Abstract
Background:
Generalized lymphatic anomalies (GLA) are complex vessel malformations that can impair lymphatic function. Potential GLA complications include lipid-rich lymph in the thoracic space or peritoneal cavity, respectively chylothorax and chylous ascites. To reduce the potential for chyle accumulation, GLA patients limit dietary fats. We hypothesized that dietary fatty acid composition impacts the potential for lymphatic dysfunction and chyle accumulation in GLA.
Methods and Results:
Adipose-specific overexpression of lymphatic growth factors has demonstrated lethal chylothorax in mice. Here, we utilized mice with inducible adipocyte overexpression of vascular endothelial growth factor-D (VD mice) to mimic lymphatic proliferation in GLA and assessed the incidence of chyle accumulation on a mixed high fat diet (HFD), high saturated fat diet (HSFD), or high unsaturated fat diet (HUSFD). Lipid transport was assessed by uptake rates of bolus oral triglyceride load and mesenteric fat analysis. Lymphatic expansion and inflammation were determined by whole mount immunofluorescence and gene expression. Body composition was assessed by MRI. HSFD 2-month wildtype groups resulted in an increase in TNF-α, IL-6, and IL-10 expression compared with chow-fed controls. The chyle accumulation incidence was highest in HFD-fed mice compared with either HSFD or HUSFD. Strikingly, increased mortality was observed irrespective of which high fat diet was consumed after administration of a bolus lipid load.
Conclusion:
Chronic HFD increases risk of chyle accumulation, however increased mortality was driven particularly by a bolus lipid load in VD mice. These findings suggest that although chronic HFD increases chyle accumulation risk, a single large meal feeding may increase risk of lethal chylothorax instances for GLA patients.
Keywords: lymphatic disease, chylous ascites, chylothorax, dietary fatty acids
Introduction
Generalized lymphatic anomalies (GLAs) in patients arise from lymphatic endothelial cell hyperplasia in the lungs, pleura, bones, or soft tissues and can result in impaired lymphatic function.1–3 Healthy lymphatic vessels transport interstitial fluid and macromolecules from organs and peripheral tissues and serve as the route of dietary fatty acid uptake from the intestine. Abnormal, hyperplastic lymphatic vessels in GLAs can cause an accumulation of interstitial fluid in the peripheral tissue or downstream lymph stasis and leakage. Retention of excess interstitial fluid in the periphery defines the condition of lymphedema, whereas transport defects in central conducting lymphatics can lead to serious complications for patients, including life-threatening chylothorax.4 Current treatments for GLA utilize surgery or target lymphatic endothelial cell proliferation, though classical management of GLAs often involves limiting dietary fat intake.5–8
In the small intestine, long chain fatty acids are formed into chylomicrons within enterocytes for transport out of the intestine. Due to the size of these lipoprotein particles, the primary means of chylomicron uptake and transport is through the lymphatic vasculature.9 Enriched in chylomicrons after a meal, milky intestinal lymph (chyle) is transported through the mesenteric lymphatics, central conducting vessels, and into the thoracic duct. With dysfunctional lymphatics, chyle leakage and accumulation in the peritoneal cavity is called chylous ascites. Accumulation of chyle in the pleural space is known as chylothorax. Unstable, hyperplasic vessels in GLA patients increase the risk of developing these conditions and often dietary management—adherence to a very low-fat diet—is used to limit chyle production.10 The interplay of lymphatic proliferation and dietary fats results in a synergistically detrimental effect in GLA patients. However, the specific fatty acids or underlying mechanisms driving this are not well understood due, in part, to lack of a viable preclinical model.
Several preclinical studies have identified that high fat feeding has the potential to reduce mesenteric lymphatic vessel function and cause chyle accumulation whereas others using acute methods have found a more limited impact.11–15 Constitutive deletions in Prox1 or Connexin43, for example, result in poorly formed, leaky lymphatic vessels that lead to chylous ascites and chylothorax, respectively, in mice.16–18 Another model utilized inducible overexpression of the lymphatic growth factor vascular endothelial growth factor (VEGF)-C in adipocytes to induce lymphangiogenesis. Interestingly, these mice quickly (<7 days) developed lethal chylothorax with the volume of chyle produced correlated to the degree of induced lymphatic hyperplasia.16 The effects of long-term high fat diet (HFD) on chylous events was not possible, however, due to the rapid onset of lethal chylothorax even on a chow (low fat) diet.16 We previously developed a similar model with inducible overexpression of VEGF-D in adipocytes (VD mice) that demonstrated both lymphatic proliferation and tolerance upto 4 months of chronic HFD for use in metabolic studies.19–21 A few mice were lost to chyle accumulation in these studies, however, making the VD mouse a potential platform to study the interaction of lymphatic hyperplasia and dietary fats.
Here, we utilized the VD mouse as a model of GLA-like lymphatic hyperplasia to test whether any interplay existed between dietary fatty acid composition and an increased risk of chylothorax. Chow diet fed VD mice were compared with those on different HFDs specifically enriched in mixed, unsaturated, or saturated fatty acids to identify the dietary effects on lymphatic integrity and chyle accumulation during lymphatic hyperplasia. Chronic high fat feeding increased the risk, but a single, high fat bolus meal was most causative of chylous appearance in this GLA model.
Materials and Methods
Animals
Tetracycline-response element promoter VEGF-D mice were crossed with Adipoq-rtTA mice to generate mice with inducible, adipose tissue-specific VEGF-D (VD mice) as previously described.19,21 Mice lacking one of the transgenes served as wildtype (WT) littermate controls. VEGF-D overexpression was induced in VD mice by providing doxycycline in the various diets throughout the experimental period. All mice received the diet to control for doxycycline effects. Experiments were performed in female mice with diets administered starting at 8–10 weeks of age with termination between 16 and 26 weeks of age depending on the applied dietary protocol. Animals were housed with ad libitum access to food and water. The timing of chylothorax or chylous ascites was recorded at necropsy or termination. All experiments were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Diet
Mice were assigned to groups and provided custom formulated diets containing 600 mg/kg of doxycycline of chow (n = 10) standard high fat chow (n = 15), high unsaturated fat (n = 7), and high saturated fat (n = 10). Standard HFD (D16042108; Bio-Serv, Flemington, NJ) consisted of 60% kcal of fat derived from 100% lard-oil (HFD). Custom high saturated fat diet (HSFD; D16042102) consisted of 60% kcal of fat derived from palm oil, and high unsaturated fat diet (HUSFD; D16042106) contained 60% kcal of fat derived from 66% safflower oil. Detailed fatty acid composition for the diets use can be found in Table 1. Mice were maintained on the assigned diet for up to 16 weeks; the majority of animals in this study used 8 weeks of special diet feeding. Food consumption for each diet was measured over a 1-week period in one cage per diet.
Table 1.
Fatty Acid Composition of the Diets Used in This Study
| Fatty acid | Chow | Standard fat (HFD) | Unsaturated fat (HUSFD) | Saturated fat (HSFD) |
|---|---|---|---|---|
| Caprylic (C8) | n.d. | 0 | 0 | 1.48 |
| Capric (C10) | n.d. | 0.37 | 0 | 1.48 |
| Lauric (C12) | n.d. | 0.74 | 0.37 | 16.29 |
| Myristic (C14) | n.d. | 10.37 | 3.33 | 14.81 |
| C15 | n.d. | 0.74 | 0.37 | 0.37 |
| Palmitic (C16) | 50 | 184.81 | 103.33 | 394.44 |
| Palmitoleic n-9 (C16:1) | 5 | 12.59 | 4.07 | 1.48 |
| C17 | n.d. | 3.33 | 1.11 | 0.74 |
| Stearic (C18) | 10 | 99.63 | 47.41 | 42.96 |
| Oleic, n-9 (C18:1) | 70 | 319.63 | 188.15 | 342.59 |
| Linoleic (C18:2) | 200 | 269.26 | 609.26 | 130.00 |
| Linolenic (C18:3) | n.d. | 21.11 | 11.48 | 21.11 |
| Arachidic (C20) | n.d. | 1.85 | 0.74 | 3.33 |
| C20:1 | n.d. | 5.93 | 1.85 | 1.85 |
| C20:2 | n.d. | 7.41 | 2.22 | 0 |
| C20:3 n-6 | n.d. | 1.11 | 0.37 | 0 |
| Behenic (C22) | n.d. | 0.37 | 0.37 | |
| Docosapentaenoic, n-3 (C22:5) | n.d. | 0.74 | 0.37 | 0 |
| Arachidonic (C20:4) | n.d. | 2.59 | 0.74 | 0 |
| Total (g) | 335.00 | 940.74 | 975.56 | 973.33 |
| Summary | ||||
| Total saturated (%) | 6 | 32.2 | 16.1 | 49.0 |
| Total monounsaturated (%) | 7.0 | 35.9 | 19.9 | 35.5 |
| Total polyunsaturated (%) | 21.0 | 31.9 | 64.0 | 15.5 |
Fatty acid compositions of the diets utilized in this study as provided by the manufacturers. Values are presented as g/kg diet. “n.d.” indicates that the value was not determined by the provider.
HFD, high fat diet; HSFD, high saturated fat diet; HUSFD, high unsaturated fat diet.
Body composition analysis
Body composition (percentage of lean, fat, and fluid mass) was measured bi-weekly for long-term studies and daily over the 5-day period before planned euthanasia by using an EchoMRI 100H (EchoMRI LLC, Houston, TX).
Bolus oral triglyceride uptake and clearance
After a 5-hour morning fast, blood glucose levels were measured by a portable glucometer before an oral gavage of 20% Intralipid (Fresenius Kabi, India) in a volume equal to 1.5% body mass. Blood was collected from the tail tip over a 240-minute period. Plasma triglycerides were measured through an Infinity reagent assay (ThermoFisher, Waltham, MA). Triglyceride uptake rate was calculated by the concentration over time to the peak plasma level per mouse and total uptake by the area under the curve over time calculated by the trapezoid method for each mouse.
Tissue isolation preparation
Mice were euthanized by exsanguination under heavy isoflurane. A loop of small intestine was gently spread and imaged on a sheet of graph paper for image analysis. The mesenteric lymph nodes were isolated from mesenteric adipose. Tissues were either immediately flash frozen in liquid nitrogen for subsequent RNA extraction or fixed overnight in 10% buffered zinc formalin for whole-mount immunofluorescence.
Mesenteric adipose streak quantification
Mesenteric vessel-associated adipose streak width was measured independently by two researchers and normalized to the graph paper scale using ImageJ software version 1.57u (NIH, Bethesda, MD). Each mesenteric spoke was measured for length and width while avoiding measurements of tissue defects. Measures were averaged per animal and then per group.
Whole-mount immunofluorescence
After fixation, tissues were transferred to PBS with 0.3% Tween for an additional 24 hours. Tissues were incubated with an antibody against mouse podoplanin (1:400; goat polyclonal No. AF3244, R&D Systems) in PBS for 48 hours and then rinsed for several hours. Tissues were then incubated with a donkey anti-goat Alexa488 antibody (1:400; donkey anti-goat No. A11055; Invitrogen) for 48 hours, rinsed, and imaged on an Olympus BX51 microscope by using an Olympus DP72 CCD camera (Olympus, Center Valley, PA).
RNA extraction and quantitative PCR
The RNA was isolated from the mesenteric lymph nodes and mesenteric adipose tissue by using the Zymo Direct-zol RNA Miniprep Plus kit in accordance with the manufacturer's instructions (Zymo Research, Irvine, CA). For adipose samples, the lipid layer was separated from the initial homogenate before the addition of chloroform. Reverse transcription of 1 μg total RNA was performed with the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA). Quantitative PCR was performed in 5 μL reactions with BioRad iTaq SYBR Green Supermix (Bio-Rad Laboratories, Inc.) in 384-well plates on an Applied Biosystems QuantStudio 6 quantitative PCR machine, with the threshold cycles automatically determined. Relative expression values are represented as 2−ΔΔCt compared with WT strains fed a chow diet. Primer sequences utilized for quantitative PCR analysis are provided in Table 2.
Table 2.
Quantitative PCR Primers Sequences Utilized in this Study
| Primers | Forward | Reverse |
|---|---|---|
| Ubc | 5′-GCCCAGTGTTACCACCAAGAAG-3′ | 5′-GCTCTTTTTAGATACTGTGGTGAGGAA-3′ |
| Cd11c | 5′-CTGGATAGCCTTTCTTCTGCTG-3′ | 5′-GCACACTGTGTCCGAACTC-3′ |
| CD206 | 5′-CAGGTGTGGGCTCAGGTAGT-3′ | 5′-TGTGGTGAGCTGAAAGGTGA-3′ |
| F4/80 | 5′-CTTTGGCTATGGGCTTCCAGTC-3′ | 5′-GCAAGGAGGACAGAGTTTATCGTG-3′ |
| IL-10 | 5′-GCTCTTACTGACTGGCATGAG-3′ | 5′-CGCAGCTCTAGGAGCATGTG-3′ |
| IL-6 | 5′-ACTCACCTCTTCAGAACGAATTG-3′ | 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ |
| TNF-α | 5′-GAGAAAGTCAACCTCCTCTCTG-3′ | 5′-GAAGACTCCTCCCAGGTATATG-3′ |
Statistics
Statistical analysis was performed by using GraphPad Prism Software (Version 8.0.1; GraphPad Prism, La Jolla, CA). Two-way ANOVA with post hoc Tukey comparisons was used to compare WT mice with VD mice littermates across their respective diets to determine genotype and diet effects. Differences in triglyceride clearance AUC and slopes between grouped WT and VD mice were determined with an unpaired t-test with Welch's correction. Data are presented as mean ± SD (graphs of TG over time use SE only for visual clarity) and reported when significant, p < 0.05.
Results
VD mice exhibit spontaneous chyle accumulation with high fat feeding
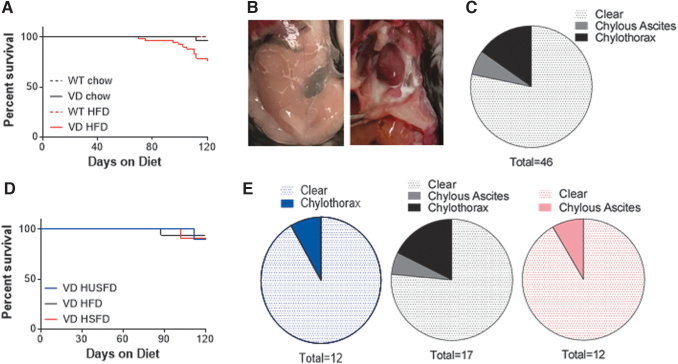
The VD mice demonstrate lymphatic hyperplasia and de novo lymphangiogenesis in adipose tissue with VEGF-D overexpression.19,21 As part of earlier diet-induced obesity metabolism studies, VD mice were provided commercial HFD (60% kcal fat) containing 600 mg/kg doxycycline (to induce VEGF-D) and ∼20% exhibited lethal chylothorax or chylous ascites at the time of termination (Fig. 1A, B). Chylothorax was more common (Fig. 1C). On a 600 mg/kg doxycycline control chow diet, only one VD mouse ever demonstrated chylothorax (Fig. 1A). No chyle accumulation was found in WT mice on either diet. The VD mice could, thus, serve as a potential model of lymphatic hyperplasia-induced chyle accumulation, with HFD causing greater incidence.
FIG. 1.
Survival and chylous incidents in VD mice. (A) Survival curves for WT and VD mice provided 600 mg/kg doxycycline in their chow diet or HFD. (B) Images of chylous ascites (left) and chylothorax (right) identified at time of sacrifice. (C) Incidence of chylous events identified through 4 months of HFD feeding in VD mice. (D) Survival curves of VD mice provided 600 mg/kg doxycycline in 60% kcal from fat HFD enriched for unsaturated fat (HUSFD), standard mixed formulation (HFD), and high saturated fat (HSFD). (E) Incidence of chylous events identified through 4 months of HFD feeding in VD mice provided HUSFD (blue, left), HFD (black, center), or HSFD (red, right). HFD, high fat diet; HSFD, high saturated fat diet; HUSFD, high unsaturated fat diet; VD, vascular endothelial growth factor-D; WT, wildtype.
To investigate whether the fatty acid composition of the diet affected the incidence of chylous events and overall survival rates in VD mice, we utilized custom diets with an altered fat source and provided HFD, HUSFD, or HSFD containing 600 mg/kg doxycycline (Table 1) for a 4-month period. Chylous events identified over 4-months of HFD, HUSFD, and HSFD feeding resulted in an overall decrease in survival, but only in 10% of mice overall (Fig. 1D). Instances of both chylous ascites and chylothorax were observed. In limited numbers, HUSFD-fed mice only developed chylothorax whereas HSFD-fed mice developed chylous ascites (Fig. 1E). With observed incidence rates on all the HFDs used far less than earlier, a key difference in the studies was identified: The historical group received a lipid gavage to study triglyceride clearance around the time that lethal chylous events occurred. We, thus, sought to couple these findings and test dietary fatty acids on the propensity for bolus lipid-driven chyle accumulation in VD mice.
Characterization of VD mice administered different dietary fatty acids over 2 months
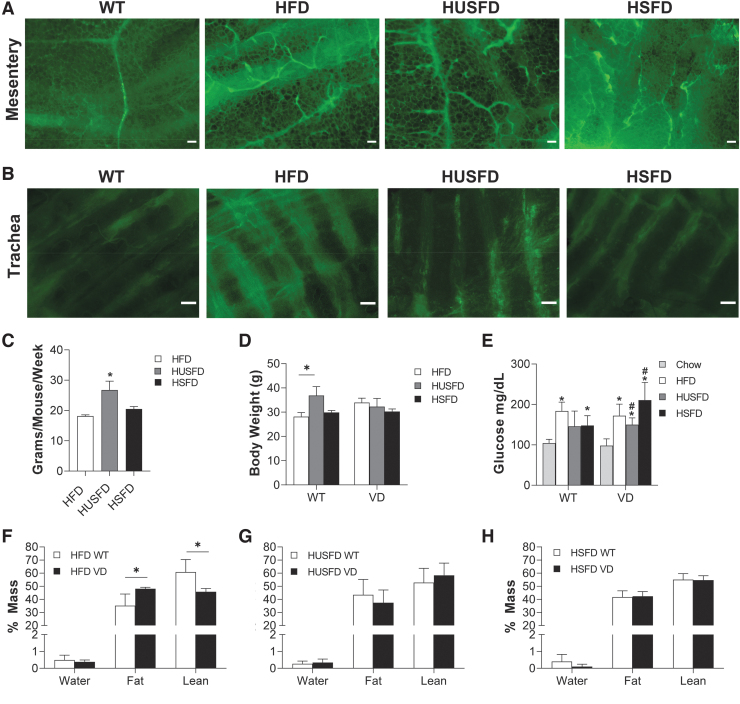
Our time-course studies identified that lethal chylous events first manifest at ∼2 months on diet, which, interestingly, coincides with the first significant lymphatic expansion measured in VD mice.19 Mice were provided custom HUSFD, HFD, and HSFD feeding as earlier (Table 1). On all of the diets used, VD mice demonstrated noted lymphatic hyperplasia in the mesenteric adipose tissue (Fig. 2A) and some in the trachea (Fig. 2B) similar to the VEGF-C model.16 Mice consumed significantly more HUSFD (Fig. 2C), resulting in slightly heavier mice in that diet group, but only among the WT cohort (Fig. 2D). Fasted glucose readings collected before fatty acid gavage were elevated in HFD, HUSFD, and HSFD mice compared with chow-fed controls, indicating early metabolic syndrome (Fig. 2E). Body composition analysis identified that VD mice exhibited significantly increased adiposity on standard HFD (Fig. 2F), but no differences were measured with HUSFD (Fig. 2G) or HSFD (Fig. 2H) across genotype. Thus, VD mice were phenotypically and metabolically similar to those characterized previously, that is, dietary fat source does not have a strong phenotypic impact.
FIG. 2.
Characterization of 2-month dietary fatty acid feeding in VD mice. (A) Immunofluorescence images of mesentery adipose in WT mice and in VD mice under unsaturated fat (HUSFD), standard mixed formulation (HFD), and high saturated fat (HSFD). Green = podoplanin. Scale bars = 200 μM. (B) Immunofluorescence images of lymphatics present in the trachea in WT mice and VD mice under different fat diets. Green = podoplanin. Scale bars = 200 μM. (C) Food consumption over a 1-week period (n = 6 WT, 5 VD). (D) Body weight in WT and VD diet conditions after 8 weeks on diet (n = 6 WT, 5 VD). (E) Five-hour fasted glucose readings recorded before 20% intralipid gavage (n = 4–10 WT, 2–9 VD). (F) End-point body mass percentages of mice at 2 months on chronic HFDs for HFD (n = 7 WT, 4 VD), (G) HUSFD (n = 5 WT, 5 VD), and (H) HSFD (n = 3 WT, 4 VD). *Indicates p < 0.05 compared with WT chow-fed mice; #Indicates p < 0.05 relative to standard HFD for the same genotype.
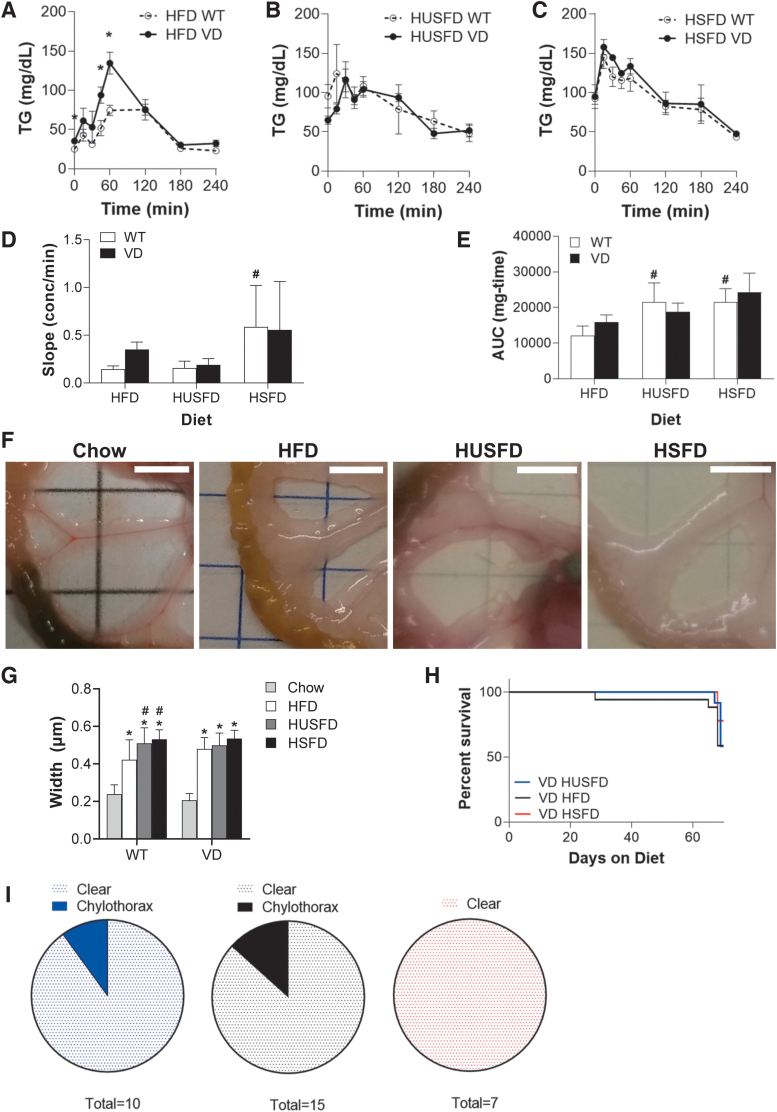
VD mice demonstrate normal lipid handling, but increased chylothorax on all HFDs
With mice phenotypically similar, a bolus lipid gavage was provided to measure lipid uptake and handling. Triglyceride uptake and clearance indicated a significant difference between WT and VD HFD mice at 45 and 60 minutes (Fig. 3A), whereas no significant differences were measured across HUSFD and HSFD groups (Fig. 3B, C). The appearance rate in serum, indicative of intestinal absorption and central lymphatic function, was significantly greater in HSFD than HFD in WT mice (Fig. 3D). No differences were measured, however, between WT and VD mice in any diet. Total area under the curves, indicative of the total mass taken up and cleared from circulation over time, indicated a significant increase in triglyceride clearance in WT HUFD and HSFD groups relative to WT HFD mice (Fig. 3E). Again, no differences were observed between WT and VD genotypes. These findings suggest that dietary fatty acid composition has subtle impacts on lipid handling regardless of VEGF-D overexpression and lymphatic expansion.
FIG. 3.
Lipid handling and instances of chylothorax in VD mice. (A) Triglyceride uptake and clearance curves of WT and VD Adipo-VD mice on (A) standard HFD (n = 8 WT, 6 VD), (B) HUSFD (n = 4 WT, 6 VD), and (C) HSFD (n = 4 WT, 2 VD). (D) Calculated appearance rate of oral triglyceride load in the plasma. (E) The calculated area under the curve for triglyceride uptake and clearance experiments. (F) Mesenteric vessel-associated adipose streaks representative images for standard chow, HFD (n = 8 WT, 7 VD), HUSFD (n = 5 WT, 5 VD), and HSFD (n = 4 WT, 2 VD). Scale bars = 5 mm. (G) Quantified average width of mesenteric vessel-associated adipose per each diet condition. (H) Survival curves of VD mice provided 600 mg/kg doxycycline in 60% kcal from fat HFD enriched for HUSFD, HFD, and HSFD. (I) Incidence of chylous events identified through 2 months of HFD feeding in VD mice provided HUSFD (blue, left), HFD (black, center), or HSFD (red, right). *Indicates p < 0.05 compared with WT; #Indicates p < 0.05 relative to standard HFD for the same genotype.
Previous studies have demonstrated increased mesenteric vessel-associated adipose streak size in models of lymphatic dysfunction, so we utilized this measure as a potential indicator of subtle, chronic chyle leakage due to vessel dysfunction.4,22 HFD, HUSFD, and HSFD mice all had markedly increased mesenteric width when compared with chow-fed mice (Fig. 3F, G). Despite similar overall adiposity (Fig. 2G, H), both HUSFD- and HSFD-fed WT mice had significantly increased mesenteric adipose accumulation when compared with WT HFD mice (Fig. 2F). As in the acute clearance studies, there were no statistically significant differences between WT and VD mice.
Before the lipid gavage test, only one VD mouse exhibited chylothorax, but from the time of gavage through the next 7 days, 20% of VD mice died of chylothorax (Fig. 3H). No chylous ascites was observed in these groups (Fig. 3I). Combined, the normal triglyceride uptake rate and lack of a mesenteric adipose phenotype suggests that chronic high dietary fatty acid exposure itself does not cause dangerous chyle accumulation, but, importantly, that a bolus fat load is a significant and powerful driver of dysfunction.
Mesenteric lymph node inflammatory markers demonstrate genotype nonspecific effects
To examine whether dietary fatty acids altered the inflammatory environment in WT and VD mice, we assessed mesenteric lymph node inflammatory markers. Analysis of lymph node inflammatory markers indicated that HSFD WT mice had increased relative expression of RNA for the cytokines TNF-α, IL-6, and IL-10 compared with WT chow-fed mice (Fig. 4A–C). The HSFD WT mice also demonstrated increased Tnfa as compared with the WT HFD-fed group. The HUSFD VD mice demonstrated significant expression of Il6 and Il10 (Fig. 4B, C). Both WT HFD and VD HFD-fed mice demonstrated increased gene expression of the macrophage marker F4/80 compared with WT chow conditions (Fig. 4D) and increased monocyte CD11c RNA expression in VD mice when compared with HUSFD and HSFD conditions (Fig. 4E). Taken together, neither the type of added dietary fat or mouse genotype had a significant impact on HFD-induced mesenteric inflammation.
FIG. 4.
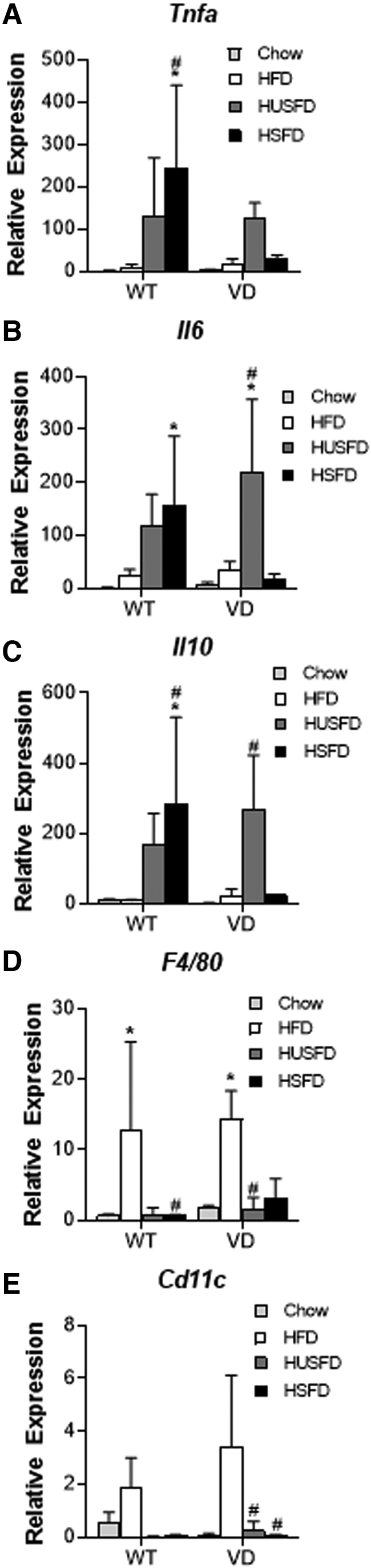
Inflammatory gene expression with high fat feeding. Relative RNA expression for the proteins (A) TNFα (Tnfa); (B) IL-6 (Il6); (C) IL-10 (Il10); (D) F4/80 (F4/80); and (E) CD11c (Cd11c) in mesenteric lymph nodes of WT and VD mice normalized to the expression in chow-fed WT. Mice were fed a standard HFD (n = 7 WT, 5 VD), HUSFD (n = 4 WT, 5 VD), and HSFD (n = 5 WT, 2 VD). *Indicates p < 0.05 compared with WT chow-fed mice; #Indicates p < 0.05 relative to standard HFD for the same genotype.
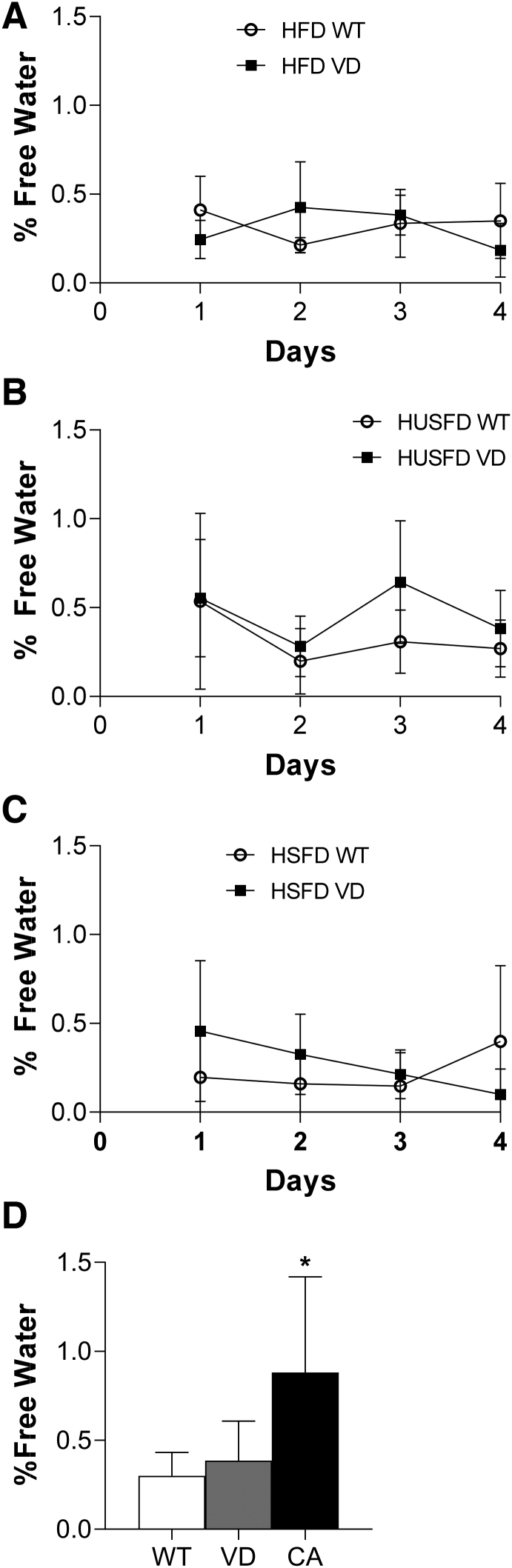
Free water accumulation increases in VD mice with chylous events
With chronic effects largely unchanged, yet a bolus lipid delivery causing chyle accumulation, we thus sought to test whether monitoring changes in free water accumulation could act as a clinical indicator ahead of health complications. Body composition was measured by MRI daily after a lipid gavage. No significance was found between percent free water in WT and VD groups on any diet across all mice measured (Fig. 5A–C). Interestingly, data from only the mice that presented with lethal chyle accumulation, as identified by postmortem necropsy, demonstrated a significant spike in free water in only their last MRI measured (Fig. 5D). The onset of chyle accumulation was, therefore, observed only after a lipid gavage, occurred very rapidly, and was significant enough in volume to be measured by body composition analysis.
FIG. 5.
Free water percentages in WT and VD mice. (A) Percent free water as measured by MRI each day for 4 days after 20% intralipid gavage in standard HFD (n = 6 WT, 5 VD), (B) HUSFD (n = 5 WT, 5 VD), and (C) HSFD-fed mice (n = 4 WT, 3 VD). (D) Group averages of percent free water in WT, VD, and mice with chyle accumulation (CA) identified post-mortem (n = 27 WT, 28 VD, 16 CA). *Indicates p < 0.05 compared with WT and VD mice.
Discussion
The inability of lymphatics to properly transport dietary lipids is a potentially dangerous clinical manifestation of GLA. The VD mice demonstrated lymphangiogenesis under chronic HFD conditions in the mesentery and trachea similar to the previously published findings in the Adipo-VC model.16 No major differences were noted in body composition, lipid handling, or inflammation across several HFD formulations or across genotype in these studies. The VD mice demonstrate chyle accumulation after chronic high fat feeding coupled with a bolus lipid gavage. Lymphatic dysfunction has been shown to increase with HFD conditions and is associated with detrimental GLA outcomes.11,23,24 These studies have confirmed a relationship between increased dietary fatty acids and the development of GLA complications and they demonstrate that VD mice have the potential to serve as a model of GLA physiology.
Chylothorax is not uncommon in mouse models that manipulate genes involved in proper lymphatic development. Many of these are embryonically lethal. Prox1 haplo-insufficient mice can survive to adulthood and exhibit poorly functioning lymphatics, chylothorax, and adipose expansion.4 Adult deletion of Rasa1 in lymphatic endothelial cells results in VEGFR-3 mediated lymphatic proliferation and chylothorax.25 PIK3CA mutations have been identified in human GLA and a mouse model, with inducible expression of a PIK3CA mutant, demonstrated chylothorax.26 Similar to the VD mouse used in this study, Nitschke et al. overexpressed VEGF-C from adipocytes and found rapid lymphatic proliferation and lethal chylothorax resulting from thoracic region lymphatic backflow and chyle leakage.16 In contrast, the VD model demonstrated long-term survival with chronic low-level VEGF-D induction, lymphatic expansion, and high fat feeding. A bolus fatty meal, thus, appears to be a potent inducer of subsequent chyle accumulation. Why there are differences in efficacy and incidence between overexpression of VEGF-C and VEGF-D are interesting speculation, but may include transgene copy number or the relative binding and activation kinetics with VEGFR-3 between the two ligands. VEGF-C also has the potential to activate VEGFR-2, whereas VEGF-D only activates murine VEGFR-3.27 As doxycycline dose-controllable models, either could titrate growth factor expression to optimize lymphatic hyperplasia for the study design.
Our findings indicate that VEGF-D-driven lymphangiogenesis or lymphatic hyperplasia alone was insufficient to cause significant chyle accumulation. Only when mice were fed a chronic HFD did any chylous incidents occur; this effect was greatly amplified on delivery of a high lipid bolus. Chronic HFD has been shown to reduce lymphatic function by decreasing phasic contractility and inducing fluid extravasation.11,28,29 A reduction in lymphatic transport has even been measured on a single acute meal.14 The mechanisms by which this occurs has been linked to direct lymphatic endothelial cell stress, changes in nitric oxide regulation, changes in shear forces, and an inflammation response.29 Lipid uptake rates from the gut were similar across mouse genotypes and diets in this study. VEGF-D induced lymphatic proliferation, coupled with the burdens of increased fatty acid transport, thus increasing the risk of spontaneous chyle leakage from lymphatics.
Due to the clinical prevalence of lymphatic diseases in female patients, we performed our controlled 2-month study on the effect of dietary fatty acids exclusively in female mice.30,31 Outcomes could, therefore, be different in males, but the preliminary data characterizing VD mice utilized males and demonstrated similar chylous manifestations and rates. Despite small group sizes, it is clear that central chylous events occur only in VD mice with lymphatic hyperplasia and not in WT mice. To identify the underlying mechanisms of inflammation-driven vessel instability, larger group sizes and more extensive study would be necessary. The 2-month timepoint was selected, because it was the first time at which any lethal chylothorax was noted in chronic metabolic studies. According to time course studies in VD mice, at 2 months lymphangiogenesis is only just becoming significant.19 Extending observations to a 3-month timepoint, and then providing a bolus lipid load, could increase the likelihood of chylous events under chronic HFD condition and permit the study of whether a specific dietary fatty acid composition or mechanism alters chylous events during marked lymphatic hyperplasia.
Clinical treatment options, which include surgical intervention and anti-proliferative pharmaceuticals, are effective yet remain unstandardized with patient specificity.32,33 The most commonly recommended management option for GLA patients is to limit their dietary fatty acid intake.8,10 It was hypothesized that the incidence of central chylous events would be dependent on dietary fatty acid composition in this study. HSFD, for example, is reportedly more inflammatory and eliminates the positive metabolic effects measured in VD mice.19,34 Two months on custom HFDs did not have a large effect on chyle accumulation events. It could be that these diets still contained a mix of fatty acids (i.e., they were not pure enough) or that the excess intake of any long chain fatty acids is detrimental to lymphatic integrity. Clinical studies have indicated that a medium chain triglyceride diet is effective in treating chylothorax.35 This is intriguing, as medium chain fats are a minimal component of chylomicrons and are absorbed into the blood directly instead of via lymphatic transport. The elimination of central chyle accumulation in VD mice, if such a diet was applied, would confirm this clinical recommendation. While a further study of how dietary components and nutritional management in GLA can improve patient outcomes is needed, our data suggest that large meal feeding most likely exacerbates lymphatic dysfunction.
Conclusion
Low fat diets are recommended for patients with central GLAs. The VD mice exhibit lymphatic hyperplasia and demonstrate spontaneous central chylous events reminiscent of GLA. Chronic HFD feeding increases the incidence of these events, whereas lethal chyle accumulation is predominantly driven by a bolus lipid load that simulates a large high fat meal. A better understanding of which fatty acid compositions and dietary profiles specifically increase the risk of chylothorax could improve GLA patients' diets, increase quality of life, and potentially identify a lymphatic signaling mechanism as a therapeutic target for GLA treatment.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
University of Pennsylvania Million Dollar Bike Ride, Lymphangiomatosis & Gorham's Disease Alliance, and the Texas A&M University College of Medicine. H.A.C. and J.M.R. are supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK119497 to J.M.R.).
References
- 1. Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly—Clinical, radiologic, and histologic differentiation. Skeletal Radiol 2013; 42:917–924. [DOI] [PubMed] [Google Scholar]
- 2. Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest 2014; 124:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luisi F, Torre O, Harari S. Thoracic involvement in generalised lymphatic anomaly (or lymphangiomatosis). Eur Respir Rev 2016; 25:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 2005; 37:1072–1081. [DOI] [PubMed] [Google Scholar]
- 5. Lai Y, Yu T, Qiao XY, Zhao LN, Chen QK. Primary intestinal lymphangiectasia diagnosed by double-balloon enteroscopy and treated by medium-chain triglycerides: A case report. J Med Case Rep 2013; 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozeki M, Nozawa A, Yasue S, et al. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet J Rare Dis 2019; 14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol 2011; 28:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marts BC, Naunheim KS, Fiore AC, Pennington DG. Conservative versus surgical management of chylothorax. Am J Surg 1992; 164:532–534; discussion 534–535. [DOI] [PubMed] [Google Scholar]
- 9. Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest 2014; 124:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen GL, Mascioli EA, Meyer LP, et al. Dietary modification of chyle composition in chylothorax. Gastroenterology 1989; 97:761–765. [DOI] [PubMed] [Google Scholar]
- 11. Blum KS, Karaman S, Proulx ST, et al. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 2014; 9:e94713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuan EL, Ivanov S, Bridenbaugh EA, et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 2015; 194:5200–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohan AB, Howles PN, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr Protoc Mouse Biol 2012; 2:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kassis T, Yarlagadda SC, Kohan AB, Tso P, Breedveld V, Dixon JB. Postprandial lymphatic pump function after a high-fat meal: A characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol 2016; 310:G776–G789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia Nores GD, Cuzzone DA, Albano NJ, et al. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 2016; 40:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nitschke M, Bell A, Karaman S, et al. Retrograde lymph flow leads to chylothorax in transgenic mice with lymphatic malformations. Am J Pathol 2017; 187:1984–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munger SJ, Davis MJ, Simon AM. Defective lymphatic valve development and chylothorax in mice with a lymphatic-specific deletion of Connexin43. Dev Biol 2017; 421:204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol 2011; 354:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chakraborty A, Barajas S, Lammoglia GM, et al. Vascular endothelial growth factor-D (VEGF-D) overexpression and lymphatic expansion in murine adipose tissue improves metabolism in obesity. Am J Pathol 2019; 189:924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakraborty A, Scogin CK, Rizwan K, Morley TS, Rutkowski JM. Characterizing lymphangiogenesis and concurrent inflammation in adipose tissue in response to VEGF-D. Front Physiol 2020; 11:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lammoglia GM, Van Zandt CE, Galvan DX, Orozco JL, Dellinger MT, Rutkowski JM. Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D. Am J Physiol Heart Circ Physiol 2016; 311:H384–H394. [DOI] [PubMed] [Google Scholar]
- 22. Gasheva OY, Tsoy Nizamutdinova I, Hargrove L, et al. Prolonged intake of desloratadine: Mesenteric lymphatic vessel dysfunction and development of obesity/metabolic syndrome. Am J Physiol Gastrointest Liver Physiol 2019; 316:G217–G227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu KF, Zhang P, Tian X, et al. The role of vascular endothelial growth factor-D in diagnosis of lymphangioleiomyomatosis (LAM). Respir Med 2013; 107:263–268. [DOI] [PubMed] [Google Scholar]
- 24. Smeltzer DM, Stickler GB, Fleming RE. Primary lymphatic dysplasia in children: Chylothorax, chylous ascites, and generalized lymphatic dysplasia. Eur J Pediatr 1986; 145:286–292. [DOI] [PubMed] [Google Scholar]
- 25. Lapinski PE, Kwon S, Lubeck BA, et al. RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Invest 2012; 122:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez-Laguna L, Agra N, Ibanez K, et al. Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly. J Exp Med 2019; 216:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baldwin ME, Catimel B, Nice EC, et al. The specificity of receptor binding by vascular endothelial growth factor-d is different in mouse and man. J Biol Chem 2001; 276:19166–19171. [DOI] [PubMed] [Google Scholar]
- 28. Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 2012; 302:H643–H653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norden PR, Kume T. The role of lymphatic vascular function in metabolic disorders. Front Physiol 2020; 11:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torre YS, Wadeea R, Rosas V, Herbst KL. Lipedema: Friend and foe. Horm Mol Biol Clin Investig 2018; 33. [Epub ahead of print]; DOI: 10.1515/hmbci-2017-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grada AA, Phillips TJ. Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol 2017; 77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 32. Goity LD, Itkin M, Nadolski G. An algorithmic approach to minimally invasive management of nontraumatic chylothorax. Semin Intervent Radiol 2020; 37:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iacobas I, Simon ML, Amir T, et al. Decreased vascularization of retroperitoneal kaposiform hemangioendothelioma induced by treatment with sirolimus explains relief of symptoms. Clin Imaging 2015; 39:529–532. [DOI] [PubMed] [Google Scholar]
- 34. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116:3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rocha G, Soares P, Azevedo I, et al. Congenital pulmonary lymphangiectasia and chylothorax—A case series. Lymphology 2017; 50:188–196. [PubMed] [Google Scholar]