Abstract
Glucose repressed hemolysin production in Vibrio vulnificus. Promoter activity of the hemolysin gene, vvh, assessed with a vvh-luxCDABE transcriptional fusion, required cyclic AMP (cAMP) and cAMP receptor protein (CRP) in Escherichia coli. Hemolysin production in V. vulnificus increased after the addition of cAMP and was undetectable in a putative crp mutant, suggesting that vvh is also regulated by cAMP-CRP in V. vulnificus.
The pathogenic marine bacterium Vibrio vulnificus has been identified as the causative agent of food-borne diseases such as gastroenteritis and life-threatening septicemia in immunocompromised individuals (3, 8). Mortality from septicemia is quite high (>50%), and death may occur within 1 to 2 days after the first signs of illness (8, 18). A variety of endotoxins and exotoxins, including polysaccharide capsules (16, 21, 23), a cytolytic hemolysin (15, 20), an elastolytic protease (10), and a phospholipase A2 (19), have been implicated as virulence factors for this organism. Among these, the cytolytic hemolysin, with a molecular mass of 51 kDa, lyses erythrocytes (RBC) from various animals by forming small pores in the cytoplasmic membrane and shows cytolytic activity against cultured cell lines (15, 20). Our laboratory demonstrated that this hemolysin caused vasodilation at a far lower dosage than that required for cytotoxicity. This indicated that hemolysin plays an important role in the pathogenesis of hypotensive septic shock (9).
A 3.4-kb fragment of V. vulnificus DNA that encodes hemolysin has been cloned, and its nucleotide sequence has been determined (22). This DNA fragment contains two genes, vvhB and vvhA, which are organized as a single transcription unit termed the vvh operon. The vvhA gene encodes the hemolysin, but the function of the vvhB gene product is still unidentified. All V. vulnificus strains tested, regardless of source, carry the vvhA gene for hemolysin production (20). A possible binding site for cyclic AMP (cAMP) receptor protein (CRP) and several sequences for the putative promoter of vvh were suggested previously on the basis of homology to a consensus sequence from Escherichia coli (22). However, regulation of the vvh operon and environmental signals which stimulate its expression have not previously been characterized. In this report, we have begun to elucidate the molecular mechanism by which the bacterium modulates the expression of vvh genes by examining the nature of the glucose effect on the synthesis of the hemolysin. The promoter activities of vvh in E. coli, deficient in either adenylate cyclase or CRP, were analyzed by using a vvh-luxCDABE transcriptional fusion. The effects of a putative crp mutation and the addition of exogenous cAMP on hemolysin production in V. vulnificus were also examined.
The bacterial strains and plasmids used in this study are listed in Table 1. Hemolytic activities in culture supernatant were determined by the method described by Shinoda et al. (15), and a hemolytic unit was defined as the reciprocal of the maximal dilution showing 50% hemolysis of human RBC (hRBC) or sheep RBC (sRBC) solution (1%, vol/vol).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| V. vulnificus | ||
| C7184 | Clinical isolate | CDCb; J. Oliver |
| MO6-24/O | Opaque | J. G. Morris, Jr. (21) |
| CMM988 | crp mutant | This study |
| E. coli | ||
| CH1105 | MG1655 lacIqZΔM15 | H. E. Choy (5) |
| CH1019 | CH1105 Δcya854 | H. E. Choy (5) |
| CH1133 | CH1105 crp::P1 | H. E. Choy (5) |
| Plasmids | ||
| pCVD702 | pBR322 with vvhBA; Apr | J. G. Morris, Jr. (22) |
| pYB9801 | pUC18 with vvhBA; Apr | This study |
| pUCD615 | luxCDABE, oripSA, oripBR322; Apr, Kmr | C. I. Kado (14) |
| pYB9802 | pUCD615 with regulatory element of vvhBA | This study |
| pHA7 | pBR322 with crp of E. coli K-12 | S. Y. Yoo (1) |
| pYB9803 | pACYC184 with crp of E. coli K-12; Tcr | This study |
Apr, ampicillin resistant; Kmr, kanamycin resistant; Tcr, tetracycline resistant. When necessary, the appropriate antibiotics were added to the medium at the following concentrations; ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; tetracycline, 10 μg/ml.
CDC, Centers for Disease Control and Prevention.
Effect of glucose on production of hemolysin in V. vulnificus.
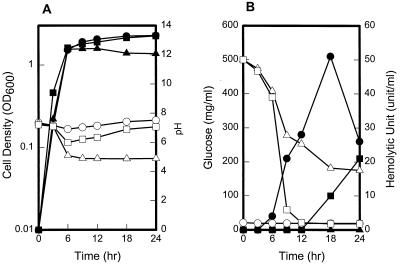
To examine whether hemolysin production of V. vulnificus C7184 is modulated by glucose, hemolytic activities of cultures grown at 37°C in heart infusion (Difco, Detroit, Mich.) broth supplemented with 2.0% NaCl were analyzed by using hRBC at indicated time intervals (Fig. 1). Hemolytic activity appeared during midexponential phase of growth and reached a maximum during stationary phase. The addition of glucose at 0.5% to this culture resulted in complete inhibition of hemolytic activity. In the presence of glucose, the pH of the culture broth decreased, the cell yield was lower, and the level of residual glucose remained at approximately 200 mg/ml during stationary phase. The residual glucose levels in the culture broth were determined with the Glucose Analyzer 2 (Beckman, Palo Alto, Calif.). In a previous report, our group showed that growth of V. vulnificus was highly pH dependent, with the highest growth rate at pH 8.0 (11). It was possible that the decrease of hemolytic activity of V. vulnificus cells with increasing glucose was due to the decline of pH and/or reduced cell growth. Therefore, 10 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES; Sigma, St. Louis, Mo.) buffering agent was added to the medium to adjust pH, and then pH change, cell growth, and the level of residual glucose were monitored (Fig. 1). When TES was added to the cells in the presence of glucose, the pH and cell mass increased approximately to the level observed in the nonglucose control culture and was accompanied by a decrease of glucose in the culture broth. However, the hemolytic activity was still completely inhibited and began to reappear 18 h later when the residual glucose in the culture broth was depleted (Fig. 1B). It is apparent from these results that the hemolytic activity is subject to repression by glucose in V. vulnificus and not regulation by growth phase or pH.
FIG. 1.
Effect of glucose on growth and hemolysin activity of V. vulnificus C7184. Cultures of V. vulnificus were grown in heart infusion broth (●, ○) or in heart infusion broth supplemented with glucose alone (▴, ▵) or with glucose and TES together (■, □). Samples removed at the indicated times were analyzed for growth and pH (A) and for hemolytic activity and residual glucose (B). Filled symbols represent growth and hemolytic activity, and open symbols represent pH and residual glucose changes in each panel. Hemolytic activities were measured by using hRBC. OD600, optical density at 600 nm.
Construction of vvh-luxCDABE transcriptional fusion and characterization of vvh promoter activity.
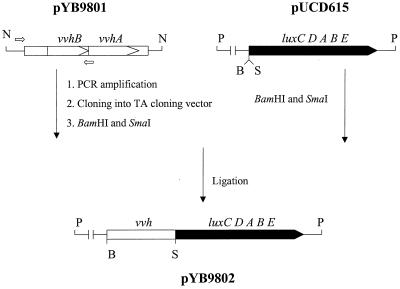
To determine if the glucose-mediated repression is exerted at the transcriptional level, we assessed the promoter activity of vvh by constructing a vvh-lux fusion reporter. Plasmid pYB9802 was constructed as outlined in the scheme in Fig. 2 by subcloning the regulatory elements of the vvh operon from pYB9801 into pUCD615 (14) carrying a promoterless luxCDABE operon. The parent plasmid, pYB9801, carries an EcoRI-HindIII fragment of the vvh operon from pCVD702 (22) in pUC18. The 0.9-kb vvh DNA fragment was amplified by PCR using two primers, SE-1 (5′-GACCATGATTACGCCAAGCTT-3′) and SE-2 (5′-AAGAGAGGTAAACAGAGTCA-3′), which were located within the multicloning site of pUC18 and the open reading frame of vvh, respectively.
FIG. 2.
Construction of vvh-lux fusion plasmid pYB9802. A PCR fragment carrying the regulatory region of the vvh operon was subcloned into pUCD615 carrying promoterless luxCDABE to create pYB9802. Filled blocks represent lux DNA, open blocks represent vvh DNA, and thin lines indicate the vector DNA used. Hybridizing locations of oligonucleotide primers used for PCR are depicted by open arrows. Abbreviations: B, BamHI; N, NdeI; P, PstI; S, SmaI.
E. coli CH1105, a MG1655 derivative known to contain functional adenylate cyclase and CRP (cya+ crp+) (2), was transformed with pYB9802, and cellular luminescence of the culture was measured. Cellular luminescence was measured with a Lumat model 9501 luminometer (Berthold, Berlin, Germany) and was expressed in the instrument's arbitrary relative light units (RLU). Cultures were grown at 30°C in Luria-Bertani broth to an optical density at 600 nm of 1.0, and 1-ml samples from each culture were taken and placed into the cuvettes. The light is produced by the products of the luxCDABE genes controlled under the vvh promoter; therefore, the luminescence level reflects the promoter activity of vvh.
For the CH1105 strain containing pYB9802, luminescence activity (defined as a wild-type level throughout this report) was present at 1,100 RLU (Table 2). This level of luminescence decreased to about a 12-fold-lower level when the culture was grown in the presence of 0.5% glucose. This indicated that the promoter activity of vvh in the vvh-luxCDABE fusion is repressed by glucose and the glucose effect on the synthesis of the hemolysin is exerted at a transcriptional level in E. coli. When cAMP was added at 5 mM to cells subjected to repression by glucose, luminescence was restored. This observation suggested that the repression of the vvh promoter by glucose resulted from the decrease of intracellular cAMP and that the hemolysin synthesis may be regulated by cAMP-controlled catabolite repression.
TABLE 2.
Luminescence in E. coli CH1105 and its isogenic cya and crp mutants containing pYB9802 under various conditions
| Supplementa | Luminescenceb
|
||
|---|---|---|---|
| CH1105 (wild type) | CH1019 (cya) | CH1133 (crp) | |
| None | 1,100 | ≤50 | ≤50 |
| Glucose (0.5%) | 90 | ≤50 | ≤50 |
| cAMP (5 mM) | 3,800 | 4,000 | ≤50 |
| crp | NDc | ≤50 | 1,200 |
| cAMP + crpd | ND | 398,000 | 421,000 |
Cultures were grown with or without respective supplements added to the final concentrations indicated. Details are in the text.
Luminescences are given as RLU authentic to the luminometer used in this study. Luminescence values less than 50 RLU were simplified as ≤50.
ND, not determined.
cAMP was added to the final concentration of 5 mM to each culture transformed with pYB9803.
Expression of vvh in E. coli with a cya or crp background.
To further examine the regulation of the vvh promoter in E. coli, luminescence production levels from vvh-luxCDABE in E. coli CH1105 and in its isogenic mutants, which lack either adenylate cyclase (cya) or CRP (crp), were compared. When transformed with pYB9802, both cya mutant CH1019 and crp mutant CH1133 cells expressed luminescence levels below the detection limit of the luminometer used (Table 2). Apparently, the promoter activity of vvh is completely repressed or very poorly expressed in the absence of active gene products of either cya or crp. Addition of cAMP to the cya mutant containing pYB9802 restored luminescence, and the level of luminescence was even higher than the wild-type level, as will be discussed later (Table 2). In contrast to this, the repressed luminescence in the crp mutant CH1133 cells was not induced at all by addition of cAMP (Table 2). The luminescence in CH1133 cells remained repressed regardless of the amount of cAMP added (data not shown). These observations revealed that the cAMP exerts its effects on the vvh promoter through CRP in E. coli.
Complementation of repression of vvhA in CH1133 with E. coli CRP protein.
As a further test of this hypothesis, we examined whether introduction of the crp gene from E. coli could complement repression of the vvh promoter of vvh-luxCDABE in CH1133 cells. For this purpose, a 1.0-kb SspI-NruI fragment carrying the crp gene of E. coli K-12 was isolated from pHA7 (1) and was subcloned into the pACYC184 vector digested with PvuII. Since it has a p15A origin, the resulting plasmid, pYB9803, is compatible with pYB9802 carrying oriS (oripSA) and pMBI (oripBR322) origins. The crp mutant CH1133 containing pYB9802 was transformed with pYB9803, and the CH1133 double transformant containing both pYB9802 and pYB9803 was constructed. The luminescence in the CH1133 double transformant was restored to a level comparable to the wild-type level, whereas the repressed level of luminescence in the cya mutant CH1019 containing pYB9802 was not restored at all by transformation with pYB9803 (Table 2). Apparently, the E. coli crp gene product introduced can recognize and activate the vvh promoter, but the CRP is not functional for activation of the vvh unless cAMP is provided. Combined with data described earlier, these findings led us to conclude that the promoter of vvh of V. vulnificus is activated by the cAMP-CRP complex.
Transformation with pYB9803 would provide CH1133 with multicopies of crp, meaning that the number of CRP molecules in the double transformant cells could be relatively higher than that in CH1105 cells, in which all CRP molecules are products of crp carried on the chromosome. However, the level of luminescence recovered by the CH1133 double transformant was not significantly higher than the wild-type level. Only when exogenous cAMP was added to this culture did the level of luminescence increase, with the highest level at 421,000 RLU (Table 2). The lack of increased luminescence in cells carrying extra copies of crp can be explained if intracellular levels of cAMP are the limiting factor rather than the number of CRP molecules needed to form a functional cAMP-CRP complex and activate the vvh promoter on vvh-luxCDABE. In agreement with this assumption, addition of cAMP to cells in which presumably a single allele of crp exists (such as CH1105 or even CH1019) increased the light production to a level higher than the wild-type level (Table 2).
Dependence of hemolysin production of V. vulnificus on cAMP and cAMP receptor protein.
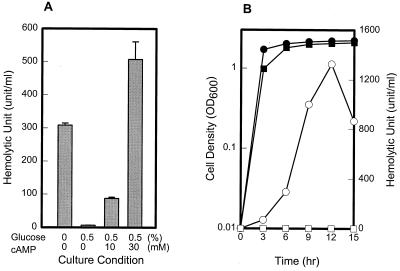
Results with E. coli suggest that vvh is activated by the cAMP-CRP complex, but it is not known if the same mechanism regulates expression of vvh in V. vulnificus. To examine if the cAMP and CRP can regulate vvh in V. vulnificus, the effects of adding cAMP or mutating crp on the production of hemolysin were examined. Cultures of V. vulnificus MO6-24/O were grown in heart infusion broth or heart infusion broth supplemented with 0.5% glucose alone or with 0.5% glucose and cAMP together. Hemolytic activities of samples removed at 12 h from each culture were analyzed and compared. To ensure accurate sensitivity, sRBC were employed to determine hemolytic activity in the supernatants of cultures. The level of hemolytic activity suppressed in the presence of glucose was recovered by the addition of cAMP (Fig. 3). When the amount of cAMP was increased to 30 mM, the level of hemolytic activity reached higher levels than that observed in the absence of glucose. This dose-dependent increase of hemolytic activity brought about by cAMP indicates that the intracellular level of cAMP is a limiting factor for hemolysin synthesis in V. vulnificus.
FIG. 3.
Dependency of hemolysin production of V. vulnificus on cAMP and CRP. (A) Cultures of strain MO6-24/O were grown in heart infusion broth with various supplements added as indicated. Samples removed at 12 h were analyzed for hemolytic activity. Error bars represent standard errors of the mean. (B) Growth and hemolytic activity of wild-type (●, ○) and crp mutant CMM988 (■, □) strains were compared. Samples removed at the indicated times from cultures were analyzed for determination of growth (filled symbols) and hemolytic activity (open symbols). For both panels, sRBC were used for determination of hemolytic activities. OD600, optical density at 600 nm.
The dependence of hemolysin production of V. vulnificus on CRP was examined by isolating a spontaneous mutant of MO6-24/O having a crp phenotype. Isolation was carried out by methods described previously (7), with only a slight modification. Briefly, the MO6-24/O strain was spread on selection medium containing 0.5% galactose, 0.5% maltose, 1% tryptone, 0.5% yeast extract, 20 mM Tris (pH 7.5), neutral red (30 μg/ml), 2.5% NaCl, and 1.5 mM phosphomycin. After overnight incubation, the mutants which showed gold to pink colony formation were transferred to selection medium containing 10 mM cAMP to differentiate cya mutants, and a desired crp mutant was selected and referred to as CMM988. The putative crp mutation of CMM988 was confirmed by testing fermentation of carbon sources on neutral red plates. The mutant exhibited slow growth and no fermentation of many sugars such as maltose and d-galactose, which is consistent with the phenotypes of a typical crp mutant. When the resulting mutant was compared with its parental type, it appeared to synthesize much less hemolysin and the levels of hemolytic activity were almost undetectable (Fig. 3). Although other explanations are possible, the observation that hemolytic activity increased by adding cAMP in a dose-dependent fashion and decreased to undetectable levels in the putative crp mutant indicates that the synthesis of hemolysin in V. vulnificus is regulated by cAMP and CRP.
Like other symbiotic and parasitic microorganisms, V. vulnificus exists in two distinct habitats, seawater and the human body. Many differences such as the type and concentration of nutrients are encountered when the organism is introduced into the human body from seawater environments. Among them, the increased level of cAMP could be one of the major stimuli that allows V. vulnificus to recognize the new environment (inside the human body) and respond by producing hemolysin.
cAMP-CRP plays a central role in carbon catabolite repression, by which a rapidly metabolizable carbon source added to the growth medium represses the synthesis of many enzymes required to metabolize other carbon sources. This global regulatory system has been well described, especially for enteric bacteria (4). Besides regulation of the synthesis of these catabolic enzymes, cAMP-CRP catabolite regulation has also been observed in the synthesis of the toxin proteins of several pathogenic bacteria. cAMP-CRP has recently been shown to activate hemolysin gene expression in avian pathogenic E. coli (12). In contrast to this, CRP protein negatively regulates the expression of cholera toxin and toxin-coregulated pilus genes in Vibrio cholerae, a species closely related to V. vulnificus (17). It has also been reported that cAMP-CRP plays a crucial role in the regulation of virulence gene expression and pathogenesis of Salmonella typhimurium (6, 13). In this report, we have shown that the expression of the V. vulnificus hemolysin gene is dependent on cAMP and crp gene function in both E. coli and V. vulnificus.
Acknowledgments
We thank J. Oliver, J. G. Morris, Jr., and H. E. Choy for providing the V. vulnificus C7184, V. vulnificus MO6-24/O, and E. coli CH strains, respectively. We are also indebted to J. G. Morris, Jr., C. I. Kado, and S. Y. Yoo for providing the plasmids pCVD702, pUCD615, and pHA7, respectively.
This study was supported by a grant to S.H.C. and J.H.R. from the KRF (GE-97-146), Republic of Korea.
REFERENCES
- 1.Aiba H, Fujimoto S, Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982;10:1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann B J. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 2460–2488. [Google Scholar]
- 3.Blake P A, Weaver R E, Hollis D G. Diseases of humans (other than cholera) caused by Vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- 4.Boxford J, Harman J G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy, H. E. 1997. Unpublished data.
- 6.Curtiss R, III, Kelly S M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:386–405. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlap P V. Regulation of luminescence by cyclic AMP in cya-like and crp-like mutants of Vibrio fischeri. J Bacteriol. 1989;171:1199–1202. doi: 10.1128/jb.171.2.1199-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klontz K C, Lieb S, Schreiber M, Janowski H T, Baldy S M, Gunn R A. Syndromes of Vibrio vulnificus infections: clinical and epidemiologic features in Florida cases, 1981–1987. Ann Intern Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 9.Kook H, Lee S E, Baik Y H, Chung S S, Rhee J H. Vibrio vulnificus hemolysin dilates rat thoracic aorta by activating guanylate cyclase. Life Sci. 1996;59:41–47. doi: 10.1016/0024-3205(96)00292-5. [DOI] [PubMed] [Google Scholar]
- 10.Kothary M H, Kreger A S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987;133:1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- 11.Lee J Y, Eun J B, Choi S H. Improving detection of Vibrio vulnificus in Octopus variabilis by PCR. J Food Sci. 1997;62:179–182. [Google Scholar]
- 12.Nagai S, Yagihashi T, Ishihama A. An avian pathogenic Escherichia coli strain produces a hemolysin, the expression of which is dependent on cyclic AMP receptor protein gene function. Vet Microbiol. 1998;60:227–238. doi: 10.1016/s0378-1135(98)00144-8. [DOI] [PubMed] [Google Scholar]
- 13.O'Byrne C P, Dorman C J. The spv virulence operon of Salmonella typhimurium LT2 is regulated negatively by the cyclic AMP (cAMP)-cAMP receptor protein system. J Bacteriol. 1994;176:905–912. doi: 10.1128/jb.176.3.905-912.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogowsky P M, Close T J, Chimera J A, Shaw J J, Kado C I. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol. 1987;169:5101–5112. doi: 10.1128/jb.169.11.5101-5112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinoda S, Miyoshi S, Tamanaka H, Miyoshi N N. Some properties of Vibrio vulnificus hemolysin. Microbiol Immunol. 1985;29:583–590. doi: 10.1111/j.1348-0421.1985.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 16.Simpson L M, White V K, Zane S F, Oliver J D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987;55:269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skorupski K, Taylor R K. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci USA. 1997;94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacket C O, Brenner F, Blake P A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 19.Testa J, Daniel L W, Kreger A S. Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect Immun. 1984;45:458–463. doi: 10.1128/iai.45.2.458-463.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright A C, Morris J G, Jr, Maneval D R, Jr, Richardson K, Kaper J B. Cloning of the cytotoxin-hemolysin gene of Vibrio vulnificus. Infect Immun. 1985;50:922–924. doi: 10.1128/iai.50.3.922-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright A C, Simpson L M, Oliver J D, Morris J G., Jr Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect Immun. 1990;58:1769–1773. doi: 10.1128/iai.58.6.1769-1773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Wright A C, Kaper J B, Morris J G., Jr The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae El Tor hemolysin gene. Infect Immun. 1990;58:2706–2709. doi: 10.1128/iai.58.8.2706-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida S I, Ogawa M, Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect Immun. 1985;47:446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]