Abstract
Summary
Vasoactive intestinal peptide-secreting tumours (VIPomas) are an extremely rare form of functional pancreatic neuroendocrine tumour with an estimated annual incidence of 1 in 10 million. Associated tumour hypersecretion of other peptides, including pancreatic polypeptide (PPomas), may also be seen. These malignancies classically present with a defined triad of refractory diarrhoea, hypokalaemia and metabolic acidosis known as Verner–Morrison syndrome. Diagnosis is frequently delayed, and the majority of patients will have metastatic disease at presentation. Symptoms are usually well controlled with somatostatin analogue administration. Here we report a case of metastatic mixed VIPoma/PPoma-induced diarrhoea causing renal failure so severe that ultrafiltration was required to recover adequate renal function.
Learning points
Profuse, watery diarrhoea is a common presenting complaint with a multitude of aetiologies. This, combined with the rarity of these tumours, makes diagnosis difficult and frequently delayed. A functional neuroendocrine tumour should be suspected when diarrhoea is unusually extreme, prolonged and common causes have been promptly excluded.
These patients are likely to be profoundly unwell on presentation. They are extremely hypovolaemic with dangerous electrolyte and metabolic abnormalities. Aggressive initial rehydration and electrolyte replacement are imperative. A somatostatin analogue should be commenced as soon as the diagnosis is suspected.
This is an extreme example of Verner–Morrison syndrome. We are unaware of another case where renal failure secondary to diarrhoea and dehydration was so severe that renal replacement therapy was required to restore adequate renal function, further emphasising how critically unwell these patients can be.
Both the primary tumour and metastases showed a remarkably good and rapid response to somatostatin analogue administration. Cystic change and involution were noted on repeat imaging within days.
Prior to his illness, this patient was extremely high functioning with no medical history. His diagnosis was an enormous psychological shock, and the consideration and care for his psychological well-being were a crucial part of his overall management. It highlights the importance of a holistic approach to cancer care and the role of the clinical nurse specialist within the cancer multidisciplinary team.
Patient Demographics: Adult, Male, White, Ireland
Clinical Overview: Pancreas, Endocrine-related cancer
Related Disciplines: Surgery
Publication Details: Unique/unexpected symptoms or presentations of a disease, August, 2022
Background
Pancreatic neuroendocrine tumours (pNETs) constitute 1–2% of all pancreatic neoplasms (1). Arising from multipotent cells in the pancreatic islets, they have the ability to secrete biologically active peptides resulting in a diverse range of clinical features and presentations (2). Vasoactive intestinal peptide (VIP)-secreting tumours (VIPomas) are an extremely rare form of functional pNET with an estimated annual incidence of 1 in 10 million (2). The secretory component of pNETs may be mixed, with concurrent hypersecretion of pancreatic polypeptide (PPoma) seen in 50% of cases, most frequently VIPomas and glucagonomas (3). The extreme watery diarrhoea, hypokalaemia and achlorhydria/metabolic acidosis resultant of excessive serum levels of VIP was first described by Verner and Morrison in 1958 (4). Here we present a case of so-called ‘pancreatic cholera,’ severe enough to cause life-threatening renal failure requiring critical care admission and renal replacement therapy.
Case presentation
A 55-year-old Caucasian male presented to our hospital with an 8-month history of severe watery diarrhoea. He was opening his bowels 8–9 times in 24 h but had no other significant symptoms. He had recently presented to his general practitioner and had undergone a normal colonoscopy 3 days prior to admission. He had no past medical or surgical history and took no regular medications. He had no significant family history to note and was a non-smoker.
On arrival to the emergency department, he was lethargic and clinically dehydrated. Initial blood results revealed an estimated glomerular filtration rate (eGFR) of 14 mL/min/1.73 m2 and a creatinine of 381 μmol/L (8-115). Serum potassium was low at 2.5 mmol/L (3.5–5.3), and serum calcium was elevated at 2.88 mmol/L (2.2–2.6). Blood gases showed a normal anion gap metabolic acidosis with a pH of 7.16 (7.35–7.45), a base excess of −14.2 mmol/L and a normal lactate at 0.8 mmol/L (0.5–2). Inflammatory markers were within the normal range. Coeliac serology, a vasculitis screen and stool cultures all subsequently came back as negative.
The patient was admitted to the intensive care unit for central potassium replacement and aggressive rehydration with i.v. fluids. Parenteral nutrition was commenced due to poor absorption from nasogastric feeding.
Investigation
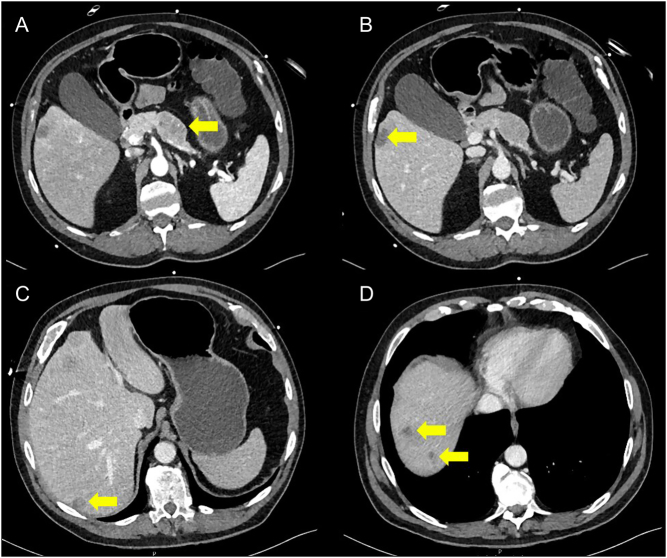
Due to ongoing diagnostic uncertainty, a contrast-enhanced CT scan of the abdomen was obtained. This showed a 32-mm heterogenous mass in the pancreatic body, as well as multiple hypo-attenuating lesions throughout the liver parenchyma. These hepatic lesions demonstrated peripheral rim enhancement on the arterial phase and were suspicious for metastases (Fig. 1). In the context of the clinical presentation, a metastatic functional pancreatic neuroendocrine tumour was suspected. Radiological stage was T2 N0 M1 (as per the European Neuroendocrine Tumour Society staging guidelines) (5).
Figure 1.
Axial sections of initial CT abdomen showing the pancreatic primary tumour (A) and liver metastases (B, C and D). The yellow arrows in panels B, C and D indicate liver metastases.
A fasting serum gut hormone profile was sent, and it showed 116 pmol/L (0–150) of somatostatin, 47 pmol/L (0–40) of gastrin, 66 pmol/L (0–50) of glucagon, 223 pmol/L (0–60) of chromogranin A and >4500 pmol/L (0–150) of C terminal end chromogranin B. VIP and PP were significantly elevated at 211 pmol/L (0–30) and 6928 pmol/L (0–300). For confirmatory purposes, a repeat profile was also sent and showed concordant results.
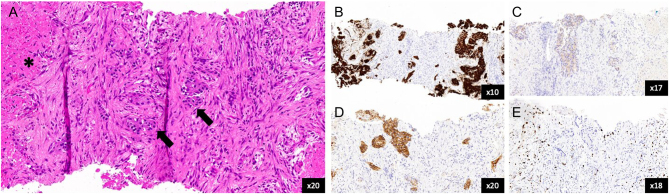
An ultrasound-guided core biopsy of a segment V liver lesion was performed. Histology was reported as mainly necrotic tissue with ‘ghosted’ tumour outlines in addition to small quantities of viable tumour cells. These expressed epithelial marker CK8/18 and neuroendocrine markers synaptophysin and CD56. Chromogranin A expression was negative. This confirmed the diagnosis of a well-differentiated grade I neuroendocrine tumour (Ki-67 proliferation index = 2.9%) with p53 WT expression (Fig. 2).
Figure 2.
Histopathology slides from core biopsy of the liver. (A) H&E stain showing nests of well-differentiated neuroendocrine tumour cells (arrows) and area of necrosis (asterisk). (B) Synaptophysin immunohistochemistry (IHC) stain. (C) Chromogranin IHC stain. (D) CD56 IHC stain. (E) Ki-67 IHC stain.
Treatment
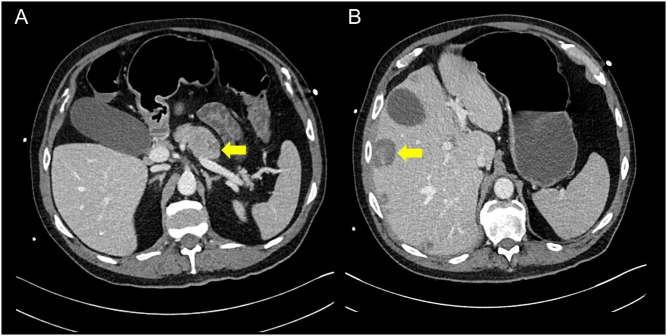
As soon as there was CT evidence of a pNET, an i.v. somatostatin analogue (SSA) (octreotide) infusion was initiated at 12.5 µg/h. Upon starting this, the patient’s diarrhoea settled completely and almost immediately. His renal function, however, still failed to improve, and several days after the liver biopsy, he began to spike high temperatures and inflammatory markers increased. A repeat CT showed that significant amounts of intra-intestinal fluid and blood cultures were positive for K lebsiella and Enterococcus. This study was also notable for significant autonecrosis of the pancreatic primary and liver lesions (Fig. 3).
Figure 3.
Axial sections of repeat CT abdomen showing cystic change and involution of the pancreatic primary tumour (A) and the liver metastases (B). The arrow in panel A indicates the pancreatic primary tumour; the arrow in panel B indicates a liver metastasis.
The patient was treated for bowel bacteria overgrowth sepsis, after other sources were excluded. Given the clinical deterioration and persistent failure to improve renal function with rehydration and control of the diarrhoea, ultrafiltration was commenced for 48 h. After this, renal function improved significantly and inflammatory markers settled. The patient was stepped down to the ward and was discharged 12 days later having been successfully converted to long-acting lanreotide injections. His eGFR at discharge was 54 mL/min/1.73 m2. He had been in hospital for 1 month.
Outcome and follow-up
Post-discharge, the patient remained under investigation for possible multiple endocrine neoplasia syndrome type 1 (MEN-1). As stated, serum calcium levels were elevated on admission and initial parathyroid hormone and prolactin levels were also raised. However, as these substances may be raised in acute illness, these will be repeated in the outpatient setting in the first instance.
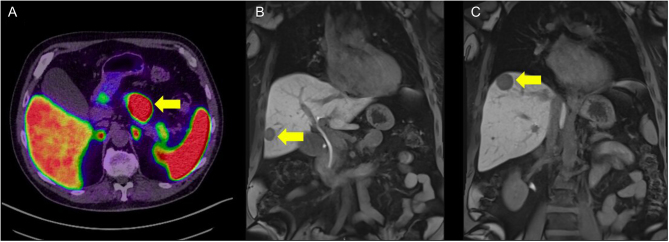
The patient has also had a Gallium-68 DOTA-TATE PET CT (Ga-68 PET/CT) and MRI of his liver (Fig. 4). These studies showed stable disease with a continued good response to SSA. There was no evidence of any disease beyond the pancreas and liver.
Figure 4.
Axial section of Ga-69 PET/CT showing PET-avid pancreatic primary (A) and coronal sections of liver MRI showing metastases (B and C)t The arrow in panel A indicates the pancreatic primary tumour; the arrows in panels B and C indicate liver metastases.
He will remain on lanreotide injections for a further 6 months before repeat Ga68 PET/CT and MRI imaging. If his disease remains stable, the plan at the time as of February 2022 was to proceed to surgical resection of the primary tumour and debulking of the liver lesions.
Discussion
VIPomas usually present as solitary tumours >3 cm in diameter (6). They most frequently occur in the pancreatic body or tail, although they have been described in extra-pancreatic locations such the liver, lung, colon, pituitary, thyroid, adrenals and sympathetic nervous system (7). Most are present in the fifth decade of life, and there is a small female preponderance (8). As in our case, 70% of VIPomas are metastatic by the time of presentation and 5% of cases are associated with MEN-1 (9, 10).
VIP is a 28-amino acid polypeptide which is structurally similar to secretin but functions exclusively as a neurotransmitter in the enteric neurones (11). It has a role in stimulating pancreatic secretion and intestinal smooth muscle, regulating enteric blood flow and inhibiting gastric acid secretion (12). In patients with a VIPoma, serum levels of VIP are abnormally and persistently elevated, resulting in cAMP-driven hypersecretion of H2O, K+, Na+, Cl– and HCO3 – throughout the gastrointestinal tract, thus accounting for Verner and Morrison’s observations (11). Hypercalcaemia and hyperglycaemia may also be seen, although the mechanism behind this is not entirely clear (7). Other nutritional and electrolyte deficiencies secondary to malabsorption are common (7). In addition, extra-intestinal features such as flushing, bloating, rash, nausea, vomiting, back pain and lethargy may be present (13).
That said, VIPoma diagnosis is difficult and frequently delayed. This is undoubtedly due to its rarity and the non-specific nature of its presentation. Plainly, chronic watery diarrhoea may be caused by a multitude of pathologies, and patients may well have had several normal investigations before a VIPoma is considered or chanced upon. Indeed, our patient underwent a colonoscopy only 3 days prior to admission on the suspicion of inflammatory bowel disease.
Clearly, VIPoma diagnosis does rely on raised serum levels of VIP. Clinicians should, however, note that false-positive serum gut hormone profiles are possible and repeat levels, taken after strict fasting and interpreted with appropriate radiological imaging, are advisable in the first instance (14). A mixed secretory component is not uncommon in pNETs and elevated levels of other substances, including PP, may be seen (3, 13). Hypersecretion of PP in isolation, however, is unusual, and these tumours represent <1% of all pNETS (15). The physiological role of PP remains somewhat unclear, and pure PPomas are ordinarily characterised as non-functional tumours as elevated levels of PP do not usually manifest as a defined clinical syndrome (16). Consequently, the diagnosis is usually only made once the tumour is large enough to cause mass-effect symptoms. Interestingly, if non-compressive clinical signs do occur with PPomas, they seem to mimic those of VIPoma and, given the significantly elevated levels of PP in this patient, it is possible that VIP was not exclusively responsible for the clinical presentation (15). Like VIPomas, PPomas are also associated with MEN-1 syndrome (15, 16).
As with all malignancies, cross-sectional imaging is vital to identify the location and size of the primary tumour and to accurately stage the disease. Contrast-enhanced CT remains the main imaging modality for pNETs, with MRI being particularly useful in differentiating smaller tumours (11). Nuclear medicine investigations such as somatostatin receptor scintigraphy and Ga-68 PET/CT are usually beneficial, as 80–90% of VIPomas are somatostatin receptor-positive (11).
Tissue diagnosis for pNETS relies heavily on immunohistochemical markers, particularly synaptophysin, chromogranin A, CD56 and Ki-67 (1). While chromogranin A is highly specific, absent or focal expression, as shown in this case, is well recognised and the diagnosis can be confirmed by positive expression of two other markers (in this case synaptophysin and CD56) (17). Histological examination enables appropriate tumour grading, usually with the World Health Organisation classification system (18).
As seen in this case, the management of VIPomas invariably requires an initial phase of aggressive fluid and electrolyte replacement. SSAs must be administered intravenously or subcutaneously and will achieve good control of diarrhoea in the majority of patients by reducing tumour polypeptide secretion (2). As described, this patient was commenced on an initial 12.5 µg/h i.v. octreotide infusion, with a plan for dose escalation if required. As it happened, he responded very well to this dosage, and dose increase was unnecessary. It should be noted, however, that much higher doses of SSA are required in patients with carcinoid crisis. Further pharmacological management with high dose steroid therapy may be required if the response to a SSA is inadequate. Ultimately, surgical resection is the preferred treatment modality and the only curative option for pNETs. Trans-arterial embolisation may be employed for hepatic disease. Where resection is not possible, continued therapy with a SSA forms the mainstay of VIPoma management (7). Although the aim of such therapy is symptom control, reduction in tumour burden has been noted (19). This was, in fact, particularly noticeable in our patient, with cystic changes and involution of the liver metastases being noted on CT just days after octreotide commencement (Fig. 3). Spontaneous tumour autonecrosis is another potential explanation for this finding, but this would be more expected in high-grade NETs rather than a grade 1 tumour.
This case clearly highlights the difficulties in VIPoma/PPoma diagnosis. It is also an extreme example of how critically unwell such patients can become before appropriate management is initiated. Indeed, we have not identified another case in literature where VIPoma/PPoma-induced diarrhoea caused renal failure so profound that ultrafiltration was required in order to restore adequate renal function. That said, this may also have been contingent on patient behaviour, with this particular patient being unwilling to seek medical advice in the first instance, as well as superimposed sepsis.
From the beginning, multidisciplinary management was essential to the management of a life-threatening and life-altering situation and demonstrates the importance of a holistic approach to cancer care. The patient was previously fit, well and very active. The diagnosis and treatment required were an enormous emotional shock, and the consideration and care for his psychological well-being were a crucial part of his overall management. It emphasises the importance of the clinical nurse specialist who was able to coordinate patient and family discussions, be an approachable point of contact and took time to fully explore the patient’s thoughts and fears.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Author contribution statement
George Brown and Anthony Mark Monaghan were involved in the hospital management of the patient and wrote the final manuscript. Rushda Rajak provided extensive input to the interpretation of pathological results, creation of histology figures and editing of sections of the manuscript relevant to pathology. Richard Fristedt, Emma Ramsey, Ma’en Al-Mrayat, Thomas Armstrong and Arjun Takhar were involved in senior management decisions as part of the neuroendocrine tumour multidisciplinary team. All contributed significantly to the case discussion and identification of learning points. Arjun Takhar was the clinician with overall responsibility for the patient. All authors have reviewed and approved the final manuscript.
References
- 1.Sun J.Pancreatic neuroendocrine tumors. Intractable and Rare Diseases Research 2017621–28. ( 10.5582/irdr.2017.01007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimitriadis GK, Weickert MO, Randeva HS, Kaltsas G, Grossman A. Medical management of secretory syndromes related to gastroenteropancreatic neuroendrocrine tumours. Endocrine-Related Cancer 201623R423–R436. ( 10.1530/ERC-16-0200) [DOI] [PubMed] [Google Scholar]
- 3.Adrian TE, Uttenthal LO, Williams SJ, Bloom SR. Secretion of pancreatic polypeptide in patients with pancreatic endocrine tumors. New England Journal of Medicine 1986315287–291. ( 10.1056/NEJM198607313150504) [DOI] [PubMed] [Google Scholar]
- 4.Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhoea and hypokalemia. American Journal of Medicine 195825374–380. ( 10.1016/0002-9343(5890075-5) [DOI] [PubMed] [Google Scholar]
- 5.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel Get al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016103153–171. ( 10.1159/000443171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bani Sacchi T, Bani D, Biliotti G. Are pancreatic vipomas paraneuron neoplasms? A clue to neuroectodermal origin of these tumors. Pancreas 1992787–97. ( 10.1097/00006676-199201000-00012) [DOI] [PubMed] [Google Scholar]
- 7.Schizas D, Mastoraki A, Bagias G, Patras R, Moris D, Lazaridis II, Arkadopoulos N, Felekouras E. Clinicopathological data and treatment modalities for pancreatic vipomas: a systematic review. Journal of B.U.ON. 201924415–423. (PMID: https://pubmed.ncbi.nlm.nih.gov/31127985/) [PubMed] [Google Scholar]
- 8.Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis and treatment. Chinese Journal of Cancer 201332312–324. ( 10.5732/cjc.012.10295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekhijian HS, O’Dorisio TM. Vipoma syndrome. Seminars in Oncology 198714282–291. (https://pubmed.ncbi.nlm.nih.gov/2820063/) [PubMed] [Google Scholar]
- 10.Parbhu SK, Adler DG. Pancreatic neuroendocrine tumors: contemporary diagnosis and management. Hospital Practice 201644109–119. ( 10.1080/21548331.2016.1210474) [DOI] [PubMed] [Google Scholar]
- 11.Ghaferi AA, Chohnacki KA, Long WD, Cameron JL, Yeo CJ. Pancreatic vipomas: subject review and one institutional experience. Journal of Gastrointestinal Surgery 200812382–393. ( 10.1007/s11605-007-0177-0) [DOI] [PubMed] [Google Scholar]
- 12.Holst JJ, Fahrenkrug J, Knuhtsen S, Jensen SL, Poulsen SS, Nielsen OV. Vasoactive intestinal peptide (VIP) in the pig pancreas: role of VIPergic nerves in the control of fluid and bicarbonate secretion. Regulatory Peptides 19848245–259. ( 10.1016/0167-0115(8490066-1) [DOI] [PubMed] [Google Scholar]
- 13.Peng SY, Li JT, Liu YB, Fang HQ, Wu YL, Peng CH, Wang XB, Qian HR. Diagnosis and treatment of vipoma in China: (case report and 31 cases review) diagnosis and treatment of vipoma. Pancreas 20042893–97. ( 10.1097/00006676-200401000-00015) [DOI] [PubMed] [Google Scholar]
- 14.Butler OL, Mekhael MM, Ahmed A, Cuthbertson DJ, Pritchard DM. Frequency and causes of false-positive elevated plasma concentrations of fasting gut hormones in a specialist neuroendocrine tumor center. Frontiers in Endocrinology 202011 606264. ( 10.3389/fendo.2020.606264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortenson M, Bold RJ. Symptomatic pancreatic polypeptide-secreting tumor of the distal pancreas (PPoma). International Journal of Gastrointestinal Cancer 200232153–156. ( 10.1385/IJGC:32:2-3:153) [DOI] [PubMed] [Google Scholar]
- 16.Kuo SC, Gananadha S, Scarlett CJ, Gill A, Smith RC. Sporadic pancreatic polypeptide secreting tumors (PPomas) of the pancreas. World Journal of Surgery 2008321815–1822. ( 10.1007/s00268-008-9499-7) [DOI] [PubMed] [Google Scholar]
- 17.Luong T, Watkins J, Chakrabarty B, Wang L. Standards and Datasets for Reporting Cancers Dataset for Histopathological Reporting of Neuroendocrine Neoplasms of the Gastroenteropancreatic Tract. London: Royal College of Pathologists, 2019. (available at: https://www.rcpath.org/profession/guidelines/cancer-datasets-and-tissue-pathways.html). Accessed on 12 May 2022. [Google Scholar]
- 18.Lloyd R, Osamura R, Kloppel G, Rosai J.Eds. WHO Classification of Tumours of Endrocrine Organs, 4th ed.Lyon, France: International; Agency; for; Research; on; Cancer, 2017. (ISBN: 978-92-832-4493-6) [Google Scholar]
- 19.Kraenzlin M, Ch’ng J, Wood S, Carr D, Bloom S. Longterm treatment of a VIPoma with somatostatin analogue resulting in remission of symptoms and possibly shrinkage of metastases. Gastroenterology 198588185–187. ( 10.1016/s0016-5085(85)80153-0) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a