Abstract
Importance
The increasing trend of delaying childbirth means that more women are being diagnosed with breast cancer before having given birth to their desired number of children. Although chemotherapy can significantly improve the prognosis of this population, it also causes ovarian damage, including premature ovarian insufficiency and infertility. Gonadotropin-releasing hormone agonists (GnRHa) have shown promising fertility protective activity in premenopausal women, but their clinical usage remains controversial.
Objective
Here, we conducted a meta-analysis to assess the efficacy of GnRHa when administered concurrently with chemotherapy that included cyclophosphamide in the prevention of chemotherapy-induced ovarian damage in premenopausal women.
Evidence Review
An extensive literature search was performed using the PubMed, Embase, and Cochrane databases. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were determined.
Findings
Eleven randomized controlled trials with a total of 1,219 participants were included in the analyses. A significantly higher number of women treated with GnRHa experienced the resumption of ovarian function after chemotherapy than those who did not receive this treatment (OR, 3.04; 95% CI, 1.87‐4.94; P < 0.001). Regarding spontaneous pregnancy, a statistically significant difference was observed only in hormone receptor-negative participants (OR, 2.06; 95% CI, 1.03‐4.11; P = 0.04).
Conclusions and Relevance
When treating premenopausal women with breast cancer, the administration of GnRHa concurrently with chemotherapy appeared to improve the resumption rate of ovarian function; however, the spontaneous pregnancy rate only improved in hormone receptor-negative patients. Thus, the use of GnRHa during chemotherapy may represent a feasible strategy for preserving ovarian function in women with breast cancer.
Key Words: Breast cancer, Chemotherapy, GnRHa, Meta-analysis, Ovarian damage
Key points
Question: Can gonadotropin-releasing hormone agonists (GnRHa) protect ovarian function during chemotherapy?
Objective: To assess the effect of GnRHa on ovarian function in women with breast cancer via a systematic review and meta-analysis of randomized controlled trials.
Findings: Administration of GnRHa concurrent with chemotherapy treatment of breast cancer in premenopausal women improved the rate of resumption of ovarian function. However, the rate of spontaneous pregnancy was only improved in hormone receptor-negative women.
Meaning: Administering GnRHa during chemotherapy might be a feasible strategy to preserve ovarian function in women with breast cancer.
Breast cancer is a common malignant tumor among women worldwide and tends to also affect younger patients. Approximately 12% of newly diagnosed breast cancer patients are women of reproductive age.1,2 Chemotherapy is an important treatment that has significantly improved the prognosis of this population.3 However, younger women who undergo chemotherapy can eventually develop premature ovarian insufficiency and infertility.4 The increasing trend of delaying childbirth means that more women are now being diagnosed with breast cancer before they have given birth to their desired number of children. Thus, it is vital to develop treatment options that can preserve reliable ovarian function and fertility in these young women.
Currently, several strategies, such as ovarian tissue, embryo, and oocyte preservation techniques, are available for fertility preservation treatments. However, these procedures are associated with high costs and delayed treatment.5,6 In addition, strategies such as embryo preservation would require the use of partner or anonymous donor sperm. Thus, fertility preservation counseling for young women with cancer is crucial.7 Preclinical data have confirmed that temporarily suppressing ovarian function using gonadotropin-releasing hormone agonists (GnRHa) during chemotherapy can reduce ovarian toxicity.8–10 However, based on currently available clinical data, the question of whether GnRHa can improve the resumption rates of menses and pregnancy in women with breast cancer remains unanswered.
Considering the importance of this topic, and the controversy regarding currently available data, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to better assess the effects of GnRHa on ovarian function in women with breast cancer.
METHODS
Literature search
We performed an extensive literature search in the PubMed, Embase, and Cochrane databases. This search was conducted from inception to January 31, 2022, without any restriction on language or publication year. The following Medical Subject Heading terms and/or text words were used for the search: “breast neoplasm,” “breast cancer,” “breast carcinoma,” “breast tumor,” “mammary cancer,” “breast malignant tumor,” “gonadotropin-releasing hormone,” “goserelin,” “triptorelin,” “buserelin,” “leuprolide,” “luteinizing hormone-releasing hormone,” and “FSH releasing hormone.” When repeated trials were identified, the most elaborate and latest articles were included. The protocol was registered with PROSPERO (CRD42021272060).
Inclusion and exclusion criteria
Only those RCTs that compared the effects of GnRHa during chemotherapy that included cyclophosphamide (GnRHa group) with chemotherapy including cyclophosphamide alone (control group) were considered eligible. Eligible RCTs had to fulfill the following inclusion criteria: (1) contained at least two treatment groups (GnRHa plus chemotherapy and chemotherapy alone), (2) participants were adult (18 years and older) breast cancer patients, (3) GnRHa was administered concurrently with chemotherapy, and (4) cyclophosphamide was included in both the experimental and control groups. The exclusion criteria were as follows: (1) studies with incomplete or missing information, (2) reviews and case reports, (3) metastatic breast cancer, and (4) inflammatory breast cancer.
Two authors performed the literature searches independently and identified eligible studies based on the aforementioned criteria. Cases of disagreement were resolved through discussion and consensus. When this was unsuccessful, a final decision was adjudicated by a third investigator.
Data extraction
The following information was extracted independently from each trial by two different authors: (1) study information, including the first author's name and year of publication; (2) participant data, including country, body mass index, mean age, hormone receptor status, and sample size; and (3) trial design, including GnRHa intervention and the duration of follow-up.
Risk of bias assessment
We assessed the risk of bias for the eligible studies in accordance with the guidelines in the Cochrane Reviewers' Handbook. We evaluated selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases, with the risk of bias being stratified into three levels: high, low, and unclear.
Publication bias
We evaluated publication bias using funnel plots that were created using Egger test and Begg test in Stata version 15.1 software (Stata, College Station, TX). In addition, we performed a t test to determine the significance of the intercept, in which a P value of <0.05 was deemed to be statistically significant.
Statistical analyses
We conducted our meta-analysis using Review Manager (version 5.3.5; Cochrane Collaboration, Oxford, United Kingdom). We used pooled odds ratios (ORs) with 95% confidence intervals (CIs) to calculate dichotomous variables. In addition, a χ2-based Q statistic test was performed to assess between-study heterogeneity. When the P value was <0.10 in the Q test, the random-effect model was performed. For all other cases, a fixed-effect model was applied. We used classic forest plots to present the meta-analysis results, with statistical significance set at P < 0.05. We used sensitivity analyses to estimate the influence of individual studies on the overall effect.
RESULTS
Search results
Based on the predefined search strategy, a total of 3,774 records were identified for evaluation. By checking the titles, abstracts, and full-texts of these records, on the basis of the inclusion and exclusion criteria listed previously, 10 records and 1,219 participants were identified as being eligible for meta-analysis. One of these 10 records involved more than two therapy regimen groups; only data regarding eligible groups were extracted, and they were considered as separate trials. Another record was divided randomly into two groups (early chemotherapy and delayed chemotherapy); the said record was treated as two trials in the final calculation. The reference flow is illustrated in Fig. 1, and the characteristics of the 11 trials included in this review are summarized in Table 1.
FIG. 1.
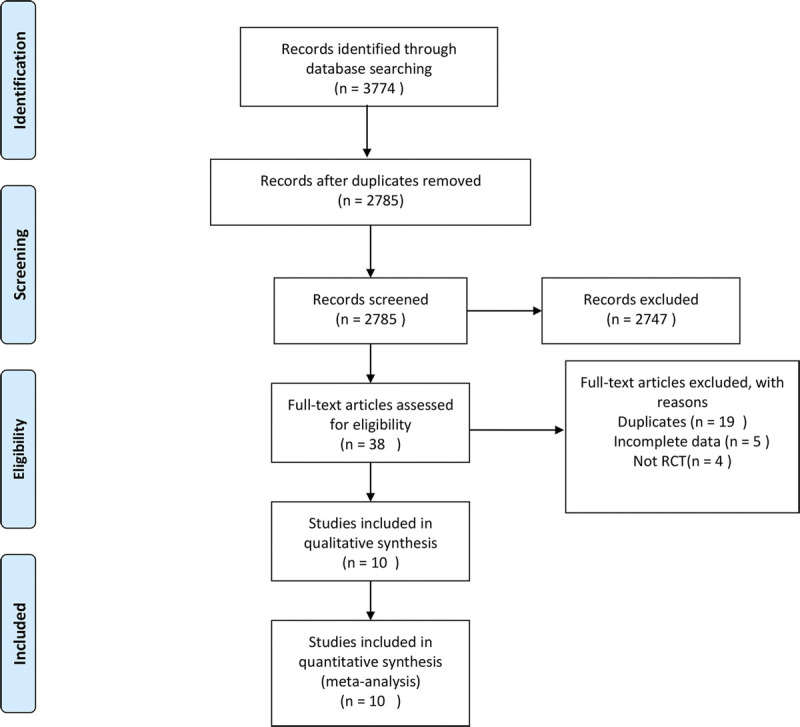
Flow chart of the literature search.
TABLE 1.
Characteristics of eligible studies considered in this meta-analysis
| Study | Year | Country | BMI, kg/m2 | Mean age, y | HR | GnRHa | Follow-up, mo | No. participants | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GnRHa | Control | GnRHa | Control | GnRHa | Control | ||||||
| Sverrisdottir et al11 | 2009 | Sweden | NA | NA | 45 | 46 | HR+/HR− | Goserelin | 36 | 22 | 20 |
| Badawy et al12 | 2009 | Egypt | NA | NA | 30 | 29.2 | HR+/HR− | Goserelin | 8 | 39 | 39 |
| Gerber et al13 | 2011 | Germany | NA | NA | 35 | 38.5 | HR− | Goserelin | 24 | 30 | 30 |
| Elgindy et al14 (A) | 2013 | Egypt | 24.9 ± 3.4 | 24.9 ± 3.0 | 33.3 | 32.3 | HR− | Triptorelin | 18 | 25 | 25 |
| Elgindy et al14 (B) | 2013 | Egypt | 24.9 ± 4.2 | 24.9 ± 3.4 | 33 | 32.8 | HR− | Triptorelin | 18 | 25 | 25 |
| Song et al15 | 2013 | China | NA | NA | 40.3 | 42.1 | HR+/HR− | Leuprorelin | 12 | 89 | 94 |
| Moore et al16 | 2015 | US | NA | NA | 37.6 | 38.7 | HR− | Goserelin | 48 | 66 | 69 |
| Karimi-Zarchi et al17 | 2014 | Iran | NA | NA | 37 | 38 | HR− | Triptorelin | 6 | 21 | 21 |
| Munster et al18 | 2012 | US | NA | NA | 39 | 38 | HR+/HR− | Triptorelin | 18 | 26 | 21 |
| Leonard et al19 | 2017 | UK | NA | NA | 37.9 | 38.8 | HR+/HR− | Goserelin | 24 | 95 | 107 |
| Zong et al20 | 2021 | China | 22.7 ± 3.0 | 22.7 ± 3.3 | 40.6 | 40.2 | HR+/HR− | Goserelin/leuprorelin | 49 | 165 | 165 |
BMI, body mass index; GnRHa, gonadotropin-releasing hormone agonists; HR, hormone receptor; NA, not available.
In total, the final selected studies featured a total of 1,219 participants, of whom 603 were in the GnRHa group and 616 were in the control group. Five studies only included participants who were hormone receptor-negative (estrogen and progesterone receptor <1%, as analyzed by immunohistochemistry); the other six studies did not consider hormone receptor status. Of the 11 studies included, only 6 reported pregnancy data.
Quality assessment
We assessed the risk of bias for the 11 included studies using the Cochrane Risk of Bias Tool. As shown in Fig. 2, all studies randomly allocated participants to their treatment arms, but three studies did not specify their exact randomization methods. None of the 11 studies specified that all trial personnel and participants were blinded throughout each study's course. Only four studies provided registration information. Taken together, these characteristics suggest that there was a moderate risk of study design bias (Fig. 3).
FIG. 2.

Quality assessment for the risk of bias for the included RCTs. RCT, randomized controlled trial.
FIG. 3.
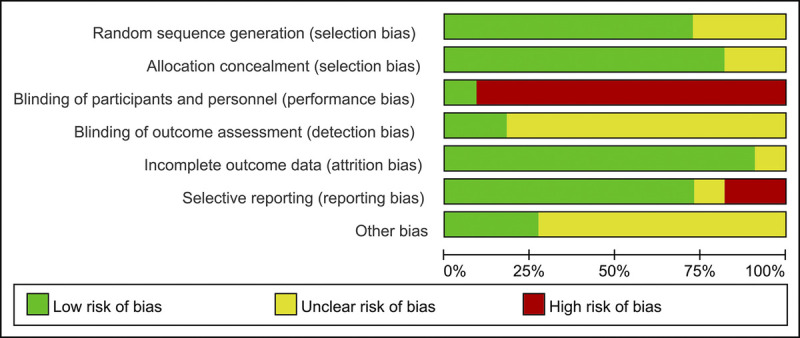
Graphs of risk of bias for the included RCTs. RCT, randomized controlled trial.
GnRHa and resumption of ovarian function
Across all 11 studies, 818 of the 1,219 participants (67.1%) resumed ovarian function; this included 474 of 603 participants (78.6%) in the GnRHa group and 344 of 616 participants (55.8%) in the control group. As shown in Fig. 4, a significant statistical difference was observed between these two groups (OR, 3.04; 95% CI, 1.87‐4.94; P < 0.001). Because the studies had high heterogeneity (I2 = 60%, P = 0.006), the random-effects model was used for evaluation.
FIG. 4.
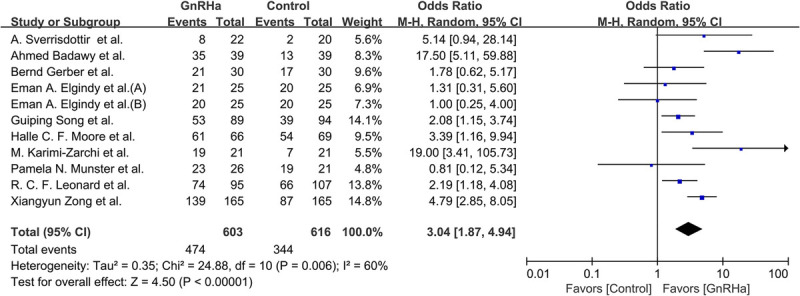
Forest plot of the rate of resumed ovarian function with GnRHa plus chemotherapy versus chemotherapy alone. GnRHa, gonadotropin-releasing hormone agonist.
Regarding hormone receptor-negative studies, 260 of 337 participants (77.2%) resumed menstruation; this involved 142 of 167 participants (85.0%) in the GnRHa group and 118 of 170 participants (69.4%) in the control group. As shown in Fig. 5, a significant statistical difference was observed between these groups (OR, 2.51; 95% CI, 1.07‐5.89; P = 0.03).
FIG. 5.
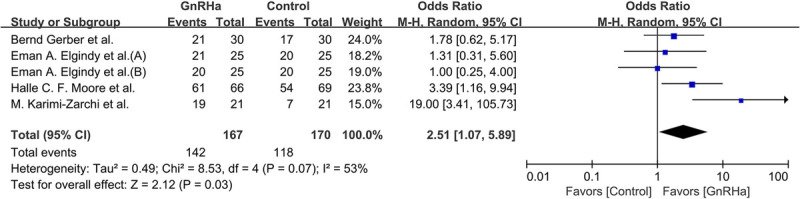
Forest plot of the rate of resumed ovarian function with GnRHa plus chemotherapy versus chemotherapy alone in hormone receptor-negative patients. GnRHa, gonadotropin-releasing hormone agonist.
GnRHa and pregnancies
Six studies reported pregnancy data (Fig. 6). Overall, 56 of the 652 participants (9.4%) reported in these studies became pregnant naturally, of whom 34 of 317 (10.7%) were in the GnRHa group and 22 of 335 (6.6%) were in the control group (OR, 1.72; 95% CI, 0.99‐2.99; P = 0.06; I2 = 0%, P = 0.65).
FIG. 6.
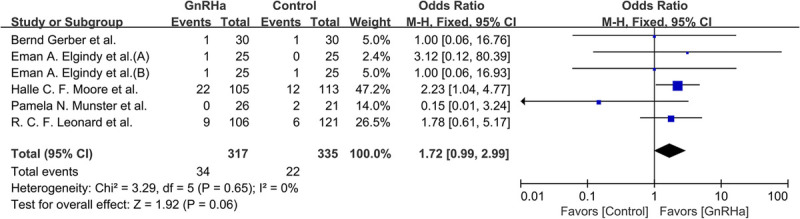
Forest plot of the rate of spontaneous pregnancy achieved with GnRHa plus chemotherapy versus chemotherapy alone. GnRHa, gonadotropin-releasing hormone agonist.
Within the hormone receptor-negative participants, 39 of 378 (10.3%) became pregnant naturally, of whom 25 of 185 (13.5%) were in the GnRHa group and 14 of 193 (7.3%) were in the control group. As shown in Fig. 7, a significant statistical difference was observed (OR, 2.06; 95% CI, 1.03‐4.11; P = 0.04; I2 = 0%, P = 0.89).
FIG. 7.
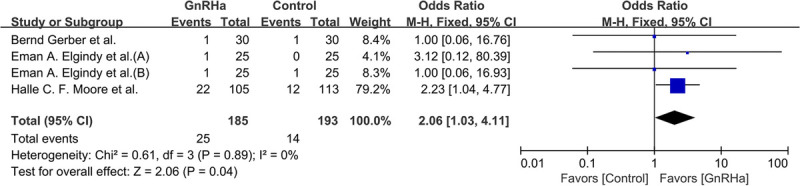
Forest plot of the rate of spontaneous pregnancy achieved with GnRHa plus chemotherapy versus chemotherapy alone in hormone receptor-negative patients. GnRHa, gonadotropin-releasing hormone agonist.
Sensitivity analysis
We conducted a sensitivity analysis by excluding each study one at a time; the results showed the stability of pooled OR estimates (Fig. 8).
FIG. 8.
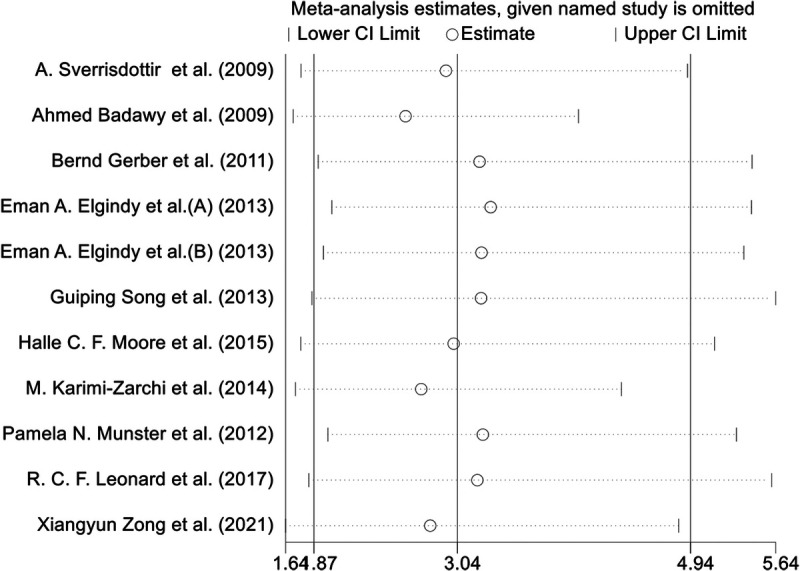
Forest plot for the sensitivity analysis.
Publication bias
As shown in Fig. 9, we did not detect any publication bias in the funnel plot (Egger test, P = 0.815; Begg test, P = 0.815).
FIG. 9.
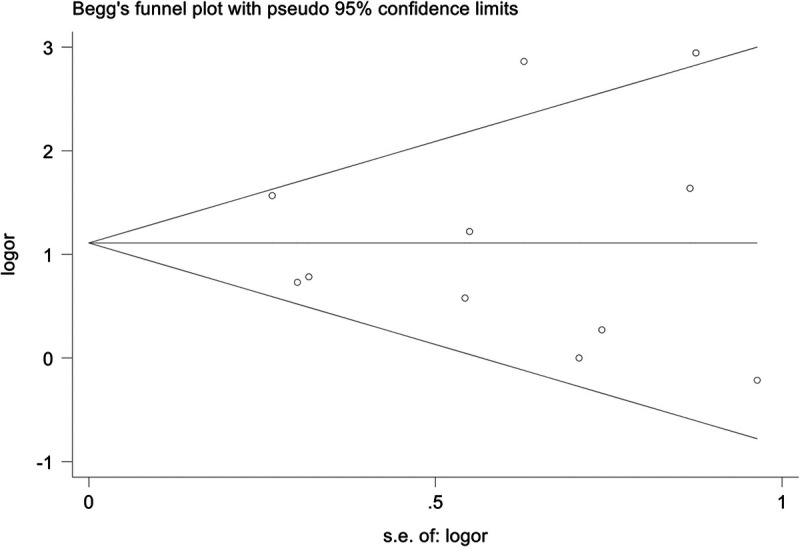
Funnel plots for publication bias.
DISCUSSION
To assess the effects of GnRHa on ovarian function and fertility preservation for premenopausal women with breast cancer undergoing chemotherapy including cyclophosphamide, we conducted a systematic review and meta-analysis of GnRHa cotreatment during chemotherapy; there was no evidence of publication bias regarding the outcomes. Our meta-analysis revealed that the inclusion of GnRHa significantly increased the overall rate of menstrual recovery, but it only improved the spontaneous pregnancy rate in hormone receptor-negative participants.
Chemotherapy drugs, especially highly toxic drugs such as cyclophosphamide, have profound and lasting effects on gonadal function.21 Animal experiments have shown that GnRHa can minimize chemotherapy-associated gonad toxicity, but its specific mechanism has not yet been elucidated completely.8 There are several possible mechanisms for the effect of GnRHa on chemotherapy-associated gonad toxicity: (1) the interruption of follicle-stimulating hormone secretion, (2) the activation of GnRH receptors, (3) a decrease in utero-ovarian perfusion, (4) the upregulation of sphingosine-1-phosphate, and (5) the protection of undifferentiated germ-line stem cells.22
Several clinical studies in related fields have reported findings that support the beneficial effect of GnRHa.11,12,15–17,19,23–27 Likewise, the current meta-analysis revealed that women with breast cancer who underwent chemotherapy containing cyclophosphamide would benefit from the addition of GnRHa. A significant increase in the resumption of ovarian function (22.8%) was observed accompanying the use of GnRHa-containing chemotherapy (OR, 3.04; 95% CI, 1.87–4.94; P < 0.001). However, two other similar studies reported findings inconsistent with our results.28,29 Specifically, these studies found that administering GnRHa concurrently with chemotherapy had no effect on the resumption of menses. The reason for this discrepancy may be because, compared with previous studies, our meta-analysis covered more participants (n = 1,219). Moreover, all 11 of the trials that we included contained cyclophosphamide, which is highly toxic. We thus implemented stricter inclusion criteria and used a larger sample size, so our results might be more reliable.
Infertility is another potential complication of chemotherapy, even if menses resume. It may be a major concern for some premenopausal women. Regarding the pregnancy rate, although we found that hormone receptor-negative patients benefited from the inclusion of GnRHa (OR, 2.06; 95% CI, 1.03‐4.11; P = 0.04), there was no statistically significant increase in the overall pregnancy rate in the current meta-analysis. This difference may arise from the fact that patients who are hormone receptor-positive need to postpone pregnancy while undergoing endocrine therapy. However, it was not possible to determine how many of the participants had attempted natural pregnancy, and the follow-up time was short. Thus, we could not fully evaluate the influence of GnRHa on fertility. GnRHa should not be used as an alternative to fertility preservation treatments.
Our meta-analysis has some important limitations that should be considered when interpreting the results. First, 10 of the 11 trials included in our meta-analysis were open label. Second, owing to the limited follow-up time of these RCTs, we were unable to determine the long-term impacts of GnRHa on the recovery of menses and fertility. Third, menses are not a reliable measure for ovarian function and fertility; hormonal changes during and after the course of treatment are also important markers. Fourth, implementing the strict inclusion and exclusion criteria detailed previously meant that we only retrieved a small number of studies. Finally, we did not conduct an individual data meta-analysis. Hence, future research should pay more attention to the design of RCTs, such as the adoption of strict blinding and allocation concealment, the performance of sufficient follow-up, and the introduction of other markers of ovarian reserve such as follicle stimulating hormone, luteinizing hormone, estradiol hormone, and anti-Müllerian hormone levels.
CONCLUSIONS
Our results showed that, when treating breast cancer in premenopausal women, the administration of GnRHa concurrently with chemotherapy improved the rate of menstrual recovery. However, GnRHa appeared to improve the spontaneous pregnancy rate only for hormone receptor-negative patients. Thus, the use of GnRHa may represent a feasible strategy for reducing the adverse impact on ovarian function of cyclophosphamide chemotherapy in women with breast cancer. However, additional high-quality RCTs are needed to support any definitive recommendations for the routine use of GnRHa in clinical practice.
Footnotes
Funding/support: None reported.
Financial disclosure/conflicts of interest: None reported.
Contributor Information
Ying-Li Dong, Email: doctordyl123@163.com.
Xiao-Zhong Cao, Email: dcotorcxz123@163.com.
Sha-Sha Ren, Email: doctorrss@163.com.
Zhen Zhang, Email: doctorzz123@163.com.
REFERENCES
- 1.Trivers KF Fink AK Partridge AH, et al. Estimates of young breast cancer survivors at risk for infertility in the U.S. Oncologist 2014;19:814–822. doi: 10.1634/theoncologist.2014-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. doi: 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–1717. doi: 10.1016/s0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 4.Tiong V, Rozita AM, Taib NA, Yip CH, Ng CH. Incidence of chemotherapy-induced ovarian failure in premenopausal women undergoing chemotherapy for breast cancer. World J Surg 2014;38:2288–2296. doi: 10.1007/s00268-014-2542-y [DOI] [PubMed] [Google Scholar]
- 5.Peccatori FA Azim HA Jr Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24Suppl 6:vi160–170. doi: 10.1093/annonc/mdt199 [DOI] [PubMed] [Google Scholar]
- 6.Loren AW Mangu PB Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500–2510. doi: 10.1200/jco.2013.49.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oktay K Harvey BE Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. doi: 10.1200/jco.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 8.Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod 1995;52:365–372. doi: 10.1095/biolreprod52.2.365 [DOI] [PubMed] [Google Scholar]
- 9.Ataya K, Ramahi-Ataya A. Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol 1993;7:22935. doi: 10.1016/0890-6238(93)90229-z [DOI] [PubMed] [Google Scholar]
- 10.Bokser L, Szende B, Schally AV. Protective effects of D-Trp6-luteinising hormone-releasing hormone microcapsules against cyclophosphamide-induced gonadotoxicity in female rats. Br J Cancer 1990;61:861–865. doi: 10.1038/bjc.1990.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat 2009;117‐:561–567. doi: 10.1007/s10549-009-0313-5 [DOI] [PubMed] [Google Scholar]
- 12.Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril 2009;91:694–697. doi: 10.1016/j.fertnstert.2007.12.044 [DOI] [PubMed] [Google Scholar]
- 13.Gerber B von Minckwitz G Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol 2011;29:2334–2341. doi: 10.1200/JCO.2010.32.5704 [DOI] [PubMed] [Google Scholar]
- 14.Elgindy EA El-Haieg DO Khorshid OM, et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol 2013;121:78–86. doi: 10.1097/aog.0b013e31827374e2 [DOI] [PubMed] [Google Scholar]
- 15.Song G, Gao H, Yuan Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol. 2013;30:667. doi: 10.1007/s12032-013-0667-8 [DOI] [PubMed] [Google Scholar]
- 16.Moore HC Unger JM Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med 2015;372:923–932. doi: 10.1056/NEJMoa1413204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimi-Zarchi M Forat-Yazdi M Vafaeenasab MR, et al. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur J Gynaecol Oncol 2014;35:59–61. [PubMed] [Google Scholar]
- 18.Munster PN Moore AP Ismail-Khan R, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol 2012;30:533–538. doi: 10.1200/JCO.2011.34.6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard RCF Adamson DJA Bertelli G, et al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol 2017;28:1811–1816. doi: 10.1093/annonc/mdx184 [DOI] [PubMed] [Google Scholar]
- 20.Zong X Yu Y Yang H, et al. Effects of gonadotropin-releasing hormone analogs on ovarian function against chemotherapy-induced gonadotoxic effects in premenopausal women with breast cancer in China: a randomized clinical trial. JAMA Oncol. 2022;8:252–258. doi: 10.1001/jamaoncol.2021.6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreuser ED, Hetzel WD, Billia DO, Thiel E. Gonadal toxicity following cancer therapy in adults: significance, diagnosis, prevention and treatment. Cancer Treat Rev 1990;17:169–175. doi: 10.1016/0305-7372(90)90043-f [DOI] [PubMed] [Google Scholar]
- 22.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044 [DOI] [PubMed] [Google Scholar]
- 23.Lambertini M Boni L Michelotti A, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA 2015;314:2632–2640. doi: 10.1001/jama.2015.17291 [DOI] [PubMed] [Google Scholar]
- 24.Li JW Liu GY Yu KD, et al. Effect of using LHRH analog during chemotherapy (CT) on premature ovarian failure and prognosis in premenopausal patients with early-stage, hormone receptor-positive breast cancer: the primary analysis of a randomized controlled phase III trial. Conference Abstract. Cancer Res 2015;75(9). doi: 10.1158/1538-7445.SABCS14-P1-12-02 [DOI] [Google Scholar]
- 25.Anderson R, Adamson D, Yellowlees A, Dunlop J, Thomas G, Leonard R. Administration of a GnRH agonist during chemotherapy for breast cancer reduces ovarian toxicity in women aged under 40 years. Journal: Conference Abstract. Human Reproduction (Oxford, England). 2016;31:i339 doi: 10.1093/humrep/31.Supplement_1.1 [DOI] [Google Scholar]
- 26.Senra JC, Roque M, Talim MCT, Reis FM, Tavares RLC. Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:77–86. doi: 10.1002/uog.18934 [DOI] [PubMed] [Google Scholar]
- 27.Zhong Y Lin Y Cheng X, et al. GnRHa for ovarian protection and the association between AMH and ovarian function during adjuvant chemotherapy for breast cancer J Cancer 2019;10:4278–4285. doi: 10.7150/jca.31859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitek WS, Shayne M, Hoeger K, Han Y, Messing S, Fung C. Gonadotropin-releasing hormone agonists for the preservation of ovarian function among women with breast cancer who did not use tamoxifen after chemotherapy: a systematic review and meta-analysis. Fertil Steril 2014;102:808–815.e1. doi: 10.1016/j.fertnstert.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 29.Yang B Shi W Yang J, et al. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast 2013;22:150–157. doi: 10.1016/j.breast.2012.12.008 [DOI] [PubMed] [Google Scholar]


