STROCSS 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 18.Kim S.Y., Kim M.J., Yoon C.S., Lee M.S., Han K.H., Lee M.J. Comparison of the reliability of two hydronephrosis grading systems: the Society for Foetal Urology grading system vs. the Onen grading system. Clin. Radiol. 2013;68(9):e484–e490. doi: 10.1016/j.crad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Turk C., Petrik A., Sarica K., Seitz C., Skolarikos A., Straub M., Knoll T. EAU guidelines on interventional treatment for urolithiasis. Eur. Urol. 2016;69(3):475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 20.DrakeT, Grivas N., Dabestani S., Knoll T., Lam T., Maclennan S., Petrik A., Skolarikos A., Straub M., Tuerk C., et al. What are the benefits and harms of ureteroscopy compared with shock-wave lithotripsy in the treatment of upper ureteral stones? A systematic review. Eur. Urol. 2017;72(5):772–786. doi: 10.1016/j.eururo.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Lotan Y., Gettman M.T., Roehrborn C.G., Cadeddu J.A., Pearle M.S. Management of ureteral calculi: a cost comparison and decision making analysis. J. Urol. 2002;167(4):1621–1629. [PubMed] [Google Scholar]
- 22.Abou Heidar N., Labban M., Bustros G., Nasr R. Inflammatory serum markers predicting spontaneous ureteral stone passage. Clin. Exp. Nephrol. 2020 Mar;24(3):277–283. doi: 10.1007/s10157-019-01807-5. [DOI] [PubMed] [Google Scholar]
- 23.Türk C., Knoll T., Petrik A. 2014. EAU Guidelines on Urolithiasis.https://www.uroweb.org Available from. 2018 Apr 04. [Google Scholar]
- 24.Ramasamy V., Aarthy P., Sharma V., Thakur A.P. Role of inflammatory markers and their trends in predicting the outcome of medical expulsive therapy for distal ureteric calculus. Urol. Ann. 2022 Jan 1;14(1):8. doi: 10.4103/ua.ua_139_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson P.H., Croal B.L., Cuthbertson B.H., Small G.R., Ifezulike A.I., Gibson G., et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am. Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.S., Han K.H., Chung H.H., Kim J.W., Park N.H., Song Y.S., et al. Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparison. Eur. J. Surg. Oncol. 2010;36:691–698. doi: 10.1016/j.ejso.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Hung H.Y., Chen J.S., Yeh C.Y., Changchien C.R., Tang R., Hsieh P.S., et al. Effect of preoperative neutrophil-lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int. J. Colorectal Dis. 2011;26:1059–1065. doi: 10.1007/s00384-011-1192-x. [DOI] [PubMed] [Google Scholar]
- 28.Azab B., Bhatt V.R., Phookan J., Murukutla S., Kohn N., Terjanian T., et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann. Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 29.Proctor M.J., Morrison D.S., Talwar D., Balmer S.M., Fletcher C.D., O'Reilly D.S., et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur. J. Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Lee K.S., Ha J.S., Koo K.C. Significance of neutrophil-to-lymphocyte ratio as a novel indicator of spontaneous ureter stone passage. Yonsei Med. J. 2017 Sep 1;58(5):988–993. doi: 10.3349/ymj.2017.58.5.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitz C., Tanovic E., Kikic Z., Fajkovic H. Impact of stone size, location, composition,impaction, and hydronephrosis on the efficacy of holmium:YAG-laser ureterolithotripsy. Eur. Urol. 2007;52:1751–1759. doi: 10.1016/j.eururo.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Ozbir S., Can O., Atalay H.A., Canat H.L., Cakır S., Otunctemur A. Formula for predicting the impaction of ureteral stones. Urolithiasis. 2019;48(4):353–360. doi: 10.1007/s00240-019-01152-y. [DOI] [PubMed] [Google Scholar]
- 33.Elibol O., Safak K.Y., Buz A., Eryildirim B., Erdem K., Sarica K. Radiological noninvasive assessment of ureteral stone impaction into the ureteric wall: a critical evaluation with objective radiological arameters. Invest. Clin. Urol. 2017;58(5):339–345. doi: 10.4111/icu.2017.58.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarica K., Kafkasli A., Yazici O., Sabuncu K., Cetinel C., Narter F. Ureteral wall thickness at the impacted ureteral stone site: a critical predictor for success rates after SWL. Urolithiasis. 2015;43:83–88. doi: 10.1007/s00240-014-0724-6. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida T., Inoue T., Omura N., et al. Ureteral wall thickness as a preoperative indicator of impacted stones in patients with ureteral stones undergoing ureteroscopic lithotripsy. Urology. 2017;106:45–49. doi: 10.1016/j.urology.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 36.Sahin C., Eryildirim B., Kafkasli A., et al. Predictive parameters for medical expulsive therapy in ureteral stones: a critical evaluation. Urolithiasis. 2015;43:271. doi: 10.1007/s00240-015-0762-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.