Abstract
Purpose:
To investigate the characteristics and rate of central visual field loss after optic disc hemorrhages (DH).
Design:
Prospective cohort study.
Methods:
343 eyes of 220 subjects who had at least 3 years of follow-up with minimum of 5 visits with 10-2 and 24-2 visual field (VF) were recruited. Rates of 10-2 mean deviation (MD) loss in each hemifield and pre-defined zones were compared using linear mixed-effects model in DH and non-DH eyes. Clustered pointwise regression analysis was also used to define central VF progressors and compared to 24-2 VF loss using Guided Progression Analysis.
Results:
39 eyes with DH and 304 eyes without DH had a mean follow-up of 5.2 years. Eyes with DH had rates of 10-2 mean deviation (MD) loss that were 3 times faster than non-DH eyes (mean difference (95% CI): −0.36 dB/year(0.54, 0.18), p<0.001) and were 3.7 times more likely to progress (p=0.002). A larger proportion of glaucomatous eyes showed central VF progression rather than peripheral VF progression in DH group (30.8% vs. 20.5%) compared to non-DH group (10.9% vs. 9.2%). In early glaucoma, the rate of 10-2 MD loss was 5.5 times faster in DH eyes than in non-DH eyes(p<0.001). Superonasal and superotemporal central VF regions progressed more rapidly than other regions, especially in DH eyes.
Conclusion:
Central visual field loss is accelerated in glaucoma eyes with DH and it corresponds topographically to the DH location. In glaucoma patients with DH, one should consider supplementing 10-2 VFs with 24-2 VFS to monitor the disease.
Keywords: central visual field, optic disc hemorrhage, glaucoma, progression
Introduction
Glaucoma is a progressive optic neuropathy characterized by the loss of retinal ganglion cells (RGCs) associated with degeneration of the optic nerve and retinal nerve fiber layer (RNFL).1 Optic disc hemorrhages (DHs) are strongly associated with the development2-4 and progression of glaucomatous damage.5-10 Although the pathophysiology of glaucomatous DHs is unknown, several hypotheses have been proposed, including vascular and mechanical theories. It is known, however, that peripapillary DHs are topographically associated with localized RNFL defects and neuroretinal rim notching. Most notably, DHs occur at the border between healthy and damaged RNFL.11
The central visual field (VF) generally was thought to be preserved until advanced stages of disease.12 However, recent studies reported that paracentral VF defects often are observed in early glaucoma.13, 14 Early detection of macular involvement and its progression is of great importance because the loss of central vision can significantly affect an individual’s quality of life. Central vision loss is associated with faster glaucoma progression and increased disability in performing daily tasks such as reading, driving and walking.15-19
It has been suggested that 24-2 testing strategies may be suboptimal VF test patterns to detect early macular involvement in glaucoma.20-26 To evaluate central vision, clinicians often employ a 10-2 VF test which uses 68 central test points that are 2 degrees apart. However, such a strategy has been questioned. In a study by West et al26, there was little benefit to also using 10-2 VF in revealing additional central VF defects in patients with early 24-2 VF defects. The authors suggested that use of 10-2 VF might be best employed for following patients with a high risk of central visual field progression.26
In recent studies, eyes with early glaucomatous defects in central areas were strongly associated with a history of DHs.27-31 However, information about the spatial relationship and rate of central VF progression in disc hemorrhages is sparse. In the current study, the characteristics, rates, and relationships of central VF loss with the location of DHs were investigated and compared to eyes without DH. We also tested whether DH eyes experience more rapid peripheral and central visual field loss than non-DH eyes as measured with the entire 24-2 VF and 10-2 VF test patterns.
Methods
Participants
In this observational cohort study, participants were included from a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma (Diagnostic Innovations in Glaucoma Study [DIGS] and African Descent and Glaucoma Evaluation Study [ADAGES]). Participants in these cohorts were longitudinally evaluated according to a pre-established protocol that included regular follow-up visits in which patients underwent a clinical examination and several imaging and functional tests. All participants from the DIGS and ADAGES study who met the inclusion criteria described below were enrolled in the current study. Informed consent was obtained from all participants. The University of California, San Diego Human Subjects Committee approved all protocols, and the methods described adhered to the tenets of the Declaration of Helsinki.
Subjects underwent annual comprehensive ophthalmologic examination, including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, dilated funduscopic examination, stereoscopic optic disc photography, and standard automated perimetry using a Full-Threshold or Swedish Interactive Threshold Standard Algorithm (Humphrey Field Analyzer; Carl Zeiss Meditec, Dublin, CA). Semi-annual examinations included standard automated perimetry (10-2 VF and 24-2 VF) and IOP measurement. Only subjects with open angles on gonioscopy at baseline were included. Subjects were excluded if they had a baseline best-corrected visual acuity less than 20/40, spherical refraction with greater than 6.0 diopters of myopia, cylinder correction greater than 3.0 diopters, or any ocular or systemic disease that could affect the optic nerve or visual field.
The study included eyes diagnosed as glaucoma or glaucoma suspect with or without history of DH at the baseline visit with a minimum follow-up time of 3 years and a minimum of five 10-2 and 24-2 VFs. Eyes were classified as glaucomatous if they had repeatable (at least 2 consecutive) abnormal VF test results or evidence of glaucomatous optic neuropathy defined as excavation, the presence of focal thinning, notching of neuroretinal rim, or localized or diffuse atrophy of the RNFL on the basis of masked grading of optic disc photographs by 2 graders or clinical examination by a glaucoma specialist. An abnormal VF test was defined as a pattern standard deviation outside of the 95% normal confidence limits or a Glaucoma Hemifield Test result outside normal limits. Glaucoma suspects were defined as having elevated IOP (≥22mmHg) or suspicious-appearing optic discs without the presence of repeatable glaucomatous VF damage.
Stereophotography
All patients had stereoscopic optic disc photographs repeated at least every 12 months during follow-up. The presence of an optic disc hemorrhage was evaluated by two experienced graders using a stereoscopic viewer (Screen-VU stereoscope; PS Manufacturing, Portland, OR). Each grader was masked to the subject’s identity and the other test results. All included photographs were judged to be of adequate quality or better. Discrepancies between the two graders were resolved by consensus or adjudication by a third experienced grader. DHs had to be located within one-half disc diameter of the optic disc border and not associated with optic disc edema, papillitis, diabetic retinopathy, central or branch retinal vein occlusion, or any other retinal disease.32, 33 The locations of DHs were categorized as located in the superior hemisphere, inferior hemisphere, or both.
Standard Automated Perimetry
The 10-2 and 24-2 VF tests were considered unreliable and excluded if there was >33% fixation losses, >33% false-positive errors, or >33% false-negative errors. Experienced graders at the University of California, San Diego Visual Field Assessment Center (VisFACT) reviewed all the results, excluding tests with eyelid or rim artifacts, fatigue or learning effects, inappropriate fixation, or evidence that the visual field results were caused by a disease other than glaucoma (e.g. homonymous hemianopia) or inattention. Patients with glaucoma were stratified into 2 categories based on the severity of their VF damage. Patients with mean deviation (MD) >−6.0 dB were classified as mild glaucoma, and patients with MD≤−6.0 were classified as moderate to severe glaucoma.34
The regions corresponding to the structure versus function map for 10-2 VFs proposed by Hood et al13 (Figure 1) were divided into five zones: the superior nasal (Zone 1), superior temporal (Zone 2), superior temporal band (Zone 3), inferior temporal (Zone 4), and inferior nasal (Zone 5). For calculation of the mean deviation (MD) in each zones, threshold sensitivity values in decibels (dB) were used. The MDs of the zones were calculated as the average threshold sensitivity values of all points tested in that region.
Figure 1.
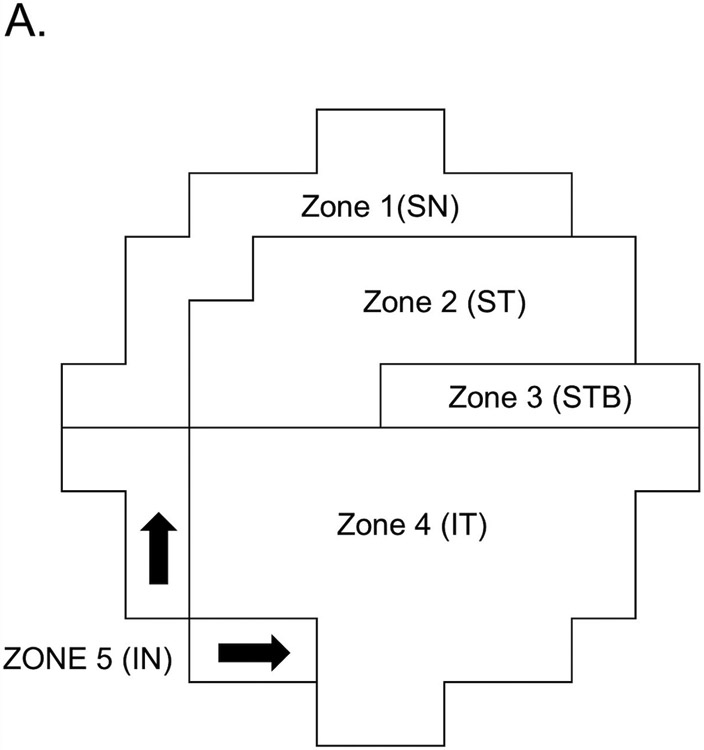
Visual field zones of 10-2 VF devised by Hood et al.13 Note that this map assumed 5 distinct VF zones based on their vulnerability to damage in the macula. Zone 1 = superior nasal zone, Zone 2 = superior temporal zone, Zone 3 = superior temporal band, Zone 4 = inferior temporal zone, Zone 5 = inferior nasal zone.
Central Visual Field Progression
Different trend-based analyses were used to characterize progression in the 10-2 VF tests are described below.
Best Linear Unbiased Prediction:
Estimates of rates of change for individual eyes and different zones were obtained by best linear unbiased prediction (BLUP). Ordinary least square estimates can be imprecise in eyes with just a few measurements available over time or with large intraindividual variability.35 Individual ordinary least square estimates (i.e., individual regression lines) also do not take into account the information provided by the whole population, whereas BLUPs are shrinkage estimates that take into account the results obtained by evaluating the whole sample of eyes, giving less weight to estimates obtained from eyes with few measurement occasions or large intraindividual variability (i.e., more “noise”).36 In eyes with a large number of measurements over time, BLUP and ordinary least square estimates give similar results. BLUPs have been used to estimate individual rates of structural change measured by different instruments in glaucoma, and rate of cognitive change in longitudinal models.37, 38
Clustered Pointwise Linear Regression:
Regression of VF parameters over time has been used to identify VF deterioration and to estimate magnitude of VF loss. Regression of individual locations or of clusters provided more information about the location of VF loss than could regression of global indices.39, 40 A VF test point was flagged as worsening if it showed a significant negative slope of at least −1 dB/year, with a significance level of p<0.01.41, 42 As in de Moraes et al., progression in a 10-2 VF was defined as at least 3 test points located in the same latent class analysis (LCA) derived 10-2 VF sector progressing faster than −1.0 dB/year at p<0.01.43
Peripheral Visual Field (24-2) Progression
24-2 VF progression was defined when there were >3 locations that showed a significant change (ie, change greater than the test-retest variabilities) compared with 2 baseline examinations for at least 3 consecutive tests (ie, “likely progression” as reported in the Guided Progression Analysis [GPA]) during the study follow-up and when the changes also were observed at the latest follow-up visit.44
Statistical analysis
Continuous and categorical data were presented as mean (95% confidence interval, CI) and count (%). Statistically significant differences in patient characteristics between the DH group and the non-DH group were determined by two-sample t-tests for continuous variables and Fisher’s exact test for categorical variables. Eye characteristics were compared using linear mixed effects models with random intercepts to account for within-subject variability. VF trajectories were estimated using linear mixed effects models with random eye-within-patient intercepts and independent random slopes-within-eye. These models include fixed effect for DH history and time interaction. Multivariable models were fit including age, mean IOP over follow-up, as well as any other ocular characteristic reported to be associated with progression in previous studies and whose p value was below 0.10 in univariable analysis.
Logistic regression was used to compare progressors versus non-progressors assessed by clustered PLR analysis or GPA analysis. Multivariable logistic regression was performed to explore factors contributing to progressive 10-2 mean deviation (MD) loss.
Linear mixed-effect models with random intercepts and random slopes were also used to compare the rate of 10-2 MD loss in each hemifield and each zone between DH and non-DH eyes. Similarly, rates of MD loss in the hemifield corresponding to DHs of DH eyes were calculated and compared to the rates of central MD loss in non-corresponding hemifields of DH eyes and non-DH eyes. All statistical analyses were conducted using Stata/IC version 13.0 (StataCorp, Texas, USA).
Results
Three hundred forty three eyes from 220 patients met our inclusion criteria and were included in this report. Mean age (95% CI) at baseline was 71.1 (67.8, 74.5) years for the disc hemorrhage (DH) group and 68.1 (66.7, 69.5) years for the non-DH group (p=0.089). The number of visits were comparable between 24-2 VF (p=0.212) and 10-2 VF (p=0.497) visits. Eyes with DH had a mean number (95% CI) of 7.8 (7.2, 8.5) 10-2 VFs and 7.9 (7.2, 8.7) 24-2 VFs over 5.1 (4.9, 5.2) years, while non-DH eyes had 7.6 (7.3, 7.8) 10-2 VFs and 7.7 (7.4, 8.0) 24-2 VFs over 5.3 (5.2, 5.5) years. Baseline 24-2 MD and 10-2 MD were similar in both groups (p=0.867 and p=0.825, respectively). Of the 39 eyes with DH, the DH was located inferiorly in 28 (71.8%) eyes, superiorly in 4 (10.3%) eyes, and in both hemispheres in 7 (17.9%) eyes. Patient characteristics are summarized in Table 1.
Table 1.
Demographics and Baseline Characteristics of DH and Non-DH Eyes
| Variables | DH group | Non-DH group | P value |
|---|---|---|---|
| By Subject (No.) | 34 | 186 | |
| Age (years) | 71.1 (67.8, 74.5) | 68.1 (66.7, 69.5) | 0.089 |
| Gender (M/F) | 12/22 | 91/95 | 0.143 |
| Race | |||
| African American/ Non-African American | 5/29 | 72/114 | 0.007 |
| Self-reported HTN, n (%) | 22 (64.7%) | 121 (65.1%) | 0.969 |
| Self-reported DM, n (%) | 3 (8.8%) | 28 (15.1%) | 0.337 |
| Systolic blood pressure (mmHg) | 131.8 (125.9, 137.6) | 132.0 (129.2, 134.7) | 0.818 |
| Diastolic blood pressure (mmHg) | 76.8 (73.3, 80.2) | 78.5 (76.9, 80.2) | 0.349 |
| By Eye (No.) | 39 | 304 | |
| MOPP (mmHg) | 54.4 (51.8, 57.0) | 54.4 (53.5, 55.4) | 0.994 |
| Axial length (mm) | 24.3 (23.9, 24.7) | 24.2 (24.0, 24.3) | 0.646 |
| CCT (μm) | 533.3 (518.8, 547.7) | 537.8 (531.7, 543.9) | 0.557 |
| Baseline IOP (mmHg) | 13.5 (12.4, 14.6) | 14.6 (14.1, 15.1) | 0.076 |
| Mean IOP during follow-up (mmHg) | 13.8 (12.8, 14.9) | 14.4 (14.0, 14.9) | 0.280 |
| Diagnosis | 0.145 | ||
| Glaucoma suspect, Eye No. (%) | 7 (18.0%) | 89 (29.3%) | |
| Mild glaucoma, Eye No. (%) | 25 (64.0%) | 145 (47.7%) | |
| Moderate/ advanced glaucoma, Eye No. (%) | 7 (18.0%) | 70 (23.0%) | |
| Total DH No. | |||
| Multiple DH, Eye No. (%) | 18 (46.2%) | ||
| DH Location | |||
| Inferior, Eye No. (%) | 28 (71.8%) | ||
| Superior, Eye No. (%) | 4 (10.3%) | ||
| Both hemispheres, Eye No. (%) | 7 (17.9%) | ||
| Baseline 24-2 MD (dB) | –4.2 (−5.8, −2.7) | −4.4 (−5.1, −3.7) | 0.867 |
| Baseline 24-2 PSD (dB) | 5.6 (4.3, 6.9) | 4.7 (4.3, 5.2) | 0.168 |
| Baseline 10-2 MD (dB) | −3.9 (−5.5, −2.4) | −3.8 (−4.4, −3.1) | 0.825 |
| Baseline 10-2 PSD (dB) | 5.2 (3.6, 6.8) | 4.1 (3.6, 4.6) | 0.183 |
| Follow-up (years) | 5.1 (4.9, 5.2) | 5.3 (5.2, 5.5) | 0.082 |
| Visits of 24-2 Visual Field, n | 7.9 (7.2, 8.7) | 7.7 (7.4, 8.0) | 0.212 |
| Visits of 10-2 Visual Field, n | 7.8 (7.2, 8.5) | 7.6 (7.3, 7.8) | 0.497 |
CCT = central corneal thickness; DH = disc hemorrhage; DM = diabetes mellitus; F = female; HTN = hypertension; IOP = intraocular pressure; M = male; MD = mean deviation; MOPP = mean ocular perfusion pressure; PSD = pattern standard deviation. Values are shown in mean (95% confidence interval), unless otherwise indicated. Statistically significant P value is shown in bold.
Central visual field (VF) mean deviation (MD) deteriorated in both DH eyes and non-DH eyes. Eyes with DH had rates of 10-2 MD loss three times faster than non-DH eyes (mean difference (95% CI): −0.36 dB/year (0.54, 0.18), p<0.001).
Differences in rates of 10-2 MD loss were also found between DH and non-DH groups when comparing the hemifields. Eyes with DH had a faster rate of 10-2 MD loss in both superior hemifields ( −0.31 dB/year (−0.50, −0.12), p=0.001) and inferior hemifields (−0.18 dB/year (−0.36, 0.01, p=0.037) compared to non-DH eyes (Table 2). Among DH eyes, faster rates of MD loss were found in the superior hemifield (mean (95% CI): −0.48 dB/year (−0.65, −0.30) compared to the inferior hemifield (−0.32 dB/year (−0.49, −0.16). Similar results were found after adjusting for age and baseline 24-2 MD. A history of DH (p<0.001) and lower baseline 24-2 MD (p<0.001) were associated with faster 10-2 MD loss in the multivariable analysis (Table 3).
Table 2.
Comparison of Rates of VF Loss between DH and Non-DH Eyes
| DH Group Mean (95% CI) |
Non-DH Group Mean (95% CI) |
Difference Mean (95% CI) |
P value (adjusted) |
|
|---|---|---|---|---|
| No. of Eyes | 39 | 304 | ||
| 24-2 MD Change Rate (dB/year) | ||||
| Global 24-2 | −0.38 (−0.54, −0.21) | −0.21 (−0.27, −0.15) | −0.17 (−0.34, 0.01) | 0.060 (0.112) |
| Central MD Change Rate (dB/year) | ||||
| Global 10-2 | −0.50 (−0.68, −0.33) | −0.15 (−0.21, −0.09) | −0.36 (−0.54, −0.18) | <0.001 (<0.001) |
| Central Hemifield MD Change Rate (dB/year) | ||||
| Superior | −0.48 (−0.65, −0.30) | −0.16 (−0.23, −0.10) | −0.31 (−0.50, −0.12) | 0.001 (0.001) |
| Inferior | −0.32 (−0.49, −0.16) | −0.14 (−0.2, −0.08) | −0.18 (−0.36, −0.01) | 0.037 (0.033) |
DH = disc hemorrhage; MD = mean deviation; VF = visual field. Values are shown in mean (95% confidence interval), unless otherwise indicated. P values were adjusted for age and baseline 24-2 MD. Statistically significant P values are shown in bold.
Table 3.
Factors Contributing to the Rate of 10-2 VF Loss Over Time in Study Participants by Univariable and Multivariable Mixed Model Analysis
| Variables | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
| β, 95 % CI | P value | β, 95 % CI | P value | |
| Age, per 10 year older | −0.03 (−0.09, 0.03) | 0.343 | −0.05 (−0.12, 0.01) | 0.105 |
| Gender: M/F | −0.05 (−0.17, 0.07) | 0.437 | ||
| Race: African American/ Non-African American | −0.07 (−0.20, 0.05) | 0.246 | ||
| Axial length, per 1mm longer | −0.03 (−0.08, 0.02) | 0.202 | ||
| CCT, per 10 μm thinner | 0.04 (−0.11, 0.20) | 0.574 | ||
| Self-reported diabetes | 0.07 (−0.10, 0.24) | 0.423 | ||
| Self-reported hypertension | 0.11 (−0.01, 0.24) | 0.068 | 0.12 (0.00, 0.24) | 0.051 |
| MOPP, per 1 mmHg higher | 0.00 (−0.01, 0.01) | 0.592 | ||
| Baseline IOP, per 1 mmHg higher | −0.01 (−0.02, 0.01) | 0.294 | ||
| Mean IOP, per 1 mmHg higher | 0.00 (−0.02, 0.02) | 0.935 | −0.01 (−0.03, 0.00) | 0.158 |
| History of disc hemorrhage | −0.36 (−0.54, −0.18) | <0.001 | −0.36 (−0.53, −0.18) | <0.001 |
| Baseline MD 10-2, per 1 dB worse | −0.01 (−0.02, 0.00) | 0.028 | ||
| Baseline MD 24-2, per 1 dB worse | −0.02 (−0.03, −0.01) | <0.001 | −0.02 (−0.03, −0.01) | <0.001 |
| Follow-up time, per 1 year longer | −0.04 (−0.08, 0.00) | 0.051 | −0.03 (−0.07, 0.01) | 0.123 |
CCT = central corneal thickness; F = female; IOP = intraocular pressure; M = male; MD = mean deviation; MOPP = mean ocular perfusion pressure; VF = visual field. Age, mean IOP and variables with a P value of less than 0.10 in the univariable analysis were included in the multivariable model. Statistically significant P values are shown in bold.
Using the clustered PLR criteria, central VF progression was seen in 12 of the 39 (30.8%) eyes in the DH group and 33 of the 304 (10.9%) eyes in the non-DH group. Although a similar proportion of 24-2 progression was found in the non-DH group (28 eyes, 9.2%), a lower proportion of DH eyes were progressors in 24-2 VF (8 eyes, 20.5%) compared to 10-2 VF.Table 4 shows the univariable and multivariable logistic regression analyses results of factors associated with 10-2 VF progression defined by clustered pointwise linear regression (PLR) of MD. In univariable analysis, history of DH (Odds Ratio (OR): (95% CI): 3.65 (1.58, 8.42); p=0.002), worse baseline 10-2 VF MD (OR: 1.04 (0.99, 1.09) per 1 dB; p=0.096) and worse baseline 24-2 VF MD (OR: 1.06 (1.02, 1.11) per 1 dB; p=0.004) were associated with 10-2 VF progression. As both baseline 10-2 MD and 24-2 MD were significantly correlated with each other, these two VF parameters were included in separate multivariable models to avoid multicollinearity. In both multivariable models, DH was significantly associated with VF progression (OR: 3.78 (1.56, 9.13), p=0.003; OR: 3.51 (1.48, 8.29), p=0.004, in models adjusting for 24-2 and 10-2 VF MD, respectively).
Table 4.
Factors Contributing to the 10-2 VF Loss Progression Assessed by clustered PLR in Study Participants using Univariable and Multivariable Logistic Regression Analyses.
| Variables | Univariate Model | Multivariable Model I | Multivariable Model II | |||
|---|---|---|---|---|---|---|
| Odds ratio, 95% CI |
P value |
Odds ratio, 95 % CI |
P value |
Odds ratio, 95 % CI |
P value |
|
| Age, per 10 year older | 10.06 (9.70, 10.44) | 0.732 | 10.17 (9.78, 10.57) | 0.404 | 10.18 (9.79, 10.59) | 0.377 |
| Gender: M/F | 0.99 (0.48, 2.02) | 0.973 | ||||
| Race: African American/ Non-African American | 1.38 (0.63, 3.00) | 0.417 | ||||
| Axial length, per 1mm longer | 1.05 (0.81, 1.36) | 0.719 | ||||
| CCT, per 10 μm thinner | 10.01 (9.93, 10.1) | 0.749 | ||||
| Self-reported diabetes | 1.39 (0.51, 3.83) | 0.519 | ||||
| Self-reported hypertension | 0.59 (0.28, 1.22) | 0.153 | ||||
| MOPP, per 1 mmHg higher | 1.01 (0.96, 1.06) | 0.788 | ||||
| Baseline IOP, per 1 mmHg higher | 1.01 (0.92, 1.10) | 0.884 | ||||
| Mean IOP, per 1 mmHg higher | 1.01 (0.91, 1.12) | 0.899 | 1.05 (0.95, 1.17) | 0.301 | 1.04 (0.93, 1.15) | 0.512 |
| History of disc hemorrhage | 3.65 (1.58, 8.42) | 0.002 | 3.78 (1.56, 9.13) | 0.003 | 3.51 (1.48, 8.29) | 0.004 |
| Baseline MD 10-2, per 1 dB worse | 1.04 ( 0.99, 1.09 ) | 0.096 | 1.05 ( 1, 1.11 ) | 0.074 | ||
| Baseline MD 24-2, per 1 dB worse | 1.06 ( 1.02, 1.11 ) | 0.004 | 1.08 ( 1.03, 1.13 ) | 0.002 | ||
| Follow-up time, per 1 year longer | 1.16 (0.98, 1.37) | 0.088 | 1.17 (0.97, 1.40) | 0.106 | 1.19 (0.97, 1.45) | 0.096 |
CCT = central corneal thickness; F = female; IOP = intraocular pressure; M = male; MD = mean deviation; MOPP = mean ocular perfusion pressure; VF = visual field. Values are shown in odds ratio (95% confidence interval), unless otherwise indicated. Age, mean IOP and variables with a P value of less than 0.10 in the univariable analysis were included in the multivariable model. Statistically significant P values are shown in bold.
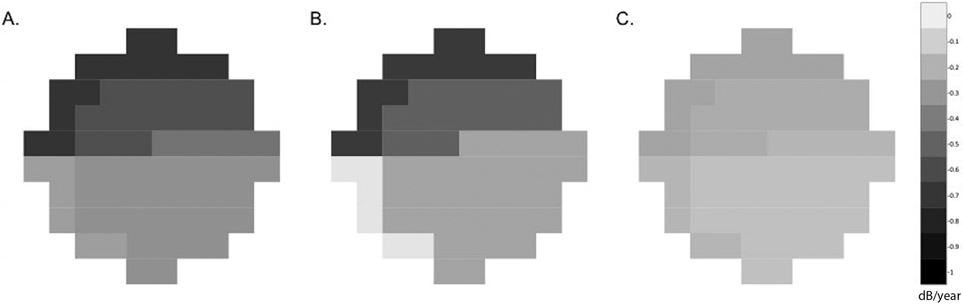
When evaluating different VF zones between DH and non-DH eyes, superior nasal (Zone 1), superior temporal (Zone 2), superior temporal band (Zone 3), and inferior nasal (Zone 5) zones had significantly faster rates of MD loss compared to the inferior temporal (Zone 4) zone (Table 5). The distributions of the mean rates of 10-2 VF loss for DH and non-DH eyes are presented in Figure 2. Similar results were found when comparing the zones in 28 eyes with inferior DH and those of non-DH eyes (Supplemental Table 1).
Table 5.
Comparison of Rates of Regional 10-2 MD Loss between DH and Non-DH Eyes
| DH Group Mean (95% CI) |
Non-DH Group Mean (95% CI) |
Difference Mean (95% CI) |
P value (adjusted) |
|
|---|---|---|---|---|
| No. of Eyes | 39 | 304 | ||
| Zonal MD Change Rate (dB/year) | ||||
| Zone 1 (superior nasal) | −0.58 (−0.79, −0.37) | −0.20 (−0.27, −0.12) | −0.39 (−0.61, −0.16) | 0.001 (<0.001) |
| Zone 2 (superior temporal) | −0.71 (−0.96, −0.46) | −0.24 (−0.34, −0.15) | −0.47 (−0.73, −0.20) | 0.001 (<0.001) |
| Zone 3 (superior temporal band) | −0.43 (−0.67, −0.20) | −0.18 (−0.26, −0.09) | −0.25 (−0.50, 0.00) | 0.047 (0.039) |
| Zone 4 (inferior temporal) | −0.27 (−0.50, −0.04) | −0.20 (−0.28, −0.11) | −0.07 (−0.32, 0.18) | 0.572 (0.527) |
| Zone 5 (inferior nasal) | −0.32 (−0.49, −0.16) | −0.14 (−0.20, −0.08) | −0.19 (−0.37, −0.01) | 0.042 (0.038) |
DH = disc hemorrhage; MD = mean deviation. Values are shown in mean (95% confidence interval), unless otherwise indicated.
P values were adjusted for age and baseline 24-2 MD. Statistically significant P values are shown in bold.
Figure 2.
Diagrams showing distributions of the mean rates of 10-2 mean deviation (MD) loss for A. DH eyes, B. Inferior DH eyes and C. Non-DH eyes. DH eyes had a faster rate of 10-2 MD loss compared to non-DH eyes. Both DH and non-DH eyes presented with a faster rate of deterioration in the superior hemifield with preservation of the superior temporal band. VFs were plotted in right eye format. Data are presented as the mean MD change rate (dB/year).
The topographic location of DH was associated with higher rates of central VF loss. Of the 28 eyes in which the DH was located in the inferior hemisphere, the mean difference between the rates of 10-2 MD loss in the corresponding hemifield (mean (95% CI): −0.24 dB/year (−0.45, −0.03), p=0.026) was faster than the non-corresponding hemifield (−0.10 dB/year (−0.29, −0.10), p=0.110).
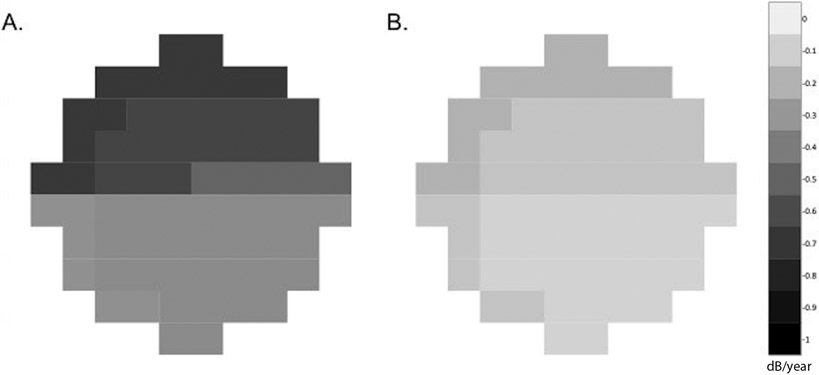
The results were more pronounced in eyes with early glaucoma (24-2 VF MD >−6 dB). Rates of 10-2 MD loss in DH eyes with early glaucoma were 5.5 times faster than non-DH eyes (mean (95% CI) −0.56 dB/year (−0.71, −0.40), vs. −0.10 dB/year (−0.16, −0.04), p<0.001). After adjusting for confounders, eyes in the DH group were 4.4 times more likely to develop 10-2 VF progression (p=0.003) (Supplemental Table 2 and 3; Figure 3).
Figure 3.
Diagrams showing distributions of the mean rates of 10-2 mean deviation (MD) loss for A. DH eyes and B. Non-DH eyes in early glaucoma (MD 24-2>−6dB). The mean rate of 10-2 MD loss between DH and non-DH eyes in early glaucoma were significantly faster for both superior and inferior hemifields. VFs were plotted in right eye format. Data are presented as the mean MD change rate(dB/year).
Although the DH eyes had faster rate of 24-2 MD deterioration compared to non-DH eyes, the difference did not reach statistical significance after adjusting for confounders in all eyes (mean difference (95% CI): −0.17 (−0.34, 0.01) dB/year, p=0.112) and was marginally significant in early glaucoma in early glaucoma (−0.15 (−0.26, −0.03) dB/year, p=0.073).
Discussion
The current study showed that rates of progressive central visual field (VF) loss in disc hemorrhage (DH) eyes were approximately three-fold faster than non-DH eyes, supporting the role of DH as an important risk factor for glaucoma progression. A larger proportion of eyes showed central, rather than peripheral, VF progression in the DH group compared to the non-DH group. Furthermore, early glaucoma patients with DH had an approximately five-fold faster rate of central mean deviation (MD) loss compared to non-DH eyes. This difference in the rate of progression was more evident in the superior hemifield with topographically associated inferiorly located DH. This information should help clinicians better understand the role of 10-2 VF as a supplement test to 24-2 VF in eyes with DH and provides clinical clues in monitoring glaucoma progression in these high-risk patients.
Associations between DH and perimetric progression,45 optic disc changes,5 retinal ganglion cell loss,46 retinal nerve fiber layer thinning,47 and vessel density dropout48 have been demonstrated in various studies. In the Ocular Hypertension Treatment Study, there was a two-fold increase in the cumulative incidence of developing primary open angle glaucoma in eyes with DH.49 De Moraes et al. studied VF progression before and after detection of DH and found that the 24-2 VF sector with the fastest progression predicted the location of future DHs in 85% of cases. The same VF sector maintained the fastest progression rate in almost all the eyes after the detection of DH.7 Although several studies have shown that the rate of RNFL thinning and VF loss increases after DH,45, 47 less was known about the rate and characteristics of central VF loss in eyes with DH. By evaluating the 10-2 VF in the current study, we demonstrated that the mean rate of central VF loss in DH eyes was −0.50 dB/year, almost three-fold faster than in non-DH eyes. Recent investigations found that DHs were associated with more central damage in 10-2 VFs.27, 50 Kono et al. characterized VF progression in eyes with DH using 24-2 VFs and found that eyes with DH were associated with greater VF progression in areas within the central 10 degrees, whereas no significant differences were found in other clusters or in the whole field.27 With analysis of progression using the clustered PLR criteria, we also confirmed prior studies and showed that the DH group had a three-fold increase in the number of eyes with central VF progression compared to non-DH eyes over 5 years of follow-up.
Hood et al. suggested that the inferior macular region was more susceptible to early glaucomatous damage than the superior macular region and coined the term “macular vulnerability zone” to describe the 50% of inferotemporal arcuate RNFL fibers prone to glaucomatous damage.13, 51 Traynis et al. showed that superior VF defects are deeper and closer to the fixation than those in the inferior VF.15 Likewise, in the present study, the rate of central VF loss was faster in the superior hemifield compared to inferior hemifield. The association of DH location with both structural and functional loss in glaucoma have been described in previous studies.52, 53 In the present study, DHs were predominantly detected in the inferotemporal region and rates of central VF deterioration were faster in the corresponding hemifield. The association between DH location and higher rates of central VF loss was further highlighted in our sub-analysis involving inferiorly located DHs; the rate of VF loss in the corresponding superior hemifield of these eyes was statistically significant higher compared to the non-corresponding inferior hemifield.
Macular involvement in early glaucoma is strongly correlated with a decline in vision-related quality of life (VRQoL) while arcuate damage outside the macula was less significant.54 A recent study on patient’s VRQoL found that it was also dependent on its hemifield location. Near activities were likely to be affected with superior field defect while distance activities with inferior field defect.55 In our sub-analysis of central VF in eyes with early glaucoma, DH eyes had significantly faster rates of progression in both superior and inferior hemifields than non-DH eyes. Additionally, in DH eyes with early glaucoma, the superior temporal zone (Zone 2), which corresponds to the MVZ, had rates of progression which were three-fold faster than the rate of non-DH eyes. Given the substantial impact of central VF on quality of life, meticulous assessment of the central VF and its inherent affected functions is recommended in managing glaucoma patients with DH. In addition, early testing of 10-2 VF has been reported to be useful in detecting patients with initial parafoveal scotoma (IPFS) in glaucoma. In a study by Park et al, in patients with IPFS, the 10-2 VF performed better in detection of progressing eyes compared with 24-2 VF.20
This study has limitations. Though the study was sufficiently powered to detect changes in the central VF in DH eyes, there were few eyes with superiorly located DHs; thus, detailed characterization of central VF progression in these eyes was not possible. Next, the frequency of optic disc photos is a possible limitation as they were acquired annually even though DHs typically resolve within 2 to 6 months.56 Therefore, it is possible that some DHs may have been missed in each group. Moreover, around 3.5 years on average elapsed between DH occurrence and baseline VF, therefore the rates of central VF loss should not be generalized to the time frame immediately following DH. In addition, the commercially analysis software built into the perimeters does not include analysis of progression of 10-2 fields which may make the use of 10-2 less relevant to most clinicians. Recently, development of a new algorithm for detecting progressive changes in 10-2 VF tests using event-based analysis has been described and validated57 and, perhaps, it will be incorporated in available perimeters in the future. Last, this was not a prospective observational study. Clinical observations of DHs and 10-2 visual field defects may have led to intensification of IOP-lowering therapy, potentially decreasing the true effect of DH on the rates of central visual field loss.
In conclusion, disc hemorrhages are an independent predictor for more accelerated central VF loss in glaucoma, especially in early stages of the disease. Central VF loss was faster in eyes with history of DH and worse visual fields at baseline. Moreover, superior hemifield defects tended to progress more rapidly than inferior hemifield defects in DH eyes. A larger proportion of eyes showed central VF progression rather than peripheral VF progression in the DH group compared the non-DH group. Therefore, examination of the central visual field using a 10-2 strategy should be considered in glaucoma patients with a history of DH for sensitive detection of disease progression.
Supplementary Material
Synopsis.
Eyes with disc hemorrhages (DH) had faster 10-2 visual field loss than those without DH.
Central visual field monitoring with 10-2 field should be considered as complementary to 24-2 field testing in eyes with DH.
Acknowledgements
Financial Support:
National Institutes of Health/National Eye Institute Grants R01EY029058, R01EY011008, U10EY14267, R01EY026574, R01EY019869, R01EY027510, R01EY025253 and 5K12EY024225; Tobacco Related Disease Research Program Grant T31IP1511; Core Grant P30EY022589; an unrestricted grant from Research to Prevent Blindness (New York, NY); and grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen. The sponsor or funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- ADAGES
African Descent and Glaucoma Evaluation Study
- BLUP
best linear unbiased prediction
- CCT
central corneal thickness
- CI
confidence interval
- dB
decibel
- DH
disc haemorrhage
- DIGS
Diagnostic Innovations in Glaucoma Study
- F
female
- IOP
intraocular pressure
- IPFS
initial parafoveal scotoma
- M
male
- MD
mean deviation
- MOPP
mean ocular perfusion pressure
- MVZ
macular vulnerability zone
- OR
odds ratio
- PLR
pointwise linear regression
- PSD
pattern standard deviation
- RGC
retinal ganglion cell
- RNFL
retinal nerve fiber layer
- VF
visual field
- VRQoL
vision-related quality of life
Footnotes
Financial Disclosures:
Ryan Caezar C. David, MD: none; Sasan Moghimi, MD: none; Jiun L. Do, MD, PhD: K12 Career Development Grant - National Eye Institute; Huiyuan Hou, MD, PhD: none; James Proudfoot, MSc: none; Linda M. Zangwill, PhD: Research support - National Eye Institute, Carl Zeiss Meditec, Heidelberg Engineering, Topcon, and Optovue; Alireza Kamalipour, MD: none; Takashi Nishida, MD, PhD: none; Carlos Gustavo De Moraes, MD, PhD, MPH: none; Christopher A. Girkin, MD, MSPH: Research support - Carl Zeiss Meditec, EyeSight Foundation of Alabama, Heidelberg Engineering, National Eye Institute, Research to Prevent Blindness, SOLX; Jeffrey M Liebmann, MD: Research support - Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Optovue, Reichert, Topcon; Consultant - Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Valeant Pharmaceuticals, Reichert, Heidelberg Engineering; Robert N Weinreb, MD: Research support - Carl Zeiss Meditec, Centervue, Heidelberg Engineering, Konan, National Eye Institute, and Optovue; Consultant - Aerie Pharmaceuticals, Allergan, Bausch & Lomb, Eyenovia, Nicox, and Novartis.
References:
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology 2006;113:2137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Song YJ, Kim YK, Jeoung JW, Park KH. Development of visual field defect after first-detected optic disc hemorrhage in preperimetric open-angle glaucoma. Jpn J Ophthalmol 2017;61:307–313. [DOI] [PubMed] [Google Scholar]
- 4.Sawada A, Manabe Y, Yamamoto T, Nagata C. Long-term clinical course of normotensive preperimetric glaucoma. Br J Ophthalmol 2017;101:1649–1653. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- 6.Keltner JL, Johnson CA, Anderson DR, et al. The association between glaucomatous visual fields and optic nerve head features in the Ocular Hypertension Treatment Study. Ophthalmology 2006;113:1603–12. [DOI] [PubMed] [Google Scholar]
- 7.De Moraes CG, Prata TS, Liebmann CA, Tello C, Ritch R, Liebmann JM. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest Ophthalmol Vis Sci 2009;50:4727–33. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros FA, Alencar LM, Sample PA, Zangwill LM, Susanna R, Jr., Weinreb RN. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology 2010;117:2061–6. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Kyung H, Azarbod P, Lee JM, Caprioli J. Disc haemorrhage is associated with the fast component, but not the slow component, of visual field decay rate in glaucoma. Br J Ophthalmol 2014;98:1555–9. [DOI] [PubMed] [Google Scholar]
- 10.Chan TCW, Bala C, Siu A, Wan F, White A. Risk Factors for Rapid Glaucoma Disease Progression. Am J Ophthalmol 2017;180:151–157. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Han JC, Kee C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog Retin Eye Res 2017;60:20–43. [DOI] [PubMed] [Google Scholar]
- 12.Henson DB, Hobley AJ. Frequency distribution of early glaucomatous visual field defects. Am J Optom Physiol Opt 1986;63:455–61. [DOI] [PubMed] [Google Scholar]
- 13.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res 2013;32:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traynis I, De Moraes CG, Raza AS, Liebmann JM, Ritch R, Hood DC. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. Jama Ophthalmol 2014;132:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman DS, Freeman E, Munoz B, Jampel HD, West SK. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology 2007;114:2232–7. [DOI] [PubMed] [Google Scholar]
- 16.Haymes SA, Leblanc RP, Nicolela MT, Chiasson LA, Chauhan BC. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci 2007;48:1149–55. [DOI] [PubMed] [Google Scholar]
- 17.Richman J, Lorenzana LL, Lankaranian D, et al. Relationships in glaucoma patients between standard vision tests, quality of life, and ability to perform daily activities. Ophthalmic Epidemiol 2010;17:144–51. [DOI] [PubMed] [Google Scholar]
- 18.Ekici F, Loh R, Waisbourd M, et al. Relationships Between Measures of the Ability to Perform Vision-Related Activities, Vision-Related Quality of Life, and Clinical Findings in Patients With Glaucoma. JAMA Ophthalmol 2015;133:1377–85. [DOI] [PubMed] [Google Scholar]
- 19.Wu N, Kong X, Gao J, Sun X. Vision-related Quality of Life in Glaucoma Patients and its Correlations With Psychological Disturbances and Visual Function Indices. J Glaucoma 2019;28:207–215. [DOI] [PubMed] [Google Scholar]
- 20.Park SC, Kung Y, Su D, et al. Parafoveal scotoma progression in glaucoma: humphrey 10-2 versus 24-2 visual field analysis. Ophthalmology 2013;120:1546–50. [DOI] [PubMed] [Google Scholar]
- 21.Hangai M, Ikeda HO, Akagi T, Yoshimura N. Paracentral scotoma in glaucoma detected by 10-2 but not by 24-2 perimetry. Jpn J Ophthalmol 2014;58:188–96. [DOI] [PubMed] [Google Scholar]
- 22.Grillo LM, Wang DL, Ramachandran R, et al. The 24-2 Visual Field Test Misses Central Macular Damage Confirmed by the 10-2 Visual Field Test and Optical Coherence Tomography. Transl Vis Sci Technol 2016;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Moraes CG, Hood DC, Thenappan A, et al. 24-2 Visual Fields Miss Central Defects Shown on 10-2 Tests in Glaucoma Suspects, Ocular Hypertensives, and Early Glaucoma. Ophthalmology 2017;124:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Medeiros FA, Weinreb RN, Girkin CA, Zangwill LM. Comparing 10-2 and 24-2 Visual Fields for Detecting Progressive Central Visual Loss in Glaucoma Eyes with Early Central Abnormalities. Ophthalmol Glaucoma 2019;2:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Medeiros FA, Weinreb RN, Zangwill LM. Performance of the 10-2 and 24-2 Visual Field Tests for Detecting Central Visual Field Abnormalities in Glaucoma. Am J Ophthalmol 2018;196:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West ME, Sharpe GP, Hutchison DM, et al. Utility of 10-2 Visual Field Testing in Glaucoma Patients with Early 24-2 Visual Field Loss, 2020/September/09 ed. Ophthalmology, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Kono Y, Sugiyama K, Ishida K, Yamamoto T, Kitazawa Y. Characteristics of visual field progression in patients with normal-tension glaucoma with optic disk hemorrhages. Am J Ophthalmol 2003;135:499–503. [DOI] [PubMed] [Google Scholar]
- 28.Park SC, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology 2011;118:1782–9. [DOI] [PubMed] [Google Scholar]
- 29.Rao A, Mukherjee S. Anatomical attributes of the optic nerve head in eyes with parafoveal scotoma in normal tension glaucoma. PLoS One 2014;9:e90554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang JW, Park B, Cho BJ. Comparison of risk factors for initial central scotoma versus initial peripheral scotoma in normal-tension glaucoma. Korean J Ophthalmol 2015;29:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias DT, Almeida I, Sassaki AM, et al. Factors associated with the presence of parafoveal scotoma in glaucomatous eyes with optic disc hemorrhages. Eye (Lond) 2018;32:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonas JB, Iester M. Disc hemorrhage and glaucoma. Ophthalmology 1995;102:365–6. [DOI] [PubMed] [Google Scholar]
- 33.Skaat A, De Moraes CG, Bowd C, et al. African Descent and Glaucoma Evaluation Study (ADAGES): Racial Differences in Optic Disc Hemorrhage and Beta-Zone Parapapillary Atrophy. Ophthalmology 2016;123:1476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. St. Louis, Mo.: Mosby, 1993:vii, 204 p., 2 p. of plates. [Google Scholar]
- 35.Beckett LA, Tancredi DJ, Wilson RS. Multivariate longitudinal models for complex change processes. Stat Med 2004;23:231–9. [DOI] [PubMed] [Google Scholar]
- 36.Robinson GK. That BLUP is a Good Thing: The Estimation of Random Effects. Statistical Science 1991;6:15–32. [Google Scholar]
- 37.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. Journal of glaucoma 2012;21:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould R, Abramson I, Galasko D, Salmon D. Rate of cognitive change in Alzheimer's disease: methodological approaches using random effects models. J Int Neuropsychol Soc 2001;7:813–24. [PubMed] [Google Scholar]
- 39.Katz J, Gilbert D, Quigley HA, Sommer A. Estimating progression of visual field loss in glaucoma. Ophthalmology 1997;104:1017–25. [DOI] [PubMed] [Google Scholar]
- 40.Gardiner SK, Mansberger SL, Demirel S. Detection of Functional Change Using Cluster Trend Analysis in Glaucoma. Invest Ophthalmol Vis Sci 2017;58:BIO180–BIO190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nouri-Mahdavi K, Caprioli J, Coleman AL, Hoffman D, Gaasterland D. Pointwise linear regression for evaluation of visual field outcomes and comparison with the advanced glaucoma intervention study methods. Arch Ophthalmol 2005;123:193–9. [DOI] [PubMed] [Google Scholar]
- 42.McNaught AI, Crabb DP, Fitzke FW, Hitchings RA. Visual field progression: comparison of Humphrey Statpac2 and pointwise linear regression analysis. Graefes Arch Clin Exp Ophthalmol 1996;234:411–8. [DOI] [PubMed] [Google Scholar]
- 43.de Moraes CG, Song C, Liebmann JM, Simonson JL, Furlanetto RL, Ritch R. Defining 10-2 visual field progression criteria: exploratory and confirmatory factor analysis using pointwise linear regression. Ophthalmology 2014;121:741–9. [DOI] [PubMed] [Google Scholar]
- 44.Heijl A, Leske MC, Bengtsson B, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial G. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol Scand 2003;81:286–93. [DOI] [PubMed] [Google Scholar]
- 45.Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology 2013;120:512–519. [DOI] [PubMed] [Google Scholar]
- 46.Gracitelli CP, Tatham AJ, Zangwill LM, Weinreb RN, Liu T, Medeiros FA. Estimated rates of retinal ganglion cell loss in glaucomatous eyes with and without optic disc hemorrhages. PLoS One 2014;9:e105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akagi T, Zangwill LM, Saunders LJ, et al. Rates of Local Retinal Nerve Fiber Layer Thinning before and after Disc Hemorrhage in Glaucoma. Ophthalmology 2017;124:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nitta K, Sugiyama K, Wajima R, Tachibana G, Yamada Y. Associations between changes in radial peripapillary capillaries and occurrence of disc hemorrhage in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol 2019;257:1963–1970. [DOI] [PubMed] [Google Scholar]
- 49.Budenz DL, Huecker JB, Gedde SJ, Gordon M, Kass M, Ocular Hypertension Treatment Study G. Thirteen-Year Follow-up of Optic Disc Hemorrhages in the Ocular Hypertension Treatment Study. Am J Ophthalmol 2017;174:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shukla AG, Sirinek PE, De Moraes CG, et al. Disc Hemorrhages Are Associated With the Presence and Progression of Glaucomatous Central Visual Field Defects. J Glaucoma 2020;29:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res 2017;57:46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsia Y, Su CC, Wang TH, Huang JY. Clinical characteristics of glaucoma patients with disc hemorrhage in different locations. Graefes Arch Clin Exp Ophthalmol 2019;257:1955–1962. [DOI] [PubMed] [Google Scholar]
- 53.Lee EJ, Han JC, Kee C. Location of Disc Hemorrhage and Direction of Progression in Glaucomatous Retinal Nerve Fiber Layer Defects. J Glaucoma 2018;27:504–510. [DOI] [PubMed] [Google Scholar]
- 54.Garg A, Hood DC, Pensec N, Liebmann JM, Blumberg DM. Macular Damage, as Determined by Structure-Function Staging, Is Associated With Worse Vision-related Quality of Life in Early Glaucoma. Am J Ophthalmol 2018;194:88–94. [DOI] [PubMed] [Google Scholar]
- 55.Chun YS, Sung KR, Park CK, et al. Vision-related quality of life according to location of visual field loss in patients with glaucoma. Acta Ophthalmol 2019;97:e772–e779. [DOI] [PubMed] [Google Scholar]
- 56.Sonnsjo B, Dokmo Y, Krakau T. Disc haemorrhages, precursors of open angle glaucoma. Prog Retin Eye Res 2002;21:35–56. [DOI] [PubMed] [Google Scholar]
- 57.De Moraes CG, Paula JS, Blumberg DM, et al. Detection of progression with 10-2 standard automated perimetry: Development and validation of an event-based algorithm. Am J Ophthalmol 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.