Background:
Apolipoprotein B (apoB) provides an integrated measure of atherogenic risk. Whether apoB levels and apoB lowering hold incremental predictive information on residual risk after acute coronary syndrome beyond that provided by low-density lipoprotein cholesterol is uncertain.
Methods:
The ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) compared the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab with placebo in 18 924 patients with recent acute coronary syndrome and elevated atherogenic lipoproteins despite optimized statin therapy. Primary outcome was major adverse cardiovascular events (MACE; coronary heart disease death, nonfatal myocardial infarction, fatal/nonfatal ischemic stroke, hospitalization for unstable angina). Associations between baseline apoB or apoB at 4 months and MACE were assessed in adjusted Cox proportional hazards and propensity score–matched models.
Results:
Median follow-up was 2.8 years. In proportional hazards analysis in the placebo group, MACE incidence increased across increasing baseline apoB strata (3.2 [95% CI, 2.9–3.6], 4.0 [95% CI, 3.6–4.5], and 5.5 [95% CI, 5.0–6.1] events per 100 patient-years in strata <75, 75–<90, ≥90 mg/dL, respectively; Ptrend<0.0001) and after adjustment for low-density lipoprotein cholesterol (Ptrend=0.035). Higher baseline apoB stratum was associated with greater relative (Ptrend<0.0001) and absolute reduction in MACE with alirocumab versus placebo. In the alirocumab group, the incidence of MACE after month 4 decreased monotonically across decreasing achieved apoB strata (4.26 [95% CI, 3.78–4.79], 3.09 [95% CI, 2.69–3.54], and 2.41 [95% CI, 2.11–2.76] events per 100 patient-years in strata ≥50, >35–<50, and ≤35 mg/dL, respectively). Compared with propensity score–matched patients from the placebo group, treatment hazard ratios for alirocumab also decreased monotonically across achieved apoB strata. Achieved apoB was predictive of MACE after adjustment for achieved low-density lipoprotein cholesterol or non–high-density lipoprotein cholesterol but not vice versa.
Conclusions:
In patients with recent acute coronary syndrome and elevated atherogenic lipoproteins, MACE increased across baseline apoB strata. Alirocumab reduced MACE across all strata of baseline apoB, with larger absolute reductions in patients with higher baseline levels. Lower achieved apoB was associated with lower risk of MACE, even after accounting for achieved low-density lipoprotein cholesterol or non–high-density lipoprotein cholesterol, indicating that apoB provides incremental information. Achievement of apoB levels as low as ≤35 mg/dL may reduce lipoprotein-attributable residual risk after acute coronary syndrome.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01663402.
Keywords: acute coronary syndrome; apolipoproteins B; cholesterol, LDL; heart disease risk factors; PCSK9 inhibitors
Clinical Perspective.
What Is New?
In patients with recent acute coronary syndrome and elevated atherogenic lipoproteins despite high-intensity or maximum-tolerated statin treatment, the risk of major adverse cardiovascular events increased with the baseline level of apolipoprotein B.
Under treatment with the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab, larger relative and absolute reductions in major adverse cardiovascular events were observed with higher baseline and lower achieved levels of apolipoprotein B.
Achieved levels of apolipoprotein B with alirocumab treatment predicted major adverse cardiovascular events after accounting for achieved low-density lipoprotein cholesterol or non–high-density lipoprotein cholesterol, but not vice versa.
What Are the Clinical Implications?
In patients with recent acute coronary syndrome receiving optimized statin therapy, a decision to treat with a proprotein convertase subtilisin/kexin type 9 inhibitor may be informed by the level of apolipoprotein B.
On treatment with the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab, achieved levels of apolipoprotein B convey information on the risk of major adverse cardiovascular events beyond that provided by achieved levels of low-density lipoprotein cholesterol or non–high-density lipoprotein cholesterol.
Treatment based on apolipoprotein B goals may further reduce lipoprotein-attributable residual risk after acute coronary syndrome.
Editorial, see p 673
Apolipoprotein B (apoB) is a key structural element of atherogenic lipoprotein particles, including low-density lipoprotein (LDL), lipoprotein(a), and triglyceride-rich lipoproteins. Although LDL cholesterol (LDL-C) is the most commonly used clinical lipid marker to stratify lipid-associated risk, all apoB-containing lipoproteins may affect the risk of major adverse cardiovascular events (MACE).1–3 Each apoB-containing lipoprotein particle contains 1 apoB molecule, so apoB concentration provides a measure of total concentration of circulating atherogenic lipoprotein particles. In patients with cardiovascular risk factors or stable cardiovascular disease, apoB levels provide incremental prognostic information on risk of incident atherosclerotic cardiovascular disease, MACE, and death, beyond that provided by LDL-C alone.3–6 Furthermore, in patients with a proatherogenic shift in lipoprotein composition not reflected by levels of LDL-C such as in type 2 diabetes mellitus or obesity, apoB concentration may reflect excess atherosclerotic risk more accurately than LDL-C.7
In patients with acute coronary syndrome (ACS), the risk of cardiovascular events is mitigated by lipid-lowering therapy with statins, ezetimibe, and inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9).8–12 Statins and ezetimibe lower the circulating concentration of LDL-C; statins additionally lower the concentration of triglyceride-rich lipoproteins; and PCSK9 inhibitors lower the concentration of all 3 types of apoB-containing lipoproteins, including lipoprotein(a), an effect integrated by apoB levels. Although apoB levels contribute beyond LDL-C levels to predict MACE in patients with established coronary heart disease,13,14 a similar association is less well established in patients with ACS.15,16 The relationship between apoB levels and MACE in ACS is important because lipoprotein(a) and triglyceride-rich lipoproteins also influence risk after ACS.17–20 Moreover, lipid-lowering drugs reduce apoB to a lesser extent than LDL-C.14 Therefore, a related question is whether on-treatment apoB or on-treatment LDL-C is a more informative indicator of lipoprotein-attributable residual risk.
In this analysis, we used data from the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab), which compared the PCSK9 inhibitor alirocumab with placebo in patients with recent ACS, to determine the relationships between baseline or achieved levels of apoB and risk of MACE and to assess whether apoB holds incremental information on risk beyond LDL-C.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. Qualified researchers may also request access to study documents, including the clinical study report, study protocol with amendments, case report forms, statistical analysis plan, and data set specifications.
Study Population
The ODYSSEY OUTCOMES trial design and primary results have been published.12,21 In brief, the trial included 18 924 patients ≥40 years of age with a recent (1–12 months before randomization) ACS and elevated atherogenic lipoprotein levels (LDL-C ≥70 mg/dL [1.81 mmol/L], non–high-density lipoprotein cholesterol [HDL-C] ≥100 mg/dL [2.59 mmol/L], or apoB ≥80 mg/dL) despite high-intensity statin therapy (atorvastatin 40–80 mg daily, rosuvastatin 20–40 mg daily) or the maximum-tolerated dose of 1 of these statins. The patients were randomly assigned to receive either 75 mg alirocumab or matching placebo subcutaneously every 2 weeks. In patients treated with alirocumab, the dose was blindly increased to 150 mg if the achieved LDL-C level was ≥50 mg/dL (1.29 mmol/L). In patients who had 2 consecutive LDL-C measurements <15 mg/dL (0.39 mmol/L), placebo was blindly substituted for alirocumab. The trial was approved by the institutional review board at each site. All patients provided informed consent.
Outcomes
The primary end point of MACE, comprising death resulting from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, and unstable angina requiring hospitalization, was adjudicated by an independent committee blinded to treatment assignment and lipid levels.
Measurement of Lipoproteins
ApoB concentration was measured in serum after an overnight fast, at randomization, at 4 months and at specified times thereafter. The samples were shipped at ambient temperature and measured at COVANCE Central Laboratories on the day of receipt with an immunonephelometry assay on the Siemens BNII Nephelometer (Siemens, Erlangen, Germany). Accuracy was validated by comparison with the American Pathologists Chemistry Survey. The interassay coefficient of variation was 2.4%, with a lower limit of detection of 35 mg/dL. LDL-C was estimated with the Friedewald formula except when triglycerides were >400 mg/dL (4.52 mmol/L) or when the Friedewald-calculated LDL-C was <15 mg/dL (0.39 mmol/L). In these cases, LDL-C was measured by β quantification. Furthermore, in select analyses, we used the Martin-Hopkins formula instead of the Friedewald formula to estimate LDL-C concentrations.22 Non–HDL-C was calculated by total cholesterol minus HDL-C. Baseline apoB was considered as a continuous variable and also as a categorical variable in 3 pre-specified strata‚ <75‚ 75–<90‚ and ≥90 mg/dL. Achieved apoB at month 4 of alirocumab treatment was considered a categorical variable in 3 post hoc strata: ≤35 mg, >35–<50, and ≥50 mg/dL. These strata were defined by the lower limit of detection of apoB (35 mg/dL) and a boundary level of 50 mg/dL that was approximately the median of samples with apoB concentration above the lower limit of detection. Strata of achieved LDL-C with alirocumab were prespecified (<25, 25–50, and >50 mg/dL).23
Statistical Analysis
Continuous variables are described by median (interquartile range [IQR]). Categorical variables are presented as counts and percentages. Distributions of apoB are described by treatment group at baseline, along with the absolute change from baseline to month 4 (122±28 days) after randomization. The last value was analyzed if a patient had multiple values within the time window. For the purposes of calculating these distributions, apoB values below the lower limit of detection were set to 35 mg/dL. At baseline, there were no missing values for apoB, and 3 patients were missing LDL-C. At 4 months, apoB was missing in 4.7% in the alirocumab group and 5.2% in the placebo group. At 12 months, apoB was missing in 8.8% of patients without a first MACE event before 12 months in the alirocumab group.
Missing postrandomization lipoprotein values were imputed by a prespecified pattern-mixture model following intention-to-treat principles.12 In brief, a missing value for a given patient within 21 days after a dose of study treatment was assumed to be influenced by the last dose of study treatment and therefore imputed from their other nonmissing values measured within 21 days after a dose, whereas missing values >21 days after a dose were imputed from their baseline value. For comparative purposes, similar assessments were made for the baseline and achieved LDL-C and non–HDL-C levels. Relative effects on first MACE are summarized by hazard ratios (HRs) with corresponding 95% CIs and P values from proportional hazards models. Event rates are expressed as the number of events per 100 patient-years of follow-up. The underlying proportional hazards assumptions of the proportional hazard models were verified by visual inspection of Kaplan-Meier graphs and plots of cumulative sums of Martingale residuals (Kolmogorov-type supremum test P>0.05 in all cases; data not shown).
Relationships between baseline apoB and first MACE in the placebo group were determined by 3 models using prespecified baseline apoB strata as the predictor variable: model A, unadjusted; model B, adjusted for demographic and nonlipid clinical variables (age, sex, race, geographic region, body mass index, smoking history, diabetes mellitus, time from index ACS to randomization); and model C, model B with the addition of baseline LDL-C. An additional analysis used LDL-C estimated by the Martin-Hopkins formula in model C. Thus, a comparison of models B and C determines whether baseline apoB provides incremental predictive information after baseline LDL-C has been taken into account. A P value was computed for linear trend in the estimated log HR across the ordered baseline apoB strata. A spline analysis of degree 2 (piecewise quadratic curve) of the relationship between continuous baseline apoB and MACE in the placebo group was performed, setting the HR to 1.00 at the overall baseline median concentration of apoB and 3 knots, located at the overall 25th percentile, median, and 75th percentile. For comparison, a spline analysis was also performed assessing the relationship between baseline non–HDL-C and MACE, with the HR set to 1.00 at the overall baseline median concentration of non–HDL-C and 3 knots located at the overall 25th percentile, median, and 75th percentile. The P value for the spline effect was based on the score test.
Heterogeneity in the relative and absolute effects of alirocumab treatment on first MACE was assessed according to baseline apoB strata. To assess heterogeneity in relative treatment effects, we constructed a proportional hazards model with baseline apoB stratum, treatment, and their interaction as predictors and computed a P value for linear trend across the estimated log HR (ie, the 3 interaction terms). To assess heterogeneity in absolute treatment effects, we constructed absolute risk reductions (ARRs) with alirocumab treatment, quantified as differences in the event rates per 100 patient-years of follow-up, along with associated 95% CIs. The cumulative incidence of first MACE by apoB strata and treatment group was estimated with Kaplan-Meier curves.
The propensity score–matched analysis included 9246 patients randomly assigned to alirocumab (including 437 [4.7%] with imputed month 4 apoB) and 9243 patients assigned to placebo (including 487 [5.2%] with imputed month 4 apoB) without a first MACE before their month 4 apoB assessment and made up 97.7% of the overall intention-to-treat population.
Month 4 values of apoB were selected because they reflect levels after several doses of alirocumab treatment and because relatively few MACE occurred before this time point.12 In relating achieved month 4 apoB levels to risk of MACE, we considered the potential for confounding attributable to differences in baseline characteristics or study medication adherence across apoB strata that were expected to be prognostic for MACE. To account for such differences, a propensity score was used to match each patient in the alirocumab group with a patient in the placebo group with similar baseline characteristics and adherence. To improve the quality of the match, the analysis used matching with replacement that allowed a given patient receiving placebo to be matched to patients receiving alirocumab in different achieved apoB stratum but not to multiple alirocumab patients within an achieved apoB stratum.24 A threshold of P<0.1 with forward selection in a logistic regression model was used to determine which characteristic differed between patients in each achieved apoB stratum of the alirocumab group and eligible patients receiving placebo. Baseline characteristics considered for matching were age, sex, and geographic region; history of diabetes mellitus, current smoking, previous coronary artery bypass grafting, previous percutaneous coronary intervention, peripheral artery disease, cerebrovascular disease, heart failure, chronic obstructive pulmonary disease, and malignancy; type of index ACS (non–ST-segment–elevation myocardial infarction, ST-segment–elevation myocardial infarction, or unstable angina), revascularization for the index ACS, and intensity of statin therapy at randomization (high intensity versus other); body mass index, systolic blood pressure, and estimated glomerular filtration rate dichotomized at 60 mL·min−1·1.73 m−2; and baseline concentrations of apoB, LDL-C, and lipoprotein(a). Adherence was assessed by the number of doses of study medication injected during the 61 days preceding the month 4 apoB measurement, as reported in patient diaries. There was no imputation of missing adherence data. Greedy matching on propensity scores was performed with caliper 0.25.
A series of spline analyses of degree 2 (piecewise quadratic curve) were performed in the alirocumab group. The estimated HR was set to 1.00 at the median month 4 concentration for the lipoprotein parameter that was related to the risk of MACE, with knots at the 25th percentile, median, and 75th percentile. The first set described the relationship between continuous apoB at month 4 and subsequent risk of MACE after adjustment for month 4 LDL-C; similar analyses adjusted for month 4 LDL-C estimated by the Martin-Hopkins formula and for month 4 non–HDL-C. A second set described the relationship between continuous month 4 LDL-C or month 4 LDL-C estimated by the Martin-Hopkins formula and subsequent risk of MACE after adjustment for month 4 apoB. Last‚ the relationship between continuous achieved non–HDL-C at month 4 and subsequent risk of MACE was described after adjustment for month 4 LDL-C.
To account for all apoB and LDL-C assessments in the study and for cumulative effects of changes in these lipid parameters over time, the relationship between continuous time-weighted moving average (TWMA) apoB, LDL-C, and MACE was analyzed within the alirocumab group. TWMA apoB and LDL-C were calculated from all available baseline and postrandomization values before a MACE event or right censoring for MACE at the last follow-up and specified as time-varying covariates in a Cox proportional hazards regression model. Separate analyses were performed with LDL-C estimated from the Friedewald and Martin-Hopkins formulas. Imputed values were excluded. This analysis therefore accounts for the potential effects of discontinuation of study medication, changes in background statin treatment, and protocol-specified blinded adjustment of alirocumab dose over the entire observation period.
Values of P<0.05 from 2-sided tests were considered statistically significant. All analyses were conducted according to intention to treat, including all patients and events from randomization to the common study end date (November 11, 2017). All analyses were conducted (by M.S.) with SAS version 9.4 (SAS Institute, Cary, NC).
Results
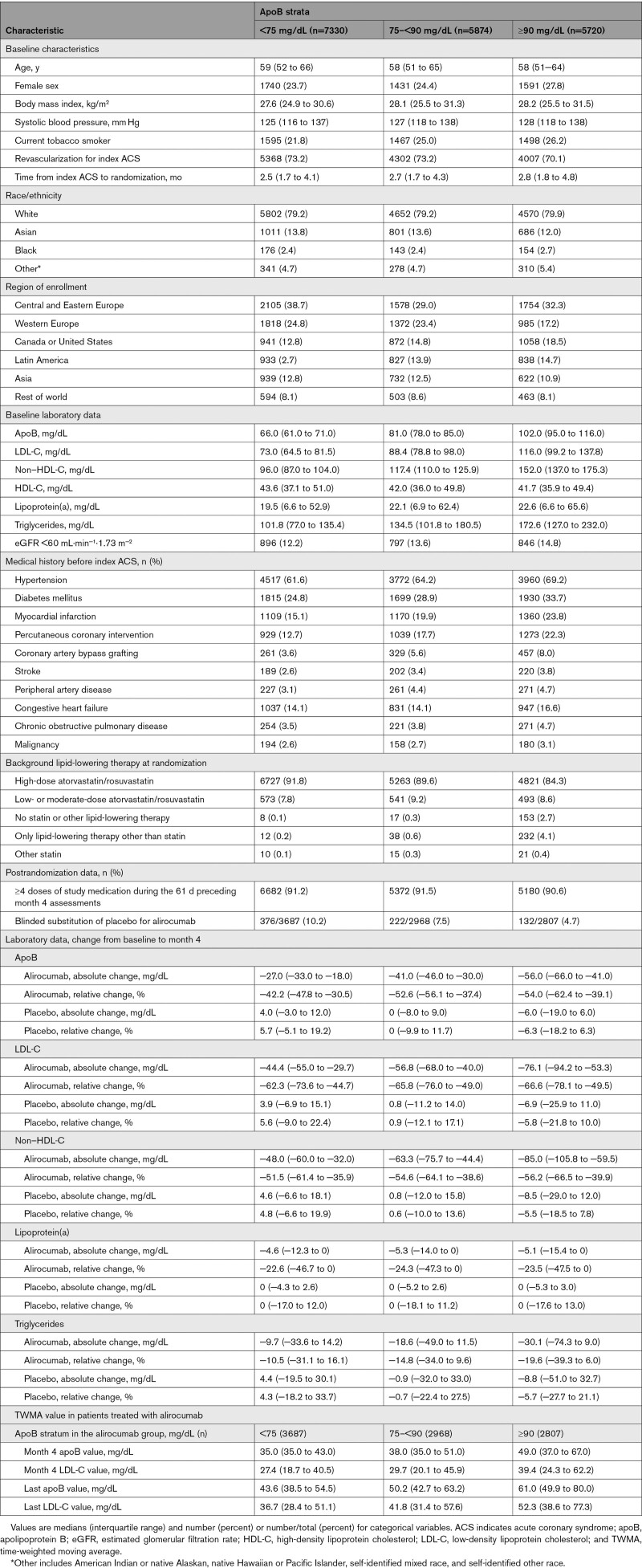
Patient Characteristics
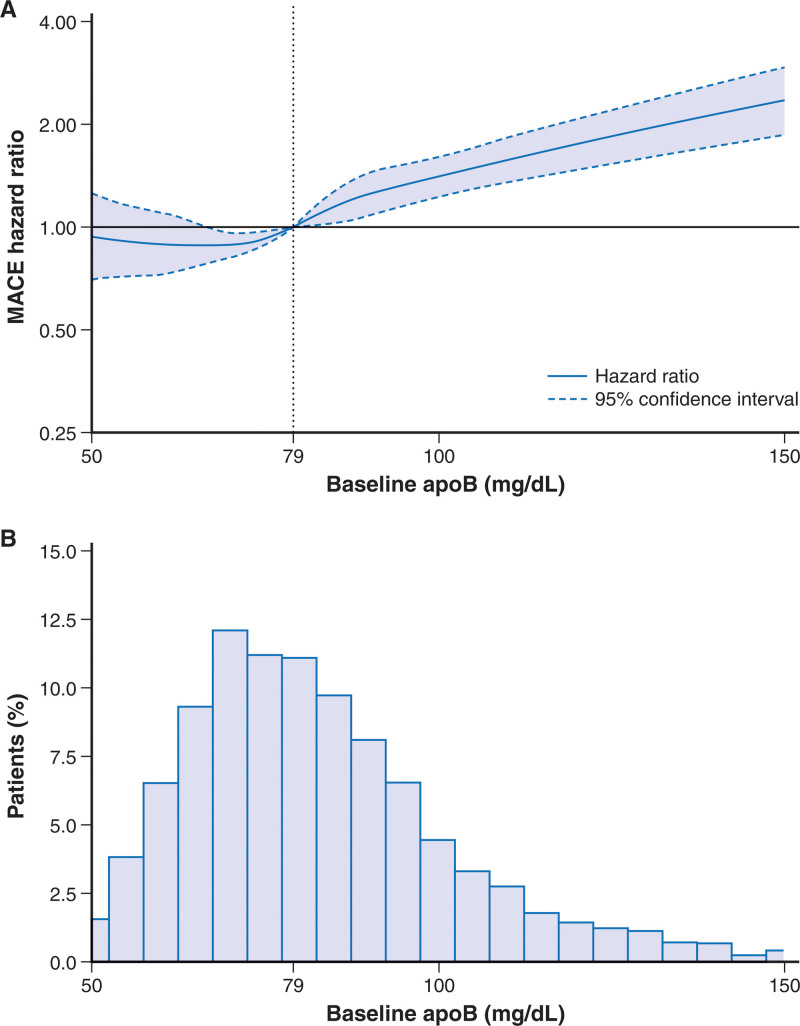
Median apoB at baseline was 79 mg/dL (IQR, 69–93 mg/dL). The distribution of baseline apoB in the placebo group is shown in Figure 1. Baseline apoB strata of <75, 75–<90, and ≥90 mg/dL comprised 7330, 5874, and 5720 patients, respectively (Table). Patients in the highest apoB strata were more likely to be women; to have diabetes mellitus, hypertension, and previous atherosclerotic events such as myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting; and less likely to be treated with a high-intensity statin regimen (Table). Patients in the lowest apoB stratum at baseline were more likely to have subsequent blinded substitution of placebo for alirocumab. In each stratum, patient characteristics were well balanced in the alirocumab and placebo groups (Table). Patients were followed up for a median of 2.8 years (IQR, 2.3–3.4 years).
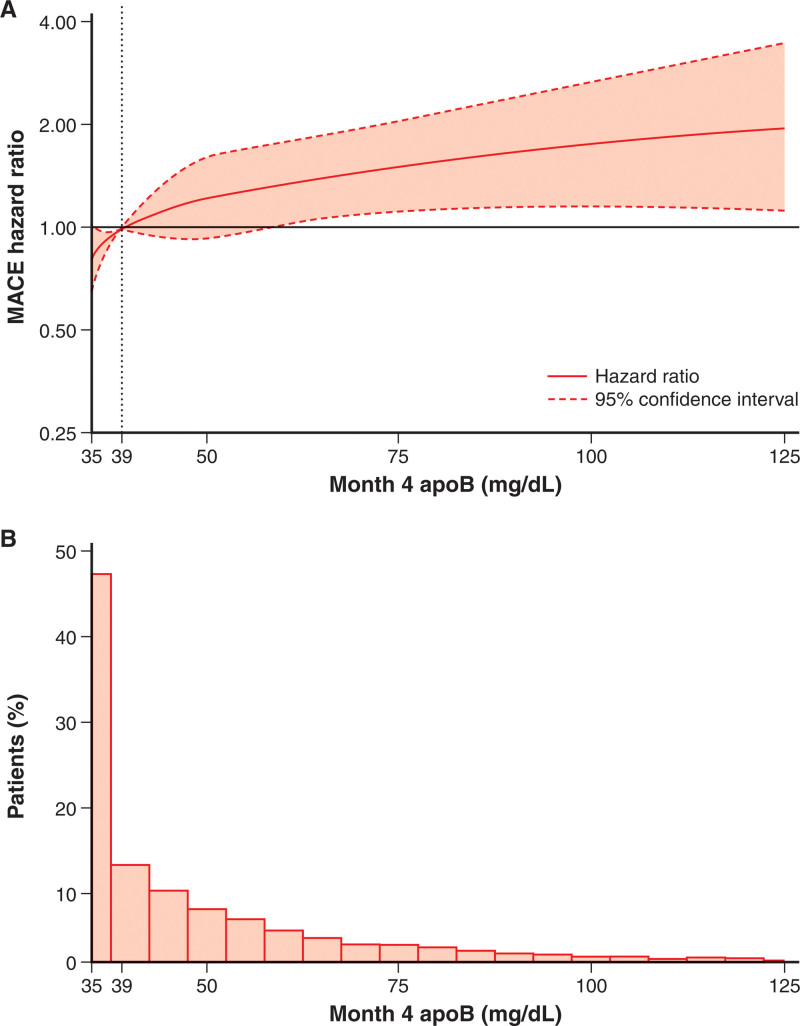
Figure 1.
Spline analysis of continuous baseline apoB and incident MACE in the placebo group. A, Hazard ratio set to 1.00 at the overall baseline median (79 mg/dL) concentration of apoB. Score test P<0.0001 for spline effect. This reflects spline of degree 2 (piecewise quadratic curve) with 3 knots, located at the 25th (69 mg/dL), 50th (79 mg/dL), and 75th (93 mg/dL) percentiles and spanning the approximate 1st (50 mg/dL) to 99th (150 mg/dL) percentiles overall. Dotted lines indicate the lower and upper bounds of the 95% CI. B, Histogram of baseline apoB in the placebo group. ApoB indicates apolipoprotein B; and MACE‚ major adverse cardiovascular events.
Effect of Alirocumab on ApoB Levels
At month 4 of treatment, the median apoB level was 39 mg/dL (IQR, 35–53 mg/dL) in the alirocumab group and 80 mg/dL (IQR, 68–95 mg/dL) in the placebo group. The median absolute change from baseline in apoB was −36 mg/dL (IQR, −47 to −24 mg/dL) in the alirocumab group versus 0 mg/dL (IQR, −9 to 10 mg/dL) in the placebo group. Patients in the highest baseline apoB stratum had the largest absolute change in apoB, −56 mg/dL (IQR, −66 to −41 mg/dL) compared with −41 mg/dL (IQR, −46 to −31 mg/dL) in the middle baseline stratum and −27 mg/dL (IQR, −33 to −18 mg/dL) in the lowest baseline stratum. The correlation between achieved apoB and achieved LDL-C is shown in Figure S1.
Among patients in the alirocumab group who achieved current US25 or European26 guideline-specified goals for LDL-C (calculated with the Martin-Hopkins formula) or non–HDL-C, we assessed the correspondence with achieved apoB quantile (Table S1). Among patients with achieved LDL-C levels <70, <55, or <40 mg/dL, 54.4%, 50.7%, and 40.7% failed to achieve an apoB level ≤35 mg/dL. We also determined the correspondence between achievement of Martin-Hopkins LDL-C or non–HDL-C in the lowest quartile (<22.0 or <40.9 mg/dL, respectively) and achieved quantiles of apoB. Of patients who achieved LDL-C in the lowest quartile, 18.2% did not achieve apoB ≤35 mg/dL. Furthermore, of those with non–HDL-C in the lowest quartile, 3.9% did not reach apoB ≤35 mg/dL.
Baseline ApoB and Incidence of MACE in the Placebo Group
In the placebo group, the incidence of MACE increased across baseline apoB strata (Figure 1). In the unadjusted model (model A), relative to the lowest baseline apoB stratum, patients in the middle and highest strata were at 24% (HR, 1.24 [95% CI, 1.06–1.45]) and 71% (HR, 1.71 [95% CI, 1.48–1.97]) higher risk for MACE, respectively (Ptrend<0.0001). With adjustment for demographic and nonlipid clinical variables (model B), these increased risks were 19% (HR, 1.19 [95% CI, 1.02–1.40]) and 64% (HR, 1.64 [95% CI, 1.41–1.90]), respectively (Ptrend<0.0001). Baseline apoB stratum predicted the risk of MACE even after further adjustment for baseline LDL-C (model C). In this model, the increased risks in the middle and highest strata were 9% (HR, 1.09 [95% CI, 0.93–1.28]) and 23% (HR, 1.23 [95% CI, 1.02–1.48]), respectively (Ptrend=0.035). When model C was adjusted for LDL-C estimated with the Martin-Hopkins formula, the results were attenuated compared with the analysis with adjustment for LDL-C estimated with the Friedewald formula, with HRs in the middle and highest apoB strata of 1.10 (95% CI, 0.92–1.30) and 1.17 (95% CI, 0.96–1.42), respectively (Ptrend=0.13). In an unadjusted model assessing the relationship between continuous baseline apoB level and MACE, there was a fairly linear association (P<0.0001 for spline effect; Figure 1). For every 10 mg/dL increment in baseline apoB, there was an 11% higher risk of MACE (HR, 1.11 [95% CI, 1.09–1.14]; P<0.0001). Spline analysis of the continuous relationship between baseline non–HDL-C and MACE (Figure S2) provided results nearly identical to those for the relationship between baseline apoB and MACE, with a 7% higher risk of MACE (HR, 1.07 [95% CI, 1.05–1.08]; P<0.0001) per 10 mg/dL increment in non–HDL-C.
Effects of Alirocumab on MACE by Baseline ApoB Stratum
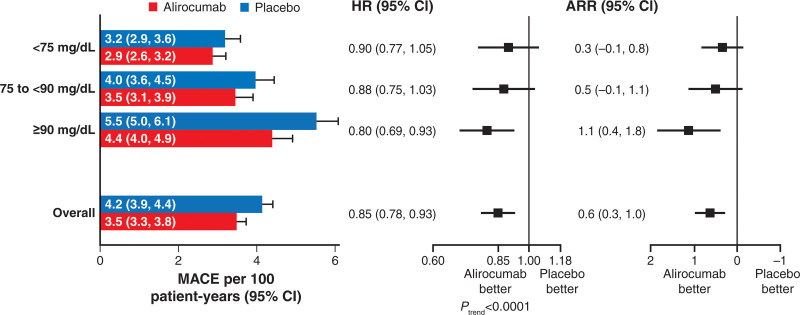
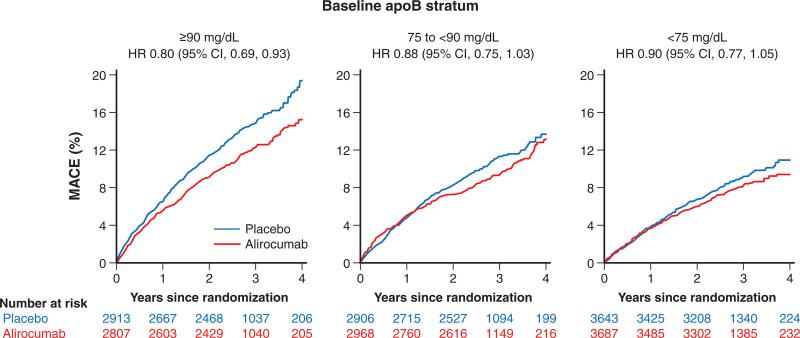
Overall, alirocumab reduced the risk of MACE compared with placebo, with an HR of 0.85 (95% CI, 0.78–0.93; P<0.001) and an ARR of 0.6 events per 100 patient-years of follow-up. There was a significant linear trend in the treatment HR for incident MACE across baseline apoB strata (P<0.0001; Figure 2), decreasing from 0.90 for <75 mg/dL to 0.80 for ≥90 mg/dL. Together with the ascending relationship between baseline apoB and absolute risk of MACE in the placebo group, the absolute reduction in risk of MACE with alirocumab increased from 0.3 events per 100 patient-years for patients with baseline apoB <75 mg/dL to 1.1 per 100 patient-years for patients with baseline apoB ≥90 mg/dL. In Figure 3, Kaplan-Meier curves show the cumulative incidence of MACE by apoB stratum and treatment allocation. Early and consistent curve separation is evident in the highest baseline apoB stratum.
Figure 2.
Relative and absolute treatment effects on the incidence rate for MACE, overall and by stratum of baseline apoB. Relative and absolute treatment effects on the incidence rate of MACE, overall and by stratum of baseline apoB. Forest plots depict relative and absolute risk reduction (ARR) with alirocumab compared with placebo. For relative risk reduction, there was a significant linear trend in the log hazard ratio (HR) across baseline strata, with point estimates progressively further <1.00 for higher strata. ApoB indicates apolipoprotein B; and MACE‚ major adverse cardiovascular events.
Figure 3.
Unadjusted cumulative incidence of the primary composite outcome across baseline apoB strata. ApoB indicates apolipoprotein B; HR‚ hazard ratio; and MACE, major adverse cardiovascular events.
Effects of Alirocumab on MACE by Achieved ApoB Stratum
Baseline characteristics, adherence, and month 4 lipoprotein levels of patients who achieved month 4 apoB ≥50, >35–<50, or ≤35 mg/dL are shown in Table S2, along with the characteristics of corresponding propensity score–matched patients from the placebo group.
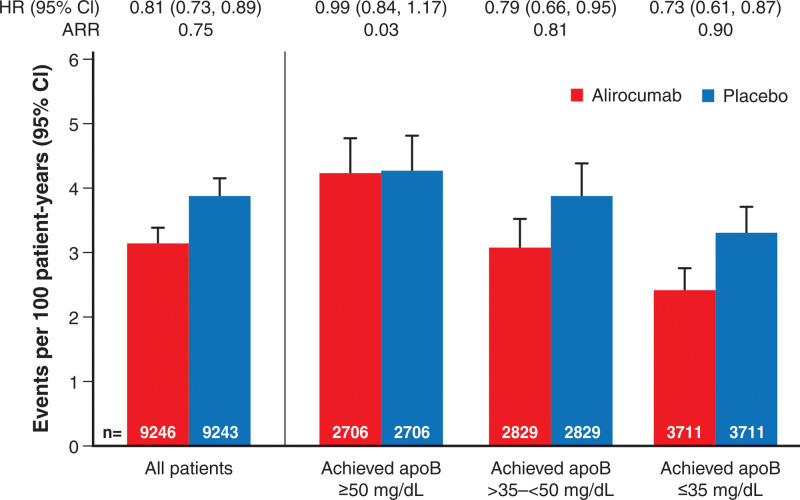
The incidence rates for MACE after month 4 in all eligible patients, in each achieved apoB stratum of the alirocumab group, and in corresponding propensity score–matched patients from the placebo group are displayed in Figure 4; the cumulative incidence of MACE is depicted in Figure S3. The analysis includes 1576 events, representing 80.6% of the events from randomization. Overall, MACE after month 4 occurred at rates of 3.16 (95% CI, 2.94–3.41) and 3.91 (95% CI, 3.66–4.18) per 100 patient-years of observation in the alirocumab and placebo groups, respectively (HR, 0.81 [95% CI, 0.73–0.89]; ARR, 0.75 events per 100 patient-years). In the alirocumab group, the incidence of MACE after month 4 decreased monotonically across apoB strata of ≥50, >35–<50, and ≤35 mg/dL, with rates of 4.26 (95% CI, 3.78–4.79), 3.09 (95% CI, 2.69–3.54), and 2.41 (95% CI, 2.11–2.76) per 100 patient-years, respectively. In the corresponding propensity score–matched patients from the placebo group, incidence rates for MACE after month 4 also decreased monotonically from 4.30 (95% CI, 3.81–4.83) to 3.90 (95% CI, 3.44–4.40) and 3.31 (95% CI, 2.94–3.71) per 100 patient-years, respectively. From these incidence rates, relative and absolute treatment benefit of alirocumab increased monotonically across decreasing strata of achieved apoB. Treatment HR was 0.99 (95% CI, 0.84–1.17; ARR, 0.03 events per 100 patient-years) with achieved apoB ≥50 mg/dL, 0.79 (95% CI, 0.66–0.95; ARR, 0.81 events per 100 patient-years) with achieved apoB >35–<50 mg/dL, and 0.73 (95% CI, 0.61–0.87; ARR, 0.90 events per 100 patient-years) with achieved apoB ≤35 mg/dL.
Figure 4.
Incidence rate for first major adverse cardiovascular events after month 4 according to achieved apoB stratum at month 4 in the alirocumab group and in propensity score–matched patients from the placebo group. ApoB indicates apolipoprotein B; ARR, absolute risk reduction; and HR, hazard ratio.
Effects of Alirocumab on MACE According to Achieved ApoB in Relation to Achieved LDL-C or Non–HDL-C
When we modeled continuous achieved apoB in spline analysis, with the HR set to 1.00 at the median apoB level (39 mg/dL) and with adjustment for achieved LDL-C, the risk of MACE increased monotonically with apoB level above the median and decreased with apoB below the median (Figure 5). Results were similar when the relationship between achieved apoB and MACE was adjusted for achieved LDL-C estimated with the Martin-Hopkins formula (Figure S4). Conversely, there was no association between continuous achieved LDL-C (calculated with either the Friedewald or Martin-Hopkins formula) and risk of MACE with adjustment for achieved apoB (Figure S5A [top] and Figure S6). Analogous findings were obtained when achieved apoB and LDL-C were considered as categorical variables. Figure S7 shows the number of patients in the alirocumab group classified into each of the 3 post hoc achieved apoB strata (≥50 mg/dL, n=2706; >35–<50 mg/dL, n=2829; and ≤35 mg/dL, n=3711) and the 3 prespecified achieved LDL-C strata (>50, 25–50, and <25 mg/dL). Overall, 6437 of the 9245 patients (69.6%) are categorized into the same ordered stratum (ie, concordance for achieved apoB and LDL-C). A total of 2808 patients (30.4%) had discordant apoB and LDL-C ordered strata. The pattern of treatment benefit across achieved LDL-C strata differed from that observed across achieved apoB strata. In each achieved LDL-C stratum, treatment benefit increased in progressively lower achieved apoB strata. In each achieved apoB stratum, treatment benefit was similar in middle and lowest quantiles of achieved LDL-C (25–50 and <25 mg/dL; Figure S7).
Figure 5.
Spline analyses of continuous achieved apoB adjusted for achieved low-density lipoprotein cholesterol, and incidence of MACE in patients treated with alirocumab. A, Hazard ratio set to 1.00 at the median concentration of apoB (39 mg/dL) achieved within the alirocumab group at month 4. Score test P=0.0009 for apoB spline effect. This reflects spline of degree 2 (piecewise quadratic curve) with 3 knots, located at the 25th percentile (35 mg/dL), median (39 mg/dL), and 75th percentile (53 mg/dL) and spanning the minimum (35 mg/dL) to ≈99th (125 mg/dL) percentiles within the alirocumab group. Dotted lines indicate the lower and upper bounds of the 95% CI. B, Histogram of month 4 apoB levels within the alirocumab group. The lower limit of detection for apoB was 35 mg/dL. ApoB indicates apolipoprotein B; and MACE‚ major adverse cardiovascular events.
Figure S5B (top) shows spline analysis of MACE after month 4 according to continuous achieved non–HDL-C at month 4 adjusted for achieved LDL-C. In contrast to the monotonic relationship for achieved apoB adjusted for achieved non–HDL-C (Figure S5C), the association between non–HDL-C adjusted for achieved apoB and MACE was not monotonic for values above the median, with confidence boundaries for the HR crossing 1.0 over most of the non–HDL-C range. In addition, the relationship between achieved apoB and MACE was monotonic throughout the evaluated range of apoB, whereas the relationship between achieved non–HDL-C and MACE was relatively flat over most of its range.
In a model within the alirocumab group consisting of TWMA apoB (constructed from 40 947 values) and LDL estimated with the Friedewald formula (constructed from 87 161 values), the MACE HR was 0.91 (95% CI, 0.87–0.96; P<0.001) per 10 mg/dL decrement in apoB and 0.96 (95% CI, 0.93–1.00; P=0.029) per 10 mg/dL decrement in LDL-C. Overall, the median last TWMA value per patient was 50.3 (IQR, 41.8–64.7) for apoB and 42.3 (IQR, 31.6–60.6) for LDL-C. Findings were similar when LDL-C was calculated by the Martin-Hopkins formula. The MACE HR per 10 mg/dL decrement was 0.93 (95% CI, 0.88–0.98; P=0.010) for apoB and 0.95 (95% CI, 0.91–0.98; P=0.004) for LDL-C. Overall, the median last TWMA value was 50.3 mg/dL (IQR, 41.8–64.7) for apoB and 42.3 mg/dL (IQR, 31.6–60.6) for LDL-C, reflecting modest increases over time after month 4 as shown by baseline apoB stratum in the Table.
Table.
Patient Characteristics by Baseline ApoB Strata and Treatment
Sensitivity Analysis
Patients in the lowest achieved apoB stratum at month 4 were more likely to have blinded, protocol-specified substitution of placebo for alirocumab after month 4 (567 of 724 patients). The association between achieved apoB strata and outcomes was assessed in a sensitivity analysis that excluded these 724 patients (Figure S8). Findings were similar to those of the main analyses. In the alirocumab group, the risk of MACE decreased across decreasing achieved apoB strata. The risk of MACE also decreased in corresponding matched patients from the placebo group. Treatment HR declined monotonically from highest to lowest achieved apoB stratum in the alirocumab group; the ARR was least in the highest stratum of achieved apoB and similar in the 2 lower strata.
Discussion
In this study of >18 000 patients on high-intensity or maximum-tolerated statin therapy and assigned to treatment with alirocumab or placebo, there were 4 key findings concerning the relationship of baseline or achieved levels of apoB with the risk of MACE after ACS. First, in the placebo group, higher baseline apoB was associated with greater risk of MACE. Second, in patients treated with alirocumab, lower achieved apoB at 4 months was associated with greater relative and absolute reductions in MACE after 4 months, with the greatest reductions observed in patients who achieved an apoB level ≤35 mg/dL. Third, the relationship between achieved apoB and MACE retained significance after adjustment for achieved levels of LDL-C or achieved levels of non–HDL-C; conversely, achieved levels of LDL-C or non–HDL-C were not predictive of MACE after taking achieved apoB into account. Fourth, among patients in the alirocumab group who achieved current US or European guideline-directed LDL-C or non–HDL-C goals for secondary prevention in very high-risk patients (ie, LDL-C <70 or <55 mg/dL, non–HDL-C <100 or <85 mg/dL),25,26 approximately half failed to achieve a simultaneous apoB level ≤35 mg/dL (Table S1). Even among those who achieved the most stringent goal of LDL-C <40 mg/dL,26 40.7% did not achieve an apoB level ≤35 mg/dL; furthermore, in patients reaching the lowest LDL-C quartile (<22 mg/dL), 18.2% did not achieve apoB levels ≤35 mg/dL. Despite correlation among categories of achieved LDL-C, non–HDL-C, and apoB, of 6510 patients who achieved a non–HDL-C level <70 mg/dL, 2849 (43.8%) failed to achieve an apoB level ≤35 mg/dL. These findings suggest that achievement of even the most stringent current LDL-C or non–HDL-C goals may not ensure that lipoprotein-attributable residual risk has been minimized. However, among those reaching the lowest non–HDL-C quartile (<40.9 mg/dL), only 4% did not achieve apoB levels ≤35 mg/dL, indicating that treating patients according to non–HDL-C levels may reduce the apoB-associated residual risk.
Although LDL-C is the principal lipid parameter used to estimate lipoprotein-attributable cardiovascular risk, all apoB-containing lipoproteins, including LDL, lipoprotein(a), and triglyceride-rich lipoproteins, may contribute to that risk in the general population and in patients with stable, established coronary heart disease.13,27 In contrast, data on the association between apoB and outcomes after ACS are limited.15,16 We show that, in analogy with non–ACS populations, a higher apoB concentration was associated with an increased risk of MACE in a fairly linear relationship, despite high-intensity or maximum-tolerated statin therapy.
Non–HDL-C, similar to apoB, includes estimates of atherogenic lipids such as LDL-C, lipoprotein(a), and triglyceride-rich lipoproteins, and both are secondary targets to LDL-C in prevention guidelines.13 In this study, the relationships between baseline (on statin) apoB or non–HDL-C and risk of MACE were similar. In contrast, on both statin and alirocumab treatment achieved apoB appeared to be more predictive of future MACE than achieved non–HDL-C, with the former displaying a positive monotonic relationship with MACE but the latter not. The reasons for this incongruity are uncertain. Although plasma levels of apoB and non–HDL-C have high correlation on a population basis, individual discordance is common, related primarily to variance in the cholesterol content of LDL particles.5,28–31 The predictive value of apoB versus non–HDL-C under treatment with both statin and PCSK9 inhibitor has not been examined previously. It is possible that under conditions of marked LDL-C reduction, a larger proportion of non–HDL-C resides in the pool of triglyceride-rich lipoproteins, which in turn may be less strongly associated with cardiovascular risk than LDL particles.32
Pharmacological lowering of atherogenic lipids with high-intensity statin therapy reduces the risk of MACE after an ACS.8,9,11,12,33 Despite achievement of a conventional LDL-C goal with statins, there is substantial residual risk for recurrent MACE after ACS that may be reduced with intensified lipid lowering with ezetimibe or a PCSK9 inhibitor.10,12,34 Ezetimibe produces an additional lowering of LDL-C but has minimal effects on lipoprotein(a) or triglyceride-rich lipoproteins. In contrast, the addition of a PCSK9 inhibitor to statin produces further reductions in LDL-C, lipoprotein(a), and triglycerides, an effect integrated by levels of apoB. In a previous analysis, we showed that reduction in the risk of MACE with alirocumab was similar when achieved LDL-C levels were <25 mg/dL compared with 25 to 50 mg/dL.23 The results of the present analysis suggest that the residual risk of MACE in patients receiving high-intensity statin treatment and alirocumab after ACS may be better gauged by levels of apoB than levels of LDL-C. Achievement of progressively lower strata of apoB, to ≤35 mg/dL, is associated with progressively lower risk of MACE even after adjustment for achieved LDL-C or non–HDL-C levels. There was also a robust association between continuous achieved apoB and the subsequent risk of MACE, even with adjustment for achieved LDL-C or non–HDL-C. Conversely, achieved LDL-C or non–HDL-C held no prognostic information if adjusted for achieved apoB.
To account for changes in apoB and LDL-C after month 4 due to factors such as discontinuation of study medication, changes in background statin treatment, and protocol-specified changes in alirocumab dose, we performed an analysis in the alirocumab group that related continuous, time-weighted apoB and continuous, time-weighted LDL-C to the risk of MACE. The interpretation of this analysis, similar to the categorical apoB analyses, is that a lower apoB level on treatment with statin and PCSK9 inhibitor predicts the risk of MACE more faithfully than a lower LDL-C level. This was true regardless of whether LDL-C was estimated with the Friedewald or the Martin-Hopkins formula.35 In sum, these findings reinforce previous data suggesting that the number of atherogenic particles (as reflected by apoB levels) predicts risk more accurately than the cholesterol content of those particles.36–39 Thus, although apoB, LDL-C, and especially non–HDL-C are correlated, apoB may be a superior predictor of lipoprotein-attributable residual risk after ACS on maximum-intensity lipid-lowering therapy.
There are several explanations for the incomplete estimation of risk with LDL-C observed in this study and in the literature, especially in statin-treated patients.3–5,26,40 First, we have shown that lipoprotein(a) is a major predictor of risk and risk reduction with alirocumab and is not taken into account by LDL-C alone.19 Other apoB-containing lipids such as triglyceride-rich lipoproteins may also contribute to risk after ACS.1,18 This is especially important in patients in whom there is a discordance between circulating cholesterol concentration and the number of apoB-containing particles. This may be the case in individuals who have relatively more triglyceride and less cholesterol in apoB-containing particles such as those with type 2 diabetes mellitus, metabolic syndrome, or obesity,7,41 with a high concentration of small, dense LDL particles, elevated apoB, but normal LDL-C.7,41 ApoB-containing particles other than LDL may assume greater importance in the determination of residual risk when LDL-C levels are low. This may explain why the relationship of baseline apoB to risk of MACE in the placebo group was attenuated by adjustment for baseline LDL-C with a mean level of ≈90 mg/dL, but the relationship of achieved apoB to risk of MACE in the alirocumab group was minimally affected by adjustment for achieved LDL-C with a mean level of ≈40 mg/dL. Assessing apoB rather than LDL-C in such patients may more accurately reflect their residual atherogenic lipoprotein burden and hence their residual cardiovascular risk.1,7,40
Strengths and Limitations
Strengths of the present analyses include the use of data from a large multinational study with detailed description of patient phenotype and with adjudicated outcomes. Furthermore, almost 90% of the patients were treated with high-intensity statin therapy. Propensity score–matched analyses to determine the relationship between achieved apoB and risk were included to overcome some potential bias related to baseline differences in patient characteristics.
However, there are some limitations. The analyses of achieved apoB levels use postrandomization data. Even adjusted Cox proportional hazards and propensity score–matched analyses may be subject to residual confounding. The quantile boundaries for baseline apoB were prespecified, but the quantile boundaries for both baseline and achieved apoB were arbitrary. For this reason, the analyses of continuous apoB are useful and corroborate the quantile analyses. Achieved apoB in the trial was influenced by protocol-specified blinded substitution of placebo for alirocumab in patients who had consecutive LDL-C levels <15 mg/dL. However, despite the fact that patients in the lowest quantile of apoB at month 4 were the most likely to undergo this substitution, they nonetheless had the lowest risk of MACE after month 4. Moreover, the findings were not substantially affected by excluding these patients from the analysis. Results from patients with recent ACS on optimal statin therapy might not be generalizable to other populations. Although ODYSSEY OUTCOMES was an international trial, 79% of the participants were White and 75% were male. Therefore, caution must be applied in generalizing the findings to non-White and female patients with ACS. Relatively few patients in this trial were treated with ezetimibe. It is uncertain whether relationships between baseline or achieved apoB and MACE would have been influenced by greater use of this drug.
A persistent limitation to the use of apoB as a risk predictor or as a therapeutic target has been the lack of a formal, universal standard for its measurement.35 The present findings reinforce the imperative to seek such standardization so that apoB measurements can be applied to guide everyday clinical practice. Estimation of LDL-C levels was performed with the Friedewald formula, which has known performance issues at high triglyceride levels and low LDL-C levels. To take this into account in a secondary analysis, apoB was adjusted by LDL-C estimated with the Martin-Hopkins formula, which has been shown to perform better than the Friedewald formula.35 The relationship between baseline apoB and risk of MACE was attenuated when LDL-C was estimated by the Martin-Hopkins rather than the Friedewald formula‚ but the relationship of achieved apoB to risk of MACE in the alirocumab group was minimally affected by such adjustment. These findings indicate that the contribution of LDL particles to residual risk diminishes when their concentration is low. Under those conditions‚ other apoB-containing particles including lipoprotein(a) or triglyceride-rich lipoproteins whose concentrations are less affected by PCSK9 inhibition‚ may assume greater importance in determining residual risk.
Last, the lower limit of quantification for apoB was 35 mg/dL; therefore, the association between continuous apoB and MACE could not be ascertained below 35 mg/dL. It is important to note that quantile analyses of achieved apoB levels were not affected by this limitation because the lowest quantile was defined as a level ≤35 mg/dL. In fact, the MACE incidence among patients receiving alirocumab with achieved apoB ≤35 mg/dL was 22% lower than that in patients with achieved apoB of >35–<50 mg/dL.
Conclusions
In patients with recent ACS receiving high-intensity or maximum-tolerated statin treatment, a higher baseline level of apoB was associated with a higher risk of MACE. Among those treated with the PCSK9 inhibitor alirocumab, lower achieved apoB levels (to ≤35 mg/dL) were associated with a lower risk of MACE. Furthermore, lower achieved levels of apoB remained predictive of MACE even after adjustment for achieved LDL-C or non–HDL-C. Conversely, achieved levels of LDL-C or non–HDL-C held no information after adjustment for achieved apoB, indicating that apoB holds incremental predictive information on residual risk beyond either LDL-C or non–HDL-C when the latter 2 levels are low. An achieved apoB concentration ≤35 mg/dL was associated with more favorable cardiovascular outcomes than higher achieved apoB levels. However, despite meeting guideline-directed secondary prevention goals for LDL-C or non-HDL-C with statin and alirocumab, a substantial proportion of patients remained at apoB levels >35 mg/dL, suggesting a potential therapeutic benefit from even more intensive lowering of atherogenic lipoproteins.
Article Information
Acknowledgments
The authors thank the patients, study coordinators, and investigators who participated in this trial. Sophie Rushton-Smith, PhD (MedLink Healthcare Communications, London), provided editorial assistance in the preparation of the manuscript (limited to editing for style, referencing, and figure and table editing) and was funded by Sanofi.
Sources of Funding
This work was supported by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosures
Dr Hagström reports research grant funding from Sanofi-Aventis, Pfizer, Amgen, and Swedish Heart and Lung Foundation, as well as consulting or honoraria from Amgen, Sanofi-Aventis, Novartis, and NovoNordisk. Dr Steg reports grants, personal fees, and nonfinancial support from Sanofi; grants and personal fees from Amarin, Servier, and Bayer; and personal fees from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Idorsia, Pfizer, and Novartis. In addition, Dr Steg has a patent on the use of alirocumab to reduce risk after ACS (royalties to Sanofi) pending. Dr Szarek reports serving as a consultant or on advisory boards (or both) for CiVi, Resverlogix, Baxter, Esperion, Sanofi, and Regeneron Pharmaceuticals, Inc. Dr Bhatt reports the following relationships: Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; chair: inaugural chair, American Heart Association Quality Oversight Committee; Data Monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, and Population Health Research Institute; honoraria: American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (editor in chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), K2P (cochair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), WebMD (CME steering committees); other: Clinical Cardiology (deputy editor), NCDR-ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); research funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, and 89Bio; royalties: Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site coinvestigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, and Svelte; trustee: American College of Cardiology; and unfunded research: FlowCo, Merck, and Takeda. Dr Bittner reports grant support from Sanofi, Regeneron Pharmaceuticals, AstraZeneca, DalCor, Esperion, and Novartis; consulting fees from Pfizer; honoraria from Medscape; and fees for participating on a Data Safety Monitoring Board or Advisory Board from the National Institutes of Health. Dr Danchin reports speaking and consulting fees or fees for participation in advisory boards, critical events committees, or data safety monitoring boards from Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, China National Clinical Research Center for Neurological Diseases Intercept, MSD, Novo-Nordisk, Pfizer, Sanofi, Servier, UCB, and Vifor. Dr Diaz reports research grants from Sanofi, DalCor Pharmaceuticals, Population Health Research Institute, Duke Clinical Research Institute, the TIMI group, Amgen, Cirius, Montreal Health Innovations Coordinating Center, and Lepetit, as well as personal fees, as a member of the Executive Steering Committee, from Amgen and Cirius. Dr Goodman reports research grant support (eg, Steering Committee or Data and Safety Monitoring Committee) and/or speaker/consulting honoraria (eg, Advisory Board) from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, GlaxoSmithKline, HLS Therapeutics, JAMP Pharma, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk A/C, Pendopharm, Pfizer, Regeneron, Sanofi, Servier, and Valeo Pharma, as well as salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, and PERFUSE Research Institute. Dr Harrington reports receiving research grants (all Data Safety Monitoring Board related) from AstraZeneca, Janssen, and Bristol-Myers Squibb; serving on the Advisory Board for Gilead (uncompensated) and WebMD; and serving on the Board of Directors (unpaid) for the American Heart Association and Stanford HealthCare. Dr Jukema reports research grants from the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, and the European Commission Seventh Framework Programme, as well as research support from Amgen, Astellas, AstraZeneca, Daiichi- Sankyo, Lilly, Merck-Schering-Plough, Pfizer, Roche, and Sanofi. Dr Liberopoulos reports personal fees from Sanofi, Servier, Boehringer-Ingelheim, MSD, Lilly, Novartis, and Chiesi, as well as personal fees and nonfinancial support from Amgen, AstraZeneca, and Bayer. Dr Marx has served as a consultant and speaker for Amgen and Sanofi-Aventis. All honoraria go the RWTH Aachen University. Drs McGinniss, Manvelian, and Pordy are employees of Regeneron Pharmaceuticals and may hold shares and/or stock options in the company. Dr Scemama is an employee of Sanofi and may hold shares and/or stock options in the company. Dr White reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial from Sanofi-Aventis and Regeneron Pharmaceuticals; for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly; for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals; for the SPIRE trial (The Evaluation of Bococizumab [PF-04950615; RN 316] in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects) from Pfizer USA; for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study (A Study to Evaluate the Effect of Long-term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc; for the dal-GenE study (Effect of Dalcetrapib vs. Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc; for the AEGIS-II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd; and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid [ETC-1002] or Placebo) from Esperion Therapeutics Inc. Dr White was on the Advisory Board for Acetelion, Sirtex, and Genentech, Inc (an affiliate of F. Hoffmann-La Roche Ltd, “Roche”; Lytics PostPCI Advisory Board at European Society of Cardiology) and received lecture fees from AstraZeneca. Dr Zeiher reports consulting fees from Novo Nordisk, Sanofi, and Boehringer Ingelheim; speaker fees from Bayer, Pfizer, Novartis, and Amgen; and travel costs from Sanofi, Boehringer Ingelheim, and Amgen. Dr Zeiher is president of the German Cardiac Society. Dr Schwartz reports research grants to the University of Colorado from Resverlogix, Sanofi, The Medicines Company, and Roch; and is coinventor of pending US patent 62/806,313 (Methods for Reducing Cardiovascular Risk) assigned in full to the University of Colorado.
Supplemental Material
Tables S1 and S2
Figures S1–S8
ODYSSEY OUTCOMES Committees and Investigators
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- apoB
- apolipoprotein B
- ARR
- absolute risk reduction
- HDL-C
- high-density lipoprotein cholesterol
- HR
- hazard ratio
- IQR
- interquartile range
- LDL
- low-density lipoprotein
- LDL-C
- low-density lipoprotein cholesterol
- MACE
- major adverse cardiovascular events
- ODYSSEY OUTCOMES
- Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- TWMA
- time-weighted moving average
Circulation is available at www.ahajournals.org/journal/circ
This manuscript was sent to Erin Michos, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The ODYSSEY OUTCOMES committee members, investigators, and contributors are listed in the Supplemental Material.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.121.057807.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 670.
Contributor Information
P. Gabriel Steg, Email: gabriel.steg@aphp.fr.
Michael Szarek, Email: michael.szarek@downstate.edu.
Deepak L. Bhatt, Email: dlbhattmd@post.harvard.edu.
Vera A. Bittner, Email: vbittner@uab.edu.
Nicolas Danchin, Email: nicolas.danchin.pro@gmail.com.
Rafael Diaz, Email: rafadiaz@me.com.
Shaun G. Goodman, Email: goodmans@chrc.net.
Robert A. Harrington, Email: robert.harrington@stanford.edu.
J. Wouter Jukema, Email: j.w.jukema@lumc.nl.
Evangelos Liberopoulos, Email: elibero@med.uoa.gr.
Nikolaus Marx, Email: nmarx@ukaachen.de.
Jennifer McGinniss, Email: jennifer.mcginniss@regeneron.com.
Garen Manvelian, Email: garen.manvelian@regeneron.com.
Robert Pordy, Email: robert.pordy@regeneron.com.
Michel Scemama, Email: Michel.Scemama@sanofi.com.
Harvey D. White, Email: harveyw@adhb.govt.nz.
Andreas M. Zeiher, Email: zeiher@em.uni-frankfurt.de.
Gregory G. Schwartz, Email: Gregory.Schwartz@va.gov.
Collaborators: Gregory G. Schwartz, Deepak L. Bhatt, Vera A. Bittner, Rafael Diaz, Shaun G. Goodman, Robert A. Harrington, J. Wouter Jukema, Michael Szarek, Harvey D. White, Andreas M. Zeiher, Pierluigi Tricoci, Matthew T. Roe, Kenneth W. Mahaffey, Jay M. Edelberg, Corinne Hanotin, Guillaume Lecorps, Angèle Moryusef, Robert Pordy, William J. Sasiela, Jean-François Tamby, Rafael Diaz, Philip E Aylward, Heinz Drexel, Peter Sinnaeve, Mirza Dilic, Renato D. Lopes, Nina N Gotcheva, Shaun G. Goodman, Juan-Carlos Prieto, Huo Yong, Patricio López-Jaramillo, Ivan Pećin, Zeljko Reiner, Petr Ostadal, Steen Hvitfeldt Poulsen, Margus Viigimaa, Markku S Nieminen, Nicolas Danchin, Vakhtang Chumburidze, Nikolaus Marx, Evangelos Liberopoulos, Pablo Carlos, Montenegro Valdovinos, Hung-Fat Tse, Robert Gabor Kiss, Denis Xavier, Doron Zahger, Marco Valgimigli, Takeshi Kimura, Hyo Soo Kim, Sang-Hyun Kim, Andrejs Erglis, Aleksandras Laucevicius, Sasko Kedev, Khalid Yusoff, Gabriel Arturo Ramos López, Marco Alings, Harvey D. White, Sigrun Halvorsen, Roger M Correa Flores, Rody G. Sy, Andrzej Budaj, Joao Morais, Maria Dorobantu, Yuri Karpov, Arsen D. Ristic, Terrance Chua, Jan Murin, Zlatko Fras, Anthony J Dalby, José Tuñón, H. Asita de Silva, Emil Hagström, Ulf Landmesser, Christian Müller, Chern-En Chiang, Piyamitr Sritara, Sema Guneri, Alexander Parkhomenko, Kausik K. Ray, Patrick M. Moriarty, Matthew T. Roe, Robert Vogel, Bernard Chaitman, Sheryl F. Kelsey, Anders G. Olsson, Jean-Lucien Rouleau, Maarten L. Simoons, Karen Alexander, Chiara Meloni, Robert Rosenson, Eric J.G. Sijbrands, Pierluigi Tricoci, John H. Alexander, Luciana Armaganijan, Akshay Bagai, Maria Cecilia Bahit, J. Matthew Brennan, Shaun Clifton, Adam D. DeVore, Shalonda Deloatch, Sheila Dickey, Keith Dombrowski, Grégory Ducrocq, Zubin Eapen, Patricia Endsley, Arleen Eppinger, Robert W. Harrison, Connie Ng Hess, Mark A. Hlatky, Joseph Dedrick Jordan, Joshua W. Knowles, Bradley J. Kolls, David F. Kong, Sergio Leonardi, Linda Lillis, Renato D. Lopes, David J. Maron, Kenneth W. Mahaffey, Jill Marcus, Robin Mathews, Rajendra H. Mehta, Robert J. Mentz, Humberto Graner Moreira, Chetan B. Patel, Sabrina Bernardez Pereira, Lynn Perkins, Thomas J. Povsic, Etienne Puymirat, Matthew T. Roe, William Schuyler Jones, Bimal R. Shah, Matthew W. Sherwood, Kenya Stringfellow, Darin Sujjavanich, Mustafa Toma, Charlene Trotter, Sean F.P. van Diepen, Matthew D. Wilson, Andrew Tze-Kay Yan, Lilia B Schiavi, Marcelo Garrido, Andrés F Alvarisqueta, Sonia A Sassone, Anselmo P Bordonava, Alberto E Alves De Lima, Jorge M Schmidberg, Ernesto A Duronto, Orlando C Caruso, Leonardo P Novaretto, Miguel Angel Hominal, Oscar R Montaña, Alberto Caccavo, Oscar A Gomez Vilamajo, Alberto J Lorenzatti, Luis R Cartasegna, Gustavo A Paterlini, Ignacio J Mackinnon, Guillermo D Caime, Marcos Amuchastegui, Oscar Salomone, Oscar R Codutti, Horacio O Jure, Julio OE Bono, Adrian D Hrabar, Julio A Vallejos, Rodolfo A Ahuad Guerrero, Federico Novoa, Cristian A Patocchi, Cesar J Zaidman, Maria E Giuliano, Ricardo D Dran, Marisa L Vico, Gabriela S Carnero, Pablo N Guzman, Juan C Medrano Allende, Daniela F Garcia Brasca, Miguel H Bustamante Labarta, Sebastian Nani, Eduardo DS Blumberg, Hugo R Colombo, Alberto Liberman, Victorino Fuentealba, Hector L Luciardi, Gabriel D Waisman, Mario A Berli, Ruben O Garcia Duran, Horacio G Cestari, Hugo A Luquez, Jorge A Giordano, Silvia S Saavedra, Gerardo Zapata, Osvaldo Costamagna, Susana Llois, Jonathon H Waites, Nicholas Collins, Allan Soward, Philip E Aylward, Chris LS Hii, Philip E Aylward, James Shaw, Margaret A Arstall, John Horowitz, Daniel Ninio, James F Rogers, David Colquhoun, Romulo E Oqueli Flores, Philip Roberts-Thomson, Owen Raffel, Sam J Lehman, Constantine Aroney, Steven GM Coverdale, Paul J Garrahy, Gregory Starmer, Mark Sader, Patrick A Carroll, Ronald Dick, Robert Zweiker, Uta Hoppe, Heinz Drexel, Kurt Huber, Rudolf Berger, Georg Delle-Karth, Bernhard Frey, Franz Weidinger, Dirk Faes, Kurt Hermans, Bruno Pirenne, Attilio Leone, Etienne Hoffer, Peter Sinnaeve, Mathias CM Vrolix, Luc De Wolf, Bart Wollaert, Marc Castadot, Karl Dujardin, Christophe Beauloye, Geert Vervoort, Harry Striekwold, Carl Convens, John Roosen, Emanuele Barbato, Marc Claeys, Frank Cools, Ibrahim Terzic, Fahir Barakovic, Zlatko Midzic, Belma Pojskic, Emir Fazlibegovic, Mirza Dilic, Azra Durak-Nalbantic, Mehmed Kulić, Dusko Vulic, Adis Muslibegovic, Boris Goronja, Gilmar Reis, Luciano Sousa, Jose C Nicolau, Flavio E Giorgeto, Ricardo P Silva, Lilia Nigro Maia, Rafael Rech, Paulo RF Rossi, Maria José AG Cerqueira, Norberto Duda, Renato Kalil, Adrian Kormann, José Antonio M Abrantes, Pedro Pimentel Filho, Ana Priscila Soggia, Mayler ON de Santos, Fernando Neuenschwander, Luiz C Bodanese, Yorghos L Michalaros, Freddy G Eliaschewitz, Maria H Vidotti, Paulo E Leaes, Roberto V Botelho, Sergio Kaiser, Euler Roberto F Fernandes Manenti, Dalton B Precoma, Jose C Moura Jorge, Pedro Silva, Jose A Silveira, Wladmir Saporito, Jose A Marin Neto, Gilson S Feitosa, Luiz Eduardo F Ritt, Juliana A de Souza, Fernando Costa, Weimar KSB Souza, Helder JL Reis, Renato D. Lopes, Leandro Machado, José Carlos Aidar Ayoub, Georgi V Todorov, Fedya P Nikolov, Elena S Velcheva, Maria L Tzekova, Haralambi O Benov, Stanislav L Petranov, Haralin S Tumbev, Nina S Shehova-Yankova, Dimitar T Markov, Dimitar H Raev, Mihail N Mollov, Kostadin N Kichukov, Katya A Ilieva- Pandeva, Nina N Gotcheva, Raya Ivanova, Maryana Gospodinov, Valentina M Mincheva, Petar V Lazov, Bojidar I Dimov, Manohara Senaratne, James Stone, Jan Kornder, Stephen Pearce, Danielle Dion, Daniel Savard, Yves Pesant, Amritanshu Pandey, Simon Robinson, Gilbert Gosselin, Saul Vizel, Gordon Hoag, Ronald Bourgeois, Anne Morisset, Eric Sabbah, Bruce Sussex, Simon Kouz, Paul MacDonald, Ariel Diaz, Nicolas Michaud, David Fell, Raymond Leung, Tycho Vuurmans, Christopher Lai, Frank Nigro, Richard Davies, Gustavo Nogareda, Ram Vijayaraghavan, John Ducas, Serge Lepage, Shamir Mehta, James Cha, Robert Dupuis, Peter Fong, Sohrab Lutchmedial, Josep Rodes-Cabau, Hussein Fadlallah, David Cleveland, Thao Huynh, Iqbal Bata, Adnan Hameed, Cristian Pincetti, Sergio Potthoff, Juan C Prieto, Monica Acevedo, Arnoldo Aguirre, Margarita Vejar, Mario Yañez, Guillermo Araneda, Mauricio Fernandez, Luis Perez, Paola Varleta, Fernando Florenzano, Laura Huidobro, Carlos A Raffo, Claudia Olivares, Leonardo Nahuelpan, Humberto Montecinos, Jiyan Chen, Yugang Dong, Weijian Huang, Jianzhong Wang, Shi’An Huang, Zhuhua Yao, Xiang Li, Lan Cui, Wenhua Lin, Yuemin Sun, Jingfeng Wang, Jianping Li, Xuelian Zhang, Hong Zhu, Dandan Chen, Lan Huang, Shaohong Dong, Guohai Su, Biao Xu, Xi Su, Xiaoshu Cheng, Jinxiu Lin, Wenxia Zong, Huanming Li, Yi Feng, Dingli Xu, Xinchun Yang, Yuannan Ke, Xuefeng Lin, Zheng Zhang, Zeqi Zheng, Zhurong Luo, Yundai Chen, Chunhua Ding, Yi Zhong, Yang Zheng, Xiaodong Li, Daoquan Peng, Shuiping Zhao, Ying Li, Xuebo Liu, Meng Wei, Shaowen Liu, Yihua Yu, Baiming Qu, Weihong Jiang, Yujie Zhou, Xingsheng Zhao, Zuyi Yuan, Ying Guo, Xiping Xu, Xubo Shi, Junbo Ge, Guosheng Fu, Feng Bai, Weiyi Fang, Xiling Shou, Xiangjun Yang, Jian’An Wang, Meixiang Xiang, Yingxian Sun, Qinghua Lu, Ruiyan Zhang, Jianhua Zhu, Yizhou Xu, Zhongcai Fan, Tianchang Li, Chun Wu, Nicolas Jaramillo, Gregorio Sanchez Vallejo, Diana C Luna Botia, Rodrigo Botero Lopez, Dora I Molina De Salazar, Alberto J Cadena Bonfanti, Carlos Cotes Aroca, Juan Diego Higuera, Marco Blanquicett, Sandra I Barrera Silva, Henry J Garcia Lozada, Julian A Coronel Arroyo, Jose L Accini Mendoza, Ricardo L Fernandez Ruiz, Alvaro M. Quintero Ossa, Fernando G Manzur Jatin, Aristides Sotomayor Herazo, Jeffrey Castellanos Parada, Rafael Suarez Arambula, Miguel A Urina Triana, Angela M Fernandez Trujillo, Maja Strozzi, Siniša Car, Davor Miličić, Martina Lovrić Benčić, Hrvoje Pintarić, Đeiti Prvulović, Jozica Šikić, Viktor Peršić, Dean Mileta, Kresimir Štambuk, Zdravko Babić, Vjekoslav Tomulic, Josip Lukenda, Stanka Mejic-Krstulovic, Boris Starcevic, Jindrich Spinar, David Horak, Zdenek Velicka, David Alan, Vilma Machova, Ales Linhart, Vojtech Novotny, Vladimir Kaucak, Richard Rokyta, Robert Naplava, Zdenek Coufal, Vera Adamkova, Ivo Podpera, Jiri Zizka, Zuzana Motovska, Ivana Marusincova, Petr Ostadal, Petr Heinc, Jiri Kuchar, Petr Povolny, Jiri Matuska, Steen H Poulsen, Bent Raungaard, Peter Clemmensen, Lia E Bang, Ole May, Morten Bøttcher, Jens D Hove, Lars Frost, Gunnar Gislason, John Larsen, Peter Betton Johansen, Flemming Hald, Peter Johansen, Jørgen Jeppesen, Tonny Nielsen, Kjeld S Kristensen, Piotr Maria Walichiewicz, Jens D Lomholdt, Ib C Klausen, Peter Kaiser Nielsen, Flemming Davidsen, Lars Videbaek, Margus Viigimaa, Mai Soots, Veiko Vahula, Anu Hedman, Üllar Soopõld, Kaja Märtsin, Tiina Jurgenson, Arved Kristjan, Markku S Nieminen, Saila Vikman, Heikki Huikuri, Juhani Airaksinen, Pierre Coste, Emile Ferrari, Nicolas Danchin, Olivier Morel, Gilles Montalescot, Jacques Machecourt, Gilles Barone-Rochette, Jacques Mansourati, Yves Cottin, Ph. Gabriel Steg, Florence Leclercq, Abdelkader Belhassane, Nicolas Delarche, Franck Boccara, Franck Paganelli, Jérôme Clerc, Francois Schiele, Victor Aboyans, Vincent Probst, Jacques Berland, Thierry Lefèvre, Vakhtang Chumburidze, Irakli Khintibidze, Tamaz Shaburishvili, Zurab Pagava, Ramaz Ghlonti, Zaza Lominadze, George Khabeishvili, Rayyan Hemetsberger, Kemala Edward, Ursula Rauch-Kröhnert, Matthias Stratmann, Karl-Friedrich Appel, Ekkehard Schmidt, Heyder Omran, Christoph Stellbrink, Thomas Dorsel, Emmanouil Lianopoulos, Hans Friedrich Vöhringer, Roger Marx, Andreas Zirlik, Detlev Schellenberg, Thomas Heitzer, Ulrich Laufs, Christian Werner, Nikolaus Marx, Stephan Gielen, Sebastian Nuding, Bernhard Winkelmann, Steffen Behrens, Karsten Sydow, Mahir Karakas, Gregor Simonis, Thomas Muenzel, Nikos Werner, Stefan Leggewie, Dirk Böcker, Rüdiger Braun- Dullaeus, Nicole Toursarkissian, Michael Jeserich, Matthias Weißbrodt, Tim Schaeufele, Joachim Weil, Heinz Völler, Johannes Waltenberger, Mohammed Natour, Susanne Schmitt, Dirk Müller-Wieland, Stephan Steiner, Lothar Heidenreich, Elmar Offers, Uwe Gremmler, Holger Killat, Werner Rieker, Sotiris Patsilinakos, Athanasios Kartalis, Athanassios Manolis, Dimitrios Sionis, Geargios Chachalis, Evangelos Liberopoulos, Ioannis Skoumas, Vasilios Athyros, Panagiotis Vardas, Frangkiskos Parthenakis, Georgios Hahalis, John Lekakis, Apostolos Hatzitolios, Sergio R Fausto Ovando, Pablo Carlos Montenegro Valdovinos, Juan L Arango Benecke, Edgar R Rodriguez De Leon, Bryan PY Yan, David CW Siu, Tibor Turi, Bela Merkely, Robert Gabor Kiss, Imre Ungi, Geza Lupkovics, Lajos Nagy, András Katona, István Édes, Gábor Müller, Iván Horvath, Tibor Kapin, Zsolt Szigeti, József Faluközy, Mukund Kumbla, Manjinder Sandhu, Sharath Annam, Naveen Reddy Proddutur, Reddy Regella, Rajendra K Premchand, Ajaykumar Mahajan, Sudhir Pawar, Atul D Abhyanakar, Prafulla Kerkar, Ravishankar A Govinda, Abraham Oomman, Dhurjati Sinha, Sachin N Patil, Dhiman Kahali, Jitendra Sawhney, Abhijeet B Joshi, Sanjeev Chaudhary, Pankaj Harkut, Santanu Guha, Sanjay Porwal, Srimannarayana Jujjuru, Ramesh B Pothineni, Minguel R Monteiro, Aziz Khan, Shamanna S Iyengar, Jasprakash Singh Grewal, Manoj Chopda, Mahesh C Fulwani, Dr. Aparna Patange, Patil Sachin, Vijay K Chopra, Naresh K Goyal, Rituparna Shinde, Gajendra V Manakshe, Nitin Patki, Sumeet Sethi, Vengatesh Munusamy, Sunil Karnaand Sunil Thanvi, Srilakshmi Adhyapak, Chandrakant Patil, Ulhas Pandurangi, Rishabh Mathur, Jugal Gupta, Suhas Kalashetti, Ajit Bhagwat, Bagirath Raghuraman, Shiv Kumar Yerra, Prasant Bhansali, Rohidas Borse, Patil Rahul, Srihari Das, Vinay Kumar, Jabir Abdullakutty, Shireesh Saathe, Priya Palimkar, Jabir Abdullkutty, Shireesh Sathe, Shaul Atar, Michael Shechter, Morris Mosseri, Yaron Arbel, Chorin Ehud, Havakuk Ofer, Chaim Lotan, Uri Rosenschein, Amos Katz, Yaakov Henkin, Adi Francis, Marc Klutstein, Eugenia Nikolsky, Robert Zukermann, Yoav Turgeman, Majdi Halabi, Alon Marmor, Ran Kornowski, Michael Jonas, Offer Amir, Yonathan Hasin, Yoseph Rozenman, Shmuel Fuchs, Vered Zvi, Osamah Hussein, Dov Gavish, Zvi Vered, Yoseph Caraco, Mazen Elias, Naveh Tov, Efrat Wolfovitz, Michael Lishner, Nizar Elias, Giancarlo Piovaccari, Annamaria De Pellegrin, Raffaella Garbelotto, Gabriele Guardigli, Valgimigli Marco, Giovanni Licciardello, Carla Auguadro, Filippo Scalise, Claudio Cuccia, Alessandro Salvioni, Giuseppe Musumeci, Michelle Senni, Paolo Calabrò, Salvatore Novo, Pompilio Faggiano, Marco Metra, Nicoletta B De Cesare, Sergio Berti, Enrico Puccioni, Marcello Galvani, Maurizio Tespili, Piermarco Piatti, Michela Palvarini, Giuseppe De Luca, Roberto Violini, Alessandro De Leo, Zoran Olivari, Pasquale Perrone Filardi, Maurizio Ferratini, Vittorio Racca, Kazuoki Dai, Yuji Shimatani, Haruo Kamiya, Kenji Ando, Yoshihiro Takeda, Yoshihiro Morino, Yoshiki Hata, Kazuo Kimura, Koichi Kishi, Ichiro Michishita, Hiroki Uehara, Toshinori Higashikata, Atsushi Hirayama, Keiji Hirooka, Yasuji Doi, Satoru Sakagami, Shuichi Taguchi, Akihiro Koike, Hiroyuki Fujinaga, Shinji Koba, Ken Kozuma, Tomohiro Kawasaki, Yujiro Ono, Masatoshi Shimizu, Yousuke Katsuda, Atsuyuki Wada, Toshiro Shinke, Takeshi Kimura, Junya Ako, Kenshi Fujii, Toshiyuki Takahashi, Koichi Nakao, Yutaka Furukawa, Hiroshi Sugino, Ritsu Tamura, Toshiaki Mano, Masaaki Uematsu, Noriaki Utsu, Kashima Ito, Takuya Haraguchi, Katsuhiko Sato, Yasunori Ueda, Akira Nishibe, Kazuteru Fujimoto, Motomaru Masutani, Akira Nishibe, Kazuteru Fujimoto, Jung Han Yoon, Sang-Hyun Kim, Hack-Lyoung Kim, Hun Sik Park, In-Ho Chae, Moo Hyun Kim, Myung Ho Jeong, Seungwoon Rha, Chongjin Kim, Hyo-Soo Kim, Hae Young Kim, Taekjong Hong, Seung-Jea Tahk, Youngkwon Kim, Arija Busmane, Natalija Pontaga, Aldis Strelnieks, Iveta Mintale, Iveta Sime, Zaneta Petrulioniene, Roma Kavaliauskiene, Ruta Jurgaitiene, Gintare Sakalyte, Rimvydas Slapikas, Sigute Norkiene, Nerijus Misonis, Aleksandras Kibarskis, Raimondas Kubilius, Stojko Bojovski, Sasko Kedev, Nensi Lozance, Aleksandar Kjovkaroski, Snezana Doncovska, Tiong Kiam Ong, Sazzli Kasim, Oteh Maskon, Balachandran Kandasamy, Khalid Yusoff, Houng B Liew, Wan Mohd Izani Wan Mohamed, Armando García Castillo, Gabriel Arturo Ramos López, Jorge Carrillo Calvillo, Pedro Fajardo Campos, Juan Carlos Núñez Fragoso, Edmundo Alfredo Bayram Llamas, Marco Antonio Alcocer Gamba, Jaime Carranza Madrigal, Luis Gerardo González Salas, Enrique López Rosas, Belinda González Díaz, Eduardo Salcido Vázquez, Alfredo Nacoud Ackar, Guillermo Antonio Llamas Esperón, Carlos Rodolfo Martínez Sánchez, María Guerrero De Leon, Rodrigo Suarez Otero, Guillermo Fanghänel Salmón, Jesús Antonio Pérez Ríos, José Angel Garza Ruíz, Marco Alings, Robert W Breedveld, Margriet Feenema-Aardema, Alida Borger-Van Der Burg, Pieter AM Hoogslag, Harry Suryapranata, Antonius Oomen, Paulus Van Haelst, Margriet Feenema-Aradema, Jacobijne J Wiersma, Dirk Basart, Ruud MA Van Der Wal, Peter Zwart, Pascalle Monraats, Henricus Van Kesteren, Ioannis Karalis, Johan Jukema, Gerardus JE Verdel, Bart RG Brueren, Roland PTh Troquay, Eric P Viergever, Nadea YY Al-Windy, Gerard L Bartels, Jan H Cornel, Walter RM Hermans, Johannes PR Herrman, Robert J Bos, Reginald GEJ Groutars, Coenraad C Van Der Zwaan, Refik Kaplan, Eelko Ronner, Bjorn E Groenemeijer, Patrick NA Bronzwaer, Anho AH Liem, Bernard JWM Rensing, Marcel JJA Bokern, Remco Nijmeijer, Ferry MRJ Hersbach, Frank F Willems, Antonius TM Gosselink, Saman Rasoul, John Elliott, Gerard Wilkins, Raewyn Fisher, Douglas Scott, Hamish Hart, Ralph Stewart, Scott Harding, Ian Ternouth, Nicholas Fisher, Samuel Wilson, Denise Aitken, Russell Anscombe, Laura Davidson, Tadeusz Tomala, Ottar Nygård, Jon Arne Sparby, Kjell Andersen, Lars Gullestad, Jarle Jortveit, Peter S Munk, Erlend gyllensten Singsaas, Sigrun Halvorsen, Ulf Hurtig, Roger M Correa Flores, Jorge R Calderon Ticona, Julio R Durand Velasquez, Sandra A Negron Miguel, Enrique S Sanabria Perez, Jesus M Carrion Chambilla, Carlos A Chavez Ayala, Reynaldo P Castillo Leon, Rolando J Vargas GonzalesC, Jose D Hernandez Zuniga, Luis A Camacho Cosavalente, Jorge E Bravo Mannucci, Javier Heredia Landeo, Nassip C Llerena Navarro, Yudy M Roldan Concha, Víctor E Rodriguez Chavez, Henry A Anchante Hernandez, Carlos A Zea Nunez, Walter Mogrovejo Ramos, Arthur Ferrolino, Rosa Allyn G Sy, Louie Tirador, Rody G. Sy, Generoso Matiga, Raul Martin Coching, Alisa Bernan, Gregorio Rogelio, Dante D. Morales, Edgar Tan, Dennis Jose Sulit, Adrian Wlodarczak, Grzegorz Skonieczny, Lidia Pawlowicz, Pawel Wojewoda, Benita Busz-Papiez, Janusz Bednarski, Aleksander Goch, Pawel Staneta, Elzbieta Dulak, Andrzej Budaj, Krzysztof Saminski, Wlodzimierz Krasowski, Wanda Sudnik, Aleksander Zurakowski, Marcin Skorski, Roman Lysek, Beata Miklaszewicz, Jan Andrzej Lipko, Edyta Kostarska-Srokosz, Marek Piepiorka, Anna Drzewiecka, Arkadiusz Stasiewski, Tomasz Blicharski, Leszek Bystryk, Michal Szpajer, Marek Korol, Tomasz Czerski, Jacek Gniot, Andrzej Lubinski, Jerzy Gorny, Edward Franek, Grzegorz Raczak, Hanna Szwed, Pedro Monteiro, Jose Mesquita Bastos, Helder H Pereira, Joao Morais, Filipe Seixo, Carlos Mendonça, Ana Botelho, Francisca Caetano, Bogdan Minescu, Octavian Istratoaie, Dan N Tesloianu, Maria Dorobantu, Gabriel Cristian, Silviu Dumitrescu, Cristian GC Podoleanu, Mircea CA Constantinescu, Cristina M Bengus, Constantin Militaru, Doina Rosu, Irinel R Parepa, Adrian V Matei, Tom M Alexandru, Mihaela Malis, Ioan Coman, Yury Shvarts, Olga Orlikova, Zhanna Kobalava, Olga L Barbarash, Valentin Markov, Nadezhda Lyamina, Alexander Gordienko, Konstantin Zrazhevsky, Alexander Y Vishnevsky, Victor Gurevich, Raisa Stryuk, Nikita V Lomakin, Igor Bokarev, Tatiana Khlevchuk, Sergey Shalaev, Larisa Khaisheva, Petr Chizhov, Inna Viktorova, Natalya Osokina, Vladimir Shchekotov, Evgenia Akatova, Galina Chumakova, Igor Libov, Mikhail I Voevoda, Tatyana V Tretyakova, Evgeny Baranov, Sergey Shustov, Sergey Yakushin, Ivan Gordeev, Niiaz Khasanov, Olga Reshetko, Tatiana Sotnikova, Olga Molchanova, Konstantin Nikolaev, Liudmila Gapon, Elena Baranova, Elena Kosmachova, Yuriy Karpov, Yuri Karpov, Anton Povzun, Liudmila Egorova, Vadim V Tyrenko, Igor G Ivanov, Masterov Ilya, Sergey Kanorsky, Dragan Simic, Nikola Ivanovic, Goran Davidovic, Nebojsa Tasic, Milika R. Asanin, Stevo Stojic, Svetlana R. Apostolovic, Stevan Ilic, Biljana Putnikovic Tosic, Aleksandar Stankovic, Aleksandra Arandjelovic, Slavica Radovanovic, Branislava Todic, Arsen D. Ristic, Jovan Balinovac, Dragan V. Dincic, Petar Seferovic, Ana Karadzic, Slobodan Dodic, Sinisa Dimkovic, Tamara Jakimov, Kian-Keong Poh, Hean Yee Ong, Justin Tang I-Shing, Karol Micko, Jan Nociar, Daniel Pella, Peter Fulop, Marian Hranai, Juraj Palka, Juraj Mazur, Ivan Majercák, Andrej Dzupina, František Fazekas, Jozef Gonsorcik, Viliam Bugan, Jan Murin, Juraj Selecky, Gabriel Kamensky, Jaroslava Strbova, Rudolf Smik, Andrej Dukat, Peter Olexa, Ivan Žuran, Janez Poklukar, Nataša Černič Šuligoj, Matija Cevc, Zlatko Fras, Henry P Cyster, Naresh Ranjith, Clive Corbett, Junaid Bayat, Ellen Makoali Makotoko, Hendrik du Toit Theron, Ilse E Kapp, Matthys M de V Basson, Hanlie Lottering, Dina Van Aswegen, Louis J Van Zyl, Peter J Sebastian, Thayabran Pillay, Jan A Saaiman, Patrick J Commerford, Soraya Cassimjee, Garda Riaz, Iftikhar O Ebrahim, Mahomed Sarvan, Joseph H Mynhardt, Anthony J Dalby, Helmuth Reuter, Rajendran Moodley, Manuel Vida, Angel R. Cequier Fillat, Vicente Bodí Peris, Francisco Fuentes Jimenez, Francisco Marín, Jose M Cruz Fernández, Rafael Jesus Hidalgo Urbano, Blas Gil-Extremera, Pablo Toledo, Fernando Worner Diz, David Garcia-Dorado, Andres Iñiguez, Jose R Gonzalez-Juanatey, Javier Fernandez Portales, Fernando Civeira Murillo, Laia Matas Pericas, Jose Luis Zamorano, Manuel De Mora Martin, Jordi Bruguera Cortada, Joaquin J Alonso Martin, Jose Maria Serrano Antolin, José R De Berrazueta Fernández, José Antonio Vázquez de Prada, Jose Francisco Díaz Fernández, José Alberto García Lledó, Juan Cosín Sales, Javier Botas Rodriguez, Gabriel Gusi Tragant, Amparo Benedicto, Carlos Gonzalez-Juanatey, Mercedes Camprubí Potau, Ignacio Plaza Perez, César Morís De La Tassa, Pablo Loma-Osorio Rincon, Javier Balaguer Recena, Juan M Escudier, Antonio Coca Payeras, Norberto Alonso Orcajo, Godwin Constantine, Ruvaiz Haniffa, Nirmali Tissera, Stanley Amarasekera, Chandrike Ponnamperuma, Nimali Fernando, Kaputella Fernando, Jayanthimala Jayawardena, Santharaj Wijeyasingam, Gotabhaya Ranasinghe, Ruvan Ekanayaka, Sepalika Mendis, Vajira Senaratne, Gnanamoorthy Mayurathan, Ajantha Rajapaksha, Thilak Sirisena, Jagath I Herath, Naomali Amarasena, Stefan Berglund, Gundars Rasmanis, Emil Hagström, Ola Vedin, Nils Witt, Georgios Mourtzinis, Peter Nicol, Ole Hansen, Stefano Romeo, Steen Agergaard Jensen, Ingemar Torstensson, Ulf Ahremark, Torbjörn Sundelin, Tiziano Moccetti, Christian Müller, Francois Mach, Ronald Binde, Ulf Landmesser, Oliver Gämperli, Chern-En Chiang, Wei- Chuan Tsai, Kwo-Chang Ueng, Wen-Ter Lai, Ming-En Liu, Juey- Jen Hwang, Wei-Hsian Yin, I-Chang Hsieh, Ming-Jer Hsieh, Wei Hsiang Lin, Jen-Yuan Kuo, Tsuei-Yuan Huang, Chih-Yuan Fang, Pinij Kaewsuwanna, Wasant Soonfuang, Woravut Jintapakorn, Apichard Sukonthasarn, Piyamitr Sritara, Nattawut Wongpraparut, Krisada Sastravaha, Nakarin Sansanayudh, Wirash Kehasukcharoen, Dilok Piyayotai, Paiboon Chotnoparatpat, Ahmet Camsari, Hakan Kultursay, Sema Guneri, Bulent Mutlu, Murat Ersanli, Mustafa Demirtas, Cevat Kirma, Ertan Ural, Lale Koldas, Oleksandr Karpenko, Alexander Prokhorov, Ihor Vakaluyk, Halyna Myshanych, Dmytro Reshotko, Valeriy Batushkin, Leonid Rudenko, Ihor Kovalskyi, Mykola Kushnir, Vira Tseluyko, Yuriy Mostovoy, Mykola Stanislavchuk, Yulian Kyiak, Yuriy Karpenko, Yaroslav Malynovsky, Andriy Klantsa, Oles Kutniy, Ekaterina Amosova, Viktor Tashchuk, Oleh Leshchuk, Alexander Parkhomenko, Mykola Rishko, Mykola Kopytsya, Andriy Yagensky, Mykola Vatutin, Andriy Bagriy, Olga M Barna, Olexiy Ushakov, Georgiy Dzyak, Borys Goloborodko, Anatolii Rudenko, Volodymyr Zheleznyy, Jasper Trevelyan, Azfar Zaman, Kaeng Lee, Andrew Moriarty, Rajesh K Aggarwal, Piers Clifford, Yuk- Ki Wong, Syed MR Iqbal, Eduardas Subkovas, Denise Braganza, David Sarkar, Robert Storey, Huw Griffiths, Sam Mcclure, Rangasamy Muthusamy, Simon Smith, John Kurian, Terry Levy, Craig Barr, Honer Kadr, Robert Gerber, Audrius Simaitis, Handrean Soran, Adrian Brodison, Mohammad Ayaz, Muhammad Cheema, Richard Oliver, Simon Thackray, Telal Mudawi, Gohar Rahma, Ayyaz Sultan, Timothy Reynolds, David Sharman, david Spriging, Rob Butler, Peter Wilkinson, Gregory YH Lip, Nicholas Ossei-Gerning, Gil Vardi, Duccio Baldari, David Brabham, Charles Treasure II, Charles Dahl, Bruce Palmer, Alan Wiseman, Abul Khan, Sanjeev Puri, Ann Elizabeth Mohart, Carlos Ince, Enrique Flores, Scott Wright, Shi-Chi Cheng, Michael Rosenberg, William Rogers, Edward Kosinski, Les Forgosh, Jonathan Waltman, Mohammad Shoukfeh, Georges Dagher, Patrick Cambier, Ira Lieber, Priya Kumar, Cara East, Perry Krichmar, Mian Hasan, Lindsey White, Thomas Knickelbine, Thomas Haldis, Eve Gillespie, Thomas Amidon, David Suh, Imran Arif, mouhamad Abdallah, Faiq Akhter, Eric Carlson, Michael D’Urso, Fadi El- Ahdab, William Nelson, Katie Moriarty, Barry Harris, Steven Cohen, Luther Carter, Daniel Doty, Kenneth Sabatino, Tariq Haddad, Sunder Rao, Angel Mulkay, Ion Jovin, Kim Klancke, Vinay Malhotra, Sai K Devarapalli, Michael Koren, Harish Chandna, George Dodds III, Tauqir Goraya, James Bengston, Matthew Janik, Joseph Moran, Andrew Sumner, John Kobayashi, William Davis, Shahram Yazdani, John Pasquini, Maitreya Thakkar, Amarnath Vedere, Wayne Leimbach, James Rider, Sarah fenton, Narendra Singh, Anil V Shah, Patrick M. Moriarty, Denise Janosik, Carl Pepine, Brett Berman, Joseph Gelormini, Christopher Daniels, Kerensky Richard, Friederike Keating, Nicholas I Kondo, Sanjay Shetty, Howard Levite, Winfried Waider, Theodore Takata, Mazen Abu-Fadel, Vipul Shah, Rahul Aggarwal, Anil Kumar, Brack Hattler, Rose Do, Chad Link, Anna Bortnick, George Kinzfogl III, Arnold Ghitis, John Larry, Edward Teufel, Peter Kuhlman, Brent Mclaurin, Wenwu Zhang, Stephen Thew, Jalal Abbas, Matthew White, Othman Islam, Sumeet Subherwal, Nandkishore Ranadive, Babak Vakili, Christian Gring, David Henderson, Timothy Schuchard, Naim Farhat, Geoffrey Kline, Sharan Mahal, Jack Whitaker, Shawn Speirs, Rolf Andersen, Nizar Daboul, Phillip Horwitz, Firas Zahr, George Ponce, Zubair Jafar, Joseph Mcgarvey, Vipul Panchal, Stephen Voyce, Thomas Blok, William Sheldon, Masoud M Azizad, Carsten Schmalfuss, Mark Picone, Robert Pederson, William Herzog, Keith Friedman, Jason Lindsey, Eichenlaub Timothy, Parilak Leonard, Norman Lepor, Mahfouz El Shahawy, Howard Weintraub, Anand Irimpen, Alvaro Alonso, Wade May, Daniels Christopher, Thomas Galski, Alan Chu, Freny Mody, Ebrahimi Ramin, Zachary Hodes, Joseph Rossi, Gregory Rose, James Fairlamb, Charles Lambert, Ajit Raisinghani, Antonio Abbate, George Vetrovec, Marilyn King, Charles Carey, Jaime Gerber, Liwa Younis, Hyeun (Tom) Park, Mladen Vidovich, Thomas Knutson, Dennis Friedman, Fred Chaleff, Arthur Loussararian, Phillip Rozeman, Carey Kimmelstiel, Jeffrey Kuvin, Kevin Silver, Malcolm Foster, Glen Tonnessen, Andrey Espinoza, Mohamadali Amlani, Andreas Wali, Christopher Malozzi, Geert T Jong, Clara Massey, Keattiyoat Wattanakit, Philip J. O’Donnell, Dinesh Singal, Naseem Jaffrani, Sridhar Banuru, Daniel Fisher, Mark Xenakis, Neal Perlmutter, Ravi Bhagwat, James Strader, Ronald Blonder, Ayim Akyea-Djamson, Ajay Labroo, Kwan Lee, H. John Marais, Edmund Claxton, Robert Weiss, Rohr Kathryn, Martin Berk, Peter Rossi, Parag Joshi, Amit Khera, Ajit S Khaira, Greg Kumkumian, Steven Lupovitch, Joshua Purow, Stephen Welka, David Hoffman, Stuart Fischer, Eugene Soroka, Donald Eagerton, Samir Pancholy, Michael Ray, Norman Erenrich, Michael Farrar, Stewart Pollock, William J French, Steve Diamantis, Douglas Guy, Lawrence Gimple, Mark Neustel, Steven Schwartz, Edward Pereira, Seals Albert, Douglas Spriggs, Janet Strain, Suneet Mittal, Anthony Vo, Majed Chane, Jason Hall, Nampalli Vijay, Kapildeo Lotun, F. Martin Lester, Ahed Nahhas, Theodore Pope, Paul Nager, Rakesh Vohra, Mukesh Sharma, Riyaz Bashir, Hinan Ahmed, Michael Berlowitz, Robert Fishberg, Robert Barrucco, Eric Yang, Michael Radin, Daniel Sporn, Dwight Stapleton, Steven Eisenberg, Joel Landzberg, Martin Mcgough, Samir Turk, Michael Schwartz, P. Sandy Sundram, Diwakar Jain, Mark Zainea, Carlos Bayron, Ronald Karlsberg, Suhail Dohad, Henry Lui, William Keen, Donald Westerhausen, Sandeep Khurana, Himanshu Agarwal, Jessica Birchem, William Penny, Mark Chang, Sherrill Murphy, John Henry, Branislav Schifferdecker, John M Gilbert, Gopal Chalavarya, Charles Eaton, John F Schmedtje, Stuart Christenson, Imran Dotani, Douglas Denham, Alexander Macdonell, Paul Gibson, Aref Rahman, Tammam Al Joundi, Nizar Assi, Gary Conrad, Purushotham Kotha, Michael Love, Gregory Giesler, Howard Rubenstein, Dawood Gamil, Laura Akright, Justine Krawczyk, Joanne Cobler, Terry Wells, James Welker, Robert Foster, Richard Gilmore, Jay Anderson, Douglas Jacoby, Bill Harris, Geraldine Gardner, Ramprasad Dandillaya, Kishor Vora, John Kostis, John Hunter, David Laxson, Eric Ball, Jay Anderson, Terry Wells, Kishor Vora, Eric Ball, James Welker, Renato D. Lopes, Flavia Egydio, Anelise Kawakami, Janaina Oliveira, Shaun G. Goodman, Julianna Wozniak, Alexander Matthews, Caroline Ratky, Janine Valiris, Lisa Berdan, Anita Hepditch, Kirby Quintero, Matthew T. Roe, Tyrus Rorick, Melissa Westbrook, Nicolas Danchin, Madeleine Bezault, Elodie Drouet, Tabassome Simon, Harvey D. White, Caroline Alsweiler, Peter Sinnaeve, Anne Luyten, Philip E Aylward, Julie Butters, Liddy Griffith, Michelle Shaw, Emil Hagström, Lena Grunberg, Michael Szarek, Shahidul Islam, Marie-France Brégeault, Nathalie Bougon, Douglas Faustino, Sylvie Fontecave, Judith Murphy, Jean- Francois Tamby, Melanie Verrier, Veronique Agnetti, Dorthe Andersen, Emmy Badreddine, Mhamed Bekkouche, Cecile Bouancheau, Imane Brigui, Maddy Brocklehurst, Joseph Cianciarulo, Dawn Devaul, Szilvia Domokos, Cecile Gache, Caroline Gobillot, Severine Guillou, Jan Healy, Megan Heath, Gayatri Jaiwal, Carine Javierre, Julien Labeirie, Myriam Monier, Ulises Morales, Asmaa Mrabti, Bicky Mthombeni, Betim Okan, Lucile Smith, Jennifer Sheller, Sebastien Sopena, Valerie Pellan, Fadela Benbernou, Nafissa Bengrait, Maud Lamoureux, Katarina Kralova, Michel Scemama, Raphael Bejuit, Anthony Coulange, Christelle Berthou, Jérôme Repincay, Christelle Lorenzato, Alexis Etienne, Valerie Gouet, Guillaume Lecorps, Virginie Loizeau, Mickael Normand, Anne Ourliac, Christelle Rondel, Antony Adamo, Pascale Beltran, Pauline Barraud, Helene Dubois-Gache, Benjamin Halle, Lamia Metwally, Maxime Mourgues, Marc Sotty, Marion Vincendet, Raluca Cotruta, Zhu Chengyue, Dominique Fournie-Lloret, Christine Morrello, Aurelie Perthuis, Patrick Picault, Isabelle Zobouyan, Helen M. Colhoun, Michael A. Dempsey, and Mark A. McClanahan
References
- 1.Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4:1287–1295. doi: 10.1001/jamacardio.2019.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borén J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. 2016;27:473–483. doi: 10.1097/MOL.0000000000000330 [DOI] [PubMed] [Google Scholar]
- 4.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247 [DOI] [PubMed] [Google Scholar]
- 5.Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol. 2021;77:1439–1450. doi: 10.1016/j.jacc.2021.01.027 [DOI] [PubMed] [Google Scholar]
- 6.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2 [DOI] [PubMed] [Google Scholar]
- 7.Taskinen MR, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–495. doi: 10.1016/j.atherosclerosis.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711 [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 13.Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, Deedwania P, Olsson AG, Boekholdt SM, Demicco DA, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002–3009. doi: 10.1161/CIRCULATIONAHA.107.713438 [DOI] [PubMed] [Google Scholar]
- 14.Sniderman AD. Differential response of cholesterol and particle measures of atherogenic lipoproteins to LDL-lowering therapy: implications for clinical practice. J Clin Lipidol. 2008;2:36–42. doi: 10.1016/j.jacl.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 15.Ray KK, Cannon CP, Cairns R, Morrow DA, Ridker PM, Braunwald E. Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: results from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2009;29:424–430. doi: 10.1161/ATVBAHA.108.181735 [DOI] [PubMed] [Google Scholar]
- 16.Meeusen JW, Donato LJ, Jaffe AS. Lipid biomarkers for risk assessment in acute coronary syndromes. Curr Cardiol Rep. 2017;19:48. doi: 10.1007/s11886-017-0863-9 [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–2275. doi: 10.1016/j.jacc.2015.03.544 [DOI] [PubMed] [Google Scholar]
- 19.Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. doi: 10.1016/j.jacc.2019.10.057 [DOI] [PubMed] [Google Scholar]
- 20.O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, Im K, Lira Pineda A, Wasserman SM, Ceska R, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. doi: 10.1016/j.ahj.2014.07.028 [DOI] [PubMed] [Google Scholar]
- 22.Martin SS, Giugliano RP, Murphy SA, Wasserman SM, Stein EA, Ceska R, Lopez-Miranda J, Georgiev B, Lorenzatti AJ, Tikkanen MJ, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749–753. doi: 10.1001/jamacardio.2018.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz GG, Gabriel Steg P, Bhatt DL, Bittner VA, Diaz R, Goodman SG, Jukema JW, Kim YU, Li QH, Manvelian G, et al. Clinical efficacy and safety of alirocumab after acute coronary syndrome according to achieved level of low-density lipoprotein cholesterol: a propensity score-matched analysis of the ODYSSEY OUTCOMES trial. Circulation. 2021;143:1109–1122. doi: 10.1161/CIRCULATIONAHA.120.049447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehejia R, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Economics Statistics. 2002;84:151–161. [Google Scholar]
- 25.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 27.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease, 1: evidence from genetic, epidemiologic, and clinical studies: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sniderman A, Williams K, Cobbaert C. ApoB versus non-HDL-C: what to do when they disagree. Curr Atheroscler Rep. 2009;11:358–363. doi: 10.1007/s11883-009-0054-2 [DOI] [PubMed] [Google Scholar]
- 29.Clark D, 3rd, Nicholls SJ, St John J, Elshazly MB, Kapadia SR, Tuzcu EM, Nissen SE, Puri R. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur Heart J. 2018;39:2551–2558. 10.1093/eurheartj/ehy209 [DOI] [PubMed] [Google Scholar]
- 30.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantey EP, Wilkins JT. Discordance between lipoprotein particle number and cholesterol content: an update. Curr Opin Endocrinol Diabetes Obes. 2018;25:130–136. doi: 10.1097/MED.0000000000000389 [DOI] [PubMed] [Google Scholar]
- 32.Heidemann BE, Koopal C, Bots ML, Asselbergs FW, Westerink J, Visseren FLJ. The relation between VLDL-cholesterol and risk of cardiovascular events in patients with manifest cardiovascular disease. Int J Cardiol. 2021;322:251–257. doi: 10.1016/j.ijcard.2020.08.030 [DOI] [PubMed] [Google Scholar]
- 33.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307 [DOI] [PubMed] [Google Scholar]
- 34.Fruchart JC, Davignon J, Hermans MP, Al-Rubeaan K, Amarenco P, Assmann G, Barter P, Betteridge J, Bruckert E, Cuevas A, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol. 2014;13:26. doi: 10.1186/1475-2840-13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson PWF, Jacobson TA, Martin SS, Jackson EJ, Le NA, Davidson MH, Vesper HW, Frikke-Schmidt R, Ballantyne CM, Remaley AT. Lipid measurements in the management of cardiovascular diseases: practical recommendations: a scientific statement from the National Lipid Association Writing Group. J Clin Lipidol. 2021;15:629–648. doi: 10.1016/j.jacl.2021.09.046 [DOI] [PubMed] [Google Scholar]
- 36.Marston NA, Giugliano RP, Melloni GEM, Park JG, Morrill V, Blazing MA, Ference B, Stein E, Stroes ES, Braunwald E, et al. Association of apolipoprotein B-containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol. 2022;7:250–256. doi: 10.1001/jamacardio.2021.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67:193–201. doi: 10.1016/j.jacc.2015.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Nomura SO, Steffen BT, Guan W, Remaley AT, Karger AB, Ouyang P, Michos ED, Tsai MY. Apolipoprotein B discordance with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in relation to coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Lipidol. 2020;14:109–121.e5. doi: 10.1016/j.jacl.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langlois MR, Sniderman AD. Non-HDL cholesterol or apoB: which to prefer as a target for the prevention of atherosclerotic cardiovascular disease? Curr Cardiol Rep. 2020;22:67. doi: 10.1007/s11886-020-01323-z [DOI] [PubMed] [Google Scholar]
- 40.Thanassoulis G, Williams K, Ye K, Brook R, Couture P, Lawler PR, de Graaf J, Furberg CD, Sniderman A. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: a meta-analysis of randomized trials. J Am Heart Assoc. 2014;3:e000759. doi: 10.1161/JAHA.113.000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.