Abstract
Background
Up to 30% of differentiated thyroid cancer (DTC) will develop advanced-stage disease (aDTC) with reduced overall survival (OS).
Objective
The aim of this study is to characterize initial diagnosis of aDTC, its therapeutic management, and prognosis in Spain and Portugal.
Methods
A multicentre, longitudinal, retrospective study of adult patients diagnosed with aDTC in the Iberian Peninsula was conducted between January 2007 and December 2012. Analyses of baseline characteristics and results of initial treatments, relapse- or progression-free survival ((RP)FS) from first DTC diagnosis, OS, and prognostic factors impacting the evolution of advanced disease were evaluated.
Results
Two hundred and thirteen patients (median age: 63 years; 57% female) were eligible from 23 hospitals. Advanced disease presented at first diagnosis (de novo aDTC) included 54% of patients, while 46% had relapsed from early disease (recurrent/progressive eDTC). At initial stage, most patients received surgery (98%) and/or radioiodine (RAI) (89%), with no differences seen between median OS (95% CI) (10.4 (7.3–15.3) years) and median disease-specific-survival (95% CI) (11.1 (8.7–16.2) years; log-rank test P = 0.4737). Age at diagnosis being <55 years was associated with a lower risk of death (Wald chi-square (Wc-s) P < 0.0001), while a poor response to RAI to a higher risk of death ((Wc-s) P < 0.05). In the eDTC cohort, median (RP)FS (95% CI) was of 1.7 (1.0–2.0) years after RAI, with R0/R1 surgeries being the only common significant favourable factor for longer (RP)FS and time to aDTC ((Wc-s) P < 0.05).
Conclusion
Identification of early treatment-dependent prognostic factors for an unfavourable course of advanced disease is possible. An intensified therapeutic attitude may reverse this trend and should be considered in poor-performing patients. Prospective studies are required to confirm these findings.
Keywords: advanced differentiated thyroid cancer, relapsing differentiated thyroid cancer, radioiodine-refractory differentiated thyroid cancer, epidemiological study, relapsing prognostic factors, survival prognostic factors
Introduction
Differentiated thyroid carcinoma (DTC) is the most frequent subtype of thyroid cancer (1). In the initial disease scenario, therapeutic approach mainly relies on surgery and treatment with I-131 radioiodine (RAI). Overall survival (OS) rate in this population is high (98.3% and 90% at 5 and 10 years, respectively) after initial diagnosis (2, 3).
However, up to 20% of patients with DTC, excluding those with microcarcinomas, present with metastasis or locally advanced disease at the time of diagnosis (4, 5, 6). Moreover, in up to 30% of patients with initial early-stage diagnosis (eDTC), disease relapses into the advanced stage (aDTC), either as locoregional unresectable or metastatic dissemination (7). Additionally, one-third of the metastatic tumours will show low avidity for iodine at the time of diagnosis and nearly two-thirds will become refractory to RAI (radioiodine-refractory differentiated thyroid cancer; RR-DTC) along treatment (8, 9).
In the aDTC situation, survival rates decrease markedly. The 10-year survival rate for RAI-responder patients is about 56%, whereas in RR-DTC patients, this is only 10% (10). In this scenario, patient’s care usually involves a multidisciplinary team whose composition is variable among countries and centres (11, 12).
In this clinical context, available data in Europe are outdated and very limited. In response to this need, the ERUDIT study aimed to characterize the natural evolution of adult aDTC in Spain and Portugal.
Specifically, this communication will describe the demographic and clinical characteristics, usage patterns, and efficacy profile of upfront therapies used to treat aDTC, either presenting at first diagnosis (de novo aDTC) or after successive relapses (recurrent/progressive eDTC). We will also explore potential prognostic factors correlating with disease relapse/progression from eDTC into aDTC and death.
Methods
Study design and setting
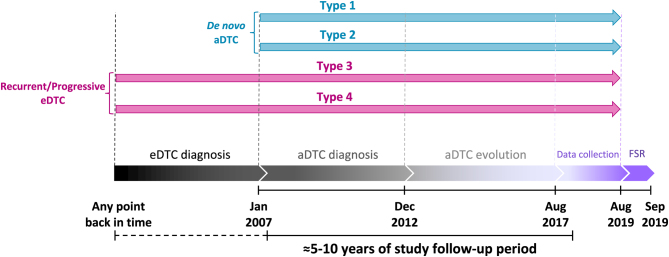
ERUDIT is a multicentre, observational, longitudinal, international, and retrospective study run in 22 representative hospitals from Spain and Portugal (Supplementary Table 1, see section on supplementary materials given at the end of this article). Clinical records from eligible patients diagnosed with aDTC from January 2007 to December 2012, both inclusive, were retrospectively reviewed until August 2017 (expected 5–10 years follow-up) and collected from August 2017 to August 2019 (Fig. 1).
Figure 1.
ERUDIT study design and evolution timeline of the aDTC patients included according to the type of their diagnosis. Type 1: de novo metastatic with resectable locoregional disease. Type 2: de novo locoregional unresectable disease. Type 3: recurrent metastatic without resectable locoregional disease. Type 4: recurrent locoregional unresectable disease, with or without distant metastases. aDTC, advanced differentiated thyroid cancer; eDTC, early stage differentiated thyroid cancer; FSR, final study report.
Patients
Patient records were considered if aDTC (papillary, follicular, Hürthle’s cell, or mixed or poorly differentiated) diagnosis was confirmed with first evidence of unresectable locally advanced and/or metastatic disease (presenting either de novo or relapsed after first treatment) being as such documented during the inclusion period. Accordingly, four patient types were predefined in the study protocol to account for most common situations at diagnosis: Type 1, de novo metastatic with resectable locoregional disease; Type 2, de novo locoregional unresectable disease; Type 3, recurrent metastatic with resectable locoregional disease; and Type 4, recurrent locoregional unresectable disease, with/without distant metastases. Patients had to be ≥18 years old at initial diagnosis and their medical records be sufficiently complete to allow data analysis until 31 August 2017, death, or lost to follow-up, whichever happened first. Patients with diagnosis of anaplastic or medullary thyroid cancer were excluded.
Patients’ informed consent was requested. The study was performed in compliance with all basic principles of the Helsinki Declaration (2013) from World Medical Association (13) and applicable national regulations. Both accredited Research Ethics Committees of Hospital Universitario Gregorio Marañón (Madrid, Spain) and National Ethics Committee for Clinical Research (Portugal) approved it.
Variables at initial diagnosis and clinical endpoints
Demographic and clinical characteristics
Gender, comorbidities, initial diagnosis of DTC (incidental post-surgery, no-incidental), diagnosis type (de novo or recurrent), histology result, biochemistry at diagnosis (patients with thyroglobulin (Tg) levels and/or anti-Tg positive in serum), tumour size (mm), lobes involved, tumour focality, tumour invasion (adjacent tissue, vascular, lymphatic nodes, absent, or no invasion), metastases at diagnosis by site, and type of image diagnosis were collected as variables of interest (Table 1 and Supplementary Table 2).
Table 1.
Demographic and clinical characteristics of the study population at initial disease presentation (N = 213).
| Parameter | Global study population | De novo aDTC | Recurrent/progressive eDTC | P-value |
|---|---|---|---|---|
| Patient, no. (%) | 213 (100) | 115 (54.0) | 98 (46.0) | NA |
| Age at initial diagnosis, median (Q1–Q3), years | 63.0 (51.0–71.0) | 67.0 (57.0–73.0) | 56.5 (45.0–67.0) | 0.0002c |
| Gender, patient no. (%) | ||||
| Female | 126 (59.2) | 65 (56.5) | 61 (62.2) | 0.3970 |
| Male | 87 (40.8) | 50 (43.5) | 37 (37.8) | |
| Comorbidities, patient no. (%) | ||||
| ≥1 comorbidity | 91 (42.7) | 50 (43.5) | 41 (41.8) | 0.7667 |
| Cardiovascular | 61 (28.6) | 33 (28.7) | 28 (28.6) | 0.9841 |
| Metabolic | 40 (18.8) | 26 (22.6) | 14 (14.3) | 0.1211 |
| Other clinically relevant | 23 (10.8) | 13 (11.3) | 10 (10.2) | 0.7965 |
| Initial diagnosis of DTC and method, patient no. (%) | ||||
| Incidental post-surgery | 45 (21.1) | 15 (13.0) | 30 (30.6) | 0.0017c |
| No incidental | 168 (78.9) | 100 (87.0) | 68 (69.4) | |
| Echography | 101 (60.1) | 54 (54.0) | 47 (69.1) | 0.0671 |
| Others | 65 (38.7) | 45 (45.0) | 20 (29.4) | |
| Not available | 2 (1.2) | 1 (1.0) | 1 (1.5) | |
| Fine needle aspiration result, patient no. (%) | 129 (60.6) | 71 (61.7) | 58 (59.2) | 0.7037 |
| Malignant | 100 (77.5) | 60 (84.5) | 40 (69.0) | 0.0085c |
| Indeterminate | 17 (13.2) | 4 (5.6) | 13 (22.4) | |
| Benign | 6 (4.7) | 5 (7.0) | 1 (1.7) | |
| Nondiagnostic | 6 (4.7) | 2 (2.8) | 4 (6.9) | |
| Histological result, patient no. (%) | 0.0497c | |||
| Papillary thyroid carcinoma | 125 (59.8) | 67 (60.4) | 58 (59.2) | |
| Follicular thyroid carcinoma | 39 (18.7) | 26 (23.4) | 13 (13.3) | |
| Hürthle cell carcinoma | 21 (10.0) | 5 (4.5) | 16 (16.3) | |
| Poorly differentiated | 8 (3.8) | 5 (4.5) | 3 (3.1) | |
| Mixed carcinoma (papillary and follicular) | 7 (3.3) | 4 (3.6) | 3 (3.1) | |
| Others | 9 (4.3) | 4 (3.6) | 5 (5.1) | |
| Biochemistry at diagnosis, a patient no. (%) | 74 (34.7) | 42 (36.5) | 32 (32.7) | 0.5545 |
| Diagnosis images, patient no. (%) | 180 (100) | 93 (51.7) | 87 (48.3) | 0.1120 |
| Measurable disease, no. (%) | 106 (58.9) | 58 (62.4) | 48 (55.2) | 0.3270 |
| Tumour size, median (mm) (Q1–Q3) | 40.0 (25.0–57.0) | 40.0 (26.0–60.0) | 38.5 (25.0–50.5) | 0.3478 |
| Lobes involved, no. (%) | 0.8248 | |||
| Left or right | 119 (66.1) | 60 (64.5) | 59 (67.8) | |
| Both | 51 (28.3) | 27 (29.0) | 24 (27.6) | |
| Not available | 10 (5.6) | 6 (6.5) | 4 (4.6) | |
| Tumour invasion,b no. (%) | ||||
| Present | 48 (26.7) | 34 (36.6) | 14 (16.1) | d |
| Absent | 34 (18.9) | 12 (12.9) | 22 (25.3) | d |
| Unknown | 78 (43.3) | 39 (41.9) | 39 (44.8) | d |
| Pathological lymph nodes | 37 (20.6) | 24 (25.8) | 13 (14.9) | d |
| Metastases by site, no. (%) | ||||
| Lung | 66 (71.0) | 66 (71.0) | d | |
| Liver | 5 (5.4) | 5 (5.4) | d | |
| Bone | 33 (35.5) | 33 (35.5) | d | |
| Others | 3 (3.2) | 3 (3.2) | d |
aRefers patients with thyroglobulin (Tg): Tg levels and/or anti-Tg positive in serum; btumour invasion refers to disease invading either adjacent tissues and structures and/or vascular spaces. Patients could be part of more than one tumour invasion category making statistical testing not feasible; cstatistically significant (P < 0.05); drecurrent/progressive eDTC patients, by definition, had no metastases at diagnosis.
aDTC, advanced differentiated thyroid cancer; eDTC, early differentiated thyroid cancer.
Therapeutic approach and response
(a) Surgery: neck dissection; microscopic complete resection (R0), macroscopic resection with microscopic residual tumour (R1), and gross macroscopic residual tumour (R2) and histological results; (b) ablative (adjuvant) I-131 radioiodine (RAI): administered dose, cumulative RAI dose by intervention, RAI refractoriness at initial diagnosis, use of human recombinant thyroid-stimulating hormone (hrTSH) per intervention, RAI response according to American Thyroid Association (ATA) (1) criteria (excellent, structural incomplete, biochemical incomplete, and indeterminate); and (c) radiotherapy: dose and response according to the standard Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 definition (objective response rate as the sum of complete response plus partial response; stable disease and progressive disease as per physician-reported best response to treatment) (Supplementary Table 3) (14).
Medical specialities involved in initial disease management
Department responsible for patient monitoring and the multidisciplinary committee for decision-making were involved.
Potential confounding variables
A total of 33 potential covariates were analysed using the Kaplan–Meier (K–M) method. Later, all covariates were entered into the multivariate Cox model following both forwards addition and backwards removal of non-significant covariates with a type I error ≤ 1. Potential covariates included variables at initial diagnosis described above (baseline characteristics and therapeutic approaches), covariates concerning relapse or progression of eDTC into aDTC (type of relapse, surgery, cumulative RAI dose, therapeutic RAI with stimulating agent (hrTSH), response to therapeutic RAI), and variables related to the advanced disease/RAI-refractory disease (RAI refractoriness (RR) criteria 1, 5, 6; RR criteria 2 + 3 + 4, RR criteria 7 + 8 (15), Eastern Cooperative Oncology Group score (16), watchful waiting (yes/no), watchful waiting duration (months), surgery for advanced/RAI-refractory disease, radiotherapy for advanced/RAI-refractory disease, use of systemic therapy, number of lines, and reason for stopping systemic therapy).
Data were obtained from medical records resulting from standard clinical practice in participating sites and collected in electronic case report forms as mandated in the ERUDIT study protocol (EIS-CDT-2017-01). Investigators received on-site training before data entry to guarantee consistency. The eCRF included a range of filters and logical controls to reduce errors, and an authorized Clinical Research Associate helped data entering at various sites.
Clinical endpoints of interest included OS and disease-specific survival (DSS), which were measured from the initial DTC diagnosis to death in the global study population. Relapse- or progression-free survival ((RP)FS) was defined as a composite time-to-event variable involving only eDTC patients that had relapsed into aDTC or died provided they were free of disease after first treatment or, alternatively, progressed into aDTC or died having residual disease after first treatment. Finally, all potential prognostic factors concerning these survival variables were explored in the global study population and the recurrent/progressive eDTC cohort.
Statistical methods
An estimated sample size of close to 300 patients was originally considered representative of nearly 20% of all aDTC diagnoses made in Spain and Portugal during the inclusion period based on available incidence data at the time of protocol writing.
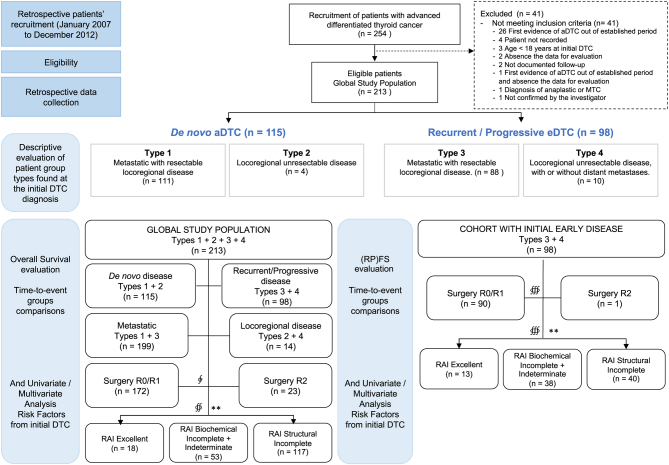
Description of baseline characteristics and therapeutic approaches received by patients was done for the total study population and for the two mutually exclusive groups de novo aDTC (Types 1 + 2) and recurrent/progressive eDTC (Types 3 + 4). Additional time-to-event analyses involved other ad hoc groups like (a) patients with metastatic disease (Types 1 + 3) vs those with the locoregional unresectable disease (Types 2 + 4) or (b) by first RAI ATA response: excellent, structural incomplete, biochemical incomplete, and indeterminate (1). Missing data were not imputed (Fig. 2).
Figure 2.
Flow diagram of eligible patients and analysed groups with evaluable data from the initial diagnosis of differentiated thyroid cancer (DTC). The (relapse/progression) free survival ((RP)FS) evaluation describes the time elapsed from an upfront treatment until death, relapse into advanced DTC (aDTC) in patients who were initially free of disease, or structural progression into aDTC in patients who had initial residual disease, respectively. n, number of patients with available data evaluable. ∲, from 208 total patients with evaluable outcome from first surgery to death, of which, 13 patients were excluded because of ‘metastases resection’ (n = 8) and ‘other results’ (n = 5). ∯, only 188 patients had evaluable response from the first RAI therapy to death. ∰, from 98 total patients in the cohort with initial early disease with evaluable outcome from first surgery to death or from the first RAI therapy to death, of which, 7 patients were excluded because of ‘metastases resection’ (n = 3) and ‘other results’ (n = 4). **, ATA RAI response.
Data were analysed using SAS Institute Inc. Version 8.2 software (Cary, NC, USA). All data were summarised using adequate descriptive statistics (mean, s.d.; median, quartiles, 95% CI; minimum and maximum for continuous variables; and absolute and relative frequencies for categorical variables). Comparisons of continuous variables were done by Wilcoxon rank-sum test, and categorical variables by chi-square test or Fisher’s exact test. Parameters based on objective response followed the standard RECIST v1.1 definition (14) as per physician-reported best response to treatment.
OS and (RP)FS survival functions were analysed and compared using K–M and Mantel–Haenszel (log-rank) methods. Prognostic risk factors for OS, (RP)FS, and time to develop aDTC/RR-DTC were analysed by univariate and multivariate Cox proportional risk-based regression model. Non-evaluable medians in K–M curves were identified as NE. All P-values were nominal and when <0.05 were considered statistically significant.
Results
A total of 213 eligible patients were identified from the initial 254 patients diagnosed with aDTC from January 2007 to December 2012 in 23 participant hospitals (22 Spanish and 1 Portuguese). Patients were grouped according to the time and disease extension at aDTC diagnosis for the descriptive and comparative analysis (Fig. 2). Fifty-four per cent (115/213) were diagnosed with de novo aDTC and 46% (98/213) with eDTC that, eventually after one or more relapses to previous interventions, became aDTC.
Demographic, clinical, and treatment characteristics of patients at initial DTC diagnosis
Study population had a median (Q1–Q3) age of 63.0 (51.0–71.0) years, and 59% (126/213) were female. Forty-three per cent (91/213) of patients had comorbidities at the time of DTC diagnosis, being cardiovascular disease in 28.6% (61/213) of them. Concerning primary tumour characteristics, median (Q1–Q3) size was 40 (25.0–57.0) mm. Almost 60% (125/209) of patients presented with papillary thyroid carcinoma, 18.7% (39/209) with follicular thyroid carcinoma, 10.0% (21/209) with Hürthle cell carcinoma, and 3.8% (8/209) with poorly differentiated carcinoma. Initial imaging techniques showed unilateral primary involvement in 66.1% (119/180), extrathyroidal invasion in 26.7% (48/180), and/or pathological lymph nodes in 20.6% (37/180). Distant metastases were evidenced in 51.7% (93/180) of patients, mostly in lung (71.0%) and bone (35.5%) (Table 1). Baseline characteristics showed that de novo aDTC patients were significantly older, the results of tumour FNA were more likely to be malignant, and with preferential follicular histology, compared to recurrent/progressive eDTC patients at initial diagnosis (Wilcoxon rank-sum test P = 0.0002, Fisher’s exact test P = 0.0085, and Fisher’s exact test P = 0.0497; respectively) (Table 1).
Standard upfront treatments were used to manage DTC after initial diagnosis (Supplementary Table 3). Concisely, at least one surgery was given to 98.1% (209/213) of patients and 75.1% (157/209) of them were subjected to any sort of neck dissection. Microscopic local complete resection R0 was achieved in 68.4% (143/209) of cases. Eighty-nine per cent (190/213) of patients received at least one RAI treatment, and 30% (57/190) had a median (Q1–Q3) cumulative dose of 450.0 (400.0–500.0) mCi after three doses. Most frequent ATA response to treatment was ‘structural incomplete,’ being 61.6% (117/190) after the first RAI dose. Ten per cent (21/213) of patients received at least one course of locoregional external beam radiotherapy, and 52% (11/21) showed subsequent ‘stable disease’ according to RECIST v1.1 (14). Significant differences were observed between results attained by de novo aDTC and recurrent/progressive eDTC, potentially favouring a better course of disease in eDTC patients (surgical results, post-RAI response, etc) (Supplementary Table 3).
Multidisciplinary committees monitored initial management in 73.2% (152/231) of the cases, the Endocrinology department being the most frequently involved followed by Nuclear Medicine and Oncology (Table 2).
Table 2.
Medical specialities involved in disease management after initial diagnosis (N = 213).
| Parameter | De novo aDTC (N = 115) | Recurrent/progressive eDTC (N = 98) | Global study population (N = 213) |
|---|---|---|---|
| Service responsible for patient monitoring, patient no. (%) | |||
| Endocrinology | 75 (65.2) | 67 (68.4) | 142 (66.7) |
| Nuclear medicine | 19 (16.5) | 15 (15.3) | 34 (16.0) |
| Oncology | 15 (13.0) | 12 (12.2) | 27 (12.7) |
| Surgery | 1 (0.9) | 1 (1.0) | 2 (0.9) |
| Others | 1 (1.1) | 1 (0.5) | |
| Not availablea | 5 (4.3) | 2 (2.0) | 7 (3.3) |
| Presence of multidisciplinary committee, patient no. (%) | |||
| No | 28 (24.3) | 22 (22.4) | 50 (23.5) |
| Yes | 82 (71.3) | 74 (75.5) | 156 (73.2) |
| Not available | 5 (4.3) | 2 (2.0) | 7 (3.3) |
aRecords could not be retrieved from the electronic case report form.
Time-to-event endpoints in the global study population
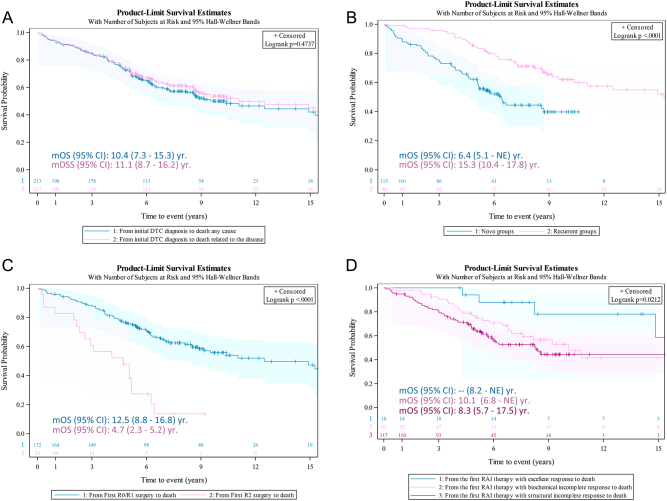
Median (Q1–Q3) study follow-up was 6.2 (4.5–9.0) years from initial DTC diagnosis and, specifically, 5.2 (3.3–7.0) years from aDTC diagnosis to end of the study. No statistical differences were observed between median OS (mOS) and median DSS (mDSS) from initial DTC diagnosis to death (mOS (95% CI): 10.4 (7.3–15.3) years and mDSS (95% CI): 11.1 (8.7–16.2) years; log-rank test P = 0.4737) (Fig. 3A).
Figure 3.
Survival Kaplan–Meier curves from the initial differentiated thyroid cancer (DTC) diagnosis to death in the global study population. (A) Comparison of overall survival (OS) from initial DTC diagnosis to death by any cause (blue) vs disease-specific survival (DSS) from initial DTC diagnosis to death related to the disease (pink). No differences were found (log-rank P = 0.474). (B) Comparison of OS from initial DTC diagnosis to death by any cause according to the time of advanced DTC (aDTC) diagnosis: de novo (blue) vs recurrent/progressive (pink). The OS from the initial DTC diagnosis was a median 8.9 years shorter in patients with de novo aDTC (log-rank test P < 0.0001). (C) Comparison of OS from first surgery: complete macroscopic resection, R0/R1 (blue) vs incomplete resection, R2 (pink). The OS from initial surgery was a median 7.8 years longer in patients with R0/R1 outcome (log-rank test P < 0.0001). (D) Comparison of OS from first RAI (±surgery) according to ATA response criteria: excellent (blue) vs biochemical incomplete (pink) vs structural incomplete response (magenta) (log-rank P = 0.0212). mOS (95% CI): median OS (95% CI) years.
mOS (95% CI) of de novo aDTC was significantly shorter compared to that in recurrent/progressive eDTC patients (6.4 (5.1–NE) vs 15.3 (10.4–17.8) years, respectively; log-rank test P < 0.0001) (Fig. 3B). However, the extension of advance disease (metastatic vs locoregional) did not have statistical impact on mOS (10.4 (7.3–16.0) vs 8.6 (0.7–12.5) years, respectively; log-rank test P = 0.1869). Additionally, mOS (95% CI) after first surgery was 10.4 (8.1–16.0) years, being 7.8 years longer in patients with complete macroscopic resection (R0/R1) compared to those with incomplete (R2) resections (12.5 (8.8–16.8) vs 4.7 (2.3–5.2) years, respectively; log-rank test P < 0.0001) (Fig. 3C). Finally, mOS (95% CI) after first RAI treatment was 10.1 (7.6–17.5) years, being significantly higher in patients achieving ATA ‘excellent response’ compared to those with ‘incomplete biochemical’ or ‘incomplete structural’ response (NE (8.2–NE) vs 10.1 (6.8–NE) vs 8.3 (5.7 - 17.5) years; log-rank test P = 0.0212) (Fig. 3D).
Time-to-event endpoints in the recurrent/progressive eDTC population
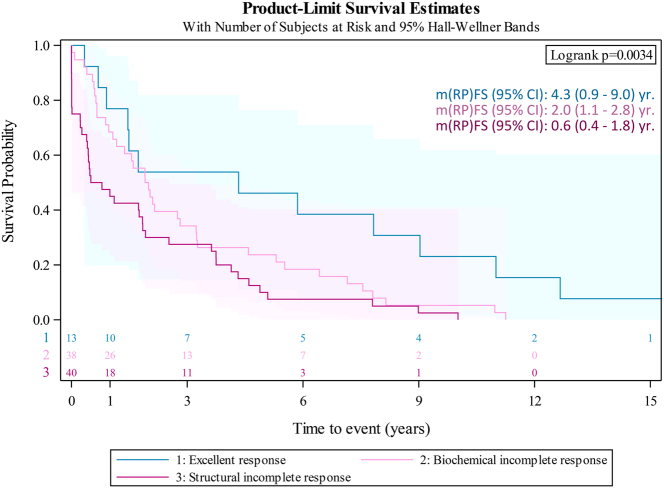
Median (RP)FS (95% CI) after initial radical surgery was 2.3 (1.7–2.9) years (n = 90), and 1.7 (1.0 - 2.0) years (n = 91) after initial RAI, being significantly higher for patients achieving ATA ‘excellent response’ compared to those with ‘incomplete biochemical’ or ‘incomplete structural’ response after this initial treatment (4.3 (0.9–9.0) vs 2.0 (1.1–2.8) vs 0.6 (0.4 - 1.8) years, respectively; log-rank test P = 0.0034) (Fig. 4).
Figure 4.
(Relapse/progression)-free survival ((RP)FS) after the first RAI treatment in patients with early disease condition stratified by ATA response (log-rank P = 0.0034). 1: excellent (blue); 2: incomplete biochemical (pink). 3: incomplete structural response (magenta). m(RP)FS (95% CI): median (95% CI) years.
Analysis of prognostic factors
OS
In the global study population, initial DTC diagnosis under 55 years was confirmed as a favourable prognostic factor associated with lower risk of death (adjusted hazard ratio (aHR): 0.39, 95% CI: 0.2–0.7; Wald chi-square P = 0.0018). In contrast, cumulative RAI dose <600 mCi, no RAI treatment, and RAI-scan positivity after the initial RAI treatment were all significantly associated with a higher risk of death (aHR (95% CI): 5.3 (2.3–12.0), Wald chi-square P = 0.0001; aHR (95% CI): 6.75 (2.4–18.9), Wald chi-square P = 0.0003; and aHR (95% CI): 2.4 (1.1–5.3), Wald chi-square P = 0.0307; respectively) (Table 3 and Supplementary Table 4).
Table 3.
Multivariate analyses for overall survival in the global study population.
| Covariatesa | N | Overall survival (Cox proportional hazard model) | |
|---|---|---|---|
| Adjusted hazard ratio (95% CI) | Wald chi-square P-value | ||
| General variables | |||
| Age at diagnosis in years | |||
| ≥55 (ref.) | 149 | ||
| <55 | 64 | 0.39 (0.214–0.703) | 0.0018d |
| Variables of initial diagnosis and first treatment | |||
| RAI cumulative dose | |||
| ≥600 mCi (ref.) | 29 | ||
| <600 mCi | 160 | 5.32 (2.352–12.037) | <0.0001d |
| No RAI treatmentb | 23 | 6.75 (2.406–18.946) | 0.0003d |
| RAI-scan positivity after initial RAI treatmentc | |||
| No (ref.) | 26 | ||
| Yes | 133 | 2.41 (1.085–5.345) | 0.0307d |
aAll covariates were entered into a Cox regression model, bpatients not receiving RAI because of it not being clinically indicated or having tumours with negative RAI scans; crefers to 6–12 months follow-up post-treatment scans in patients that could have received surgery ± RAI as first therapeutic approach; dStatistically significant (P < 0.05).
(ref.), reference category; OS, overall survival; RAI, radioactive iodine (I-131).
(RP)FS
In the recurrent/progressive eDTC cohort, attaining R0/R1 surgical result and being eligible for rescue surgeries was associated to a longer time to disease relapse or progression into aDTC/RR-DTC compared to having an R2 outcome (aHR (95% CI): 0.003 (0.00–0.05), Wald chi-square P = 0.0001 and aHR (95% CI): 0.003 (0.00–0.05), Wald Chi-square P = 0.0001; respectively). Regarding RAI therapy, both cumulative RAI doses <600 mCi and use of a pre-RAI hrTSH were positively associated to shorter time to relapse, progression, or death aDTC (aHR (95% CI): 2.45 (1.4–4.4), Wald chi-square P = 0.0025 and aHR (95% CI): 3.78 (1.1–12.6), Wald chi-square P = 0.0304; respectively). Resulting in ATA incomplete response (biochemical, structural, or both) after the first RAI treatment was also associated with an increased risk of earlier relapse, progression, or death (aHR (95% CI): 4.3 (2.0–9.2), Wald chi-square P = 0.0002 and aHR (95% CI): 2.47 (1.2–5.3), Wald chi-square P = 0.0188; respectively) (Table 4 and Supplementary Table 5).
Table 4.
Multivariate analyses for (relapse/progression) free survival and time to develop aDTC/RR-DTC in the eDTC cohort (N = 98).
| Covariatesa | N | (RP)FS (Cox proportional hazard model) | Time to develop aDTC/RR-DTC (Cox proportional hazard model) | ||
|---|---|---|---|---|---|
| Adjusted hazard ratio (95% CI) | Wald chi-square P-value | Adjusted hazard ratio (95% CI) | Wald chi-square P-value | ||
| Variables of initial diagnosis and first treatment | |||||
| Surgical outcome | |||||
| R2 (ref.) | 1 | ||||
| R0/R1 | 90 | 0.003 (0.000–0.048) | <0.0001f | 0.002 (0.000–0.034) | <0.0001f |
| Othersb | 7 | 0.003 (0.000–0.048) | <0.0001f | 0.004 (0.000–0.064) | 0.0001f |
| RAI cumulative dose | |||||
| ≥600 mCi (ref.) | 5 | ||||
| <600 mCi | 87 | 3.78 (1.13–12.60) | 0.0304f | ||
| No RAI treatmentc | 5 | 2.8 (0.53–14.76) | 0.2237 | ||
| RAI with stimulating agent at first instanced | |||||
| No (ref.) | 71 | ||||
| Yes | 18 | 2.45 (1.37–4.38) | 0.0025f | 1.82 (1.02–3.26) | 0.0438f |
| RAI Response at first instance ATA criteriae | |||||
| Excellent (ref.) | 13 | ||||
| Biochemistry incomplete + indeterminate | 38 | 2.47 (1.16–5.26) | 0.0188f | 2.34 (1.08–5.07) | 0.0315f |
| Structural incomplete | 40 | 4.32 (2.03–9.21) | 0.0002f | 3.35 (1.55–7.23) | 0.0021f |
aAll covariates were entered into a Cox regression model; bpathological lymph node dissections or metastasectomies; cpatients not receiving RAI because of it not being clinically indicated or having tumours with negative RAI scans; dfollowing standard practice in Spain and Portugal, patients not receiving hrTSH prior to RAI, were deprived of LT4; eHaugen et al., 2016;(1) fstatistically significant (P < 0.05).
(ref.), reference category; (RP)FS, relapse/progression-free survival; aDTC, advanced differentiated thyroid cancer; R0, microscopic complete resection; R1, macroscopic resection with microscopic residual tumour; R2, gross macroscopic residual tumour; RAI, radioactive Iodine (I-131); RR-DTC, radioiodine-refractory differentiated thyroid cancer.
Time to aDTC/RR-DTC
Additionally, we analysed prognostic factors associated with the time of eDTC relapse or progression into aDTC or RR-DTC. Same as with (RP)FS, attaining R0/R1 surgical result and being eligible for rescue surgeries was associated with a longer time to relapse or progression into aDTC/RR-DTC compared to the R2 result (aHR (95% CI): 0.002 (0.00–0.03), Wald chi-square P = 0.0002 and aHR (95% CI): 0.004 (0.00–0.06), Wald chi-square P = 0.0001; respectively). Finally, both use of pre-RAI hrTSH (aHR (95% CI: 1.8 (1.0–3.2); Wald chi-square P = 0.0438) and incomplete response to first RAI were positively associated with earlier relapse or progression into aDTC/RR-DTC (‘incomplete biochemical’ aHR (95% CI): 2.3 (1.1–5.1), Wald chi-square P = 0.0315 and ‘incomplete structural’ aHR (95% CI): 3.35 (1.5 - 7.2); Wald chi-square P = 0.0021) (Table 4).
Discussion
ERUDIT is a retrospective and longitudinal study of Spanish and Portuguese aDTC patients that reviewed, among others, their relevant demographic and clinical data at the time of their initial diagnosis and the therapeutic management that ensued. Prognostic factors impacting their survival as well as their relapse/progression from early into advanced stages have been also examined.
In total, 213 advanced DTC patients were retrospectively followed for a median of 6.2 years from their initial DTC diagnosis. The median age at diagnosis was 63 years, being more frequent in females than males, and with half of patients presenting at least one comorbidity, in most cases, a cardiovascular disorder. Papillary thyroid carcinoma was the most common histological type. Despite our study expressly selecting aDTC patients, these characteristics at initial diagnosis did not differ much from those previously reported in European or North American series covering a wider range of population (2, 17, 18, 19, 20).
Half (54%) of our patients were diagnosed with de novo aDTC and, of them, 98% presented with distant metastases affecting mainly lungs and bones. These findings are consistent with other studies also describing half of the advanced patients present with distant metastases at first diagnosis (4, 21, 22). In addition, more recent studies reported a higher prevalence of lung metastasis (49–75%) compared to bone metastasis (17–39%) in patients with aDTC at presentation regardless of the histological type (23, 24, 25). The remaining 46% in our series were eDTC patients who progressed into aDTC stages after receiving standard therapy (surgery ± RAI).
Initial DTC treatment followed standard recommendations, thus relying on surgery and RAI treatment (1). Consequently, different medical specialities were involved in disease management under a multidisciplinary team in over 73% of the cases after initial diagnosis. It is known that the implementation of multidisciplinary teams can help to optimise therapeutic results both for patients and for the healthcare system itself (11, 12) and, according to our observations, this aspiration was already becoming the standard in the Iberian Peninsula even before this was established as usual clinical practice (11, 26). ERUDIT data show that Endocrinology was the main responsible department followed by Nuclear Medicine and Oncology departments.
In this study, both mOS and mDSS measured since the initial DTC diagnosis were around 10 years in the global study population with no statistical differences observed between them. This observation implies that aDTC is the leading cause of death in these patients.
OS analyses by groups showed that recurrent/progressive eDTC patients had longer survival expectancy than those with advanced de novo aDTC diagnosis, independently of their disease extension. As previously reported, multivariate Cox model revealed that initial DTC diagnosis <55 years, RAI responsiveness (no evidence of post-RAI uptake and initial cumulative RAI (≥600 mCi)) were positive independent prognostic risk factors of longer OS in the global study population. Unlike other studies, gender, histological type, and metastatic localizations had no statistically significant impact on OS (21, 22, 25, 27). These discrepancies might be explained by the heterogeneity of the global population analysed, our limited sample size, and the many differences found in the published literature (21, 22, 25, 27). For example, Schlumberger et al. (22) in a similar study with 394 metastatic patients showed that age at the discovery of metastases and radioiodine avidity were favourable predictors of survival. Other studies found that older age at diagnosis, male sex, metastatic site (lung), and histology (follicular type) were significant factors for poor prognosis in patients with metastases at presentation (23, 24, 25, 27).
Regarding the recurrent/progressive eDTC cohort, we found an m(RP)FS of 2.3 years and an mOS of 15 years. Most relevant risk factors found in univariate and multivariate analysis showed that R0/R1 initial surgery and being eligible for rescue surgeries (local or metastatic) were protective factors for death, early relapse, or progression into aDTC/RR-DTC. However, this observation must be taken cautiously due to the small sample classified in the R2 group. Additionally, poor RAI responsiveness (low cumulative RAI doses, need for use of pre-RAI hrTSH, and ATA biochemical and structural incomplete RAI responses) after initial treatment was positively associated with a higher likelihood of death, premature relapse, or progression into advanced stages. Interestingly, not only patients achieving structural incomplete (0.6 years) but even biochemical incomplete responders (2.0 years) had a shorter m(RP)FS compared to those attaining excellent response (4.3 years), somewhat speaking of the precocious biological aggressiveness of the persistent tumours identified within our series. These observations not just confirm the prognostic relevance of the dynamic ATA risk stratification system (28) but also provide real estimates of the expected evolution that high-risk patients may have. This, in addition, to contradict the widely accepted evidence on the similar prognosis of these two populations (biochemical incomplete and excellent ATA response), should encourage second thoughts when first dealing with high-risk tumours in daily practice. In line with this, Sciuto et al. (19) retrospectively analysed 1553 subjects diagnosed with DTC and highlighted the relevance of RAI responsiveness as the strongest predictor of a good outcome in terms of survival. Several studies have described other independent predictors of disease persistence/recurrence which include male gender, diagnosis age >45, histology, high serum Tg levels, and the presence of distant metastases (19, 27, 29, 30). In our study, age at diagnosis, gender, and disease extension were statistically significant only in univariate analysis but not in the adjusted multivariate Cox model, probably due to sample size limitations and the aggressive behaviour that characterize our sample selection. However, common sense dictates early identification of these poor prognostic indicators since the initial DTC diagnosis and rapid implementation of commensurate therapeutic measures could greatly change the time to relapse, progression, or eventually death of these patients with aggressive tumour profile.
ERUDIT study presents several limitations and strengths. The main limitations involve its retrospective nature and limited sample size that very likely have underrepresented the actual aDTC incidence in the Iberian Peninsula despite this being outside the scope of the study. The reduced sample size, however, could have affected groups’ distribution, statistical power of uni- and multivariate analyses, and potentially introduced a selection bias mainly in the recurrent/progressive eDTC cohort. On the other hand, this study longitudinally follows for the first time relevant clinical data of European aDTC patients first diagnosed de novo or as recurrent/progressive from initial eDTC that have resulted in descriptive and prognostic information useful in everyday practice decision-making.
Conclusion
ERUDIT is the first epidemiological study that reviews the natural history of aDTC in Spain and Portugal. It has revealed interesting findings such that most aDTC tumours (93%) present with metastases at diagnosis of advanced disease (de novo or recurrent/progressive), patients die from their disease and not from other causes, and early disease diagnosis (eDTC) can utterly extend survival compared to advanced ones (de novo aDTC). Additionally, the study identified early poor treatment-dependent prognostic factors impacting both the pace at which early disease progresses into advanced stages and the overall survival expectancy of aDTC patients once they are as such diagnosed. These observations should be promptly considered and translated into a proactive follow-up and an intensified therapeutic attitude in this subpopulation. This could, at least potentially, reverse its doomed prognosis and positively impact disease evolution in many cases.
Supplementary Material
Declaration of interest
This article is the authors’ own work, and not an official position of the institutions or funder. Dr Juan Antonio Vallejo Casas reports grants, consultant fees and non-financial support from Eisai, during the conduct of the study; consultant fees and non-financial support from Sanofi, consultant fees from Bayer, consultant fees and non-financial support from Novartis, outside the submitted work; Dr Marcel Sambo reports grants and consultant fees from Eisai, during the conduct of the study; Dr Carlos López López reports grants and consultant fees from Bayer, grants and consultant fees from Eisai, grants and consultant fees from Pfizer, grants and consultant fees from Ipsen, grants and consultant fees from Novartis, grants and consultant fees from Lilly, during the conduct of the study; Dr Lorenzo Orcajo Rincón and Dr Julio Rodríguez-Villanueva García report employees from Eisai, with interests outside the submitted work; Dr Marta Llanos reports consultant fees from Roche, consultant fees from Amgen, consultant fees from Eisai, consultant fees from Ipsen, consultant fees from Servier, consultant fees from Lilly, consultant fees from Merck, outside the submitted work; Dr Elena Navarro-González reports consultant fees from Eisai, during the conduct of the study; Dr Javier Aller reports consultant fees and non-financial support from Eisai, during the conduct of the study; consultant fees from Eisai, consultant fees and non-financial support from Ipsen, outside the submitted work; Dr Guillermo Crespo reports grants from Ipsen, grants from Roche, grants from BMS, grants from Eisai, grants from Sanofi, grants from Janssen, grants from Eusa Pharma, grants from Pierre Fabre, outside the submitted work; Dr Cintia González reports other from Hospital Santa Creu i Sant Pau, during the conduct of the study; Dr Miguel Navarro reports consultant fees and non-financial support from Ipsen, non-financial support from Novartis, consultant fees and non-financial support from Bayer, outside the submitted work; Dr Javier Valdivia reports consultant fees from Novartis, MSD, Roche and Eisai during the conduct of the study; Dr Manel Puig-Domingo reports non-financial support from Eisai during the conduct of the study and, Dr María José Villanueva reports consultant fees from Novartis, and GSK, outside the submitted work. The remaining authors Dr Manuel Durán-Poveda, Dr Rita Joana Santos, Dr Virginia Pubul, Dr Sonsoles Guadalix, Dr Carles Zafón, Dr Javier Santamaría-Sandi, Dr Ángel Segura, Dr Pablo Gajate, Dr Marcelino Gómez-Balaguer, Dr Juan Carlos Galofré, Dr Beatriz Castelo, and Dr Iñaki Argüelles have nothing to disclose.
Funding
ERUDIT study was sponsored and funded by Eisai Farmacéutica S.A. (Madrid, Spain).
Author contribution statement
J A V C, C L L and M S contributed equally to methodology design, critical review of the study proposal, results and interpretation, data collection and writing of the original draft. M D P contributed to the critical review of results and interpretation. L O R and J R V G the conceived the idea, methodology design, coordination, execution of the research, and provided the financial support leading to this publication. J A V C and L O R contributed as corresponding authors on behave of the ERUDIT Study Group of Co-authors. R J S, M L, E N G, J A, V P, S G, G C, C G, C Z, M N, J S S, A S, P G, M G B, J V, M P D, J C G, B C, M J V and I A contributed to data collection and final draft review. All the above authors confirm that the study was performed in compliance with all basic principles of the Helsinki Declaration (2013) and applicable national regulations. Both accredited Research Ethics Committees of Hospital Universitario Gregorio Marañón (Madrid, Spain) and National Ethics Committee for Clinical Research (Portugal) approved it.
Acknowledgements
The authors of the ERUDIT Study Group thank Eisai Farmacéutica S.A. (Madrid, Spain) for funding the study support, to Lidesec S. L. (Madrid, Spain) for the operational management, to Medicxact S. L. (Madrid, Spain) for the statistical analysed support and Cristina Gil Roda Medical Writing (Barcelona, Spain) for the editorial assistance.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger Met al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016261–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dal Maso L, Tavilla A, Pacini F, Serraino D, van Dijk BAC, Chirlaque MD, Capocaccia R, Larrañaga N, Colonna M, Agius Det al. Survival of 86,690 patients with thyroid cancer: a population-based study in 29 European countries from EUROCARE-5. European Journal of Cancer 201777140–152. ( 10.1016/j.ejca.2017.02.023) [DOI] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology and End Results (SEER) P rogram , National Institutes of Health. Cancer stat facts: thyroid cancer, 2017. (available at: https://seer.cancer.gov/statfacts/html/thyro.html) [Google Scholar]
- 4.Haugen BR, Sherman SI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocrine Reviews 201334439–455. ( 10.1210/er.2012-1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kebebew E, Clark OH. Locally advanced differentiated thyroid cancer. Surgical Oncology 20031291–99. ( 10.1016/s0960-7404(0300032-x) [DOI] [PubMed] [Google Scholar]
- 6.Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A, Shah JP, Ganly I. Operative management of locally advanced, differentiated thyroid cancer. Surgery 2016160738–746. ( 10.1016/j.surg.2016.04.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capdevila J, Galofré JC, Grande E, Zafón Llopis C, Ramón Y Cajal Asensio T, Navarro González E, Jiménez-Fonseca P, Santamaría Sandi J, Gómez Sáez JM, Riesco Eizaguirre G. Consensus on the management of advanced radioactive iodine-refractory differentiated thyroid cancer on behalf of the Spanish Society of Endocrinology Thyroid Cancer Working Group (GTSEEN) and Spanish Rare Cancer Working Group (GETHI). Clinical and Translational Oncology 201719279–287. ( 10.1007/s12094-016-1554-5) [DOI] [PubMed] [Google Scholar]
- 8.Laetitia G, Sven S, Fabrice J. Combinatorial therapies in thyroid cancer: an overview of preclinical and clinical progresses. Cells 20209 830. ( 10.3390/cells9040830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tumino D, Frasca F, Newbold K. Updates on the management of advanced, metastatic, and radioiodine tefractory differentiated thyroid cancer. Frontiers in Endocrinology 20178 312. ( 10.3389/fendo.2017.00312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire Fet al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. Journal of Clinical Endocrinology and Metabolism 2006912892–2899. ( 10.1210/jc.2005-2838) [DOI] [PubMed] [Google Scholar]
- 11.Díez JJ, Galofré JC, Oleaga A, Grande E, Mitjavila M, Moreno P. Results of a nationwide survey on multidisciplinary teams on thyroid cancer in Spain. Clinical and Translational Oncology 2019211319–1326. ( 10.1007/s12094-019-02056-4) [DOI] [PubMed] [Google Scholar]
- 12.Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, Papotti MG, Berruti A. & ESMO Guidelines Committee. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019301856–1883. ( 10.1093/annonc/mdz400) [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association. WMA Declaration of Helsinki – ethical principles for medical research involving human subjects . (available at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/) [PubMed] [Google Scholar]
- 14.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey Jet al. RECIST 1.1-update and clarification: from the RECIST Committee. European Journal of Cancer 201662132–137. ( 10.1016/j.ejca.2016.03.081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, Dillehay G, Draganescu C, Flux G, Führer Det al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 201929461–470. ( 10.1089/thy.2018.0597) [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 19825649–655. ( 10.1097/00000421-198212000-00014) [DOI] [PubMed] [Google Scholar]
- 17.European Network of Cancer Registries. Thyroid cancer (TC) factsheet, 2017. (available at: https://www.encr.eu/) [Google Scholar]
- 18.Lee YK, Hong N, Park SH, Shin DY, Lee CR, Kang SW, Lee J, Jeong JJ, Nam KH, Chung WYet al. The relationship of comorbidities to mortality and cause of death in patients with differentiated thyroid carcinoma. Scientific Reports 20199 11435. ( 10.1038/s41598-019-47898-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Annals of Oncology 2009201728–1735. ( 10.1093/annonc/mdp050) [DOI] [PubMed] [Google Scholar]
- 20.Surveillance Epidemiology and End Results (SEER) Program, National Institutes of Health. Thyroid Cancer. Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2017 Observed Rates by Subtype. SEER*Explorer Application, 2020. (available at: (https://seer.cancer.gov/explorer/application.html?site=80&data_type=1&graph_type=2&compareBy=subtype&chk_subtype_651=651&chk_subtype_650=650&sex=1&race=1&age_range=1&stage=101&hdn_rate_type=1&advopt_precision=1&advopt_display=2) [Google Scholar]
- 21.Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, Patel SG, Ganly I. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 201222884–889. ( 10.1089/thy.2011.0535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F, Ricard M, Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. Journal of Nuclear Medicine 199637598–605. [PubMed] [Google Scholar]
- 23.Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clinical Endocrinology 20056387–93. ( 10.1111/j.1365-2265.2005.02304.x) [DOI] [PubMed] [Google Scholar]
- 24.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 20071101451–1456. ( 10.1002/cncr.22956) [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Kim HI, Kim SW, Jung J, Jeon MJ, Kim WG, Kim TY, Kim HK, Kang HC, Han JMet al. Prognosis of differentiated thyroid carcinoma with Initial distant metastasis: a nulticenter study in Korea. Endocrinology and Metabolism 201833287–295. ( 10.3803/EnM.2018.33.2.287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallardo E, Medina J, Sánchez JC, Viúdez A, Grande E, Porras I, Ramón Y Cajal T, Trigo J, Iglesias L, Capdevila J. SEOM clinical guideline thyroid cancer (2019). Clinical and Translational Oncology 202022223–235. ( 10.1007/s12094-019-02284-8) [DOI] [PubMed] [Google Scholar]
- 27.Cushing SL, Palme CE, Audet N, Eski S, Walfish PG, Freeman JL. Prognostic factors in well-differentiated thyroid carcinoma. Laryngoscope 20041142110–2115. ( 10.1097/01.mlg.0000149442.22393.e2) [DOI] [PubMed] [Google Scholar]
- 28.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010201341–1349. ( 10.1089/thy.2010.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BY, Choi JE, Lee E, Son YI, Baek CH, Kim SW, Chung MK. Prognostic factors for recurrence of locally advanced differentiated thyroid cancer. Journal of Surgical Oncology 2017116877–883. ( 10.1002/jso.24740) [DOI] [PubMed] [Google Scholar]
- 30.Kim Y, Roh JL, Song D, Cho KJ, Choi SH, Nam SY, Kim SY. Predictors of recurrence after total thyroidectomy plus neck dissection and radioactive iodine ablation for high-risk papillary thyroid carcinoma. Journal of Surgical Oncology 2020122906–913. ( 10.1002/jso.26090) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a