Abstract
Summary
Leptin is secreted by adipocytes in response to fat storage and binds to its receptor (LEPR), which is ubiquitously expressed throughout the body. Leptin regulates energy expenditure and is anorexigenic. In this study, we describe the clinical and hormonal findings of three siblings with a personal history of rapid weight gain during the first months of life. They had delayed puberty, high levels of FSH (15.6 ± 3.7 mUI/mL; reference: 1.5–12.4) and LH (12.3 ± 2.2 mUI/mL; reference: 1.7–8.6), normal oestradiol and total testosterone and successful fertility. None of the patients had dyslipidemia, diabetes or thyroid disease. Next-generation sequencing identified a pathogenic homozygous variant c.2357T>C, p.(Leu786Pro) in LEPR. Their parents and children were heterozygous for this mutation. We compared clinical and biochemical findings of homozygous carriers with first-degree heterozygous family members and ten randomly selected patients with adult-onset morbid obesity. Homozygous carriers of the mutation had significantly higher BMI (32.2 ± 1.7 kg/m2 vs 44.5 ± 7.1 kg/m2, P = 0.023) and increased serum levels of leptin (26.3 ± 9.3 ng/mL vs 80 ± 36.4 ng/mL, P = 0.028) than their heterozygous relatives. Compared with the ten patients with adult-onset morbid obesity, serum levels of leptin were not significantly higher in homozygous carriers (53.8 ± 24.1 ng/mL vs 80 ± 36.4 ng/mL, P = 0.149), and thus serum levels of leptin were not a useful discriminative marker of LEPR mutations. We described a rare three-generation family with monogenic obesity due to a mutation in LEPR. Patients with early onset obesity should be considered for genetic screening, as the identification of mutations may allow personalized treatment options (e.g. MC4R-agonists) and targeted successful weight loss.
Learning points
The early diagnosis of monogenic forms of obesity can be of great interest since new treatments for these conditions are becoming available.
Since BMI and leptin levels in patients with leptin receptor mutations are not significantly different from those found in randomly selected morbid obese patients, a careful medical history is mandatory to suspect this condition.
Loss of leptin receptor function has been associated with infertility. However, our patients were able to conceive, emphasizing the need for genetic counselling in affected patients with this condition.
Patient Demographics: Adolescent/young adult, Female, Male, White, Portugal
Clinical Overview: Adipose tissue, Hypothalamus, Pituitary, Obesity
Related Disciplines: Genetics, Paediatrics, Surgery
Publication Details: Unique/unexpected symptoms or presentations of a disease, August, 2022
Background
Leptin is a 167 aminoacid product of the human leptin gene (1) produced mainly in adipocytes in response to fat storage. It binds to the leptin receptor (LEPR) which is ubiquitously expressed, particularly in hypothalamic neurons. The activation of LEPR elicits the critical role of leptin in energy balance and body weight homeostasis. Specifically, when leptin binds to LEPR in the hypothalamus, it generates α-melanocyte-stimulating hormone that activates the melanocortin-4 receptor (MC4R) inducing satiety (2). Mutations in LEPR cause a monogenic form of obesity characterized by hyperphagia and rapid weight gain starting in the first months of life. Its prevalence is estimated to be 3% of childhood-onset obesity (3), although the mean age at genetic diagnosis is 18 years old (4). A recent review estimated a prevalence of 1.34 per 1 million inhabitants in Europe (5) (95% CI: 0.95–1.72). Until now, at least 38 mutations of the human LEPR have been described (2), with 88 patients published worldwide (5). Not all of these mutations promote severe obesity, and no genotype–phenotype correlation has been ascertained to date (2). Another important clinical finding associated with LEPR mutations is hypogonadotropic hypogonadism (HH) (2), as leptin has a critical role in modelling – through the LEPR – the pulsatility of gonadorelin in the hypothalamus and the normal function of the hypothalamic–pituitary–gonadal axis.
Herein, we describe clinical and biochemical findings of a three-generation family with non-syndromic obesity caused by a mutation in LEPR, where the three probands were homozygous carriers. We compared their clinical and biochemical parameters with their heterozygous relatives for this mutation, and with patients with sporadic, severe adult-onset obesity, in an attempt to ascertain the discriminative value of these parameters for the presence of LEPR mutations. As far as we know, this work is one of the few publications in the world where the characteristics of a three-generation family with mutations in the LEPR are described. This is also one of the few reports where the genetic condition of all family members is known.
Case presentation
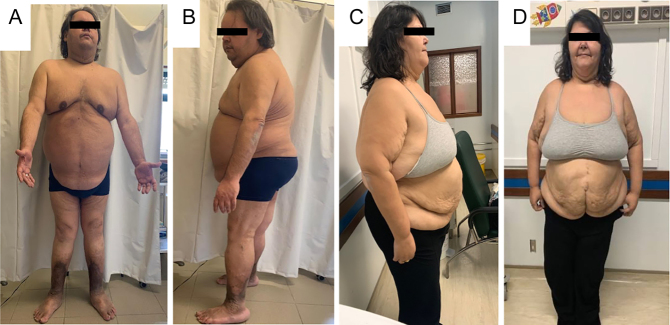
Three adult siblings of 36, 44 and 46 years old were first seen at our Endocrinology Department due to severe obesity, with BMI of 36.7, 46.1, and 50.7 kg/m2, respectively. They were born through eutocic deliveries from a non-consanguineous couple. Retrospectively, the three siblings had a normal birth weight, but during their first months of life, they presented permanent hyperphagia with rapid weight gain. In early childhood, affected individuals developed abnormal eating behaviors such as hoarding food and eating in secret. They used to hide food in their bedrooms to eat during the night. From 2 to 10 years old, their BMI increased from 25 to above 40 kg/m2 (>+3s.d.), despite keeping the linear growth within the expected target height. No dysmorphic features or cognitive impairment was perceived (Fig. 1A, B, C and D). The affected girl had her menarche at 16 years old and since then has regular menses. She got pregnant, spontaneously, at 36 years old. The boys started their puberty at 15 and 16 years old. Since then they have maintained FSH and LH levels above the upper limit of normal, although oestrogen and testosterone were within the normal range (Table 1). The oldest proband developed obstructive sleep apnoea during his fourth decade but refused to use a continuous positive airway pressure device. The probands parents are both obese and have type 2 diabetes. The father has a BMI of 31.0 kg/m2 and is taking metformin 1 g twice daily. He suffered a myocardial infarction at 62 years old. The mother has a BMI of 33.1 kg/m2 and is medicated with metformin 1 g twice daily plus gliclazide 60 mg/day.
Figure 1.
Photographs of family members homozygous for the leptin receptor (LEPR) mutation; (A and B) This 45-year-old male patient exhibits a exuberant abdominal fat apron and extensive lesions of acanthosis nigricans in the neck and axillary regions. There is evidence of lower extremity deformations associated with severe obesity as bilateral genu valgum and heavy pigmentation of the legs due to obesity-associated chronic venous insufficiency; (C and D) Phenotype of a 44-year-old female homozygous patient. As her older brother, she also presented a predominant abdominal obesity. The deformation in abdominal midline is due to a scar of a gastrointestinal by-pass surgery.
Table 1.
Clinical and laboratorial features of the three homozygous patients.
| Parameters | Mean ± s.d. | Normal range |
|---|---|---|
| Age (years) | 38.7 ± 6 | |
| BMI (kg/m2) | 44.5 ± 7.1 | 18.5–24.9 |
| Weight (kg) | 134.6 ± 16.9 | |
| Leptin (ng/mL) | 80 ± 34.6 | 2.0–60.0 |
| FSH (mUI/mL) | 15.6 ± 3.7 | 1.5–12.4 |
| LH (mUI/mL) | 12.3 ± 2.2 | 1.7–8.6 |
| Estradiol (pg/mL) | 36.4 ± 16.1 | 11–44 |
| Total testosterone (ng/dL) | 445 ± 401 | 249–836 |
| Insulin levels (mU/L) | 26.4 ± 15.8 | 5–10 |
| Glycemia (mg/dL) | 112.1 ± 33.9 | 80–110 |
| TSH (mIU/L) | 2.03 ± 1.4 | 0.5–4.5 |
| T4L (ng/dL) | 0.97 ± 1.0 | 0.8–1.8 |
| T3L (pg/mL) | 2.98 ± 0.59 | 2.3–4.2 |
| HbA1c (%) | 5.8 ± 1.0 | 4.0–5.6 |
| ACTH (pg/mL) | 41.4 ± 18.9 | 10–60 |
| IGF1 (ng/mL) | 140.9 ± 33.2 | 72–246 |
Investigation
The three siblings were insulin-resistant as ascertained by a HOMA_IR index of 7.19 (reference: <2.52) (Table 1). However, none of them had diabetes or dyslipidaemia. Thyroid function tests were also within the normal range. Next-generation sequencing identified a missense mutation in exon 16 of LEPR, c.2357 T> C, p. (Leu786Pro), in homozygosity, in the three siblings. This mutation was predicted to alter the LEPR protein sequence and is classified as ‘possibly damaging’ and ‘deleterious’ by the PolyPhen (http://genetics.bwh.har- vard.edu/pph2/) and the Sift (http://sift.jcvi.org/) web sites, respectively (4). Exons 15–19 of LEPR appear to be responsible for the translation of the binding site II of the LEPR, which is required for leptin binding to its receptor and interaction with the LEPR CRHII domain, which leads to the conformational changes in the leptin–LEPR complex that are paramount for further downstream signalling (2). The mutation was detected in heterozygosity in their parents and children (Fig. 2). BMI and serum leptin levels were significantly higher in homozygous compared with heterozygous carriers of LEPR mutation (Table 2). Women had higher BMI (42.05 ± 12.2 kg/m2 vs 37.9 ±7.5 kg/m2, P = 0.496) and also higher percentage of fat mass (46.4 ± 3.1% vs 34.9 ± 6.8%, P = 0.108). The fat mass was evaluated by bioelectric impedance analysis using a Tanita® BIA Technology (Tokyo, Japan). In brief, an electrical signal is sent through both feet and hands. It passes easily through water but not through fat. This resistance known as impedance is measured and subsequently validated to calculate body composition. We compared the clinical and hormonal findings of these subjects with those of their first-degree heterozygous relatives (Table 2). Additionally, we also compared the same parameters of homozygous carriers with those of ten randomly selected patients with adult-onset morbid obesity (Table 2). We found no significant differences regarding BMI and serum leptin levels in this last comparison (Table 2).
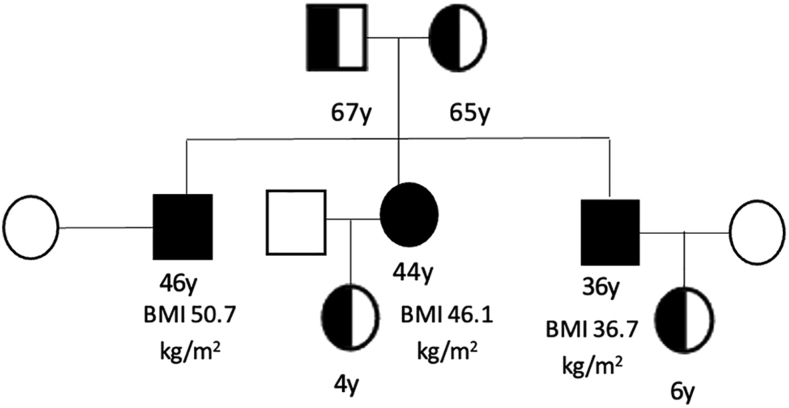
Figure 2.
Pedigree of the family showing affected (homozygous) and non-affected members (heterozygous) carriers of the leptin receptor (LEPR) mutation. BMI of the homozygous patients is shown. Two of the three homozygous siblings had children.
Table 2.
Comparison between family members harbouring the LEPR mutation and between homozygous patients for the LEPR mutation and ten randomly selected patients with adult-onset morbid obesity.
| Homozygous, n = 3 | Heterozygous, n = 4 | P | Homozygous, n = 3 | Controls, n = 10 | P | |
|---|---|---|---|---|---|---|
| Age (years) ± s.d. | 38.7 ± 6.0 | 35.5 ± 34 | 1.00 | 38.7 ± 6.0 | 41.2 ± 15 | 0.914 |
| BMI (kg/m2) ± s.d. | 44.5 ± 7.1 | 32.2 ± 1.7 | 0.023 | 44.5 ± 7.1 | 43.3 ± 5.6 | 0.876 |
| Weight (kg) ± s.d. | 134.6 ± 16.9 | 89.2 ± 15.2 | 0.021 | 134.6 ± 16.9 | 135.3 ± 21.9 | 0.212 |
| Leptin (ng/mL) ± s.d. | 80 ± 34.6 | 26.3 ± 9.3 | 0.028 | 80 ± 34.6 | 53.8 ± 24.1 | 0.149 |
| Fat mass (%) ± s.d. | 41.8 ± 6.8 | 36.2 ± 11.6 | 0.412 | 41.8 ± 6.8 | 48.3 ± 8.5 | 0.302 |
LEPR, leptin receptor.
Treatment
All patients were enrolled in nutrition consultation. The female proband was submitted to gastric bypass at 29 years old; she lost 27 kg during the first 6 months postoperatively. However, 1 year later, she regained weight to her preoperative state.
Outcome and follow-up
All members of this family have been followed by a multidisciplinary team of endocrinologists, psychologists and nutritionists at our Department for the last 20 years. Several nutritional plans were tried, always with reasonable patient compliance. Despite the efforts of our multidisciplinary team, maintenance of weight loss was never seen, and weight regain was the rule. The failure of bariatric surgery in one of the family members discouraged their relatives from accepting weight loss surgery.
Discussion
Herein, we report a family pedigree characterized by early onset obesity harbouring a LEPR mutation, with three homozygous probands, inherited from their non-consanguineous parents. Non-consanguineous parents are rare among families with LEPR mutations (2, 3, 4). Although the parents of our probands are allegedly non-consanguineous, the fact that they share the same mutation and their ancestry lives for centuries in a close geographical area of an island renders probable that these parents are related at some point of their pedigree. Unfortunately, we could not perform proper genetic ancestry surveys to elucidate this hypothesis. In physiologic conditions, a small rise in serum leptin levels reduces appetite. LEPR mutations cause a leptin-resistant state with permanent hyperphagia leading to severe obesity. The anorexigenic effect of leptin is also functionally disrupted in patients with sporadic obesity, indicating that leptin resistance is common in these individuals. Indeed, serum leptin levels were found to correlate positively with the amount of body fat in non-genetic obesity (1, 2). Our finding that serum leptin levels in homozygous patients were not significantly different from that of randomly selected patients with adult-onset morbid obesity underlines that serum leptin concentration is not a useful discriminative marker between sporadic cases and monogenic causes of obesity, as in LEPR mutations. This fact, combined with the low prevalence of monogenic obesity due to LEPR mutations in the population (which prevents the study of larger case series), explains the difficulty of the medical community in establishing criteria and thresholds to define leptin resistance (6). There is a previous published male patient harbouring the same LEPR mutation that was found in our kindred. This patient had a diagnosis of obesity at 18 months of age, which aggravated over the years – related to hyperphagia – to a final BMI of 45.2 kg/m2. He did not developed diabetes (HOMA_IR: 1.6) or dyslipidemia, but high serum leptin was noted. As an adult, he was submitted to a gastroplasty and loosed 44% of weight, but only 9 months of follow-up were reported. He was also diagnosed with HH (4).
LEPR mutations are associated with hyperphagia, which leads to severe obesity evidenced before 5 years old or earlier (e.g. during the first months of life). Additionally, another important clinical finding associated with LEPR mutations is HH (2). It is currently admitted that adequate leptin signalling is necessary for the onset of puberty and for pubertal growth (5). The pulsatile secretion of GnRH, which triggers LH and FSH secretion and gonadal activation, is altered in these patients, as leptin is a crucial modulator of a suitable pulsatility of GnRH that activates the hypothalamic–pituitary–gonadal axis (2). Interestingly, the patients herein described had delayed puberty and elevated LH and FSH values but could complete their puberty and achieve successful fertility. The prevalence of HH in patients with LEPR mutations is reported to be between 56 and 70% (4, 5). It is admitted that the rationale for this prevalence lies, in part, in the maintenance of some residual receptor activity associated with certain LEPR mutations (5), indicating that some LEPR mutations portent incomplete penetrance or at least some tissues of these affected patients are more sensitive to the leptin action than others. Further studies in this area are warranted to ensure proper genetic counselling for these patients and families. Thus, screening for monogenic causes of obesity should be considered in individuals with severe, early (<5 years old) onset obesity, particularly if hyperphagia is present and/or if there is a family history of severe obesity (7). Delaying the genetic diagnosis postpones the implementation of a personalized treatment that could induce significant weight loss in patients with mutations in several ‘obesity genes’, and thus may not reverse obesity-associated comorbidities and prevent premature death, which is especially relevant in adults with childhood-onset obesity (7). Additionally, submitting individuals with monogenic causes of severe obesity to standard treatments such as bariatric surgery has doubtful efficacy. In a small survey where six patients with LEPR mutations were submitted to bariatric surgery, three were not able to maintain the weight loss induced by surgery (2). The field of nutrigenomics also has not been able to provide an answer in terms of halting the progressive weight gain of patients with mutations in LEPR (8). Thus, finding a genetic cause for severe obesity may yield significant benefits for patients, as new therapies that reverse some degree of the pathogenic mechanism leading to obesity are becoming available. Setmelanotide, an MC4R agonist, was recently approved in the United States for the treatment of patients older than 6 years with monogenic obesity caused by pathogenic or likely pathogenic mutations of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1) and LEPR genes (9). In an investigator-initiated phase 2 trial, treatment with setmelanotide was evaluated in three patients with severe obesity with LEPR mutations. Substantial amelioration of hyperphagia was shown by reductions in hunger scores, and height losses of more than 10% of body weight were noticed. Interestingly, one of these patients had the same mutation in LEPR presented in our family, and he maintained a weight loss of more than 20% after 60 weeks of treatment (9). In accordance with these results, an open-label, phase 3 trial of setmelanotide including 11 patients with severe obesity caused by LEPR mutations (homozygous or compound heterozygous) showed a reduction of 44% in hunger scores and more than 10% weight loss in 45% of patients (10), with no serious adverse effects reported.
In conclusion, LEPR mutations should be considered in all patients with severe obesity starting in the first months of life, as targeted therapy is available with evidence for clinical benefit. Contrary to the majority of patients with LEPR mutations, pubertal development and successful fertility were achieved in our patients, making genetic counselling for these patients challenging.
Patient’s mother perspective
During several decades, I felt guilty and insecure because I was often worried that I was blamed for my children obesity. Was I overfeeding them? After all, many people including doctors told me that I was a bad mother by letting them become severely obese. Was this all my fault? The fact that they were permanently hungry raised suspicion of a familial condition, but nothing came out from the medical studies undertaken. Only recently it was found that all three have a mutated gene leading to increase appetite. It was a relief for me and for my children to finally understand their problem and explain it to family and friends.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent was obtained from the patients and parents of the patients included in this work.
Author contribution statement
Carolina Chaves planned and wrote the case report and performed literature search for writing discussion with references. João Anselmo and Teresa Kay planned and critically reviewed the final version of the manuscript.
References
- 1.Zhou Y, Rui L. Leptin signaling and leptin resistance. Frontiers of Medicine 20137207–222. ( 10.1007/s11684-013-0263-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunziata A, Funcke JB, Borck G, von Schnurbein J, Brandt S, Lennerz B, Moepps B, Gierschik P, Fischer-Posovszky P, Wabitsch M. Functional and phenotypic characteristics of human leptin receptor mutations. Journal of the Endocrine Society 2019327–41. ( 10.1210/js.2018-00123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. New England Journal of Medicine 2007356237–247. ( 10.1056/NEJMoa063988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huvenne H, Le Beyec J, Pépin D, Alili R, Kherchiche PP, Jeannic E, Frelut ML, Lacorte JM, Nicolino M, Viard A, et al. Seven novel deleterious LEPR mutations found in from Reunion Island, France, suggests a founder effect. Journal of Clinical Endocrinology and Metabolism 2015100E757–E766. ( 10.1210/jc.2015-1036) [DOI] [PubMed] [Google Scholar]
- 5.Kleinendorst L, Abawi O, van der Kamp HJ, Alders M, Meijers-Heijboer HEJ, van Rossum EFC, van den Akker ELT, Haelst MM. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. European Journal of Endocrinology 202018247–56. ( 10.1530/EJE-19-0678) [DOI] [PubMed] [Google Scholar]
- 6.Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 201912191–198. ( 10.2147/DMSO.S182406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovsky JA. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2017102709–757. ( 10.1210/jc.2016-2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crovesy L, Rosado EL. Interaction between genes involved in energy intake regulation and diet in obesity. Nutrition 201967–68 110547. ( 10.1016/j.nut.2019.06.027) [DOI] [PubMed] [Google Scholar]
- 9.Markham A.Setmelanotide: first approval. Drugs 202181397–403. ( 10.1007/s40265-021-01470-9) [DOI] [PubMed] [Google Scholar]
- 10.Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, De Waele K, Farooqi IS, Gonneau-Lejeune J, Gordon G, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet: Diabetes and Endocrinology 20208960–970. ( 10.1016/S2213-8587(2030364-8) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a