Abstract
OBJECTIVES
The adoption of robot-assisted thoracic surgery (RATS) has helped to overcome some of the challenges associated with surgeons performing conventional video-assisted thoracic surgery. The Versius Surgical System (CMR Surgical, Cambridge, UK) has been developed iteratively in line with surgical team feedback to improve the surgeon’s experience and patient outcomes. The goal of this study was to assess the use of the device in RATS in a preclinical setting and to fulfil Idea, Development, Exploration, Assessment, Long-Term Follow Up–Devices stage 1 (Idea).
METHODS
Four cadaveric sessions were conducted between November 2018 and December 2020, during which device performance in a range of thoracic operations was assessed. Procedures were categorized as either completed or not completed, and surgeons evaluated the device’s ability to successfully complete necessary surgical steps. Port and bedside unit positions were recorded.
RESULTS
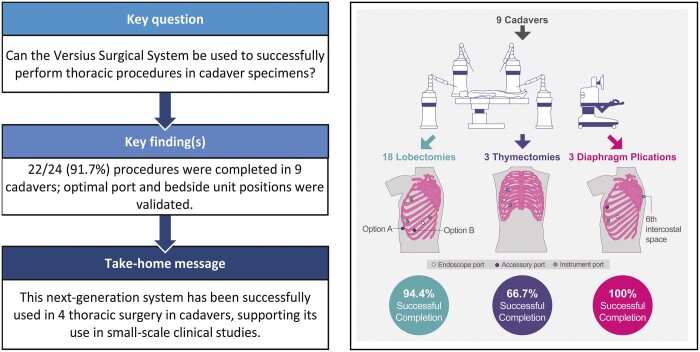
In total, 22/24 (91.7%) thoracic procedures were successfully completed, including 17/18 lobectomies, 2/3 thymectomies and 3/3 diaphragm plications, in 9 cadaver specimens. One thymectomy could not be completed due to cadaver anatomy and 1 lobectomy was not completed due a console system fault. Port and bedside unit configurations were successfully validated for all procedures, and lead surgeons deemed the device to be well-suited for thoracic surgery.
CONCLUSIONS
This preclinical study demonstrated the successful use of the device in RATS in cadaveric models and supports progression to small-scale clinical studies, as part of Idea, Development, Exploration, Assessment, Long-Term Follow Up–Devices stage 2a (Development).
Keywords: Diaphragm, Robot-assisted thoracic surgery (RATS), Video-assisted thoracic surgery (VATS)
Video-assisted thoracic surgery (VATS) is increasingly used to perform thoracic procedures that previously required a thoracotomy, including lobectomy, thymectomy and diaphragm plication [1–3].
Meeting Presentation: N/A
Trial registration: N/A
INTRODUCTION
Video-assisted thoracic surgery (VATS) is increasingly used to perform thoracic procedures that previously required a thoracotomy, including lobectomy, thymectomy and diaphragm plication [1–3]. Compared with a thoracotomy, VATS is associated with fewer complications, lower intraoperative blood loss, shorter hospital stays, reduced postoperative pain and improved cosmesis [1, 2, 4, 5].
In recent years, the use of surgical robots has improved surgical site visualization, surgical dexterity and the precision of instrument manoeuvrability in VATS [2, 6]. The adoption of robot-assisted thoracic surgery (RATS) has improved tissue handling of large, fragile pulmonary vessels and helped overcome the anatomical constraints of operating within the thoracic cavity [2]. RATS has extended the feasibility of minimal access surgery in complex procedures within narrow anatomical areas, such as the retrosternal space, and in complex cases, such as patients with a high body mass index, previous thoracic surgery or advanced-stage disease [2, 7]. Furthermore, robot-assisted lobectomies have been associated with fewer intraoperative blood transfusions, increased rates of lymph node removal, shorter hospital stays and decreased 30-day mortality compared with lobectomies performed using conventional thoracoscopic techniques [8, 9].
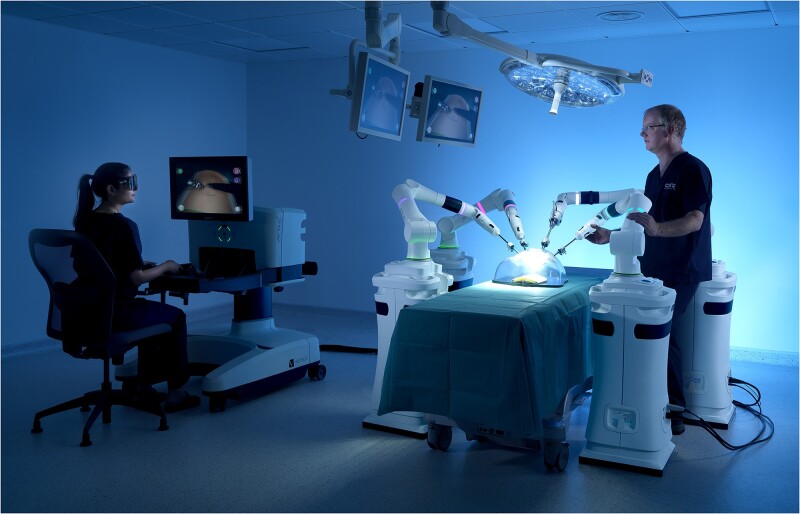
The Versius Surgical System (CMR Surgical Ltd, Cambridge, UK) is a teleoperated robotic surgical system designed to assist surgeons in performing minimal access surgery. The device was designed with the goal of improving end-user experience and surgical outcomes and was developed in line with feedback from surgical teams [10–14]. The device arms were designed to mimic the motion of a human arm, and each arm features 8 articulating joints, with wristed instruments, which enhance surgical access and provide 7 degrees of freedom within the patient [11, 12]. Mobile and practically sized bedside units (BSUs) can be positioned flexibly in existing operating rooms (OR) [11, 12]. The device’s open-console is designed to enable clear communication between the lead surgeon and the rest of the surgical team and allows the lead surgeon quick in-person access to the surgical site (Fig. 1) [11, 12, 15]. Furthermore, the console may be adjusted to either a standing or sitting operating position and has ‘game controller’ hand grips that have been developed for surgeon comfort [11, 12]. These ergonomic components are designed to alleviate the physical burden associated with performing both conventional and robot-assisted endoscopic surgery and to reduce surgeon musculoskeletal fatigue and injury [16–20].
Figure 1:
Overview of the Versius Surgical System. Reproduced from Haig et al. [11] A real-world image of the Versius set-up.
The IDEAL-D (Idea, Development, Exploration, Assessment, Long-term follow-up—Devices) framework has been developed as a guideline for compiling a thorough evidence base during each step of surgical innovation [21, 22]. Stages 0 and 1 (Ideas) incorporate initial technology development and design followed by preclinical studies designed to demonstrate proof of concept. Clinical studies of increasing size are required to align with later stages [21, 22]. Previous studies have described the design and development of the device and fulfilled stage 0, [11, 12] and demonstrated proof of concept of the device in other soft-tissue surgical indications [10, 13, 14].
This preclinical study was designed to meet stage 1 of the IDEAL-D framework, demonstrating proof of concept for the use of the device in thoracic surgery. The primary objective of the study was to demonstrate sufficient surgical access and reach to complete robot-assisted lobectomy, thymectomy and diaphragm plication in cadavers. Secondary objectives were to evaluate participating surgeons’ ability to complete milestone steps using robotic assistance for lobectomy, thymectomy and diaphragm plication in cadavers and to investigate the optimal BSU set-up and port placements required to complete thoracic procedures.
MATERIAL AND METHODS
Ethics statement
All cadavers were donated with consent.
Study design
Cadaver procedures were conducted at the Evelyn Cambridge Surgical Training Centre, UK, between 20 November 2018 and 11 December 2020.
Surgical team
The team comprised a lead surgeon and surgical assistants. The lead surgeon performed the surgical steps for the procedure and evaluated the robot’s performance in assisting each step. Assistant surgeons manipulated the robotic arms and completed additional manual tasks under instruction from the lead surgeon. The 4 lead surgeons who participated in this study were practicing, accredited, high-volume (>50 cases per annum) consultant thoracic surgeons who had experience with VATS and RATS. All surgeons were trained to use the device through a specifically designed training program that comprised online modules followed by time spent on the Versius trainer [23]. Additional personnel were also present to capture relevant information and outcomes.
Cadaver studies
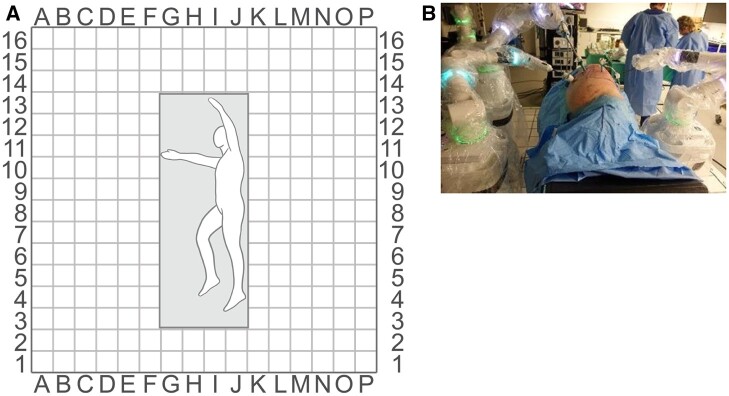
Procedures were performed in 9 cadaver specimens (torso to mid femur) that had not undergone previous thoracic surgery and were suitable for thoracoscopic surgery. Procedures included lobectomy, thymectomy and diaphragm plication. All procedures were performed in a replicated OR to reflect a true clinical setting. Port and BSUs were initially placed based on lead surgeon preference and experience, and these placements were then adapted between procedures to enhance surgical access and reach. BSU positions were recorded for each procedure using a 20-cm grid system laid out on the OR floor and port positions were drawn on a scaled printout body diagram (Fig.2). Positions were deemed suitable if good access to the surgical site was achieved without arm clashing and there was minimal need to reposition BSUs.
Figure 2:
Grid laid out on the OR floor to record port and BSU positions. Grid laid out on OR floor to record BSU positions (A) and a sample OR set-up with a grid (B). BSU: bedside unit; OR: operating room.
The key surgical steps for each procedure included accurate dissection, ligation of blood vessels and placement of sutures (Table 1). Operations were considered completed when specific milestone steps were reached. For a lobectomy, this step occurred when the pulmonary artery, vein and bronchus had been divided and the fissure had been completed. Thymectomy was considered completed when the thymus and the surrounding anterior mediastinal fatty tissue were fully mobilized from the anterior mediastinum. Diaphragm plication was considered completed once sufficient mattress sutures had been placed into the diaphragm to displace it caudally.
Table 1:
Examples of surgical steps in end-to-end procedures
| Procedure | Surgical steps |
|---|---|
| Lower lobectomy |
|
| Middle lobectomy |
|
| Upper lobectomy |
|
| Thymectomy |
|
| Diaphragm plication |
|
Carbon dioxide was used for insufflation.
Procedure success was judged by the lead surgeons, based on their satisfaction with the device’s ability to perform the steps necessary to complete the procedure. For each surgical step, the instruments used (including manual laparoscopic instruments) and the endoscope angle were recorded. The instruments used for each type of procedure are listed in Supplemental Table 1. Any additional surgeon feedback was collected through informal discussion.
RESULTS
Cadaver anatomy and range of procedures
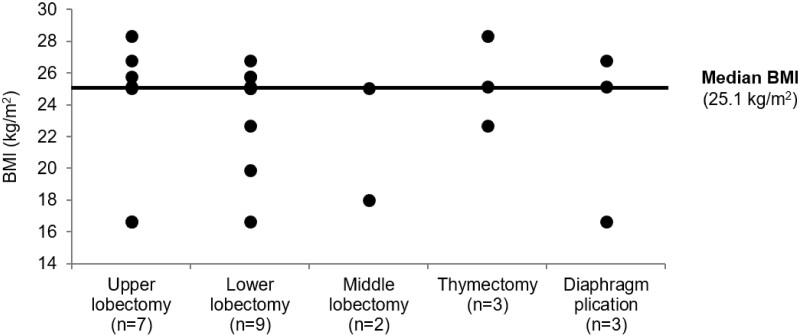
In total, 24 thoracic procedures were performed on 9 cadavers with robot assistance. The cadaver specimens reflected a wide range of human anatomy, with a body mass index ranging from 16.7–28.4 kg/m2 (median: 25.1 kg/m2; Fig. 3). Procedures ranged from relatively simple (e.g. diaphragm plication) to more complex (e.g. lobectomy; Table 2). Of the 18 lobectomies performed, 8 were left-sided lobectomies (5 lower and 3 upper lobes) and 10 were right-sided lobectomies (4 lower, 2 middle and 4 upper lobes).
Figure 3:
Range of cadaver BMIs used for thoracic surgical procedures. BMI: body mass index.
Table 2:
Summary of procedures performed and successfully completed in cadavers
| Procedure | Number performed | Number Successfully completed | Number of lead surgeons | Number of unique port configurations |
|---|---|---|---|---|
| Lobectomy a | 18 | 17 | 4 | 5 |
| Upper | 7 | 7 | 3 | 4 |
| Middle | 2 | 2 | 2 | 2 |
| Lower | 9 | 8 | 4 | 3 |
| Thymectomy | 3 | 2 | 2 | 2 |
| Diaphragm plication | 3 | 3 | 3 | 3 |
| TOTAL | 24 | 22 | 4 | 10 |
Of 18 lobectomies performed, 8 were left-sided (5 lower and 3 upper lobes) and 10 were right-sided (4 lower, 2 middle and 4 upper lobes).
In total, 22/24 (91.7%) procedures were successfully completed. One thymectomy could not be completed because left lung fusion, caused by adhesions, prevented dissection medially to the left phrenic nerve. The surgeons did not express any concern regarding the device during this procedure. One lower left lobectomy was not completed due to a console system fault. This occurred following a successful upper lobectomy in the same cadaver on the same day early in the study.
Surgical steps in end-to-end procedures
Generally, procedures were completed using the device-specific monopolar hook, bipolar Maryland grasper, fenestrated grasper, curved scissors and needle holders. All lead surgeons determined the device able to provide sufficient reach and access to complete a thoracic surgical procedure. Endoscope angles were recorded for 17/24 procedures. The angle was 30° down in 15 procedures, and 0° in 2 procedures.
Port and bedside unit positions
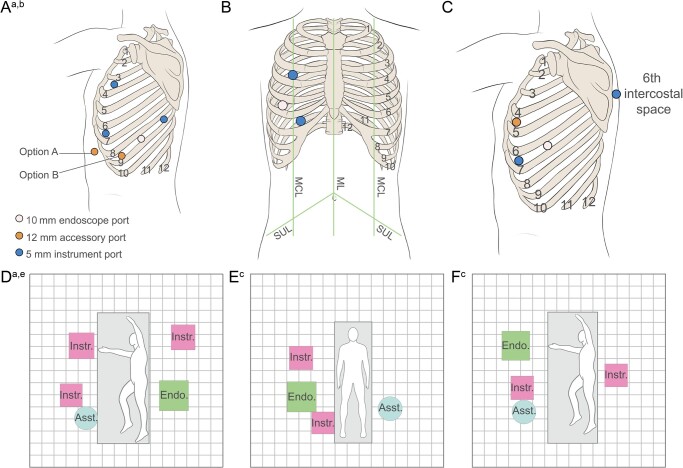
Initially, surgeons elected to place ports and BSUs based on their previous experience with VATS and RATS. These placements were adapted to overcome problems encountered while operating, including clashes between arms, inadequate surgical reach and access and impingement of the surgical arm on the table and the cadaver arm. Model port and BSU positions for lobectomy, thymectomy and diaphragm plication are illustrated in Fig. 4A-F.
Figure 4:
Model port positions for thoracic procedures in cadavers. Positions of ports and BSUs for lobectomy (A and D), thymectomy (B and E) and diaphragm plication (C and F). aPort and BSU positions shown for left lobectomy can be mirrored to perform right lobectomy. bAn accessory port could be placed either in the subxiphoid space (option A) or in the sternal eighth intercostal space (option B); stapling was performed via the accessory port. cEach square measures 20 cm × 20 cm. Asst.: assistant; BSU: bedside unit; Endo: endoscope; Instr: instrument.
The most commonly performed procedure was a lobectomy. All cadavers were placed in a lateral position for this operation and 4 different port configurations were used. A ‘V-shaped’ configuration was used most frequently (10/18 lobectomies; Fig. 4A), which surgeons agreed was the optimum port set-up for this procedure. Two BSUs were placed anterior to the patient. One was placed level with the head and controlled an instrument through a port placed sternally in the third intercostal space. A second BSU was placed at the level of the hip and controlled a second instrument through a port placed sternally in the sixth intercostal space. There was sufficient distance between the ports to prevent arm clashes experienced when initially using port placements based on previous surgeon VATS or RATS preference. A further 2 BSUs were placed posterior to the patient. One BSU was placed just above the level of the head and controlled an instrument placed through a port in the posterior eighth intercostal space. The visualization BSU, located below the level of the hip, controlled the endoscope, which entered through a port in the middle of the eighth intercostal space.
In addition, a subxiphoid accessory port or an accessory port in the sternal eighth intercostal space could have been placed to enable suitable access for a bedside assistant standing anterior to the patient. In all operations, minor adaptations to BSU position were made due to the anatomy of the individual cadaver. The bedside assistants were able to carry out all supporting tasks required without any difficulties with access.
DISCUSSION
Overall, this study demonstrated proof of concept for the use of the robotic surgical system across a range of thoracic surgical indications. Lead surgeons validated the device’s ability to assist with key surgical steps in full, end-to-end upper, middle and lower lobectomies, thymectomies and diaphragm plications. This range of procedures was chosen to demonstrate the versatility of the device. The modular BSU design allowed for a variety of port placements providing adequate surgical access and reach for a range of thoracic procedures. Surgeons were able to effectively use the majority of their existing preferred port placements for VATS and RATS. Furthermore, ports did not have to be placed along a single line, which made a flexible port configuration possible. The most posterior port in the thorax could be positioned more anteriorly. This positioning option could potentially reduce pain in thoracic surgery. The manoeuvrability of the device’s BSUs allowed their placement to be swiftly corrected intraoperatively to ensure maximum surgical reach of the instruments.
The design of the device had not been finalized for use in thoracic surgery ahead of this study. Incremental changes were made to instruments, hardware and software in line with surgeon feedback to improve the design of the robot. One operation could not be completed due to a system fault. This procedure took place at the beginning of the study, using an earlier device model that has since been refined and is substantially different from the final device used in later studies. The goal of this iterative development process during preclinical testing was to ensure that device design and surgical techniques are perfected ahead of their implementation in clinical studies.
Procedures were undertaken in a simulated environment. Although the goal of the study was to replicate as many elements of a genuine surgical procedure as reasonably possible, a preclinical study cannot provide the full experience of a live procedure. Cadavers are anatomically relevant to human thoracic surgery; however, there are significant physical differences between a cadaver and a live human body [24]. Cadavers are limited in simulating live tissue handling, dissection, surgical plane identification and haemostasis [24]. Specifically, in this study, lead surgeons commented that post-mortem lungs were filled with embalming fluid and therefore did not collapse upon entry to the thoracic cavity as they would in a living patient. Additionally, there is the possibility that a novel operating situation may have affected surgeon performance. Surgeons must also address the psychological difference between operating on a cadaver compared to a living person [24].
CONCLUSION
The Versius Surgical System successfully assisted a range of thoracic operations in cadavers. BSU and port positions were validated for each procedure to ensure optimal surgical reach and access. This preclinical study provides evidence for IDEAL-D stage 1 (Idea) and supports progression of the device for use in thoracic operations within small clinical studies as part of IDEAL-D stage 2a (Development) [21, 22].
SUPPLEMENTAL MATERIALS
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the individuals who donated their bodies to medical research and the investigators and their teams who took part in this study. The authors also acknowledge Tim Jones, VetMB, Oliver Palmer, BSc (Hons), and Emma Phillips, PhD, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Funding
This work was supported by CMR Surgical. Support for third-party writing assistance for this article was funded by CMR Surgical in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Conflict of interest: GA: Has received honoraria from CMR for preceptorship training. JD: Has received honoraria from CMR for preceptorship training. TR: None declared. PB: None declared. MS: Chief Medical Officer and Founder of CMR Surgical.
Authors’ contributions
GA: Conceptualization; Investigation; Methodology; Validation; Writing—review and editing. JD: Conceptualization; Investigation; Methodology; Validation; Writing—review and editing. TR: Conceptualization; Investigation; Methodology; Validation; Writing—review and editing. PB: Conceptualization; Methodology; Validation; Writing—review and editing. MS: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Supervision; Validation; Writing—review and editing.
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.
Glossary
Abbreviations
- BSUs
Bedside units
- IDEAL-D
Idea, Development, Exploration, Assessment, Long-Term Follow Up—Devices
- OR
Operating room
- RATS
Robot-assisted thoracic surgery
- VATS
Video-assisted thoracic surgery
REFERENCES
- 1. Friedant AJ, Handorf EA, Su S, Scott WJ.. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol 2016;11:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar A, Asaf BB.. Robotic thoracic surgery: the state of the art. J Minim Access Surg 2015;11:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stamenovic D. New technique of diaphragmatic plication by means of uniportal video-assisted thoracoscopic surgery. Interact CardioVasc Thorac Surg 2017;25:162–3. [DOI] [PubMed] [Google Scholar]
- 4. Bendixen M, Jørgensen OD, Kronborg C, Andersen C. Bjørn Licht P. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–44. [DOI] [PubMed] [Google Scholar]
- 5. Lim E, Batchelor T, Dunning J, Shackcloth M, Anikin V, Naidu B. et al. PL02.06 In hospital clinical efficacy, safety and oncologic outcomes from VIOLET: a UK multi-centre RCT of VATS versus open lobectomy for lung cancer. J Thorac Oncol 2019;14:S6. [Google Scholar]
- 6. Morris B. Robotic surgery: applications, limitations, and impact on surgical education. MedGenMed 2005;7:72. [PMC free article] [PubMed] [Google Scholar]
- 7. Ricciardi S, Zirafa CC, Davini F, Melfi F.. How to get the best from robotic thoracic surgery. J Thorac Dis 2018;10:S947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farivar AS, Cerfolio RJ, Vallières E, Knight AW, Bryant A, Lingala V. et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10–5. [DOI] [PubMed] [Google Scholar]
- 9. Novellis P, Bottoni E, Voulaz E, Cariboni U, Testori A, Bertolaccini L. et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carey M, Bali A, Pandeva I, Pradhan A, Slack M.. Preclinical evaluation of a new robot-assisted surgical system for use in gynecology minimal access surgery. Gynecol Surg 2020;17:2. [Google Scholar]
- 11. Haig F, Medeiros ACB, Chitty K, Slack M.. Usability assessment of Versius, a new robot-assisted surgical device for use in minimal access surgery. BMJ Surg Interv Health Technol 2020;2:e000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hares L, Roberts P, Marshall K, Slack M.. Using end-user feedback to optimize the design of the Versius Surgical System, a new robot-assisted device for use in minimal access surgery. BMJ Surg Interv Health Technol 2019;1:e000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morton J, Hardwick RH, Tilney HS, Gudgeon AM, Jah A, Stevens L. et al. Preclinical evaluation of the Versius Surgical System, a new robot-assisted surgical device for use in minimal access general and colorectal procedures. Surg Endosc 2021;35:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas BC, Slack M, Hussain M, Barber N, Pradhan A, Dinneen E. et al. Preclinical evaluation of the Versius Surgical System, a new robot-assisted surgical device for use in minimal access renal and prostate surgery. Eur Urol Focus 2021;7:444–52. [DOI] [PubMed] [Google Scholar]
- 15. Schiff L, Tsafrir Z, Aoun J, Taylor A, Theoharis E, Eisenstein D.. Quality of Communication in Robotic Surgery and Surgical Outcomes. JSLS 2016;20:e2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armijo PR, Huang CK, High R, Leon M, Siu KC, Oleynikov D.. Ergonomics of minimally invasive surgery: an analysis of muscle effort and fatigue in the operating room between laparoscopic and robotic surgery. Surg Endosc 2019;33:2323–31. [DOI] [PubMed] [Google Scholar]
- 17. Janki S, Mulder EEAP, IJzermans JNM, Tran TCK.. Ergonomics in the operating room. Surg Endosc 2017;31:2457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plerhoples TA, Hernandez-Boussard T, Wren SM.. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg 2012;6:65–72. [DOI] [PubMed] [Google Scholar]
- 19. Reyes DA, Tang B, Cuschieri A.. Minimal access surgery (MAS)-related surgeon morbidity syndromes. Surg Endosc 2006;20:1–13. [DOI] [PubMed] [Google Scholar]
- 20. Stucky CH, Cromwell KD, Voss RK, Chiang YJ, Woodman K, Lee JE. et al. Surgeon symptoms, strain, and selections: systematic review and meta-analysis of surgical ergonomics. Ann Med Surg (Lond) 2018;27:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Balliol Collaboration et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105–12. [DOI] [PubMed] [Google Scholar]
- 22. Sedrakyan A, Campbell B, Merino JG, Kuntz R, Hirst A, McCulloch P.. IDEAL-D: A rational framework for evaluating and regulating the use of medical devices. BMJ 2016;353:i2372. [DOI] [PubMed] [Google Scholar]
- 23. Butterworth J, Sadry M, Julian D, Assessment of the training program for Versius, a new innovative robotic system for use in minimal access surgery. [In Press].2020. [DOI] [PMC free article] [PubMed]
- 24. Stefanidis D, Yonce TC, Green JM, Coker AP.. Cadavers versus pigs: which are better for procedural training of surgery residents outside the OR? Surgery 2013;154:34–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.