Summary
The thalamus is known to process information from various brain regions and relay it to other brain regions, serving an essential role in sensory perception and motor execution. The thalamus also receives inputs from basal ganglia nuclei (BG) involved in value-based decision making, suggesting a role in the value process. We found that neurons in a particular area of the rhesus macaque posterior thalamus encoded the historical value memory of visual objects. Many of these value-coding neurons were located in the suprageniculate nucleus (SGN). This thalamic area directly received anatomical input from the superior colliculus (SC), and the neurons showed visual responses with contralateral preferences. Notably, the value discrimination activity of these thalamic neurons increased during learning, with the learned values stably retained even more than 200 days after learning. Our data indicate that single neurons in the posterior thalamus not only processed simple visual information but also represented historical values. Furthermore, our data suggest a SC-posterior thalamus-BG-SC subcortical loop circuit that encodes the historical value, enabling a quick automatic gaze by bypassing the visual cortex.
eTOC
Kim et al. finds a subcortical loop circuit that generates habitual actions quickly. The posterior thalamus in the loop circuit stably retains reward value memories, even more than 200 days after learning. Historical values can be processed through this short subcortical circuit bypassing the visual cortex, thus guiding fast-automatic actions.
Introduction
A role of the thalamus is thought to be a relay center connecting different brain areas, generally making loop circuits [1,2]. The thalamus connects areas of the cortex and subcortex, including structures in the basal ganglia that play important functions in object value processing and value-guided decisions [3]. These anatomical connections suggest the thalamus may be involved in object value processing for choice behavior.
Encoding historical values of objects in the brain is critical for automatic gaze [4]. The historical object value is encoded by neurons in the caudal regions of the basal ganglia structures, including the tail of caudate nucleus (CDt), the caudal-ventral part of the globus pallidus externus (cvGPe), and the caudal-dorsal-lateral part of the substantia nigra pars reticulata (cdlSNr) [5–8]. These structures in the basal ganglia guide automatic gaze of macaque monkeys.
The visual information for automatic gaze is known to go through a pathway to the basal ganglia: through the retina, lateral geniculate nucleus (LGN), visual cortex, and temporal cortex to the CDt [9–11]. Given that automatic behavior is fast and accurate to maximize efficiency, the number of synaptic nodes in the pathway should be minimized. Notably, the caudal region of the striatum receives direct anatomical inputs from both the sensory cortex and the sensory thalamus that process the visual information [10,12,13]. This leads to a hypothesis that the automatic gaze pathway minimizes synaptic nodes by bypassing the sensory cortex and relaying the visual information directly from the sensory thalamus to the caudal striatum.
To examine the visual sensory thalamus and its role in value processing, we first identified an area of the thalamus that receives a direct input from the superior colliculus (SC) and investigated its visual response properties. We then tested whether the thalamic neurons only processed simple visual information or also processed the values of visual objects.
Results
Visual neurons in the posterior thalamus
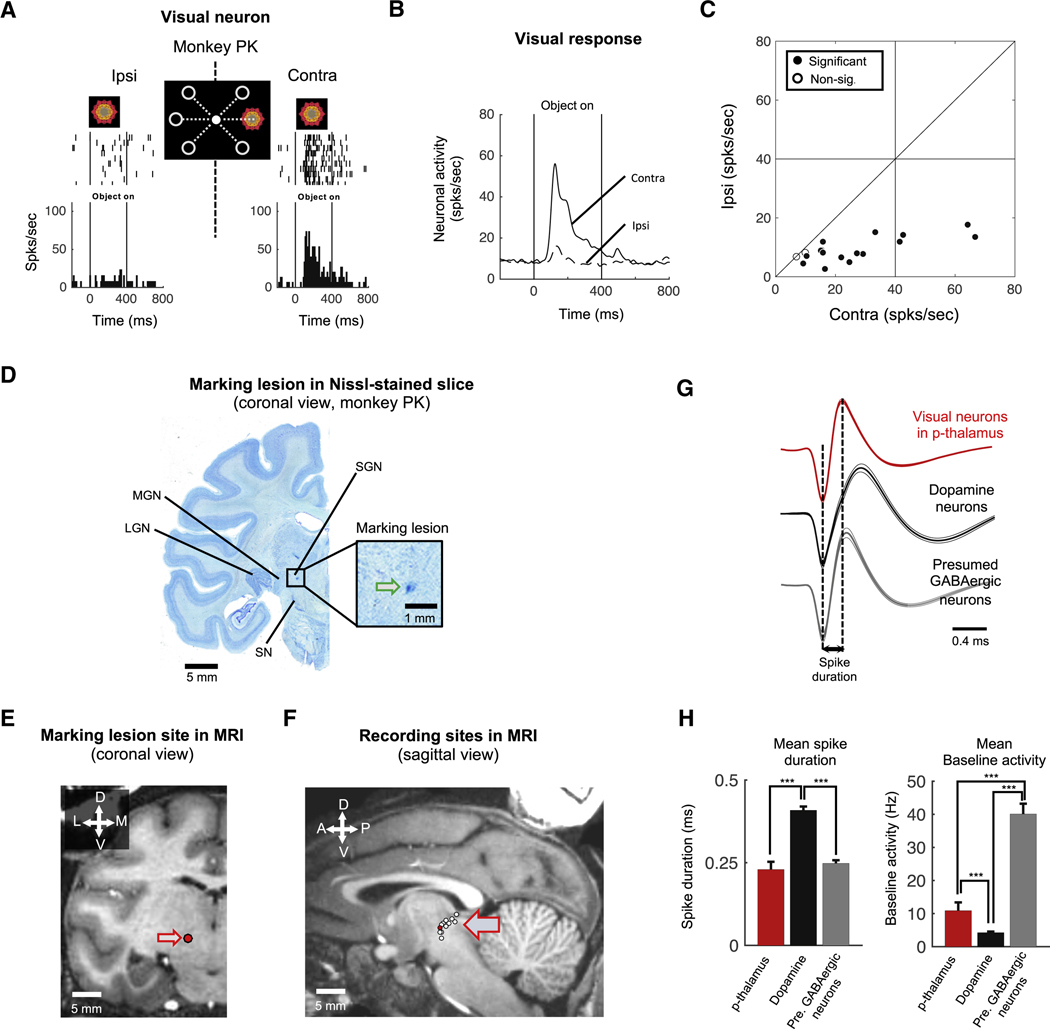
To identify visually responsive thalamus neurons, we recorded single-unit neuronal activity from the thalamus of two monkeys (monkey PK and DW) while the monkeys performed a spatial preference mapping task. In this task, a fractal object was presented sequentially at different peripheral positions as the monkey kept fixated on a central white dot (Figure 1A, middle). An example neuron from monkey PK showed a preference for contralateral visual stimuli (Figure 1A). As a population, visual neurons in the thalamus were more excited by objects presented in the contralateral visual field than ipsilateral field (Figure 1B). 15 out of 17 visual neurons tested in the spatial preference mapping task (88.2%; p < 0.05, two-tailed t-test) responded more strongly to the contralateral objects (Figure 1C).
Figure 1. Visually responsive posterior thalamus neurons.
A. Visual response of an example neuron in the p-thalamus of monkey PK. The example neuron showed stronger responses to a fractal object presented in the contralateral visual hemifield than in the ipsilateral hemifield. B. Population response of visual neurons (n = 17). Neurons in the p-thalamus more strongly responded to contralateral visual stimuli than ipsilateral stimuli. C. Spatial selectivity of visual response. Each data point indicates the responses of a neuron to ipsilateral (ordinate) and contralateral (abscissa) objects. Filled dots indicate neurons whose spatial selectivity is statistically significant (Wilcoxon rank-sum test, p < 0.05). Non-sig., non-significant. D. Marking lesion site of the example neuron in figure 1A. The electrical marking lesion indicated the example neuron was dorsal to the substantia nigra and medial to the medial geniculate nucleus in the posterior region of the thalamus (Blue dot indicated by green arrow). SGN: Suprageniculate nucleus, MGN: Medial geniculate nucleus, LGN: Lateral geniculate nucleus, SN: Substantia nigra. E. Marking lesion site in MR image. The marking lesion site was reconstructed in MR image (Red dot indicated by arrow). F. Recording sites of visual neurons in stereotaxic coordinates. Visual neurons were plotted by white dotes on the sagittal MR image. Red dot indicates the position of marking lesion. G-H. Electrophysiological properties. Spike shapes of visual neurons in the p-thalamus, dopamine neurons, and GABAergic neurons in the substantia nigra (G). Bar graphs show mean spike duration and mean baseline activity of each group of neurons (H).
Such visual neurons were found in the posterior region of the thalamus (p-thalamus) (Figure 1D-F). MRI reconstruction of the recorded neurons indicated that these visual neurons were located in a broad region of the posterior thalamus (Figure 1F). To more precisely identify our recording sites, we made an electrical marking lesion in monkey PK after recording from the example neuron (Figure 1A). After coronally sectioning the brain, we found the lesion located dorsal to the substantia nigra (SN) and medial to the medial geniculate nucleus (MGN) (Figure 1D and E), in the suprageniculate nucleus (SGN) [12,14,15].
We further confirmed our recording location by comparing the electrophysiological properties of the presumed p-thalamic neurons to neurons in the SN, which is located just below the p-thalamus (Figure 1D). The p-thalamic neurons were different in both spike shape and baseline firing rate from SN neurons (Figures 1G and 1H). Compared with the SN dopamine neurons, the p-thalamic neurons had narrower spikes and lower baseline firing rate (one-way ANOVA with post-hoc multiple comparisons, F(2, 134) = 77.78 and F(2, 135) = 56.36, respectively, p < 0.0001) (Figure 1H). Compared to the presumed GABAergic neurons in the SN, the p-thalamic neurons had lower baseline firing rate (one-way ANOVA with post-hoc multiple comparisons, p < 0.0001) (Figure 1H, right panel).
Superior colliculus inputs to the posterior thalamus
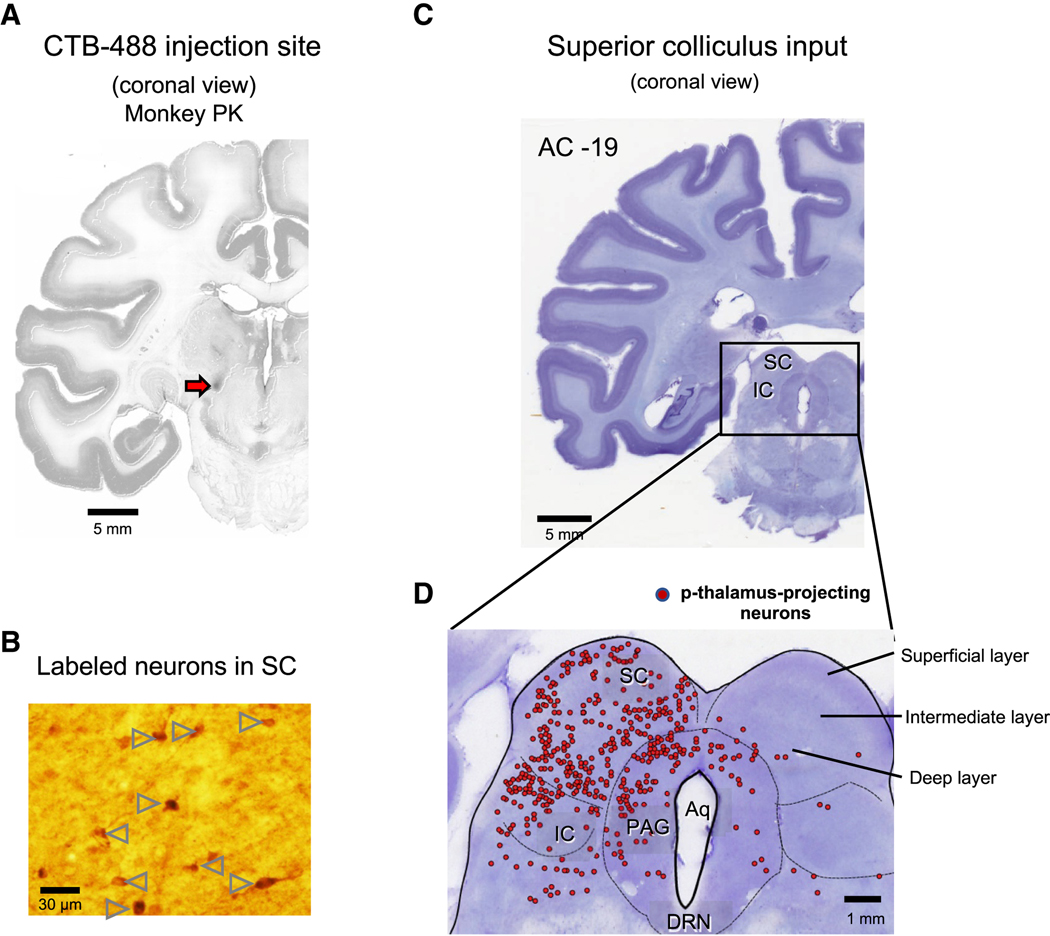
We next investigated the inputs to the p-thalamic neurons anatomically. We injected the neurotracer Alexa Fluor 488-conjugated cholera toxin subunit B (CTB-488) in monkey PK at the recording site of the example neuron in Figure 1A (Figure 2A). After waiting 2 weeks for sufficient retrograde transport of the neurotracer, we sacrificed PK and performed immunohistochemistry. We used an antibody against Alex Flour 488 (anti-AF488) to immunolabel the CTB-488 signals. The CTB-488 injection signal was mainly confined to the electrical marking lesion site (Figures 2A and 1D). Notably, we found neurons labeled by anti-AF488 antibody in the superior colliculus (SC), the inferior colliculus (IC), the periaqueductal grey (PAG), and the pedunculopontine tegmental nucleus (PPTg) of the brain stem (Figures 2B-D and Figure S1B). We also found immunolabeled cells in other brain areas (Figure S1). Most of the labeled neurons in the SC were found in the hemisphere ipsilateral to the injection site (Figures 2C and 2D). The labeled neurons in the SC were widely distributed in the superficial, intermediate, and deep layers (Figure 2D).
Figure 2. Superior colliculus projects to the posterior thalamus.
A. Injection site of retrograde tracer. We injected cholera toxin subunit B conjugated with Alexa Fluor-488 (CTB-488) into the region of the p-thalamus where the marking lesion was made (Red arrow). Slices were stained by anti-Alexa Fluor 488 antibody to detect the CTB-488 with high sensitivity. B. Retrogradely labeled neurons in the superior colliculus (SC). Arrow heads indicate the SC neurons projecting to the p-thalamus labeled by anti-Alexa Fluor 488 antibody. C. Coronal view of brain stem including the SC, inferior colliculus (IC), and periaqueductal grey (PAG). AC: Anterior commissure. D. Retrogradely labeled neurons in the SC, IC, and PAG. Red dots indicate the p-thalamus-projecting neurons in the SC, IC, and PAG.
See also Figure S1.
Value discrimination activity in the posterior thalamus during object value learning
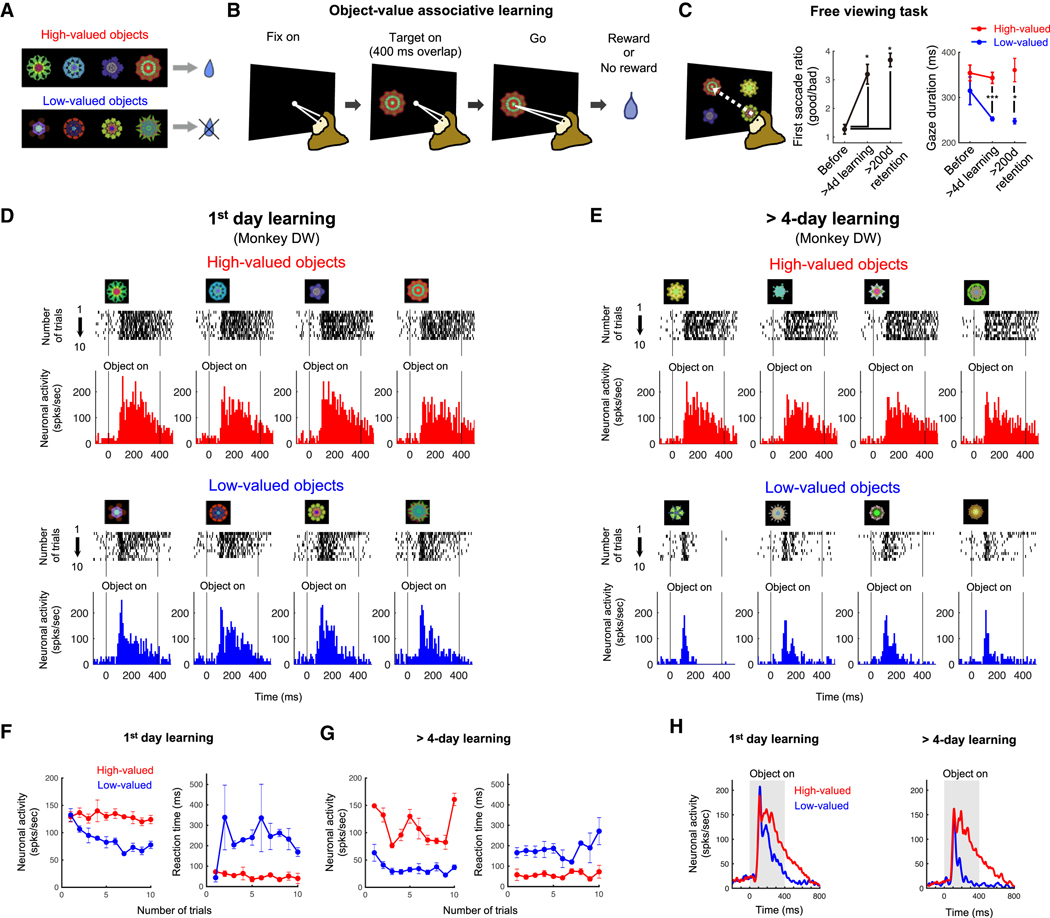
So far, we found visually responsive neurons with spatial selectivity in the p-thalamus, and that these neurons received inputs from areas of the brain stem involved in sensory processing, e.g. the SC and IC. We then asked whether these visual neurons were sensitive to the values associated with different visual objects. To examine neuronal encoding of object value during learning, we used the stable value learning task (Figures 3A and 3B). For the task, a set of 8 visual fractal objects is divided into 2 groups: high-valued objects (consistently associated with a reward) and low-valued objects (consistently associated with no reward) (Figure 3A). Neuronal response for the value learning was examined while the monkey fixated at a central white dot during object presentation (400 ms overlap period) (Figure 3B). After the fixation dot disappeared, the monkey made a saccade to the fractal object to get the predetermined reward outcome. To test the behavioral effect of the object-reward association, monkeys performed a free viewing task (Figure 3C). 4 objects from a set of 8 objects were chosen randomly and presented on the screen, and the monkeys looked at them freely for 2 seconds (Figure 3C, left panel). No reward outcome was delivered during free viewing. After more than 4 days of object-value associative learning, the monkeys showed gaze bias toward high-valued objects: increased percentage of first saccades to high-valued objects and shortened gaze duration on low-valued objects (p < 0.05 and p < 0.0001, respectively, two-tailed t-test) (Figure 3C, middle and right panels). The monkeys showed clear gaze bias toward high-valued objects, even 200 days after the last learning session (Figure 3C).
Figure 3. Neural representation of object value during object-value associative learning.
A. Fractal objects associated with a reward (high-valued) or no reward (low-valued). Monkey PK and DW learned 136 and 152 fractal objects, respectively. Among the learned objects, 32 and 80 objects were long-term learned (> 4 days) for each monkey, respectively. B. Object-value associative learning task. A fractal object was presented at a neuron’s preferred position. The monkey made a saccade to it after the central fixation point turned off. Reward or no reward was given depending on the assigned object-value association. A set of 8 objects (as shown in A) was used in each learning session. C. Free viewing task for testing automatic gaze to previously learned objects. Four of eight objects in one set was randomly chosen and presented, and the monkey freely looked around without direct reward outcome. After more than 1-day or 200-days retention time since the last day of long-term learning (>4 learning days), the monkeys showed gaze preference for the high-valued objects (mean ± SE). *p < 0.05 and ***p < 0.0001. D and E. Responses of an example neuron to 8 objects during learning of novel objects and long-term experienced objects. Spike (black ticks) were aligned to object presentation time, and the average responses to 4 high-valued object (red) and 4 low-valued object (blue) were plotted in the histogram graphs. F and G. Value discrimination and saccade reaction time difference during the first learning day and learning of long-term experienced objects. Neuronal activities and saccade reaction times to high-valued (red) and low-valued (blue) objects were plotted against the number of trials. H. Value discrimination activities of the example neuron from Figures 1D-E to the 1-day learned and long-term learned objects during the learning task. Neuronal responses to the high-valued (red) and low-valued (blue) objects were shown using peristimulus time histograms (PSTH). See also Figure S2 and S3.
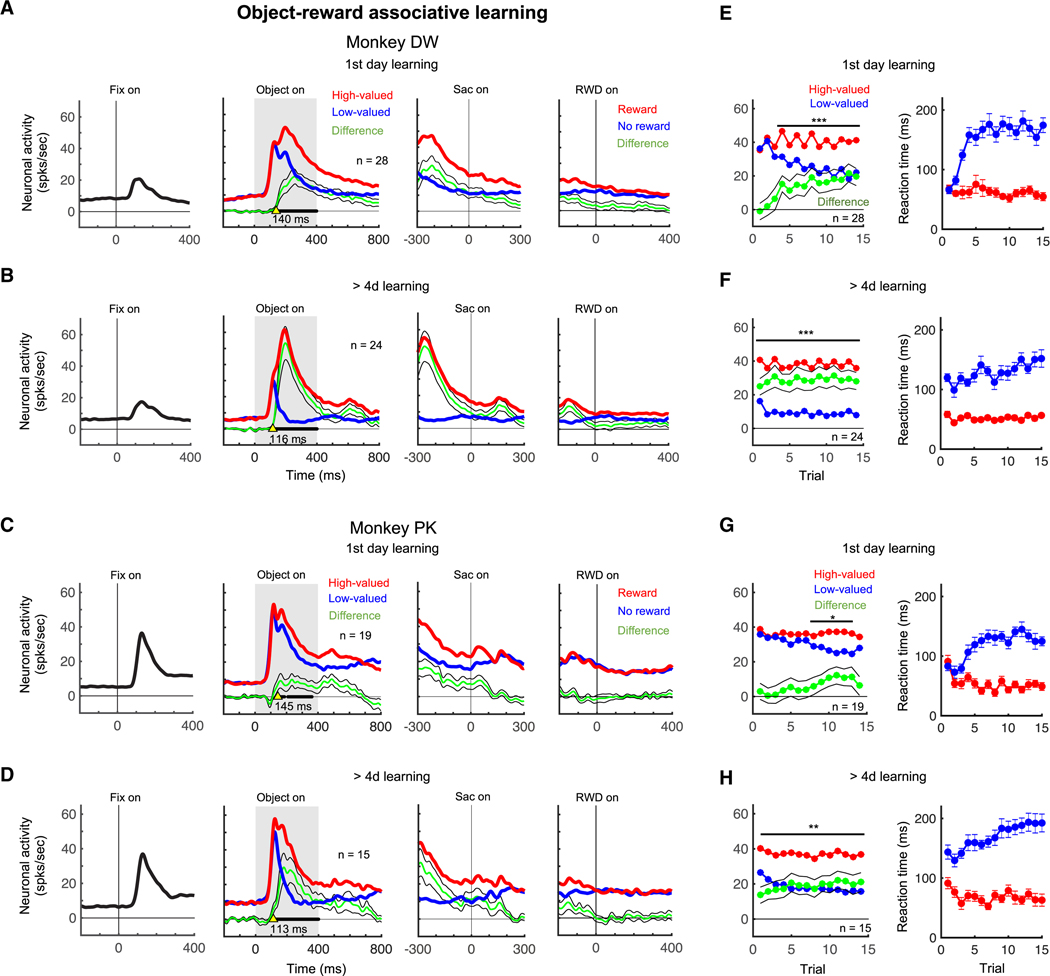
In the first day of learning, an example visual neuron in the p-thalamus responded to these objects differently depending on their associated reward values (Figure 3D, monkey DW). The value discrimination developed gradually: similar responses to high-valued and low-valued objects in the first 2–3 trials, but the responses to low-valued objects decreased in later trials (Figure 3F-left). Saccade reaction time to the high-valued and low-valued objects was not different at the first trial (Figure 3F-right).
Importantly, each monkey had learned many other sets of visual objects before this experiment, often more than several times for each set. Here, we examined the neuronal response to these learned object sets in the object-value association learning task. Figure 3E shows one example where the monkey DW had previously learned the object set 21 times. The neuron, which was the same as tested in Figure 3D, showed clearer value discrimination in response to the long-term (= 21 days) learned objects, and did so from the first trial (Figures 3E and 3G-left). Saccade reaction time to the high-valued and low-valued objects was also different from the first trial (Figure 3G-right). The neuronal activity averaged across all trials showed greater value discrimination during long-term learning than 1-day learning (Figure 3H). These data suggest the historical effects of long-term learning on the neuronal response in the p-thalamus and the behavioral response with saccade.
More learning, more value discrimination in the posterior thalamus
We performed the same set of experiments for visual neurons in the p-thalamus using visual fractal objects for 1-day learning (fractals: n = 136 in monkey PK, n = 152 in monkey DW) and long-term learning (> 4 days) (fractals: n = 32 in monkey PK, n = 80 in monkey DW) (Figure S2). Population data are shown in Figure 4 for each monkey. We aligned the neuronal activity at three-time points: fixation dot presentation, target presentation, saccade onset, and reward delivery. These neurons responded to the visual stimuli of the fixation dot and the fractal objects, but not to the saccade onset or the reward outcome (Figures 4A-D). The highest activity occurred during the object presentation, and this neuronal activity discriminated objects by their value differences in both 1-day and long-term (> 4 days) learning sessions: higher responses to high-valued objects and lower responses to low-valued objects (Figures 4A-D, middle panels).
Figure 4. Population neuronal activities of value discrimination in the p-thalamus during object-value associative learning.
A -D. Neuronal responses to the central fixation dot, fractal objects, and reward outcomes in the first learning (A and C) and in the learning of long-term experienced objects (B and D). Neuronal activities to high-valued and low-valued objects are indicated by red and blue lines, respectively. Green line indicates the difference of the activities to high-valued and low-valued objects, showing the value discrimination. Black lines in bold indicate times that show statistically significant value discrimination activity in the middle panels (paired t-test, p < 0.05). Yellow arrows indicate the initiation time of value discrimination. The initiation times are shown under yellow arrows. E-H. Value discrimination activity (left) and saccade reaction time difference (right) against the number of trials during the first day of learning (E and G) and during learning of the long-term experienced objects (F and H). Red and blue lines indicate the neuronal responses to high-valued and low-valued objects, respectively. Saccade reaction times to high-valued and low-valued objects are plotted with red and blue lines, respectively. Green line indicates the difference between neuronal activities to high-valued and low-valued objects. The number of neurons (n) is shown. *p < 0.05, **p < 0.01, ***p < 0.001 by paired t-test.
Figure 4E-H show the time course of the neuronal responses to the objects (left) and saccade reaction time (right) across learning trials. During the first day of learning, the neuronal responses to the high-valued and low-valued objects were initially similar but after 2 and 6 trials, the value discrimination activity became statistically different in monkeys DW and PK respectively (p < 0.001 and p < 0.05 respectively, paired t-test) (Figures 4E and 4G, left). Monkeys initially showed no difference in saccade reaction time to high-valued and low-valued objects, but the difference became significant after the initial several trials (Figures 4E and 4G, right). Long-term learning (> 4 days) generated greater value discrimination activity compared to the neuronal activity during the first day learning (Figures 4A-D, Object on). This was due to the larger value discrimination after long-term learning in all trials including the first trial (p < 0.001 and p < 0.01 monkey DW and PK respectively, paired t-test) (Figures 4F and 4H, left). Each monkey also showed a clear difference in saccade reaction time to high-valued and low-valued objects from the first trial (Figures 4F and 4H, right). Notably, the value discrimination started earlier after long-term learning (yellow arrows in Figures 4A-D, middle panels). These data indicate that value discrimination activity of visual neurons developed in 1-day training and became greater and stable after days of learning.
Encoding the previously learned values of objects in the posterior thalamus
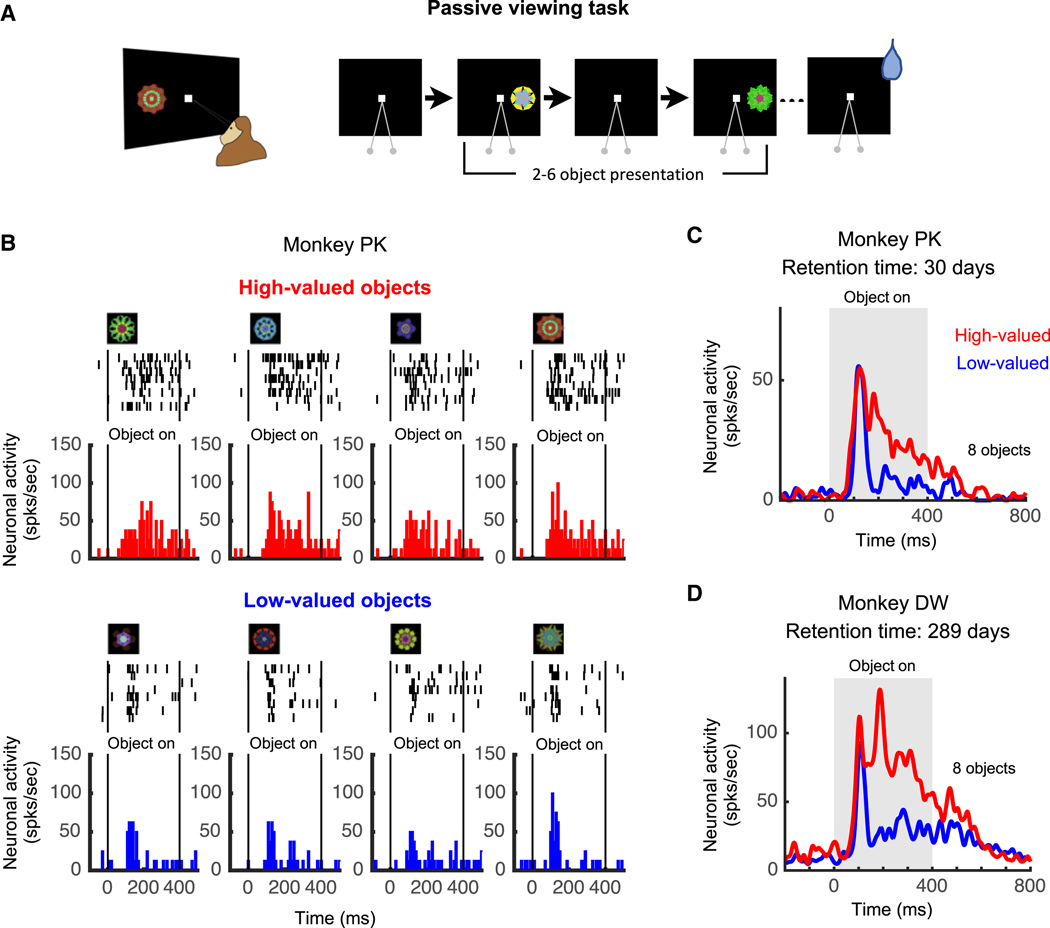
After long-term learning, neurons in the p-thalamus discriminated object values during the first trial before the reward was delivered (see the first trials in Figures 4F and 4H). This suggested neurons in the p-thalamus change their visual responses based on old learning, in addition to new learning. To test this, we examined these neurons using the passive viewing task (Figure 5A). In this task, previously learned objects (see Figures 3A and 3B) from a set of 8 visual objects were chosen randomly in each trial and presented sequentially while the monkey fixated a central dot (Figure 5A). Reward was delivered after 2–6 object presentation, and thus the fractal objects were not differentially associated with the reward outcome during the passive viewing task. Therefore, this passive viewing task does not cause new learning, but reflects only the effect of old learning (historical object value).
Figure 5. Neuronal representation of the historical value memory in the p-thalamic neuron.
A. Passive viewing task. Learned objects were presented sequentially in each neuron’s preferred position while the monkey fixated on the central white dot. A reward was delivered independently of the presented objects. B. Responses of an example neuron to 8 objects in the passive viewing task. This set of objects was not experienced for 30 days after long-term learning (> 4 days). Spikes (black ticks) were aligned at the object presentation time, and the average responses to each high-valued object (red) and each low-valued object (blue) were plotted in the histograms. C. Average response to the high-valued and low-valued objects. Red and blue lines indicate the neuronal activities to high-valued and low-valued objects, respectively. D. Average response to the high-valued and low-valued objects after 289 days of retention following the last learning. Red and blue lines indicate the neuronal activities to high-valued and low-valued objects, respectively.
See also Figure S3.
Figure 5B shows the activity of an example neuron recorded in the marking lesion site of monkey PK (Figure 1D). The neuron responded more strongly to high-valued objects than low-valued objects based on old learning (Figure 5B). Its averaged neural activity seems to be composed of two phases (Figure 5C): 1) a phasic visual response that was unrelated to object value, and 2) a subsequent value-differential response. Importantly, this experiment was done 30 days after the last learning (Object-value associative learning task, Figures 3A and 3B), which we call ‘retention time’. We also tested a learned object set with a retention time of about 9 months (289 days), and the example neuron from Figure 3 still clearly responded more strongly to high-valued objects than low-valued objects (Figure 5D). These data suggest that visual neurons in the p-thalamus encoded historical object values.
These responses to the high- and low-valued objects were positively correlated with the gaze duration in free viewing: monkeys gazed more at the objects to which the neurons more actively responded (Pearson correlation, r = 0.57, p < 0.00001) (Figure S3A). We then examined this neuronal-behavioral relationship in each group of objects (Figures S3B and S3C). There was a weak, but statistically significant, positive correlation in low-value objects (Pearson correlation, r = 0.24, p < 0.0001) (Figure S3C), but not in high-valued objects (Figure S3B). These results suggest that p-thalamus neurons may contribute to the value-based change in gaze duration using their population activity, rather than their individual neuronal activity.
Long-lasting value memory in the posterior thalamus
Next, we examined neuronal responses to the previously learned objects using the passive viewing task at different stages relative to the object-value learning (Figure 6A): 1) before learning, 2) within 1 day after the first learning, 3) short-term retention (≤ 30 days), and 4) long-term retention (> 200 days). The population activity is shown in Figure 6C-H for each monkey.
Figure 6. Value memory representation of neurons in the p-thalamus many days after the last learning.
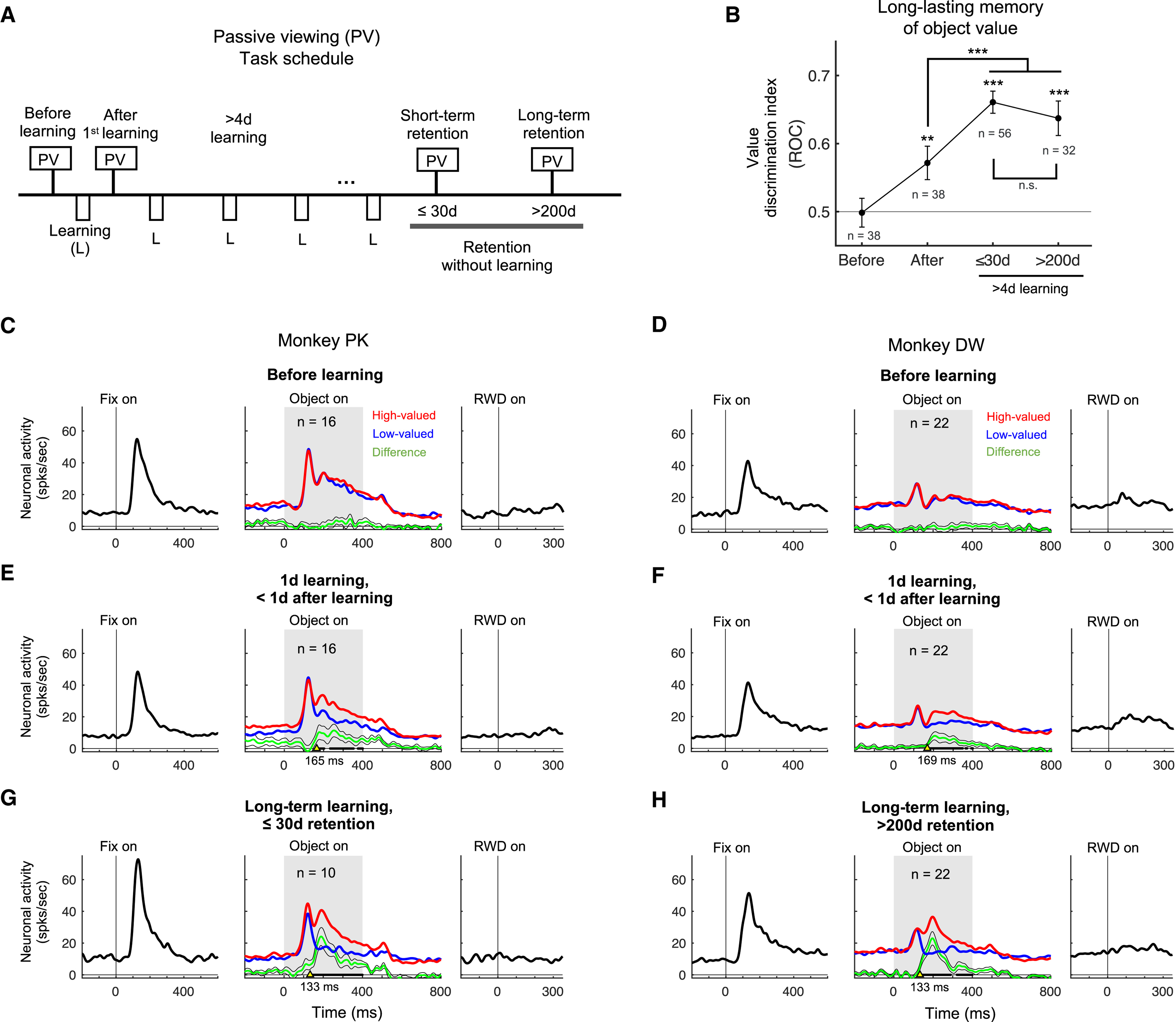
A. Passive viewing (PV) task schedule across days. The value memory was tested at 4 stages of object learning: (1) before learning, (2) within the first day of learning, (3) after short-term retention (≤ 30 days) with long-term learned objects, and (4) after long-term retention (> 200 days) with long-term learned objects. B. Value discrimination indices for each learning phase. The number of neurons (n) is shown at each data point. ** p< 0.001, ***p<0.0001 by one-way ANOVA and multiple comparison test. C-H. Neuronal responses to the central fixation dot, fractal objects, and reward outcomes in the PV task for different learning phases: before object-value associative learning (C and D), within the first day of learning (E and F), and long-term retention after the last learning (G and H). Red and blue lines indicate the neuronal activities to high-valued and low-valued objects, respectively. Green line indicates the difference of neuronal activities to high-valued and low-valued objects. Black lines in bold indicate times that show statistically significant value discrimination activity in the middle panels (paired t-test, p < 0.05). Yellow arrows indicate the initiation time of value discrimination. The initiation times are shown under yellow arrows. The number of neurons (n) is shown.
Before the first learning (Figures 6C and 6D), the p-thalamic neurons showed excitatory visual responses to the fixation dot and the fractal objects, but little responses to the reward outcome. After the first learning of object-value association (Figures 6E and 6F), the population average showed value discrimination responses to the learned objects, even though no reward was delivered during the passive viewing task (p < 0.05, paired t-test). 43.8% and 38.4% of neurons (n = 7/16 and n = 9/22) in monkeys PK and DW showed statistically significant positive value-coding after the first learning, respectively (p < 0.05, Wilcoxon rank-sum test). The value discrimination indices after the first learning significantly increased compared to the indices before the first learning (p < 0.0001, F(3, 209) = 27.21, one-way ANOVA; p < 0.01, post-hoc Tukey-Kramer pairwise comparison) (Figure 6B). After long-term learning (> 4 days), population activity of the p-thalamic neurons showed greater and faster value discrimination activity than the activity after the first learning (p < 0.05, paired t-test) (Figures 6G and 6H). The value discrimination indices after long-term learning significantly increased compared to the indices before the first learning (p < 0.0001, post-hoc Tukey-Kramer pairwise comparison) (Figure 6B).
We then tested whether the historical value-coding is sustained for a long time. To that end, we asked the monkeys to perform the passive viewing task a few or many days after the last learning (Figure 6A). Figure 6G and H show the population activity after short-term (≤ 30 days in monkey PK) and long-term retention (208 to 347 days in monkey DW), respectively. The value discrimination indices after long-term retention were statistically greater than the indices before the first learning (p < 0.0001, post-hoc Tukey-Kramer pairwise comparison) (Figure 6B). There was no significant difference in the value discrimination indices between short-term and long-term retention days (Figure 6B). Our data indicate that neurons in the p-thalamus encoded historical values of visual objects, even many days after the last learning.
Discussion
We found that the visually responsive neurons in the posterior region of the thalamus received anatomical input from the SC, and these neurons represented the changing values of visual objects during learning and encoded the long-lasting historical object values. The value-coding activity in the p-thalamic neurons suggests that this area is involved in the value learning and memory processes.
Anatomical position of the p-thalamus including the SGN
We found neurons encoding values of visual objects in the posterior thalamus, including within the SGN. Anatomical connections of this thalamic area suggest its involvement in cognitive functions and relaying sensory information [16,17]. In cats, the SGN is known to receive input directly from the SC and respond to visual stimuli [12,18–21]. SGN neurons project to the frontal eye field, amygdala, and striatum, which are areas involved in visual, emotional, and value processes [12,22–24]. Interestingly, the SGN neurons selectively project to the caudal region of the striatum [12,25] where the historical value memory of visual objects is encoded for automatic behavior [5,6]. Overall, the p-thalamus is at an anatomical position well suited to link visual information directly to the historical value memory system.
Fast action loop of subcortical pathway for automatic behavior
Based on the anatomical connections, we propose a SC-p-thalamus-CDt-cdlSNr-SC loop circuit (Figure 7). When a visual object is presented, two brain areas, the LGN and the SC, receive visual information directly from the retina. In the first pathway (right arrow from the retina), the visual information arrives in the CDt after passing through the LGN, visual cortex, and temporal cortex (cortical pathway). In the second, shorter pathway (left arrow from the retina), the visual information arrives in the CDt after only passing through the SC and p-thalamus (subcortical pathway) [12,25,26]. Importantly, the CDt neurons selectively encode long-term value memory of visual objects whereas the caudate head (CDh) neurons selectively encode short-term value memory of visual objects. The monkeys are able to choose high-valued objects based on both short-term and long-term memories processed in the CDh and CDt [6]. Eventually, the cdlSNr neurons receive inputs from the CDt and send the long-term object value information to the intermediate layer of the SC to generate automatic saccade [27,28]. This loop circuit is different from the ventromedial thalamus circuit involved in visual value-encoding [29]. Neurons in the p-thalamus innervate the basal ganglia to control eye movement, whereas neurons in the ventromedial thalamus mainly innervate the cortex [30–32].
Figure 7. Fast action loop through the p-thalamus for automatic gaze.
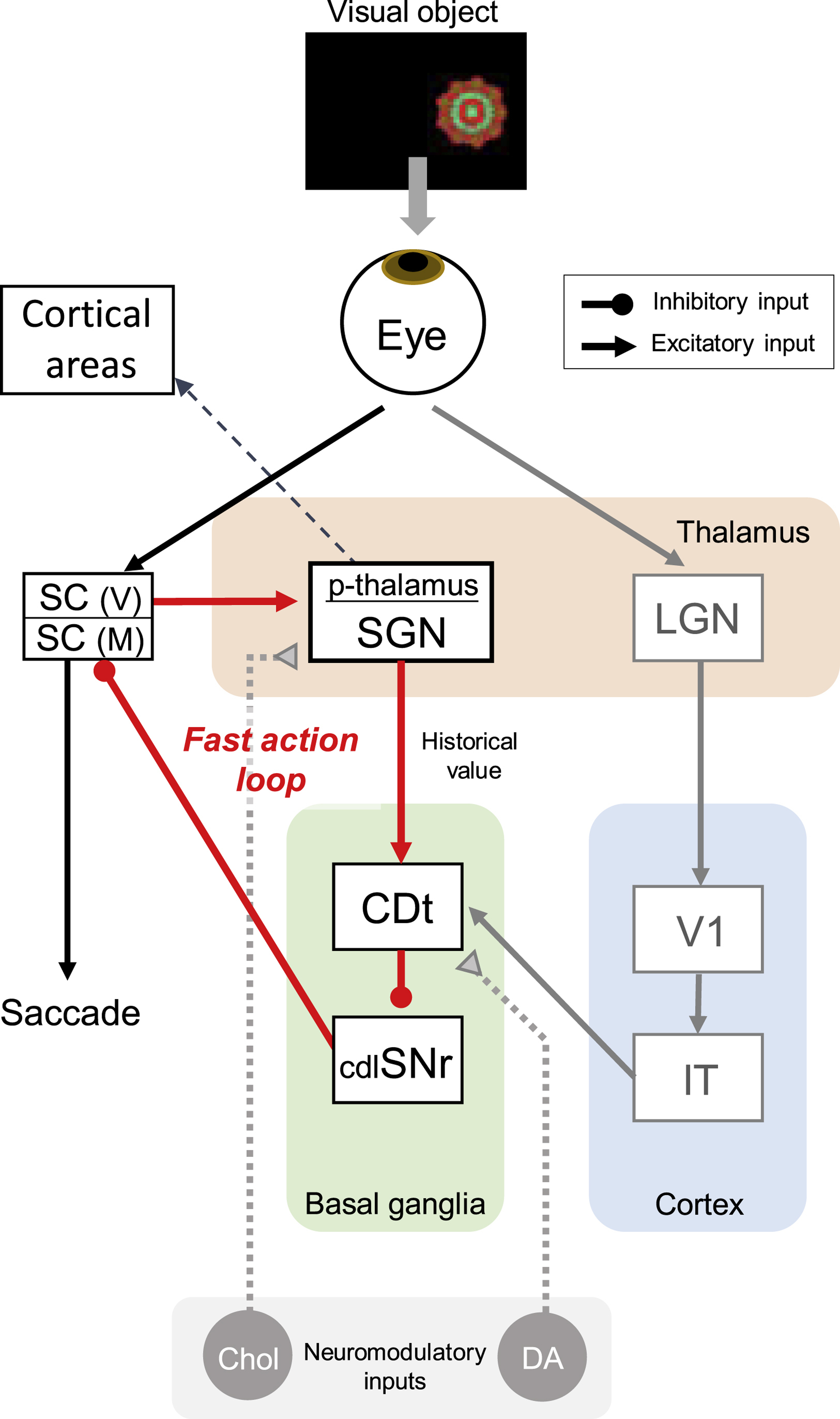
In this circuit model, inhibitory and excitatory neurons are shown by circle and arrow ends, respectively. Visual information is separately processed through the lateral geniculate nucleus (LGN) and the superior colliculus (SC). In the first pathway, the p-thalamus receives visual information from visual SC neurons and processes historical values of visual objects. This value information is then processed through the tail of caudate nucleus (CDt) and the caudal-dorsal-lateral part of the substantia nigra pars reticulata (cdlSNr). The excitation of CDt neurons to high-valued objects leads to disinhibition of saccadic neurons in the SC, facilitating saccades to high-valued objects. SC(V) and SC(M) indicate visual area and motor area of the superior colliculus, respectively. In the second pathway, visual information is progressively processed by LGN, areas of the visual cortex (V1), and inferior temporal cortex (IT) before reaching the CDt. Both pathways have a similar role in facilitating automatic saccades to high-valued objects, but the subcortical pathway through the p-thalamus is shorter than the cortical pathway, allowing for faster action (‘fast action loop’). Dopamine neurons (DA) and cholinergic neurons (Chol) innervate the CDt and the p-thalamus, respectively. The p-thalamus projects to prefrontal cortex regions, such as the ventrolateral prefrontal cortex and the frontal eye field.
We note that the p-thalamus may bypass the cortex and send the object information directly to the CDt. This subcortical pathway is shorter than the cortical pathway and may guide faster behaviors. In addition, our data show the p-thalamus encoded the historical values of visual objects, indicating that the subcortical pathway processed the object value at a very early stage of visual processing. This subcortical pathway is thus suitable for controlling action and object skills that require rapid automatic actions, suggesting a ‘fast action loop’ (Figure 7). Moreover, previous anatomical studies showed that prefrontal areas, including the ventrolateral prefrontal cortex and the frontal eye field, receive direct inputs from the p-thalamus, including the SGN [22,33]. Thus, this historical value information in the p-thalamus may also be sent to cortical areas involved in automatic choice behavior.
However, how the SGN neurons discriminated the fractal objects remains a question. Individual CDt output neurons are highly object-selective, only responding to a limited number of fractal objects [34]. This object selectivity is probably based on selective inputs from visual cortical areas [35,36]. The CDt output neurons likely transfer this object information to the p-thalamus through cdlSNr and SC. However, some SC neurons respond to complex objects differentially and quickly, likely before inputs from the cortex are available [37,38]. This suggests the direct connections from the brainstem (SC, IC, PAG) to the p-thalamus (Figure 2) may be important for conveying visual and value information. Object-selective signals are generated in individual brainstem neurons based on multiple sensory inputs. These signals are then transformed to object-value signals through the combined brainstem connections to individual p-thalamus neurons. The value-coding mechanism in this subcortical circuit remains unknown. We need to test these hypotheses in the near future.
Learning and memory process of the p-thalamus
It is known that regions of the p-thalamus are involved in sensory processes and are part of the sensory thalamus [16]. A traditional function of the sensory thalamus is to relay sensory information from subcortical areas to the cortex. In addition to this traditional role, our data suggest a function of the p-thalamus in learning and memory process. Single neurons in the p-thalamus discriminated the difference of object values during learning and sustained the learned values for a long time (>200 days). This neural activity, which represents the value memory, may be important for learned attention or salience-guided behaviors. Another possibility is that p-thalamic neurons send value teaching signal during learning and historical values of objects after learning to downstream structures, such as the CDt. In the proposed circuit (Figure 7), excitatory neurons in the p-thalamus may increase the neural activity of the CDt. The CDt then transfers this value signal to the cdlSNr through inhibitory GABAergic connections. This inhibition of cdlSNr results in disinhibition of the SC and eventually guides the eye gaze more toward high-valued objects.
It remains an important question whether the value memory is physically stored in p-thalamic neurons or whether other areas, such as the SC, store the value memory and relay the value information through the p-thalamus. Dopaminergic input is thought to send the learning signal to the striatum and modulate the synaptic strength between the cortex and the striatum for memory formation [39,40]. However, we did not find labeled neurons projecting to the p-thalamic neurons from dopaminergic midbrain areas, including the substantia nigra pars compacta (SNc) and ventral tegmental area (unpublished observation). Instead, this region of the p-thalamus receives strong input from PPTg cholinergic neurons (Figure S1) [41]. The cholinergic input to the thalamus is thought to control and modulate the neural activity during distinct behavioral states [42,43]. In addition, neurons in the PPTg encode the reward value signal [44]. It suggests that the cholinergic neurons encoding reward value signal in the PPTg may modulate the synaptic strength of the p-thalamus for memory formation (Fig 7).
STAR Methods
Resource Availability
Lead Contact
Further requests for information, reagents, data and code should be directed to, and will be fulfilled by, the Lead Contact, Hyoung F. Kim (hfkim@snu.ac.kr).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The data and computer code used in this paper will be provided upon request to the lead contact as they are government property.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal model
Two adult male monkeys (Macaca mulatta, 8–14 years old, male, 8–12 kg), DW for neuronal recording and PK for neuronal recording and histology, were used in the experiments. All animal care and experimental procedures were approved by the National Eye Institute Animal Care and Use Committee and complied with the Public Health Service Policy on the humane care and use of laboratory animals.
Method Details
General procedure
We implanted a plastic head holder and plastic recording chambers on the skull of each monkey under general anesthesia and sterile surgical conditions. We targeted the posterior thalamus, using a 25-degree laterally tilted recording chamber. During this surgery, we surgically implanted search coils under the conjunctiva of both eyes to record eye movements. After the monkeys fully recovered from surgery, the monkeys began training with the object-value associative learning and passive viewing tasks.
Single unit recording
While the monkey was performing a task, we recorded neuronal activity using conventional methods. The target regions were determined using a 1 mm spacing grid system, with the aid of MR images (4.7 T, Bruker) obtained along the direction of the chamber. We performed single-unit recording using glass-coated electrode (Alpha-Omega). The electrode was guided by a stainless-steel tube inserted through the dura mater and the electrode was advanced by an oil-driven micromanipulator (MO-97A, Narishige). The electric signals from the electrode were amplified and band-pass filtered (0.2–10 kHz; BAK). Neuronal spikes were isolated online using a custom voltage-time window discrimination software (BLIP, window version of MEX, VEX, and REX previously developed by LSR/NEI/NIH; available at www.robilis.com/blip) and their timings were detected at 1kHz sampling rate. The waveforms of individual spikes were collected at 50 kHz.
Behavioral procedure
We controlled behavioral procedures using a window-based data acquisition system, BLIP. The monkey sat in a primate chair, facing a frontoparallel screen in a sound-attenuated and electrically shielded room. Visual stimuli generated by a projector (PJ550, ViewSonic) were rear projected on the screen. The visual stimuli were created using fractal geometry [34]. Sizes of the fractal objects were ~8 degree x 8 degree.
Behavioral procedures consisted of two phases. Object-value associative learning used a learning task while testing the neuronal representation of long-term value memory used a passive-viewing task. An important point for examining the long-term memory is that no reward was delivered during passive viewing of visual objects. Details are explained below.
Object-value associative learning
To generate long-term value memories, each fractal object was consistently associated with a reward outcome (liquid reward or no reward) across all learning days. A set of 8 computer-generated fractal objects was used in each session of learning task. While the monkey was fixating at a central white dot, one of the objects was presented at right or left position pseudorandomly (15 degree from center). The center dot extinguished 400 ms later, and the monkey made a saccade to the object to proceed to the next trial. Half of the objects were associated with a liquid reward (high-valued objects), whereas the other half were associated with no reward (low-valued objects). The reward was delivered 600 ms after monkeys held their gaze on the object. One training session consisted of 80 trials (10 trials per object). Each set was learned in one learning session per day. The same sets of objects were repeatedly learned with the same value associations over 4 days.
Passive viewing task
To examine how the neurons encoded historical values of the learned fractal objects, we used the passive viewing task. While the monkey was gazing at the central fixation dot, some of the learned fractal objects (2–6 at once) were sequentially presented at a neuron’s preferred position. The duration of each object presentation was 400 ms. Reward was independently delivered 300 ms after the last object was extinguished. The activity representing the value memory was tested over 1-day retention period after the last learning.
Free viewing task
To test the behavioral response of each monkey to the previously learned objects, we used a task in which no instruction was required while the learned fractal objects were presented. After the monkey fixated on a central white dot for 300 ms, four objects were chosen pseudorandomly and presented simultaneously in four symmetric positions (15 degree from center). The monkey was free to gaze at them for 2 s without any reward outcome. To maintain the motivation of monkeys, a reward-associated white dot was presented at one of eight positions on half of the trials. If the monkeys made a saccade to it and held the gaze on it for 600 ms, a reward was delivered. Each object was presented at least 16 times in one session.
Spatial preference mapping task
To test the spatial preference, we presented fractal objects in either left or right position (15 degree from center) while the monkey maintained fixation on a center dot. The task was similar to passive viewing task, but only one familiar fractal was used.
Tracer injection
Before injecting the retrograde tracer, we identified the injection site by recording single-unit activity during behavioral tasks. We used a custom-made injectrode, consisting of an epoxy-coated tungsten microelectrode (FHC) for neuron recording and a silica tube (outer/inner diameter: 155/75 mm; Polymicro Technologies) for tracer injection. We used a 10 µL Hamilton syringe to inject 0.3 µL of cholera toxin subunit B conjugated with Alexa Fluor 488 (CTB-488 in phosphate buffer, pH 7.4) at a speed of 0.01 µl/min. After the injection, we left the injectrode in place for 1 hr to minimize tracer diffusion along the injectrode track.
Histology
Two weeks after the injection, monkey PK was anesthetized with an overdose of sodium pentobarbital. Saline and 4% paraformaldehyde were perfused transcardially. The head was fixed to the stereotaxic frame, and the brain was cut into blocks in the coronal plane, including the thalamus and brainstem structures. Each block was post-fixed overnight at 4°C and cryoprotected for 4 days in increasing gradients of glycerol solution (5, 10, and 20% glycerol in phosphate-buffered saline) before being frozen. Each frozen block was cut every 50 µm using a sliding microtome.
Immunohistochemistry
To more sensitively visualize the CTB-488 signals, we labeled the tissue slices with anti-Alexa Fluor 488 antibody (anti-AF488 antibody; A-11094, Life Tech.). After 30 min permeabilization by 0.5% Triton X-100 in PBS, the slices were blocked with 3% normal goat serum and 2% bovine serum albumin in PBS (pH 7.4). After blocking, the slices were incubated with rabbit anti-AF488 antibody (1:500) overnight at room temperature. On the second day, the incubated slices were washed three times with PBS and incubated with goat biotinylated anti-rabbit IgG antibody (ABC kit, PK6101, Vector labs) for 30 min at room temperature. We followed the ABC kit protocol to detect the chromogen inside the cell bodies. The chromogenic reaction was terminated when the staining intensity developed specifically in the soma of neurons.
Electric marking lesion
To confirm the recording location, 13 µA of negative current was passed for 30 s after recording from a long-term value-coding neuron (Figure 5). We later identified the recording site in a Nissl-stained section.
Quantification and Statistical Analysis
Analysis of the visual and value-coding responses
To examine the neurons’ visual responses, the numbers of spikes were counted within test and control windows in the spatial preference mapping task and passive viewing task. The test window was 50–400 ms after the object onset, and control window was 400–0 ms before the object onset. We compared the numbers of spikes between the control and test windows in individual trials for each object.
Statistical significance and initiation time of value discrimination were investigated by a moving window analysis. The 1-ms window was moved with the 1-ms time bin, and the value discrimination activity (high-valued object – low-valued object activity) at each time point was compared to the baseline (0) by paired t-test.
To test if the neurons encoded the long-term value memories of objects, we compared its responses to high-valued and low-valued objects. In both cases, the statistical significance was examined using Wilcoxon rank-sum test. The degree of value coding was computed by the area under the receiver operating characteristic (ROC) based on the response magnitudes of the neurons to high-valued versus low-valued objects.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-Alexa Fluor 488 antibody | Life Tech. | A-11094 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cholera Toxin Subunit B, Alexa Fluor488 conjugate | Fisher | C22841 |
| Experimental Models: Organisms/Strains | ||
| Macaca mulatta | NIH | N/A |
| Software and Algorithms | ||
| Matlab | MathWorks | www.mathworks.com |
| Blip | NIH, Simon Hong | www.simonhong.org |
Highlights.
Complex visual objects activate the posterior thalamus, including the SGN.
Posterior thalamus receives subcortical visual inputs and projects to the striatum.
Posterior thalamic neurons retain reward values of learned objects.
The posterior thalamus guides fast habitual actions by bypassing the visual cortex.
Acknowledgements
We thank M.K. Smith, D. Parker, I. Bunea, G. Tansey, A.M. Nichols, T.W. Ruffner, and A.V. Hays for technical assistance. This research was supported by the Intramural Research Program at the National Institutes of Health, National Eye Institute, and the National Research Foundation (NRF) of Korea grant funded by the Korea government (MSIT) (NRF-2019R1A2C2005213).
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander GE, DeLong MR, and Strick PL. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. [DOI] [PubMed] [Google Scholar]
- 2.Sherman SM, and Guillery RW. (2002). The role of the thalamus in the flow of information to the cortex. Philos. Trans. R. Soc. B Biol. Sci. 357, 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrero MT, Barcia C, and Navarro JM. (2002). Functional anatomy of thalamus and basal ganglia. Child’s Nerv. Syst. 18, 386–404. [DOI] [PubMed] [Google Scholar]
- 4.Kim HF, and Hikosaka O. (2015). Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards. Brain 138, 1776–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto S, Kim HF, and Hikosaka O. (2013). Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J. Neurosci. 33, 11227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HF, and Hikosaka O. (2013). Distinct Basal Ganglia circuits controlling behaviors guided by flexible and stable values. Neuron 79, 1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HF, Amita H, and Hikosaka O. (2017). Indirect Pathway of Caudal Basal Ganglia for Rejection of Valueless Visual Objects. Neuron 94, 920–930.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda M, Yamamoto S, and Hikosaka O. (2012). Robust Representation of Stable Object Values in the Oculomotor Basal Ganglia. J. Neurosci. 32, 16917–16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman SM, and Spear PD. (1982). Organization of visual pathways in normal and visually deprived cats. Physiol. Rev. 62, 738–855. [DOI] [PubMed] [Google Scholar]
- 10.Griggs WS, Kim HF, Ghazizadeh A, Gabriela Costello M, Wall KM, and Hikosaka O (2017). Flexible and Stable Value Coding Areas in Caudate Head and Tail Receive Anatomically Distinct Cortical and Subcortical Inputs. Front. Neuroanat. 11, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeterian EH, and Pandya DN. (1995). Corticostriatal connections of extrastriate visual areas in rhesus monkeys. J. Comp. Neurol. 352, 436–57. [DOI] [PubMed] [Google Scholar]
- 12.Takada M, Itoh K, Yasui Y, Sugimoto T, and Mizuno N. (1985). Topographical projections from the posterior thalamic regions to the striatum in the cat, with reference to possible tecto-thalamo-striatal connections. Exp. Brain Res. 60, 385–396. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, and Kim HF. (2018). Anatomical Inputs From the Sensory and Value Structures to the Tail of the Rat Striatum. Front. Neuroanat. 12, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanciego JL, and Vázquez A. (2012). The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton H, and Jones EG. (1976). The posterior thalamic region and its cortical projections in new world and old world monkeys. J Comp Neurol 168, 249–301. [DOI] [PubMed] [Google Scholar]
- 16.Komura Y, Tamura R, Uwano T, Nishijo H, Kaga K, and Ono T. (2001). Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature 412, 546–549. [DOI] [PubMed] [Google Scholar]
- 17.Sommer MA. (2003). The role of the thalamus in motor control. Curr. Opin. Neurobiol. 13, 663–670. [DOI] [PubMed] [Google Scholar]
- 18.Katoh YY, and Benedek G. (1995). Organization of the colliculo- suprageniculate pathway in the cat: A wheat germ agglutinin- horseradish peroxidase study. J. Comp. Neurol. 352, 381–397. [DOI] [PubMed] [Google Scholar]
- 19.Hicks TP, Stark CA, and Fletcher WA. (1986). Origins of afferents to visual suprageniculate nucleus of the cat. J. Comp. Neurol. 246, 544–554. [DOI] [PubMed] [Google Scholar]
- 20.Paróczy Z, Nagy A, Márkus Z, Waleszczyk WJ, Wypych M, and Benedek G. (2006). Spatial and temporal visual properties of single neurons in the suprageniculate nucleus of the thalamus. Neuroscience 137, 1397–1404. [DOI] [PubMed] [Google Scholar]
- 21.Hicks TP, Watanabe S, Miyake A, and Shoumura K. (1984). Organization and properties of visually responsive neurones in the suprageniculate nucleus of the cat. Exp. Brain Res. 55, 359–367. [DOI] [PubMed] [Google Scholar]
- 22.Leichnetz GR. (1982). Connections between the frontal eye field and pretectum in the monkey: an anterograde/retrograde study using HRP gel and TMB neurohistochemistry. J. Comp. Neurol. 207, 394–404. [DOI] [PubMed] [Google Scholar]
- 23.Linke R, De Lima AD, Schwegler H, and Pape HC. (1999). Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: Possible substrates of a subcortical visual pathway to the amygdala. J. Comp. Neurol. 403, 158–170. [PubMed] [Google Scholar]
- 24.LeDoux JE, and Farb CR. (1991). Neurons of the acoustic thalamus that project to the amygdala contain glutamate. Neurosci. Lett. 134, 145–149. [DOI] [PubMed] [Google Scholar]
- 25.Guirado S, Real MÁ, and Dávila JC. (2005). The ascending tectofugal visual system in amniotes: New insights. Brain Res. Bull. 66, 290–296. [DOI] [PubMed] [Google Scholar]
- 26.McHaffie JG, Stanford TR, Stein BE, Coizet V, and Redgrave P. (2005). Subcortical loops through the basal ganglia. Trends Neurosci. 28, 401–7. [DOI] [PubMed] [Google Scholar]
- 27.Yasuda M, and Hikosaka O. (2015). Functional territories in primate substantia nigra pars reticulata separately signaling stable and flexible values. J. Neurophysiol. 113, 1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amita H, Kim HF, Smith MK, Gopal A, and Hikosaka O. (2019). Neuronal connections of direct and indirect pathways for stable value memory in caudal basal ganglia. Eur. J. Neurosci. 49, 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda M, and Hikosaka O. (2019). Medial thalamus in the territory of oculomotor basal ganglia represents stable object value. Eur. J. Neurosci. 49, 672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanibuchi I, Kitano H, and Jinnai K. (2009). Substantia nigra output to prefrontal cortex via thalamus in monkeys. I. Electrophysiological identification of thalamic relay neurons. J. Neurophysiol. 102, 2933–45. [DOI] [PubMed] [Google Scholar]
- 31.Middleton FA, and Strick PL. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 266, 458–461. [DOI] [PubMed] [Google Scholar]
- 32.Ilinsky I. a, Jouandet ML, and Goldman-Rakic PS. (1985). Organization of the nigrothalamocortical system in the rhesus monkey. J. Comp. Neurol. 236, 315–30. [DOI] [PubMed] [Google Scholar]
- 33.Asanuma C, Andersen RA, and Cowan WM. (1985). The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: Divergent cortical projections from cell clusters in the medial pulvinar nucleus. J. Comp. Neurol. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto S, Monosov IE, Yasuda M, and Hikosaka O. (2012). What and where information in the caudate tail guides saccades to visual objects. J. Neurosci. 32, 11005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeterian E, and Van Hoesen G. (1978). Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 139, 43–63. [DOI] [PubMed] [Google Scholar]
- 36.Saint-Cyr JA, Ungerleider LG, and Desimone R. (1990). Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J. Comp. Neurol. 298, 129–56. [DOI] [PubMed] [Google Scholar]
- 37.Rizzolatti G, Buchtel HA, Camarda R, and Scandolara C. (1980). Neurons with complex visual properties in the superior colliculus of the macaque monkey. Exp. Brain Res. 38, 37–42. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen MN, Matsumoto J, Hori E, Maior RS, Tomaz C, Tran AH, Ono T, and Nishijo H. (2014). Neuronal responses to face-like and facial stimuli in the monkey superior colliculus. Front. Behav. Neurosci. 8, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen W, Flajolet M, Greengard P, and Surmeier DJ. (2008). Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 321, 848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calabresi P, Picconi B, Tozzi A, and Di Filippo M. (2007). Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Cogn. Sci. 30, 211–219. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino K, Hicks TP, Meguro R, Hirano S, Kase M, and Norita M. (1997). Cholinergic innervation of the lateralis medialis-suprageniculate nuclear complex (LM-Sg) of the cat’s thalamus: A double labeling immunohistochemical study. Brain Res. 747, 151–155. [DOI] [PubMed] [Google Scholar]
- 42.Beierlein M. (2014). Synaptic mechanisms underlying cholinergic control of thalamic reticular nucleus neurons. J. Physiol. 592, 4137–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y-G, Pita-Almenar JD, Wu C-S, Renger JJ, Uebele VN, Lu H-C, and Beierlein M. (2013). Biphasic Cholinergic Synaptic Transmission Controls Action Potential Activity in Thalamic Reticular Nucleus Neurons. J. Neurosci. 33, 2048–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong S, and Hikosaka O. (2014). Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience 282C, 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and computer code used in this paper will be provided upon request to the lead contact as they are government property.