Abstract
A chemically inducible acetamidase promoter-sigF fusion gene was integrated into the chromosome of Mycobacterium bovis BCG. Two-dimensional protein gel analysis permitted the identification of a number of protein spots whose expression was SigF related. One spot upregulated by inappropriate induction of sigF expression corresponded to the 16-kDa antigen alpha-crystallin.
Despite effective antimicrobial therapy, tuberculosis remains a leading cause of death worldwide. Identifying important regulatory genes which enable Mycobacterium tuberculosis to survive environmental stress could have important therapeutic implications for a disease for which treatment is prolonged and multidrug resistance a daunting problem worldwide. Alternate sigma factors are activated under specific stress conditions and control the expression of specific regulons (6). Several M. tuberculosis sigma factors, including SigB, SigC, SigE, SigF, and SigH, appear to be responsive to stress conditions (11, 12, 20).
The sigF gene in M. tuberculosis was discovered by using degenerate PCR and appears to be upregulated in stationary phase (4). It shows significant homology with stress response sigma factors in Streptomyces coelicolor and Bacillus subtilis (3). Since stress responses in vitro are likely to reflect the array of mycobacterial genes expressed upon exposure to the host's immune system, the identification of genes controlled by these sigma factors may provide insight into the pathogenesis of M. tuberculosis. Using a chromosomally integrated, chemically inducible Mycobacterium smegmatis acetamidase promoter fused to the sigF gene, we were able to express SigF inappropriately during exponential phase when it is absent in wild-type cultures of both Mycobacterium bovis BCG and M. tuberculosis (10, 13, 14, 16). By comparing two-dimensional gel protein patterns of SigF-expressing strains with noninduced cultures and wild-type M. bovis BCG, we sought to identify proteins that were SigF dependent. This conditionally expressing construct has been particularly useful as we have previously attempted to overproduce SigF by using other promoters and showed that high-level, constitutive production of SigF is lethal in M. bovis BCG (3).
Induction of sigF expression with the inducible acetamidase promoter.
A 0.7-kb sigF-containing NdeI-SpeI segment from pYZ99 (Table 1) (4) and a 3-kb acetamidase promoter locus-containing BamHI-NdeI fragment from pAMI1 (10) were ligated to one another and cloned into the BamHI-SpeI-digested pNBV1 to produce pCK0218. This fusion contained the four open reading frames upstream of the M. smegmatis acetamidase gene up to the ATG of the acetamidase gene. The acetamidase ATG was followed by sigF codon 2 (ACG) in the correct translational reading frame. The BamHI-SpeI acetamidase promoter-sigF fusion gene fragment was excised, ligated to PacI linkers, and cloned into PacI-digested pMH94, a mycobacterial integrative vector, to yield pCK0275 (9). Using M. bovis BCG (Pasteur strain) and M. tuberculosis CDC1551 (18) transformed with pCK0275, which results in the single-copy integration of the acetamidase promoter-sigF fusion gene (Pace::sigF), we tested whether acetamide induction produced inappropriate SigF expression during logarithmic phase.
TABLE 1.
Plasmids and bacterial strains used
| Plasmid | Description | Reference |
|---|---|---|
| pAMI1 | 4.2-kb BamHI fragment of M. smegmatis genomic DNA in pUC18 (acetamidase promoter) | 10 |
| PYZ99 | 2.8-kb BamHI fragment of M. tuberculosis genomic DNA (sigF) | 4 |
| pCK0218 | 3.7-kb acetamidase promoter::sigF fusion gene in pNBV1 | This paper |
| pCK0230 | 3.7-kb fusion gene in pNEB193 | This paper |
| pMH94 | 2.0-kb insert with attP and int into pUC119 | 9 |
| pCK0275 | 3.7-kb fusion gene in pMH94 | This paper |
| M. tuberculosis 102509 | ΔsigF HyRM. tuberculosis CDC1551 | Chen et al.a |
Chen et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.
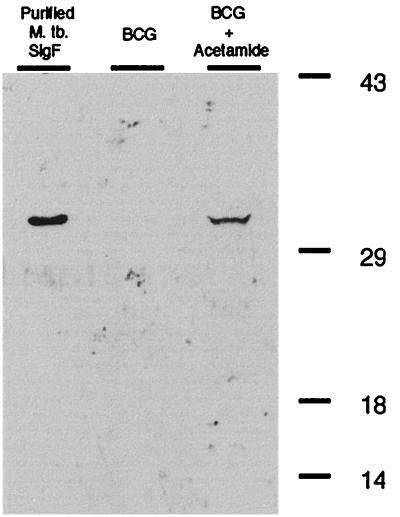
Whole-cell lysates of log-phase M. bovis BCG (optical density [OD], 0.5) containing an integrated copy of the fusion gene with and without acetamide induction were separated on a sodium dodecyl sulfate – 10% polyacrylamide gel electrophoresis minigel. Figure 1 shows a Western blot of lysates from these bacteria developed with anti-SigF antibodies. A 30-kDa band consistent with expression of SigF in exponential phase was seen in the acetamide-induced strain (Fig. 1, right lane) but not in the uninduced cells (middle lane). Purified SigF is shown in the left lane. A similar level of induction of SigF expression was seen with M. tuberculosis containing the fusion gene (data not shown). Purified SigF and polyclonal rabbit antibodies against SigF were prepared as described previously (3).
FIG. 1.
Western blot of M. bovis BCG culture lysates incubated with anti-SigF antibody showing expression of SigF by use of the inducible acetamidase promoter from M. smegmatis. Lanes: left, purified M. tuberculosis SigF; middle, exponential-phase (OD, 0.5) uninduced BCG containing the Pace::sigF fusion gene construct; right, exponential-phase BCG containing the Pace::sigF fusion gene construct induced with acetamide.
Comparison of protein expression in BCG Pace::sigF with and without acetamide induction using 2-D gel electrophoresis.
Recently, 2-D gels have been used extensively to examine culture filtrates as well as cell-associated proteins expressed by M. tuberculosis (15, 17, 19). In addition, 2-D gels have been used to compare bacterial proteins expressed during macrophage infection and under certain stress conditions (1, 8). We sought to identify SigF-dependent proteins by using 2-D gel electrophoresis. We compared protein patterns in whole-cell lysates of exponential-phase M. bovis BCG harboring the Pace::sigF fusion gene with or without acetamide induction. Only small differences in spot intensity were noted on Coomassie blue staining of gels. Hence, we opted for a pulse-labeling approach to visualize recently synthesized proteins. For metabolic labeling, 250 μCi of [35S]methionine and cysteine was added per 10 OD units of culture. Immediately thereafter, half of each labeled culture was exposed to 0.2% acetamide. After a 4-h incubation, bacteria were pelleted by centrifugation, washed twice with sterile phosphate-buffered saline (PBS), and then resuspended with lysis solution (3 M urea, 0.5% Triton X-100, 3.25 μM dithiothreitol, 2% Pharmalyte, phenylmethylsulfonyl fluoride [100 μg/ml], leupeptin [2 μg/ml]). By using 0.1-mm-diameter glass beads, the samples were homogenized twice. The samples were centrifuged, and 250 μg of soluble protein was rehydrated overnight into freeze-dried isoelectric focusing strips (Pharmacia Biotech). First-dimension electrophoresis and second-dimension electrophoresis with 8 to 18% gradient gels were carried out in accordance with the manufacturer's instructions.
Proteins initially absent in exponential phase but inducible with the addition of acetamide were readily apparent by this approach. Figure 2 shows 2-D gels of exponential-phase (OD, 0.6) Pace::sigF M. bovis BCG lysates induced with acetamide (Fig. 2A) and uninduced lysates (Fig. 2B). Several proteins appeared to be upregulated in the induced culture. The appearance of these spots was dependent upon the Pace::sigF fusion as they were not present in acetamide-treated wild-type cell lysates (data not shown). In addition, proteins which were absent in the induced culture were noted in the uninduced cell lysates.
FIG. 2.
2-D polyacrylamide gel electrophoresis of Pace::sigF M. bovis BCG whole-cell culture lysates without acetamide (B) and after 4 h of induction with acetamide (A). All cultures were treated for 4 h with [35S]cysteine-methionine. Arrows denote protein pattern differences between gels.
In another experiment, stationary-phase cultures were diluted only 1:50 and grown to ODs at 600 nm of 0.2 and >1.0. Figure 3 shows 2-D electrophoresis of these whole-cell lysates. A 16-kDa protein with an estimated isoelectric point of 5 was visible during early exponential phase with the addition of acetamide (Fig. 3A) although absent under the same conditions in uninduced lysates (Fig. 3B) and in wild-type lysates (Fig. 3C). In stationary-phase cultures where the native sigF gene is known to be expressed, the 16-kDa protein spot is uniformly present in all cell lysates. It appears to be relatively upregulated in acetamide-induced cell lysates of M. bovis BCG containing the fusion gene (Fig. 3D). This inducible spot correlates with the predicted molecular weight and isoelectric point of the alpha-crystallin homologue, Acr (21).
FIG. 3.
A comparison of wild-type M. bovis BCG and the Pace::sigF strain during early exponential phase (OD, 0.2) and stationary phase (OD, >1.0) with and without acetamide. Whole-cell lysates labeled with [35S]methionine-cysteine of both strains were compared by 2-D gel electrophoresis. (A) Exponential-phase Pace::sigF BCG with acetamide; (B) exponential-phase Pace::sigF BCG without acetamide; (C) exponential-phase wild-type BCG with acetamide; (D) stationary-phase Pace::sigF BCG with acetamide.
Analysis of 16-kDa protein.
We then sought to determine if SigF was necessary for the elaboration of the 16-kDa protein in aerobically grown cultures. Using polyclonal rabbit antibodies directed against Acr, we compared 2-D Western blots of wild-type M. tuberculosis stationary-phase cultures (Fig. 4A) and stationary-phase cultures of an M. tuberculosis sigF knockout strain (M. tuberculosis ΔsigF) (Fig. 4B) (P. Chen, R. E. Ruiz, and W. R. Bishai, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. U-101, p. 653, 1999). After gel electrophoresis, proteins were transferred to nitrocellulose membranes by gravity diffusion overnight at 4°C and then blocked with 5% nonfat milk in PBS with 0.1% Tween 20 (PBS-T) for 1 h. Membranes were then incubated overnight in PBS-T with rabbit-specific polyclonal antibody at the appropriate concentration at 4°C. After washing the membranes, they were incubated with anti-rabbit horseradish peroxidase-conjugated antibody for 2 h and then with a chemiluminescent substrate for autoradiograph exposure. Polyclonal rabbit antibodies against Acr were the gift of C. E. Barry (Bethesda, Md.).
FIG. 4.
2-D gel Western blot of M. tuberculosis culture lysates incubated with anti-alpha-crystallin antibody. (A) Western blot of the stationary-phase M. tuberculosis sigF knockout strain lysate; (B) Western blot of stationary-phase wild-type M. tuberculosis culture lysate. The arrows show the location of the alpha-crystallin protein.
While the anti-alpha-crystallin antibody nonspecifically binds to other heat shock protein complexes, it shows a discrete spot at the predicted molecular weight and isoelectric point of 5 for Acr. This 16-kDa protein uniformly appears only in wild-type cultures and is completely absent in M. tuberculosis ΔsigF culture lysates.
To further establish the identity of the 16-kDa protein, we excised the protein and performed sequence analysis on the gel piece through the Harvard Microchemistry Facility where samples are proteolytically digested, separated by microcapillary high-pressure liquid chromatography, and analyzed with tandem mass spectrometry. Correlating these results with known sequences by using the algorithm Sequest, three unique polypeptide protein fragments, including a hypothetical protein (Rv3686c), lumazine synthase (RibH, Rv1416) involved in the riboflavin synthesis pathway, and Acr (HspX, Rv2031c), were identified in the excised gel piece (5). The molecular weights and isoelectric points of all three of these proteins are similar. In summary, the 16-kDa SigF-dependent spot was both immunoreactive with anti-Acr antibodies and found by mass spectrometry to contain peptide sequences matching Acr.
The 16-kDa alpha-crystallin homologue, Acr, is a member of the small heat shock protein superfamily. Proteins in this family act as molecular chaperones preventing the thermal aggregation of proteins and suppressing denaturation (2). The M. tuberculosis Acr protein has been shown to accumulate in the transition to stationary phase similar to SigF. However, as noted by Yuan et al., SigF may not be the sole regulator of alpha-crystallin expression as many of the conditions shown to upregulate SigF expression have no effect on the expression of the 16-kDa protein (12, 21). Moreover, the expression of the acr gene has been shown to be complex, with evidence for a strong promoter and a repressor binding site modulating expression (Y. Yuan, D. D. Crane, D. R. Sherman, M. Hickey, and C. E. Barry, Proceedings of the Thirty-Second U.S.-Japan Cooperative Medical Science Program, Tuberculosis-Leprosy Research Conference, Cleveland, Ohio). In other recent data from Hu and Coates, Acr appears to be posttranscriptionally modified to account for its accumulation in stationary phase rather than solely transcriptionally regulated (7).
In agreement with Yuan et al., our data show that in ordinary exponential-phase culture, very little alpha-crystallin can be detected even with SigF induction using the acetamidase promoter (21). In contrast, in cultures recently diluted from stationary phase, SigF induction leads to alpha-crystallin expression. Furthermore, in M. tuberculosis sigF knockout strains, alpha-crystallin is absent in stationary-phase cultures. Overall, alpha-crystallin expression appears to be controlled by transcriptional and translational mechanisms of expression and accumulation which may be modulated by different stress conditions such as anaerobiosis. Our data suggest that alpha-crystallin requires SigF in conjunction with other stationary-phase elements in order to be produced at detectable levels in stationary phase under aerobic culture conditions.
We have used the M. smegmatis-inducible acetamidase promoter fused to sigF to produce the transcription factor at a nonphysiologic time. 2-D gel electrophoresis comparisons reveal several candidate proteins that may be regulated by SigF either as a direct transcriptional effect or indirectly through other regulatory proteins controlled by SigF. In addition, Western blots of 2-D gels offer an alternative method for separating and distinguishing individual proteins. Using these techniques, we have shown a putative link between two genes which are upregulated in the transition to stationary phase: sigF, encoding an alternative sigma factor, and acr, encoding the alpha-crystallin homologue.
Acknowledgments
Y.M. is supported by a Heiser Foundation Research Grant. This work is also supported by NIH grants AI36973 and AI37856.
REFERENCES
- 1.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 2.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chiu W, Gilbert H F, Quiocho F A. Mycobacterium tuberculosis 16-kDa antigen (Hsp 16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- 3.DeMaio J, Zhang Y, Ko C, Bishai W R. Mycobacterium tuberculosis sigF is part of a gene cluster with similarities to the Bacillus subtilis sigF and sigB operons. Tuberc Lung Dis. 1997;78:2–12. doi: 10.1016/s0962-8479(97)90010-1. [DOI] [PubMed] [Google Scholar]
- 4.DeMaio J, Zhang Y, Ko C, Young D B, Bishai W R. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eng J K, McCormick A L, Yates J R I. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 6.Gomez J E, Chen J M, Bishai W R. Sigma factors of Mycobacterium tuberculosis. Tuberc Lung Dis. 1997;78:175–183. doi: 10.1016/s0962-8479(97)90024-1. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Coates A R M. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J Bacteriol. 1999;181:1380–1387. doi: 10.1128/jb.181.5.1380-1387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee B-Y, Horwitz M A. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J Clin Investig. 1995;96:245–249. doi: 10.1172/JCI118028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M H, Pascopella L, Jacobs W R, Hatfull G F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and Bacille Calmette-Guerin. Proc Natl Acad Sci USA. 1991;88:3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahenthiralingam E, Draper P, Davis E O, Colston M J. Cloning and sequencing of the gene which encodes the highly inducible acetamidase of Mycobacterium smegmatis. J Gen Microbiol. 1993;139:575–583. doi: 10.1099/00221287-139-3-575. [DOI] [PubMed] [Google Scholar]
- 11.Manganelli E, Dubnau E, Tyagi S, Kramer F R, Smith I. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol Microbiol. 1998;31:715–724. doi: 10.1046/j.1365-2958.1999.01212.x. [DOI] [PubMed] [Google Scholar]
- 12.Michele T, Ko C, Bishai W R. Exposure to antibiotics induces expression of the Mycobacterium tuberculosis sigF gene: implications for chemotherapy against mycobacterial persistors. Antimicrob Agents Chemother. 1999;43:218–225. doi: 10.1128/aac.43.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parish T, Mahenthiralingam E, Draper P, Davis E O, Colston M J. Regulation of the inducible acetamidase gene of Mycobacterium smegmatis. Microbiology. 1997;143:2267–2276. doi: 10.1099/00221287-143-7-2267. [DOI] [PubMed] [Google Scholar]
- 14.Parish T, Stoker N G. Development and use of a conditional antisense mutagenesis system in mycobacteria. FEMS Microbiol Lett. 1997;154:151–157. doi: 10.1111/j.1574-6968.1997.tb12637.x. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triccas J A, Parish T, Britton W J, Gicquel B. An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol Lett. 1998;167:151–156. doi: 10.1111/j.1574-6968.1998.tb13221.x. [DOI] [PubMed] [Google Scholar]
- 17.Urquhart B L, Atsalos T E, Roach D, Basseal D J, Bjellqvist B, Britton W L, Humphery-Smith I. 'Proteomic contigs' of Mycobacterium tuberculosis and Mycobacterium bovis (BCG) using novel immobilised pH gradients. Electrophoresis. 1997;18:1384–1392. doi: 10.1002/elps.1150180813. [DOI] [PubMed] [Google Scholar]
- 18.Valway S E, Sanchez M P C, Shinnick T F, Orme I, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 19.Weldingh K, Rosenkrands I, Jacobsen S, Rasmussen P B, Elhay M J, Andersen P. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect Immun. 1998;66:3492–3500. doi: 10.1128/iai.66.8.3492-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Q L, Kong D, Lam K, Husson R N. A mycobacterial extracytoplasmic function sigma factor involved in survival following stress. J Bacteriol. 1997;179:2922–2929. doi: 10.1128/jb.179.9.2922-2929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Y, Crane D D, Barry C E. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]