Abstract
The 5′ pufQ mRNA segment and the pufLMX mRNA segment of Rhodobacter capsulatus exhibit different stabilities. Degradation of both mRNA segments is initiated by RNase E-mediated endonucleolytic cleavage. While Rhodobacter RNase E does not discriminate between the different sequences present around the cleavage sites within pufQ and pufL, Escherichia coli RNase E shows preference for the sequence harboring more A and U residues.
The polycistronic puf operon of Rhodobacter capsulatus (Fig. 1) encodes the pigment binding proteins of the light harvesting I antenna complex (LHI) (PufB and PufA) and of the reaction center complex (PufL and PufM) and the proteins PufQ and PufX, which do not bind pigments but are required for the formation of photosynthetic complexes. The stoichiometry of LHI and reaction center complexes in the membrane is in part determined by the different stabilities of individual puf mRNA segments (13, 15). The processing of this polycistronic mRNA species has been extensively studied over the last decade (reviewed in reference 14). It was shown that decay of the 2.7-kb pufBALMX mRNA species (half-life of around 8 min under low oxygen tension) is initiated by endonucleolytic cleavage by RNase E at a specific recognition sequence within the pufL coding region (9). After initial cleavage, extremely rapid decay occurs in the 3′-to-5′ direction as well as in the 5′-to-3′ direction. A highly stable intercistronic secondary structure localized between pufA and pufL protects the pufBA mRNA segments against 3′-to-5′ exonucleases and is responsible for the higher stability of the pufBA mRNA (around 30 min) and consequently for the 15:1 molar excess of LHI versus reaction center complexes (13). PufQ is a protein that most likely serves a regulatory function, and it is present in the cells in very small amounts (7). It is encoded by the 5′ puf mRNA segment that undergoes rapid turnover (half-life of less than 1 min) (11). Decay of the primary puf transcript is initiated by RNase E at a specific sequence at the 3′ end of the pufQ coding region (11a). The RNase E recognition sequences which are involved in rate-limiting endonucleolytic cleavage within the pufQ and pufL coding sequences both resemble the consensus recognition sequence suggested for Escherichia coli RNase E, A/GAUUA/U (5), but are not identical. Initial RNase E-mediated cleavage at the 3′ end of pufQ occurs at the sequence GAUUUU; within the pufL coding region, RNase E cleaves the sequence GGCUUU. In order to find out whether the different decay rates of the 5′ puf mRNA segment encompassing the pufQ gene and the pufLMX mRNA segment are due to the differences in RNase E recognition sequences, we expressed a puf mRNA that carries the GAUUUU sequence at the 3′ end of the pufQ coding region as well as around position 1205 within the pufL coding region.
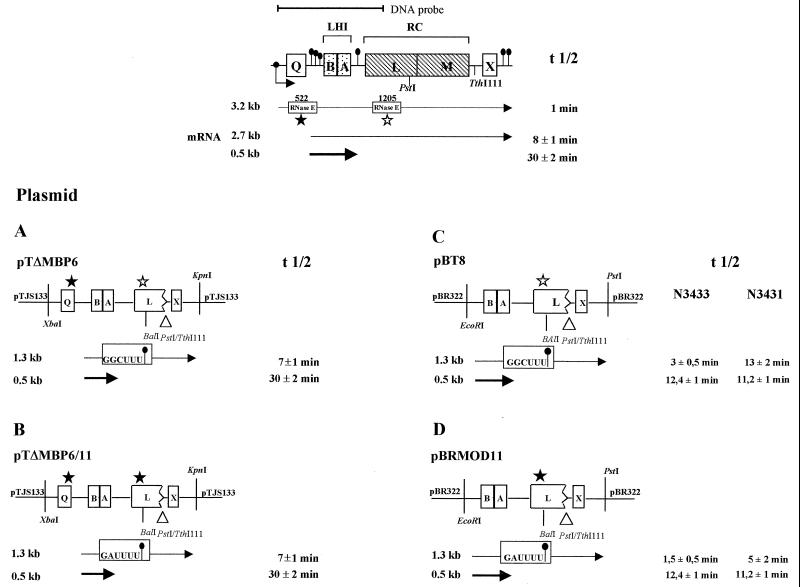
FIG. 1.
Schematic map of the R. capsulatus wild-type puf operon and the puf DNA segments present in the plasmids used in this study. The primary transcript, the stable 0.5-kb pufBA, and the 2.7-kb pufBALMX mRNA segments are indicated by arrows, and their half-lives (t 1/2) are on the side. The location of the oxygen-dependent puf promoter upstream of pufQ is shown by the bent arrow. The hairpin-loop regions forming a secondary structure at the mRNA level are indicated by pins. The RNase E cleavage sequence at position 522 is marked by a filled star, and the sequence of the RNase E cleavage site at position 1205 is signified by an open star. All plasmids investigated in this study carried a PstI-TthI111 deletion, and the positions of these restriction sites are given in the schematic map. Plasmids named pT are derivatives from pTJS133 (19, 23). Plasmids named pB are derived from pBR322 (2). mRNA segments detectable in Northern blots with a 1.7-kb DNA probe (shown on top) are located below the plasmid maps, and their respective half-lives are on the side. All half-lives were measured at least three times, and the means ± standard deviations are indicated.
Effect of modification of the RNase E recognition sequence at position 1205 within pufL on the rate of puf mRNA decay in R. capsulatus.
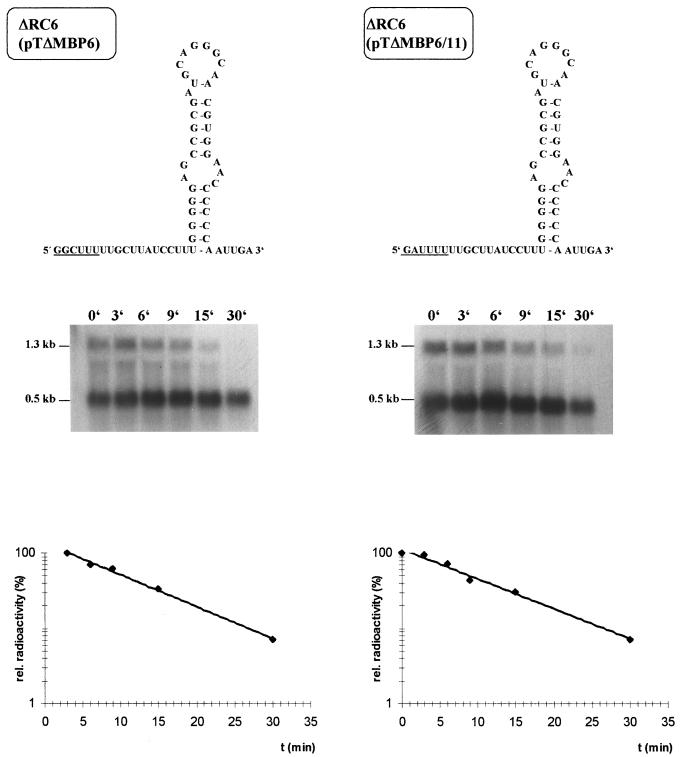
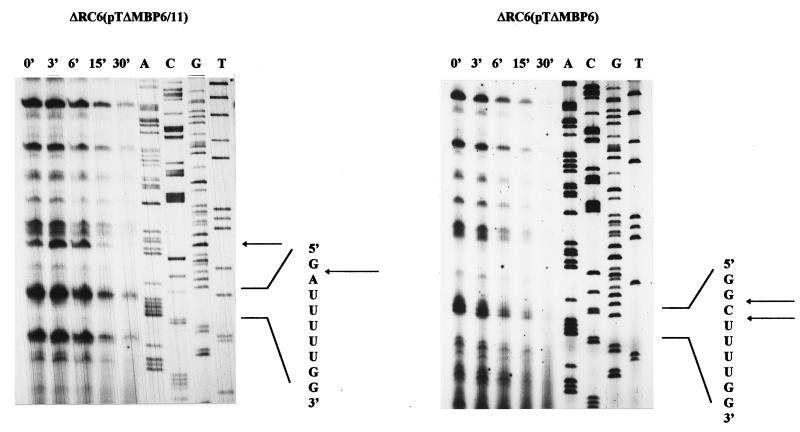
To study the effect of different sequences on RNase E-mediated mRNA cleavage, we constructed a number of plasmids with modifications of the puf DNA sequence (Fig. 1). All positions mentioned relate to the puf transcriptional start (+1). For analysis of the RNase E cleavage site within pufL, plasmid pTΔMBP6 (Fig. 1) was used in previous studies (9). This plasmid allows the expression of a puf mRNA which has a 1.2-kb segment with putative additional RNase E cleavage sites removed but the RNase E cleavage site around position 1205 present. The RNA sequence around the RNase E cleavage site is changed from GGCUUUUUGCUUAUCCUU to GGCUUUUGGCCAAUCCUU. This sequence modification showed no effect on the stability of the puf mRNA species in vivo (9) but created a new BalI recognition site, which was used for the introduction of further sequence modifications. Further modification of this sequence to AUCGAUUGGCCAAUCCA resulted in the prolongation of the half-life of the 2.7-kb pufBALMX mRNA in R. capsulatus from 8 to 20 min (9). We then constructed plasmid pTΔMBP6/11, which contains the sequence GAUUUUUGGCCAAUCCUU around position 1205. The sequence GAUUUU is identical to the sequence found directly at the RNase E recognition site at position 522 within pufQ. Both the RNase E recognition sequence around position 522 and the recognition sequence around position 1205 are followed by a hairpin loop structure with similar predicted stabilities (ΔG°′, −18.1 and −14.3 kcal/mol, respectively). Our cloning strategy retained the original locations of the hairpin loop structures. After a triparental conjugational transfer (12) with pRK2013 as a helper plasmid (8) into the Rhodobacter recipient strain ΔRC6 (3), which has the puf operon deleted from the chromosome, we analyzed the half-lives of the pufBAL/X mRNA in strains ΔRC6(pTΔMBP6) and ΔRC6(pTΔMBP6/11) by Northern blotting (Fig. 2). In both strains, the 1.3-kb pufBAL/X mRNA decayed with a half-life of 7 ± 1 min, indicating that the nucleotide sequence itself is not responsible for the different decay rates of the 5′ puf mRNA segment and the pufLMX mRNA segment. We also performed primer extension analysis in order to map the 5′ ends within and around the RNase E recognition sequences (Fig. 3). In strain ΔRC6(pTΔMBP6), the RNase E recognition sequence is cleaved at two sites, GG/C/UUUU. Cleavage within the recognition sequence G/AUUUUU also occurs in strain ΔRC6(pTΔMBP6/11). A similar cleavage pattern was observed for the cleavage motif GAUUUU at its original position, 522, in vitro. Cleavage sites for three 5′ ends within the putative RNase E recognition sequence were determined: between G/A, A/U, and U/U (data not shown). Additional 5′ ends at some distance from position 1205 are most likely the result of successive endonucleolytic cleavages which are involved in further mRNA degradation after rate-limiting cleavages have taken place (reviewed in reference 22). Surprisingly, an additional 5′ end occurs 13 nucleotides (nt) upstream of the RNase E cleavage site in strain ΔRC6(pTΔMBP6/11) (Fig. 3). This suggests that the sequence alteration around position 1205 can cause minor changes in the mRNA decay steps following rate-limiting cleavage.
FIG. 2.
Decay of pufBAL/X mRNA segments in control strain ΔRC6(pTΔMBP6) and mutant strain ΔRC6(pTΔMBP6/11). The wild-type RNase E site of strain ΔRC6(pTΔMBP6) and the modified RNase E site of strain ΔRC6(pTΔMBP6/11) are shown on top. Total RNA was isolated at various time points after addition of rifampin to a culture in the logarithmic growth phase (11). puf-specific mRNA segments were monitored by Northern blot analyses with a pufQBA DNA fragment as the probe as previously described (11). The half-life of the 1.3-kb pufBAL/X mRNA was determined by quantification of the radiolabelled bands by laser densitometry and plotting the values on a semilogarithmic scale as a function of time. The half-life for the pufBAL/X mRNA in both strains was measured to be 7 ± 1 (mean ± standard deviation) min.
FIG. 3.
Primer extension analyses to map the 5′ ends within and adjacent to the wild-type RNase E site at position 1205 in strain ΔRC6(pTΔMBP6) or the modified RNase E site in strain ΔRC6(pTΔMBP6/11) at the same position. After addition of rifampin, total RNA was isolated at the time points indicated. Each reaction contained 10 μg of total RNA. For primer extensions and the sequencing reaction as described previously (11, 16), we used the primer 5′-GGCGGCGGAAAGATGGAGATC-3′. The arrows mark the 5′ ends within the RNase E cleavage sites [between G/C and C/U in strain ΔRC6(pTΔMBP6) and G/A in strain ΔRC6(pTΔMBP6/11)]. An additional 5′ end 13 nt upstream of the modified RNase E site in strain ΔRC6(pTΔMBP6/11) was mapped, which is also marked with an arrow.
Effect of modification of the RNase E recognition sequence at position 1205 within pufL on the rate of puf mRNA decay in E. coli.
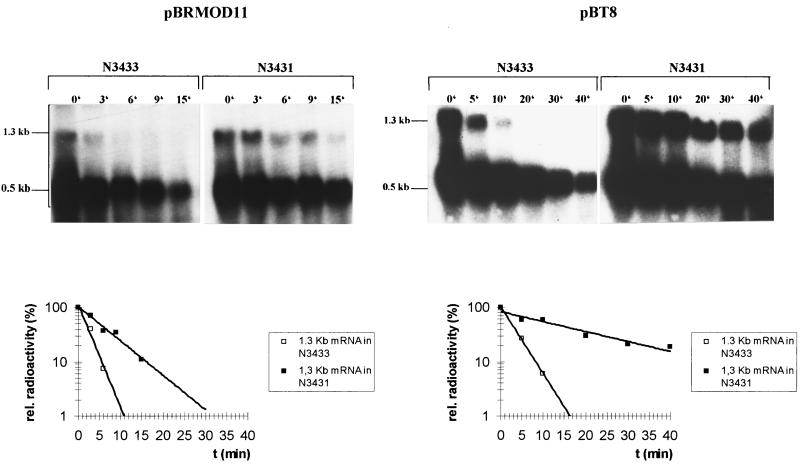
We also expressed the puf operon in E. coli in order to study the influence of the sequence alteration within the RNase E cleavage site around position 1205. To this end, we used plasmids pBPT8 and pBRMOD11 (Fig. 1), which allow transcription of the puf operon from the upstream bla promoter of plasmid pBR322 in E. coli. We transformed both plasmids into E. coli strain N3431, which expresses a temperature-sensitive RNase E, and into the isogenic wild-type strain N3433 (10). The decay rate of the 1.3-kb pufBAL/X mRNA was dependent on the sequence at the RNase E cleavage sites around position 1205 in both strains. While the presence of the sequence that naturally occurs at this position of pufL resulted in a half-life of the pufL/X mRNA segment of 3 ± 0.5 (mean ± standard deviation) min in strain N3433, the presence of the sequence that is identical to the RNase E cleavage site at position 522 within the pufQ coding region decreased the half-life to 1.5 ± 0.5 min (Fig. 4). The difference in cleavage rates was also observed in strain N3431 [rne(Ts)] at the nonpermissive temperature, at which only low RNase E activity is present. The 1.3-kb pufBAL/X mRNA exhibited a half-life of 13 ± 2 min in strain N3431(pBPT8) but one of only 5 ± 2 min in strain N3431(pBRMOD11) (Fig. 4). These results indicate that RNase E from E. coli discriminates between the two sequences, GGCUUU and GAUUUU, while RNase E from R. capsulatus does not. It is conceivable that RNase E from E. coli, an organism whose genome is 50% AT, has a stronger preference for sequences containing more A and U residues than RNase E from R. capsulatus, an organism whose genome is only 32% AT. The preference of E. coli RNase E for AU-rich sequences is in agreement with results from previous studies (5, 6, 18). When we analyzed the 5′ ends around the RNase E cleavage site at position 1205 of the puf mRNA by primer extension for both sequences expressed in E. coli, we found bands identical to those shown for the R. capsulatus strains ΔRC6(pTΔMBP6) and ΔRC6(pTΔMBP6/11) (data not shown).
FIG. 4.
puf mRNA decay in E. coli. After transferring plasmids pBT8 and pBRMOD11 into the E. coli strains N3433 (rne+) and N3431 [rne(Ts)], total RNA was isolated at different time points after addition of rifampin. An amount of 10 μg of total RNA in each lane was separated on a 1% agarose formaldehyde gel and transferred to a nylon membrane. After hybridization with the puf probe, we measured a half-life of 3 ± 0.5 (mean ± standard deviation) min for the 1.3-kb pufBAL/X mRNA in strain N3433(pBT8), and a half-life of 13 ± 2 min was determined for the same mRNA segment in strain N3431(pBT8). The 1.3-kb mRNA species in strain N3433(pBRMOD11) decayed with a half-life of 1.5 ± 0.5 min, whereas this mRNA segment of strain N3431(pBRMOD11) degraded with a half-life of 5 ± 2 min.
A number of polycistronic mRNAs have been shown to undergo processing that results in differences of the stabilities of individual mRNA segments (1, 4, 20, 21). However, it was not shown for any of these polycistronic transcripts which mechanisms initiate the decay of the individual mRNA segments. We showed previously that the decay of two puf mRNA segments exhibiting very different stabilities is initiated by the same mechanism: internal cleavage within a puf coding region at a specific recognition sequence by RNase E (9, 11a). Here we show that the differences in the rate of rate-limiting cleavage occurring within the 5′ puf mRNA segment and the pufLMX mRNA segment are not determined by the sequence directly surrounding the RNase E cleavage site. Structural analysis of the RNA (11) revealed that the cleavage site around pufL is in a single-stranded region, as is predicted for the cleavage site in pufQ by computer analysis (24) (the high instability of the 5′ puf mRNA segment does not allow an unambigous structural analysis [data not shown]). This suggests that additional factors, like ribosome density or tertiary RNA structure, are involved in determining the cleavage rates by RNase E. Context-dependent cleavage rates for RNase E for some monocistronic transcripts were described previously (17, 18). The sequence alteration of the RNase E recognition site within pufL which did not affect the half-life of the pufL/X mRNA segment in R. capsulatus showed, however, a clear influence on the stability of this mRNA segment in E. coli. Our data indicate that the RNase E enzymes from R. capsulatus and E. coli differ in regard to their preferences for the RNA recognition sequence. This observation will be of interest for future studies addressing the RNA-protein interaction that is the molecular basis for RNA recognition and cleavage by RNase E.
Acknowledgments
This work was supported by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Baga M, Goransson M, Normark S, Uhlin B E. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell. 1988;52:197–206. doi: 10.1016/0092-8674(88)90508-9. [DOI] [PubMed] [Google Scholar]
- 2.Bolivar F, Rodriguez R L, Greene P L, Betlach M L, Heynecker H L, Boyer H W, Crosa H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 3.Chen C Y, Beatty J T, Cohen S N, Belasco J G. An intercistronic stem-loop structure functions as an mRNA decay terminator necessary but insufficient for puf mRNA stability. Cell. 1988;52:609–619. doi: 10.1016/0092-8674(88)90473-4. [DOI] [PubMed] [Google Scholar]
- 4.Eddy C K, Keshav K F, An H, Utt E A, Mejia J P, Ingram L O. Segmental message stabilization as a mechanism for differential expression from the Zymomonas mobilis gap operon. J Bacteriol. 1991;173:245–254. doi: 10.1128/jb.173.1.245-254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehretsmann C P, Agamemnon J C, Krisch H M. Specificity of E. coli endoribonuclease E in in vivo and in vitro analysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev. 1992;6:149–159. doi: 10.1101/gad.6.1.149. [DOI] [PubMed] [Google Scholar]
- 6.Ehretsmann C P, Carpousis A J, Krisch H M. mRNA degradation in procaryotes. FASEB J. 1992;6:3186–3192. doi: 10.1096/fasebj.6.13.1397840. [DOI] [PubMed] [Google Scholar]
- 7.Fidai S, Kalmar G B, Richards W R, Bodford T J. Recombinant expression of the pufQ gene of Rhodobacter capsulatus. J Bacteriol. 1993;175:4834–4842. doi: 10.1128/jb.175.15.4834-4842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid pRK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritsch J, Rothfuchs R, Rauhut R, Klug G. Identification of an mRNA element promoting rate-limiting cleavage of the polycistronic puf mRNA in Rhodobacter capsulatus by an enzyme similar to RNase E. Mol Microbiol. 1995;15:1017–1029. doi: 10.1111/j.1365-2958.1995.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldblum B K, Apirion D. Inactivation of the ribonucleic acid-processing enzyme ribonuclease E blocks cell-division. Cell. 1978;15:128–132. doi: 10.1128/jb.146.1.128-132.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heck C, Rothfuchs R, Jäger A, Rauhut R, Klug G. Effect of the pufQ-pufB intercistronic region on puf mRNA stability in Rhodobacter capsulatus. Mol Microbiol. 1996;20:1165–1178. doi: 10.1111/j.1365-2958.1996.tb02637.x. [DOI] [PubMed] [Google Scholar]
- 11a.Heck, C., A. Balzer, O. Fuhrmann, and G. Klug. Initial events in the degradation of the polycistronic puf mRNA in Rhodobacter capsulatus and consequences for further processing steps. Mol. Microbiol., in press. [DOI] [PubMed]
- 12.Klug G, Drews G. Construction of a gene bank of Rhodopseudomonas capsulata using a broad host range cloning system. Arch Microbiol. 1984;139:319–325. doi: 10.1007/BF00408373. [DOI] [PubMed] [Google Scholar]
- 13.Klug G, Adams C W, Belasco J G, Dörge B, Cohen S N. Biological consequences of segmental alterations in mRNA stability: effects of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J. 1987;6:3515–3520. doi: 10.1002/j.1460-2075.1987.tb02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klug G. The role of mRNA degradation in the regulated expression of bacterial photosynthesis genes. Mol Microbiol. 1993;9:1–7. doi: 10.1111/j.1365-2958.1993.tb01663.x. [DOI] [PubMed] [Google Scholar]
- 15.Klug G, Cohen S N. Combined actions of multiple hairpin loop structures and sites of rate-limiting endonucleolytic cleavage determine differential degradation rates of individual segments within polycistronic puf operon mRNA. J Bacteriol. 1990;172:5140–5146. doi: 10.1128/jb.172.9.5140-5146.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klug G, Jäger A, Heck C, Rauhut R. Identification, sequence analysis, and expression of the lepB gene for a leader peptidase in Rhodobacter capsulatus. Mol Gen Genet. 1997;253:666–673. doi: 10.1007/s004380050370. [DOI] [PubMed] [Google Scholar]
- 17.Lin-Chao S, Wong T T, McDowall K J, Cohen S N. Effects of nucleotide sequence on the specificity of rne-dependent and RNase E-mediated cleavages of RNA I encoded by the pBR322 plasmid. J Biol Chem. 1994;269:10797–10803. [PubMed] [Google Scholar]
- 18.McDowall K J, Lin-Chao S, Cohen S N. A+U content rather than a particular nucleotide order determines the specificity of RNase E cleavage. J Biol Chem. 1994;269:10790–10796. [PubMed] [Google Scholar]
- 19.Narro M L, Adams C W, Cohen S N. Isolation and characterization of Rhodobacter capsulatus mutants defective in oxygen regulation of the puf operon. J Bacteriol. 1990;172:4549–4554. doi: 10.1128/jb.172.8.4549-4554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newbury S F, Smith N H, Higgins C F. Differential mRNA stability controls relative gene expression within a polycistronic operon. Cell. 1987;51:1131–1143. doi: 10.1016/0092-8674(87)90599-x. [DOI] [PubMed] [Google Scholar]
- 21.Oelmueller U, Schlegel H G, Friedrich C G. Differential stability of mRNA species of Alcaligenes eutrophus soluble and particulate hydrogenases. J Bacteriol. 1990;172:7057–7064. doi: 10.1128/jb.172.12.7057-7064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauhut R, Klug G. mRNA degradation in bacteria. FEMS Microbiol Rev. 1999;23:353–370. doi: 10.1111/j.1574-6976.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 23.Schmidhauser T J, Helinski D R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985;164:446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981;9:133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]