Abstract
We identified five single amino acid exchanges in CcpA that lead to permanent repression of the xylose utilization genes in the absence of glucose. Other proteins from the CcpA regulon also show glucose-independent regulation in the mutants. The mutant CcpA proteins bind to the DNA target catabolite responsive elements without the corepressor HPr-Ser-P.
Catabolite control protein CcpA is the central regulator of carbon catabolite repression (CCR) in Bacillus megaterium, Bacillus subtilis, and other gram-positive bacteria of low G+C content (5, 11, 12, 21, 22, 25). Genes and operons coding for the utilization of less favorable carbon sources, such as xylose, are regulated by CcpA on the level of transcription in the presence of rapidly metabolizable sugars like glucose or fructose (12). The mechanism of CCR is distinct from the one described for Escherichia coli (reviewed in reference 27). CcpA can either repress or activate transcription. Activation was shown in two cases, ackA in B. subtilis (31) and the las operon of Lactococcus lactis (22). Repression by CcpA was demonstrated for multiple genes and operons in B. subtilis, B. megaterium, and other gram-positive bacteria (10).
CcpA binds to DNA target sites termed catabolite responsive elements (CRE). Repression depends on the presence of HPr-Ser-P or Crh-Ser-P; the former is a component of the phosphoenolpyruvate:sugar phosphotransferase system whose phosphorylation state reflects glycolytic activity (30). The requirement for a corepressor for CcpA was confirmed by DNA footprinting studies involving CRE sequences from the xyl and gnt operons. In addition to HPr-Ser-P, glucose-6-phosphate also triggered CRE binding by CcpA in both systems in in vitro assays (7, 9, 24). Similar experiments with the xynB CRE showed that Crh-Ser-P can substitute for HPr-Ser-P as a corepressor, and both Crh-Ser-P and HPr-Ser-P can trigger CcpA-regulated CCR of the lev operon in vivo (8, 23). In contrast to these results, in vivo CCR of amyE is independent of phosphorylated HPr (14, 33), and even though CcpA-CRE interaction was strengthened by a combination of HPr-Ser-P and fructose-1,6-bisphosphate or NADP, it did not improve repression in in vitro transcription (15). Accordingly, CcpA is thought to receive signals from HPr-Ser-P or Crh-Ser-P and possibly from other effectors.
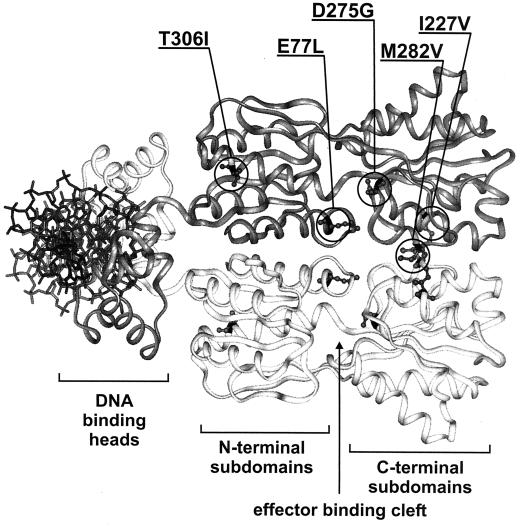
A direct interaction of CcpA with HPr-Ser-P has been demonstrated (3, 13), and a putative HPr-Ser-P binding site on CcpA was recently identified (17). CcpA is a member of the LacI-GalR family of bacterial repressors, and sequence similarities, limited proteolysis, and mutational data suggest a common three-dimensional fold for CcpA, LacI, and PurR (13, 16, 17, 34). On the other hand, HPr-Ser-P does not bind in the effector binding cleft where isopropyl-β-d-thiogalactopyranoside (IPTG) binds to LacI and hypoxanthine binds to PurR (see Fig. 4). It is therefore interesting to collect further evidence about the activation of CcpA for CRE binding.
FIG. 4.
Ribbon representation of a model of CcpA based on the PurR structure. One monomer is dark grey, and the other one is white; a stick model of the complexed DNA is shown in black, and the functional domains are named underneath. Circles in the darker monomer mark positions mutated in CcpAk variants, and the respective amino acid substitutions are shown above the model. The side chains of the original amino acids at these positions are depicted as ball-and-stick models in both monomers.
CcpA mutagenesis and screen for glucose-independent repression.
We have conducted a screen for CcpA variants which repress the xylose utilization genes of B. megaterium in the absence of a repressing carbon source. A plasmid library of mutated ccpA alleles was generated by in vitro mutagenesis with nitrite treatment, as described previously (18). It was transformed into B. megaterium WH353 [lac ΔccpA gdh2Φ(xylA-spoVG-lacZ) ΔxylR], which carries an in-frame ccpA deletion and a xylA-lacZ fusion as a probe for catabolite repression activity. An additional chromosomal deletion in xylR ensured that repression of xylA-lacZ transcription is only caused by the plasmid-encoded CcpA. We screened the transformants on M9 minimal medium containing succinate, which is neutral in CCR, as a carbon source and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) to identify CcpA mutants which permanently repress xyl expression, thus displaying the repression phenotype ccpAk.
Screening about 12,000 colonies yielded 58 white or light blue colonies which were colony purified. The plasmids were isolated, passaged through E. coli, and retransformed into B. megaterium WH353. If the original phenotype was retained we quantified repression by determining β-galactosidase activities. A total of 28 of the original clones were regarded as glucose independent in CCR (ccpAk phenotype), since they repressed xylA-lacZ expression in succinate to 50% or less of wild-type expression in the absence of glucose. Total cell extracts from the mutants were analyzed by immunoblotting with anti-CcpA antiserum as described previously (16, 19). All of the mutant proteins were present at levels similar to that of wild-type CcpA expressed from the same vector (data not shown).
Sequence analysis revealed that 13 of the 28 ccpAk alleles carried distinct mutations and that most of them caused multiple amino acid substitutions. They were separated by subcloning utilizing unique restriction sites in ccpA. The subclones were rescreened as described above. From the original screen and the subcloning we obtained the following five CcpA mutants, each with a single amino acid substitution, which exert permanent, glucose-independent repression: CcpAkE77L, CcpAkI227V, CcpAkD275G, CcpAkM282, and CcpAkT306I.
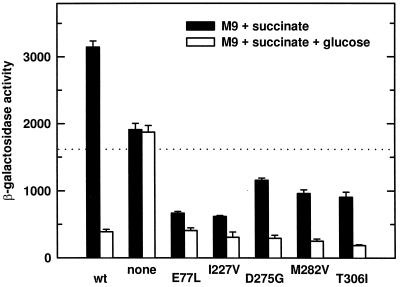
None of the mutants represses xyl expression to the level obtained by the wild type with glucose, and they all show increased repression upon addition of glucose (Fig. 1). Thus, the mutants are only partially independent of glucose. We have observed severe growth deficiencies in all of the ccpAk strains (data not shown). A more complete, permanent regulation phenotype might not show up in our screen if the phenotype is linked to poor growth.
FIG. 1.
β-Galactosidase activities expressed from xylA-lacZ in B. megaterium WH353 regulated by different CcpA mutants. Cells were grown in M9 medium with 0.5% succinate or with 0.5% succinate plus 0.2% glucose. The dotted line marks 50% of the wild-type (wt) expression with succinate. The amino acid substitutions in CcpA are shown under the respective columns.
2D gel electrophoresis of protein extracts from wild-type and ccpAk mutant cells.
To analyze the effects of the ccpAk mutations on the entire CcpA regulon we used two-dimensional (2D) gel electrophoresis. Total soluble protein extracts were prepared from the B. megaterium ccpA deletion mutant WH353 carrying the empty vector pWH1509K (26) or derivatives of pWH2051 carrying the genes for wild-type CcpA or one of the five single amino acid CcpAk mutants. Protein extract (100 mg) was then subjected to 2D protein electrophoresis as described by Völker et al. (32), and the protein profiles of the different strains were compared after silver staining. A typical gel is shown in Fig. 2 (left panel).
FIG. 2.
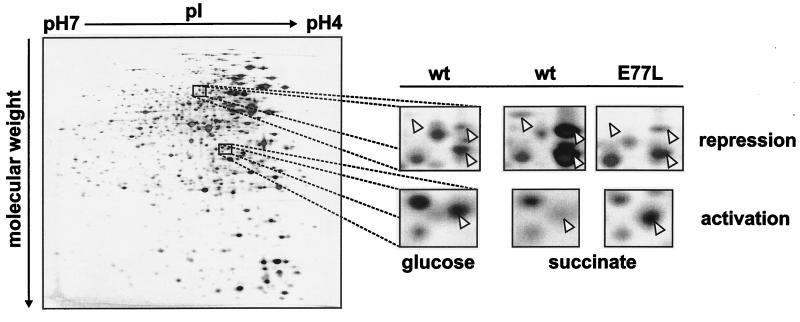
CcpA-mediated changes in the protein synthesis profile of B. megaterium. The panel on the left shows a silver nitrate-stained 2D gel loaded with 100 μg of crude protein extract from B. megaterium WH353(pWH2051) expressing wild-type (wt) CcpA which had been grown on glucose. The small panels on the right display enlarged regions from this gel and the corresponding regions from gels prepared with extracts of the same strain grown on succinate and of CcpAkE77L grown on succinate. Arrowheads indicate proteins showing the expected CCR or carbon catabolite activation pattern, and glucose-independent regulation in CcpAkE77L.
If a strain expressing wild-type CcpA is grown in glucose, spots for proteins whose expression is CCR dependent should be absent or reduced in intensity compared to those of the same strain grown in succinate, i.e., without the repressing carbon source. A comparison with a strain carrying only the vector revealed high expression of those proteins in the presence of glucose (data not shown), demonstrating that the regulation was mediated by CcpA. Using this approach, we recognized 39 spots showing the pattern for CcpA-dependent repression. Examples are shown in Fig. 2 (small panels, top row). Furthermore, we identified four proteins showing CcpA-dependent activation of expression (Fig. 2, small panels, bottom row). These spots were almost undetectable in the absence of glucose or if the cells were lacking CcpA. These numbers of proteins do not reflect the total size of the CcpA regulon because the expression of many proteins needs to be specifically induced, and we only looked at soluble proteins in the absence of inducers. The limited detectability of intensity differences in silver-stained gels further reduces the number of regulated proteins that are recognized.
CcpA-mediated regulation should be independent of glucose in the CcpAk mutants. Spot intensities of cells of CcpAk mutants grown in succinate resemble those found with cells of the wild type grown in glucose. Examples obtained with CcpAkE77L are shown in Fig. 2 (rightmost small panels). The CcpAkE77L strain showed the expected pattern for 37 of 39 repressed proteins and for 3 of 4 activated proteins identified in the wild type. Similar results were obtained with the strains with other single amino acid mutations, as follows: for CcpAkI227V, 35 of 39 and 4 of 4; for CcpAkD275G, 31 of 39 and 4 of 4; for CcpAkM282V, 30 of 39 and 4 of 4; and for CcpAkT306I, 35 of 39 and 3 of 4 (results are the numbers of repressed and activated proteins, respectively). In summary, more than 75% of the 43 proteins regulated in a glucose-dependent fashion in the wild type were regulated in the absence of glucose in each of the CcpAk strains. Therefore, permanent, glucose-independent repression by the five CcpA variants is not limited to xylA.
Purification of mutant proteins and PAGE DNA retardation assays.
We tested DNA binding of the mutant proteins with and without the corepressor HPr in vitro. For this, the five ccpAk alleles leading to single amino acid substitutions were cloned into overexpression vectors, and the proteins were overproduced in B. megaterium and purified by column chromatography (data not shown) as has been described for the wild-type protein (9). The apparently homogeneous preparations were then used for DNA retardation experiments. To obtain the corepressor HPr-Ser-P, the gene for HPr from B. megaterium was cloned into the same overexpression system, and overproduced in B. megaterium (33a). Purification was carried out essentially as described previously for HPr from Staphylococcus aureus (1). The protein was subsequently phosphorylated at Ser46 with partially purified HPr kinase from B. megaterium; the protocol was taken from Deutscher and Saier, Jr. (4), with minor adaptations. The preparation contained more than 90% HPr-Ser-P as estimated by nondenaturating polyacrylamide gel electrophoresis (PAGE) (data not shown).
For the PAGE DNA retardation assays, a synthetic double-stranded oligonucleotide containing CRE (26mer, as described in reference 13) was mixed with purified CcpA and HPr-Ser-P at concentrations of 5 μM (DNA), 10 μM (CcpA), and 10 μM (HPr-Ser-P) in a buffer containing 100 mM Tris-HCl (pH 7.5), 1 mM EDTA, and 10% glycerol. The mixture was incubated at 37°C for 10 min prior to the loading of 5 μl of it on a nondenaturing 5% polyacrylamide minigel (9 by 6 cm). The gel was run in 100 mM Tris-HCl (pH 7.5)–1 mM EDTA at 110 V for 45 min and stained with ethidium bromide.
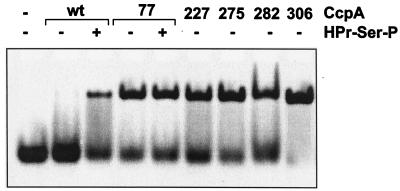
Under the conditions employed, the wild-type CcpA protein could only form a complex with the DNA fragment if the corepressor HPr-Ser-P was present, as shown in Fig. 3. This effect is specific as it cannot be induced by the addition of unphosphorylated HPr (data not shown). Figure 3 shows that the same fragment is complexed by all five CcpAk proteins in the absence of the corepressor. The addition of HPr-Ser-P does not increase the amount of complex formed (only shown for CcpAkE77L). Thus, all CcpAk proteins exhibit HPr-Ser-P-independent DNA binding in vitro.
FIG. 3.
Retardation of CRE by wild-type (wt) and mutant CcpA. Purified CcpA and HPr-Ser-P were combined with a 26mer oligonucleotide containing CRE as indicated above the lanes. Numbers indicate the position of the amino acid substitution in each CcpA mutant. Samples were incubated at 37°C for 10 min prior to loading and run on a non-denaturing 5% polyacrylamide gel. DNA was visualized with ethidium bromide.
Position of the mutations in the three-dimensional structure.
It was surprising that the five amino acid substitutions causing the ccpAk phenotype are located at distant positions on the protein chain. To further assess their location we took advantage of the putative common three-dimensional fold of CcpA and the LacI-GalR family of bacterial regulators (13, 16). Sequence comparisons of CcpA proteins to other family members showed that they constitute a distinct subgroup among LacI-GalR regulators (17), but overall structural similarities should be sufficient to evaluate amino acid location. The model of CcpA shown in Fig. 4 is based on the three-dimensional structure of PurR (29) (Protein Data Bank entry 1PNR). The positions mutated in the CcpAk proteins are indicated. The most striking common feature is their location in the protein core and not in the DNA binding heads. They highlight different regions of the protein with possible functional importance: the dimerization surface, the effector binding cleft, and the corepressor binding site.
Two of the mutations, those in CcpAkI227V and CcpAkM282V, affect residues whose analogs in LacI (Phe226 and Tyr282) and PurR (Tyr227 and Tyr282) play a role in the dimerization of the C-terminal subdomains of the protein core (6, 29). The structure and relative positions of the C-terminal subdomains do not change significantly upon switching between the DNA binding and nonbinding conformations. They form the support for a hinge movement of the two N-terminal subdomains which opens and closes the effector binding clefts (20, 28). It is therefore not obvious how a mutation in this region could lead to permanent repression by CcpA. The mutations introduce no drastic changes in the chemical properties of the affected residues in terms of size or hydrophobicity. These small changes might be the reason that dimerization is still possible, and their effector-independent DNA-binding implies a function of the C-terminal CcpA subdomain more pronounced than those recognized for LacI and PurR.
The E77L and D275G substitutions are close to the effector binding cleft (Fig. 4) which is involved in ligand recognition and binding in PurR and LacI and undergoes structural changes in switching between the DNA binding and nonbinding states (20, 28). The side chain of Glu77 faces the effector binding cleft. It is flanked by other residues making direct contact with the effectors such as the analogous residues to Asp275 of CcpA which is mutated in CcpAkD275G. Asp is well conserved at this position among the members of the LacI-GalR family and a general role in ligand binding is assumed (17, 34). The occurrence of ccpAk mutations in the effector binding cleft indicates that low-molecular-weight effectors such as glucose-6-phosphate and NADP (9, 15, 24) could bind in that region, but a direct interaction of these compounds with CcpA and their physiological function in this regulation remain to be proven.
Thr306, which is changed to Ile in CcpAkT306I, is in a solvent, exposed position, neighboring the proposed binding surface for HPr-Ser-P (17). Amino acid exchanges in that surface result in a loss of CCR in vivo and no or reduced interaction with HPr-Ser-P (17). In contrast, CcpAkT306I leads to permanent repression and binding of DNA. The amino acid at position 306 is conserved among CcpA-like proteins but not among other members of the LacI-GalR family (17). Only one protein of the CcpA subfamily, RegA from Clostridium acetobutylicum, carries an Ile at this position, as does CcpAkT306I. Interestingly, RegA complements a B. subtilis ccpA mutant to constitutive repression of amyE (2). There is no explanation why a change of the hydrophilic Thr to the hydrophobic Ile at this surface position renders CcpA regulation glucose independent, but CcpAkT306I gives further evidence for the functional importance of this region.
In summary, the five amino acid substitutions conferring permanent, glucose-independent regulation by CcpA indicate that mutations in three different regions of the protein can alter DNA binding. Mutations in the corepressor binding cleft are consistent with the assumption that CcpA may be triggered by low-molecular-weight effectors. The binding site for HPr-Ser-P has no equivalent in related repressors, but CcpA-specific conservation and the gain-of-function mutation characterized here emphasize its special role for CcpA-mediated regulation.
Acknowledgments
We thank Kerstin Mahr for help with some experiments, Sabine Pöhlmann for a gift of purified HPr-Ser-P protein, Alexandra Kraus and Richard Brennan for fruitful discussions, and Alexandra Schütz for expert technical assistance with 2D protein electrophoresis.
This study was supported by the EU Biotech Program, the Deutsche Forschungsgemeinschaft through SFB 473, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Beyreuther K, Raufuss H, Schrecker O, Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977;75:275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- 2.Davison S P, Santangelo J D, Reid S J, Woods D R. A Clostridium acetobutylicum regulator gene (regA) affecting amylase production in Bacillus subtilis. Microbiology. 1995;141:989–996. doi: 10.1099/13500872-141-4-989. [DOI] [PubMed] [Google Scholar]
- 3.Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 4.Deutscher J, Saier M H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci USA. 1983;80:6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egeter O, Brückner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- 6.Friedman A, Fischmann T, Steitz T. Crystal structure of lac repressor core tetramer and its implications for DNA looping. Science. 1995;268:1721–1727. doi: 10.1126/science.7792597. [DOI] [PubMed] [Google Scholar]
- 7.Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol Microbiol. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- 8.Galinier A, Deutscher J, Martin-Verstraete I. Phosphorylation of either Crh or HPr mediates binding of CcpA to the Bacillus subtilis xyn cre and catabolite repression of the xyn operon. J Mol Biol. 1999;286:307–314. doi: 10.1006/jmbi.1998.2492. [DOI] [PubMed] [Google Scholar]
- 9.Gösseringer R, Küster E, Galinier A, Deutscher J, Hillen W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol. 1997;266:665–676. doi: 10.1006/jmbi.1996.0820. [DOI] [PubMed] [Google Scholar]
- 10.Henkin T M. The role of the CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1996;135:9–15. doi: 10.1111/j.1574-6968.1996.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 11.Henkin T M, Grundy F J, Nicholson W L, Chambliss G H. Catabolite repression of α-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacI and galR repressors. Mol Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 12.Hueck C J, Kraus A, Schmiedel D, Hillen W. Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol Microbiol. 1995;16:855–864. doi: 10.1111/j.1365-2958.1995.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones B E, Dossonnet V, Küster E, Hillen W, Deutscher J, Klevit R E. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J Biol Chem. 1997;272:26530–26535. doi: 10.1074/jbc.272.42.26530. [DOI] [PubMed] [Google Scholar]
- 14.Kim J H, Guverner Z T, Cho J Y, Chung K-C, Chambliss G H. Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA. J Bacteriol. 1995;177:5129–5134. doi: 10.1128/jb.177.17.5129-5134.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J H, Voskuil M I, Chambliss G H. NADP, corepressor for the Bacillus catabolite control protein CcpA. Proc Natl Acad Sci USA. 1998;95:9590–9595. doi: 10.1073/pnas.95.16.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus A, Hillen W. Analysis of CcpA mutations defective in carbon catabolite repression in Bacillus megaterium. FEMS Microbiol Lett. 1997;153:221–226. doi: 10.1111/j.1574-6968.1997.tb10485.x. [DOI] [PubMed] [Google Scholar]
- 17.Kraus A, Küster E, Wagner A, Hoffmann K, Hillen W. Identification of a corepressor binding site in catabolite control protein CcpA. Mol Microbiol. 1998;30:955–964. doi: 10.1046/j.1365-2958.1998.01123.x. [DOI] [PubMed] [Google Scholar]
- 18.Küster E, Hilbich T, Dahl M, Hillen W. Mutations in catabolite control protein CcpA separating growth effects from catabolite repression. J Bacteriol. 1999;181:4125–4128. doi: 10.1128/jb.181.13.4125-4128.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Küster E, Luesink E J, de Vos W M, Hillen W. Immunological crossreactivity to catabolite control protein CcpA from Bacillus megaterium is found in many Gram-positive bacteria. FEMS Microbiol Lett. 1996;139:109–115. doi: 10.1111/j.1574-6968.1996.tb08188.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis M, Chang G, Horton N, Kercher M, Pace H, Schumacher M, Brennan R, Lu P. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- 21.Lokman B C, Heerikhuisen M, Leer R J, van den Broek A, Borsboom Y, Chaillou S, Postma P W, Pouwels P H. Regulation of expression of the Lactobacillus pentosus xylAB operon. J Bacteriol. 1997;179:5391–5397. doi: 10.1128/jb.179.17.5391-5397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luesink E, van Herpen R, Grossiord B, Kuipers O, de Vos W. Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol Microbiol. 1998;30:789–798. doi: 10.1046/j.1365-2958.1998.01111.x. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Verstraete I, Deutscher J, Galinier A. Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon. J Bacteriol. 1999;181:2966–2969. doi: 10.1128/jb.181.9.2966-2969.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miwa Y, Nagura K, Eguchi S, Fukuda H, Deutscher J, Fujita Y. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol Microbiol. 1997;23:1203–1213. doi: 10.1046/j.1365-2958.1997.2921662.x. [DOI] [PubMed] [Google Scholar]
- 25.Monedero V, Gosalbes M, Perez-Martinez G. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol. 1997;179:6657–6664. doi: 10.1128/jb.179.21.6657-6664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rygus T, Hillen W. Catabolite repression of the xyl operon in Bacillus megaterium. J Bacteriol. 1992;174:3049–3055. doi: 10.1128/jb.174.9.3049-3055.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saier M H, Jr, Ramseier T M, Reizer J. Regulation of carbon utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1325–1343. [Google Scholar]
- 28.Schumacher M A, Choi K Y, Lu F, Zalkin H, Brennan R G. Mechanism of corepressor-mediated specific DNA binding by the purine repressor. Cell. 1995;83:147–155. doi: 10.1016/0092-8674(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher M A, Choi K Y, Zalkin H, Brennan R G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by α helices. Science. 1994;266:763–770. doi: 10.1126/science.7973627. [DOI] [PubMed] [Google Scholar]
- 30.Stülke J, Hillen W. Coupling physiology and gene regulation in bacteria: the phosphotransferase sugar uptake system delivers the signals. Naturwissenschaften. 1998;85:583–592. doi: 10.1007/s001140050555. [DOI] [PubMed] [Google Scholar]
- 31.Turinsky A J, Grundy F J, Kim J H, Chambliss G H, Henkin T M. Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter. J Bacteriol. 1998;180:5961–5967. doi: 10.1128/jb.180.22.5961-5967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Völker, A., H. Antelmann, R. Schmid, A. Schütz, M. Hecker, and U. Völker. Conversion of the 2-D protein index Sub2D of Bacillus subtilis from carrier ampholyte gels to IPG-gels. Submitted for publication.
- 33.Voskuil M I, Chambliss G H. Significance of HPr and CcpA on catabolite repression of α-amylase. J Bacteriol. 1996;178:7014–7015. doi: 10.1128/jb.178.23.7014-7015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Wagner, A., S. Pöhlmann, and W. Hillen. Unpublished data.
- 34.Weickert M J, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]