Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused challenges in the management of patients living with multiple sclerosis (PLwMS). We investigated the occurrence and severity of COVID-19 infection post-vaccination among PLwMS treated with ocrelizumab and enrolled in the Maccabi Health Services (MHS) (n = 289) or followed at the Hadassah Medical Center (HMC) (n = 80) in Israel. Most patients were fully vaccinated (MHS n = 218; HMC n = 76) and confirmed infection post-vaccination was low (3.7% and 2.6%, respectively). MHS: infection was more severe (hospitalization/intensive care unit/death) in non-vaccinated (33.3%) vs vaccinated patients (25%). HMC: one vaccinated patient required hospitalization with COVID-19 vs two unvaccinated patients. These data from two Israel cohorts suggest that occurrence of COVID-19 after mRNA vaccination is low and limited in severity.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused major challenges in the management of patients living with multiple sclerosis (PLwMS), rendering the assessment of clinical outcomes and related risk factors for severe disease of utmost importance for PLwMS. Whilst several published studies characterize the immunological response to SARS-CoV-2 vaccination, evidence of clinical protection is lacking. We investigated COVID-19 infection and vaccination in PLwMS treated with ocrelizumab enrolled in the Maccabi Health Services (MHS) or followed at the Hadassah Medical Center (HMC) in Israel from February 2020 to November 2021, focusing on the occurrence and severity of COVID-19 post-vaccination (i.e., vaccine breakthrough cases).
2. Materials and methods
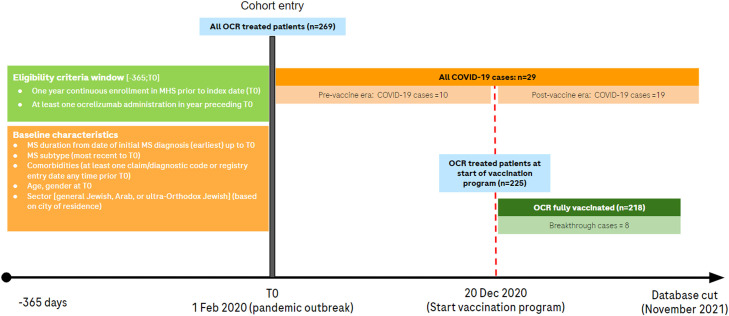
We conducted a non-interventional, retrospective cohort study using the centralized data of MHS. MHS serves as an insurer and healthcare service provider for a quarter of Israel's population; thereby collecting and storing comprehensive patient records from various clinical settings. The research period for this study spanned February 1, 2020 (index date, start of pandemic) to November 1, 2021 (data cut-off date) (Fig. 1 ) (Schneeweiss et al., 2019). Eligible patients had ≥1-year continuous MHS membership and ≥1 ocrelizumab administration in the 12 months preceding the index date. MS diagnosis was identified in the MHS database using the 9th Revision of the International Classification of Diseases (i.e., ICD-9 CM 340 and 340.9). In addition, Maccabi Health Services specific codes were used to identify MS subtypes (i.e., Y12601 Multiple sclerosis, Y21012 Multiple Sclerosis Remitting Relapsing, Y21013 Multiple Sclerosis Progressive Form, Y21014 Multiple sclerosis Progressive Primary, Multiple Sclerosis Progressive Secondary). COVID-19 cases were then identified based on ≥1 record of confirmed SARS-CoV-2 infection (polymerase chain reaction or antigen test), or hospitalization with a COVID-19 diagnosis. In a subgroup analysis, we included all ocrelizumab-treated patients vaccinated with ≥2 doses of a SARS-CoV-2 messenger RNA (mRNA) vaccine by the end of the study period and examined the occurrence of vaccine breakthrough cases, defined as COVID-19 infection diagnosed ≥14 days after recommended SARS-CoV-2 initial immunization or after the third booster dose given ≥6 months after the second vaccine dose. Clinical outcomes were described as COVID-19 infection, COVID-19-related hospitalization, COVID-19 intensive care unit (ICU) admission or COVID-19-related death. This study was approved by the MHS Institutional Review Board.
Fig. 1.
Study design Maccabi Health Services. Source: figure developed based upon information from Schneeweiss et al. (2019), COVID-19, coronavirus disease 2019; MHS, Maccabi Health Services; MS, multiple sclerosis; OCR, ocrelizumab.
Additionally, PLwMS treated with ocrelizumab followed at the HMC neurology department were contacted by their treating neurologist to identify those who had received a COVID-19 diagnosis since the start of the pandemic and up to November 30, 2021, the severity and outcome of their infection and their SARS-CoV-2 vaccination status. Patients who had ≥1 ocrelizumab administration in the 12 months preceding a COVID-19 diagnosis were included. MS diagnosis was made by a neurologist following McDonald 2017 criteria. Vaccine breakthroughs were defined similarly to the analyses conducted in MHS. COVID-19 clinical outcomes were described according to the main categories of the World Health Organization COVID-19 clinical progression scale (WHO, 2020). The Hadassah Medical Organization Ethics Committee approved this study (study no. 975–20 -HMO).
3. Results
Among 2,290,564 MHS beneficiaries, we identified a total of 269 eligible PLwMS treated with ocrelizumab, of which 57.2% were females, mean age (standard deviation [SD]) was 50.8 (11.7) years, and 52.0% had a progressive form of MS (Table 1 ). There were 225 patients on ocrelizumab treatment at the start of the SARS-CoV-2 vaccination campaign in Israel (December 20, 2020). Of these, 218 (96.9%) were fully vaccinated with the initial immunization scheme of two doses for mRNA vaccines by November 1, 2021, of which 114 patients (52.3%) received a third booster dose.
Table 1.
Overall patient characteristics and for COVID-19 cases in PLwMS treated with ocrelizumab at Maccabi Health Services.
| Characteristic | Ocrelizumab cohort All patients (n = 269) | Ocrelizumab cohort COVID-19 cases (n = 29) |
|---|---|---|
| Female sex | 154 (57.2%) | 16 (55.2%) |
| Age (years) | ||
| Mean (SD) | 50.8 (11.7) | 49.4 (11.9) |
| MS subtype* | ||
| Relapsing-remitting MS | 104 (38.7%) | 10 (34.5%) |
| Primary progressive MS | 95 (35.3%) | 11(37.9%) |
| Progressive form (unspecified) | 45 (16.7%) | 6 (20.7%) |
| MS general (unspecified) | 25 (9.3%) | 2 (6.9%) |
| MS disease duration (years) | ||
| Mean (SD) | 12.0 (6.8) | 12.2 (7.2) |
| Ocrelizumab exposure time (years) | ||
| Mean (SD) | 2.0 (0.3) | 2.0 (0.4) |
| Underlying conditions | ||
| Current cancer or history of cancer | 14 (5.2%) | 1 (3.4%) |
| Moderate liver disease | 31 (11.5%) | 3 (10.3%) |
| Hemiplegia/paraplegia | 35 (13.0% | 4 (13.8%) |
| Dementia | 18 (6.7%) | 1 (3.4%) |
| Cardiovascular disease | 16 (5.9%) | 4 (13.8%) |
| Hypertension | 55 (20.4%) | 9 (31.0%) |
| Diabetes | 17 (6.3%) | 4 (13.8%) |
| COPD | 21 (7.8%) | 2 (6.9%) |
| Chronic kidney disease | 23 (8.6%) | 4 (13.8%) |
| COVID-19 outcomesa | ||
| All cases | NA | 29 |
| Mild/moderate (non-hospitalized) | 20 (69.9%) | |
| Severe (Hospitalized/ICU/death) | 9 (31.0%) | |
| Hospitalized | 5 (17.2%) | |
| ICU admission | 3 (10.3%) | |
| Death | 1 (3.4%) | |
| Pre-vaccination era/partially vaccinated | NA | 21 |
| Mild/moderate (non-hospitalized) | 14 (66.7%) | |
| Severe (Hospitalized/ICU/death) | 7 (33.3%) | |
| Hospitalized | 3 (14.3%) | |
| ICU admission | 3 (14.3%) | |
| Death | 1 (4.8%) | |
| Fully vaccinated | NA | 8 |
| Mild/moderate (non-hospitalized) | 6 (75.0%) | |
| Severe (Hospitalized/ICU/death) | 2 (25.0%) | |
| Hospitalized | 2 (25.0%) | |
| ICU admission | 0 | |
| Death | 0 |
COPD,chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; MS, multiple sclerosis; PLwMS, patients living with multiple sclerosis; SD, standard deviation; NA, not applicable.
Categories are mutually exclusive and based on recorded worse outcome by patient (i.e., death > ICU > hospitalization > non hospitalized).
Based on Maccabi Health Services specific codes for MS subtypes (i.e., Y12601 Multiple sclerosis, Y21012 Multiple Sclerosis Remitting Relapsing, Y21013 Multiple Sclerosis Progressive Form, Y21014 Multiple sclerosis Progressive Primary, Multiple Sclerosis Progressive Secondary).
During the study observation period, a total of 29 patients (10.8% among the full eligible cohort at index date) were diagnosed with COVID-19, of which eight were vaccine breakthroughs (3.7% of the fully vaccinated cohort), with two cases occurring after the third booster dose. The median time between the last vaccine injection and the diagnosis date of breakthrough infection was 119 days (quartile 1–3: 51.75, 195.0 days). The remaining 21 cases occurred in unvaccinated patients (i.e., prior to the initiation of the vaccination campaign on December 20, 2020 (n = 10) or in partially vaccinated patients (n = 11)). The following crude rates were reported in vaccinated and unvaccinated patients, respectively: all severe COVID (hospitalization/ICU/death), two (25.0%) vs seven (33.3%); hospitalizations, two (25.0%) vs three (14.0%); ICU admission, zero vs three (14.0%); death, zero vs one (4.8%). The mean [SD] length of stay for all COVID-19 related hospitalizations was 7.2 (11.4) days (range 1–13) and 43.7 (17.3) days (range 29–68) for the three patients admitted to the ICU.
As of November 30, 2021, 80 PLwMS treated with ocrelizumab were identified at HMC. The majority (66.2%) of patients were female, with a mean age of 46.5 years, and 58.7% had a progressive form of MS. In total, 76 patients (95.0%) completed the initial immunization scheme with two doses of mRNA vaccine. Thirteen patients were diagnosed with COVID-19 (16.3% among all patients), of which two were vaccine breakthroughs (2.6% among fully vaccinated patients). The remaining 11 cases occurred in unvaccinated patients (all during pre-vaccination era), and COVID-19 was asymptomatic/mild in seven (63.6%) patients, one (9.1%) patient was hospitalized with moderate COVID-19 and one (9.1%) was hospitalized with severe disease. For the two vaccine breakthroughs, the first patient was a male aged 59 years, with secondary progressive MS, an Expanded Disability Status Scale (EDSS) of 7.5, a disease duration since diagnosis of 22.6 years, and coronary heart disease. He started ocrelizumab therapy in May 2019, having his last infusion 4.3 months before COVID-19 infection. He was hospitalized with moderate COVID-19 and fully recovered. The second patient was a pregnant woman aged 37 years, with relapsing remitting MS, an EDSS of 5.5, a disease duration since diagnosis of 14 years, and no underlying conditions reported. She was 36-weeks pregnant at time of COVID-19 infection. She started ocrelizumab therapy in May 2019 and discontinued ocrelizumab due to pregnancy (her last ocrelizumab infusion was 11 months before COVID-19). COVID-19 severity was mild, not requiring hospitalization, and she fully recovered.
4. Discussion
This study on two cohorts of PLwMS treated with ocrelizumab in Israel who largely completed full vaccination scheme suggests that the occurrence of SARS-CoV-2 infection after COVID-19 mRNA vaccination (i.e., vaccine breakthroughs) was low (3.7% and 2.6%, respectively) and limited in severity (no ICU admission and no deaths observed) compared to COVID-19 cases diagnosed among unvaccinated patients. Several real-world studies conducted during the pre-vaccination era of the pandemic found that PLwMS treated with anti-CD20 agents including ocrelizumab and rituximab have an increased risk of COVID-19-related hospitalization (Salter et al., 2021; Sormani et al., 2021a, 2021b; Simpson-Yap et al., 2021). The impact of DMTs on the clinical effectiveness of SARS-CoV-2 vaccines in preventing infections and severe disease remains to be determined. A large study on the entire population of PLwMS receiving DMTs in England found that SARS-CoV-2 vaccines offered less protection against infection (symptomatic and asymptomatic) to patients taking ocrelizumab or fingolimod (Garjani et al., 2022). An Italian study also reported similar results of a higher breakthrough infection rate in PLwMS treated with fingolimod and ocrelizumab than patients on other DMTs, but did not observe a significant reduction in hospitalization rate after vaccination in patients on ocrelizumab compared to those on the same treatment in the pre-vaccination era (16.7% post-vaccination vs. 19.4% pre-vaccination era, relative reduction=14%, RR=0.86, 95% CI=0.38– 1.91, p = 0.74) (Schiavetti et al., 2022). However, the Italian CovaXiMS (Covid-19 vaccine in Multiple Sclerosis) study found that the rate of infection requiring hospitalization was significantly reduced after vaccination, including in PLwMS treated with ocrelizumab with hospitalization rate reduced by 70% as compared to pre-vaccination hospitalization rate (Sormani et al., 2022). Disease-modifying therapies affect the immunological response to SARS-CoV-2 vaccines (Gombolay et al., 2022). It has been suggested that increased risk of breakthrough SARS-CoV-2 infections is associated with impaired humoral immune response, while preserved cellular immune response appears to play a critical role in preventing severe infections requiring hospitalization, also for SARS-CoV2 variants of concerns (Sormani et al., 2022; Ledford, 2022). Attenuated humoral response to SARS-CoV-2 mRNA vaccines has been reported, while a robust T-cell response was induced in patients treated with ocrelizumab (Brill et al., 2021; Madelon et al., 2021; Apostolidis et al., 2021). Such a T-cell response was significantly boosted after a third vaccine dose (Brill et al., 2022), and preserved against SARS-CoV-2 Delta or Omicron variants (Madelon et al., 2022). A better understanding of immunity against SARS-CoV-2 upon infection or vaccination is critical to determine the role and importance of humoral and cellular immune responses in conferring appropriate clinical protection against severe COVID-19.
In conclusion, despite the potential limitations of our study (particularly the small cohort size and few events), our findings appear to provide preliminary information suggesting a benefit of COVID-19 vaccination in PLwMS treated with ocrelizumab. Further studies are warranted to confirm that clinical protection against severe COVID-19 from SARS-CoV-2 vaccination is not impaired in PLwMS treated with ocrelizumab.
Declaration of Competing Interest
Erwan Muros-Le Rouzic, Spyros Roumpanis, Sharon Ehrlich, Nikki Jessop, and Rosetta Pedotti are employees of F. Hoffman-La Roche. Janick Weberpals was an employee of F. Hoffmann-La Roche Ltd during completion of the work related to this manuscript.
Acknowledgments
The development of this manuscript was funded by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance, under the direction of Stephanie Gibson, was provided by Rosie Durant, of Ashfield MedComms, an Ashfield Health company, and was funded by F. Hoffmann-La Roche Ltd.
References
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., Markowitz C.E., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., Rechtman A., Zveik O., Haham N., Oiknine-Djian E., Wolf D.G., Levin N., Raposo C., Vaknin-Dembinsky A. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., Raposo C., Rechtman A., Zveik O., Levin N., Oiknine-Djian E., Wolf D.G., Vaknin-Dembinsky A. Severe acute respiratory syndrome coronavirus 2 third vaccine immune response in multiple sclerosis patients treated with ocrelizumab. Ann. Neurol. 2022;91(6):796–800. doi: 10.1002/ana.26343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garjani A., Patel S., Bharkhada D., Rashid W., Coles A., Law G.R., Evangelou N. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103458. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombolay G.Y., Dutt M., Tyor W. Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis. Ann. Clin. Transl. Neurol. 2022 doi: 10.1002/acn3.51628. Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. 'Killer' immune cells still recognize Omicron variant. Nature. 2022;601(7893):307. doi: 10.1038/d41586-022-00063-0. Jan. [DOI] [PubMed] [Google Scholar]
- Madelon N., Lauper K., Breville G., Sabater Royo I., Goldstein R., Andrey D.O., Grifoni A., Sette A., Kaiser L., Siegrist C.A., Finckh A., Lalive P.H., Didierlaurent A.M., Eberhardt C.S. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab954. ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelon N., Heikkilä N., Sabater Royo I., Fontannaz P., Breville G., Lauper K., Goldstein R., Grifoni A., Sette A., Siegrist C.A., Finckh A., Lalive P.H., Didierlaurent A.M., Eberhardt C.S. Omicron-specific cytotoxic T-cell responses after a third dose of mRNA COVID-19 vaccine among patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2022;79(4):399–404. doi: 10.1001/jamaneurol.2022.0245. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78:699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavetti I., Cordioli C., Stromillo M.L., Teresa Ferrò M., Laroni A., Cocco E., Cola G., Pasquali L., Rilla M.T., Signoriello E., Iodice R., Di Sapio A., Lanzillo R., Caleri F., Annovazzi P., Conte A., Liberatore G., Ruscica F., Docimo R., Bonavita S., Ulivelli M., Cavalla P., Patti F., Ferraro D., Clerico M., Immovilli P., Di Filippo M., Salvetti M., Sormani M.P. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult. Scler. 2022 doi: 10.1177/13524585221102918. Jun 23. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S., Rassen J.A., Brown J.S., Rothman K.J., Happe L., Arlett P., Dal Pan G., Goettsch W., Murk W., Wang S.V. Graphical depiction of longitudinal study designs in health care databases. Ann. Intern. Med. 2019;170(6):398–406. doi: 10.7326/M18-3079. [DOI] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Salvetti M., Labauge P., et al. DMTs and COVID-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol. 2021;8:1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., Visconti V., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Cocco E., Frau J., Ferrò M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Pizzorno M., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A., CovaXiMS study group Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]