Abstract
Background:
Despite documented benefits of diabetes technology in managing type 1 diabetes, inequities persist in the use of these devices. Provider bias may be a driver of inequities, but the evidence is limited. Therefore, we aimed to examine the role of race/ethnicity and insurance-mediated provider implicit bias in recommending diabetes technology.
Method:
We recruited 109 adult and pediatric diabetes providers across 7 U.S. endocrinology centers to complete an implicit bias assessment composed of a clinical vignette and ranking exercise. Providers were randomized to receive clinical vignettes with differing insurance and patient names as proxy for Racial–Ethnic identity. Bias was identified if providers: (1) recommended more technology for patients with an English name (Racial–Ethnic bias) or private insurance (insurance bias), or (2) Race/Ethnicity or insurance was ranked high (Racial–Ethnic and insurance bias, respectively) in recommending diabetes technology. Provider characteristics were analyzed using descriptive statistics and multivariate logistic regression.
Result:
Insurance-mediated implicit bias was common in our cohort (n = 66, 61%). Providers who were identified to have insurance-mediated bias had greater years in practice (5.3 ± 5.3 years vs. 9.3 ± 9 years, P = 0.006). Racial–Ethnic-mediated implicit bias was also observed in our study (n = 37, 34%). Compared with those without Racial–Ethnic bias, providers with Racial–Ethnic bias were more likely to state that they could recognize their own implicit bias (89% vs. 61%, P = 0.001).
Conclusion:
Provider implicit bias to recommend diabetes technology was observed based on insurance and Race/Ethnicity in our pediatric and adult diabetes provider cohort. These data raise the need to address provider implicit bias in diabetes care.
Keywords: Type 1 diabetes, Insulin pumps, Continuous glucose monitoring
Introduction
The introduction of diabetes devices, such as insulin pumps and continuous glucose monitoring (CGM) in the management of type 1 diabetes (T1D), has transformed care and outcomes of individuals with T1D.1–4 Multiple studies have demonstrated that diabetes devices improve glycemic control and long-term outcomes in pediatric and adult patients.5–9 In addition, these devices have improved quality of life, have high patient satisfaction, and are cost effective.10–12
Despite these benefits, there are significant inequities in diabetes technology use by race/ethnicity and socioeconomic status (SES).13 When compared with non-Hispanic White individuals, non-Hispanic Black and Hispanic individuals use diabetes technology less frequently.14,15 Individuals from high SES and non-Hispanic White groups were more likely to be started on insulin pumps within the first year of diagnosis when compared with those who were non-Hispanic Black, Hispanic, or of lower SES.16
The attitudes, assumptions, and behaviors of providers have been identified as some of many factors contributing to health disparities.17–19 Specifically, explicit bias and implicit bias have an impact on health outcomes. In explicit bias, an individual is aware of their prejudices or preferences toward a subgroup. However, in implicit bias, these prejudices or preferences are automatic and unconscious.20,21 These biases are likely to impact diagnosis and treatment decisions at all levels of care, including diabetes technology recommendations.20–22 Studies have demonstrated a disconnect between providers' perceived barriers to diabetes technology use and those experienced by persons with T1D.23 Additionally, perceived discrimination, cultural congruence, and limited English proficiency likely exacerbate this disconnect between providers and patients.24,25
To evaluate the role of insurance-mediated provider bias, Addala et al. developed the Diabetes Provider Implicit Bias (D-PIB) Tool in the GatekeeperStudy.22 This study was the first to demonstrate that insurance-mediated provider bias to prescribe technology recommendations based on patient insurance was common among pediatric providers and increased with more years in practice.22 To our knowledge, studies have neither evaluated racial–ethnic-mediated implicit bias among pediatric or adult diabetes providers, nor have they evaluated insurance-mediated implicit bias in adult diabetes providers.
We aim to investigate factors associated with an implicit bias to recommending diabetes devices based on race/ethnicity and insurance status in a representative cohort of U.S. pediatric and adult endocrinologists. We hypothesize that we will identify insurance-mediated and implicit racial–ethnic bias to recommend diabetes technologies in our cohort, irrespective of being a pediatric or adult provider.
Methods
This study is a multicenter equity-focused study, a design that ensures equal opportunity for patients who are historically excluded.26 The study was conducted among diabetes clinics in the T1D Exchange Quality Improvement Network (T1DX-QI),27 as part of an ongoing Health Equity project to identify and address contributors to health inequities among patients with T1D.26,28 T1DX-QI identified seven diabetes centers (five pediatric and two adult centers) from the learning network to participate in a virtual training module on health inequities and implicit bias. The providers participating in this survey were all from states with public insurance coverage for diabetes technology, however, the exact criteria to secure diabetes technology coverage likely varied by state. This assessment was conducted before any training or improvement activities.
We obtained written informed consent for all participating team members. The providers completed the assessment through an online survey system (www.qualtrics.com). The survey was completed in June to July 2021 and the analysis was completed in September 2021. This study was reviewed and approved by the Western Institutional Review Board.
Participants
There were 75 pediatrics and 34 adult providers, including endocrinologists, advanced practice practitioners (APP), and certified diabetes care and education specialists (CDCES) from seven participating centers (five pediatric and two adult centers) in New York, Ohio, Tennessee, Georgia, and Alabama. The providers' basic demographic information, including age, gender, role, duration of practice, and personal diagnosis of T1D, were collected.
D-PIB tool
The rationale, design, and validity of the D-PIB tool have been earlier described.22 In this study, we modified the D-PIB tool to include adult providers and to evaluate implicit racial–ethnic bias. The D-PIB includes two components: a hypothetical clinical vignette and a ranking exercise of patient factors that providers consider to be important in the recommendation of diabetes technology.
The modification of the clinical vignette component of the previously published D-PIB (Supplement A) for this study is to evaluate implicit racial–ethnic bias in addition to insurance-mediated bias. This expansion and modification of the tool was led by the A.A. who developed and validated the previously published D-PIB. Consistent with prior methodology,22 we applied an incomplete factorial survey design to develop the components included in the clinical vignette for this modified D-PIB. Incomplete factorial design is an established, unbiased survey methodology that allows for the inclusion of the likely subset of combination seen in clinical situations and decision-making trees. To balance the length of the clinical vignette with the inclusion of all key and necessary components in a clinician's decision to recommend technology, we utilized subject matter expert to determine which clinical components should be included in the vignette.
During the development of the D-PIB, we engaged a total of 12 content matter experts in the development of this tool—eight endocrinologists, three clinical psychologists, three persons with diabetes, three diabetes disparities experts, and four survey design experts (of note, content experts may have more than one qualification). We developed and refined the clinical vignette with the incomplete factorial survey design and the content matter experts. To address validity of the survey measure, we used the standard method of cognitive testing29,30 (also referred to as cognitive interviewing), whereby the content experts reviewed the survey items to ensure clarity, understanding, and validity of each survey item.
For the clinical vignette component of the D-PIB, the providers were randomized to receive questions that varied by insurance status and patient names, as a proxy to racial identity.28,30 To evaluate validity of this expansion to include race/ethnicity, we applied cognitive testing once again with our content experts to select the approach of utilizing names for the identification of racial–ethnic bias.31–33 For the expansion to adult providers, we solicited our colleagues in adult endocrinology to finalize the age of a 33 year old. Pediatric providers received a vignette of a 13-year-old female with T1D who is adequately engaged but not meeting standardized treatment targets for hemoglobin A1c (HbA1c), whereas adult providers evaluated a 33-year-old female.
Providers ranked patients' factors in the order of consideration for initiating technology therapy (1 = most important to 7 = least important). The patient factors ranked were race/ethnicity, the family's income, age, HbA1c, type of insurance coverage, self-monitoring blood glucose (SMBG), and patient/family preference. Providers were required to rank all seven patient factors in order of importance. In addition to ranking these factors, providers also listed factors they considered to be the most and the least important in their decision to recommend insulin pump therapy or CGM. After the modification of the D-PIB, and in a final step of cognitive testing to ensure the reliability of the tool, we solicited input from diabetes psychologists, and pediatric and adult endocrinologists to evaluate if the revised measure questions met the intended purpose.
To evaluate the role of explicit racial–ethnic bias, and other types of explicit bias or preexisting beliefs, providers were presented with the statement “I am able to recognize my own bias” with a five-point Likert-scale response ranging from strongly agree to strongly disagree. The rationale to include this question was to evaluate a provider's conscious awareness, or perception of their own bias is the definition of explicit bias.34 Studies suggest that awareness, knowledge, and education of one's bias may not always be protective against the presence of implicit bias.35 Thus, this question was included to understand the relationship between explicit and implicit bias in diabetes providers. Providers who agreed with the statement, “I am able to recognize my own bias,” a measure of self-perception of identifying bias, were categorized as able to recognize their own explicit bias.
Definitions of bias
Racial–ethnic-mediated implicit bias
For the clinical vignettes, the a priori definition of implicit racial–ethnic bias was met when patients in the vignettes with non-Hispanic name (Amy) were offered more technology than patients in the vignettes with common Hispanic names (Juanita) or common African American names (Keisha). For the ranking exercise, the a priori definition of implicit racial–ethnic bias was met when the patient's race/ethnicity was considered important (ranked in the top two factors) to recommend technology. If a provider demonstrated implicit racial–ethnic bias in one or both parts of the assessment, they were categorized as demonstrating bias.
Insurance-mediated implicit bias
For the clinical vignettes, the a priori definition of insurance-mediated provider bias was met when patients with private insurance were offered more technology than patients in the vignettes with public insurance. For the ranking exercise, the a priori definition of insurance-mediated provider bias was met when insurance was considered important (ranked in the top two factors) to recommend technology. If a provider demonstrated insurance-mediated bias in one or both parts of the assessment, they were categorized as demonstrating bias.
Explicit bias
Explicit bias is being aware of one's pre-existing beliefs about a specific group of people, and makes intentional decisions based on these beliefs. Asking direct, self-reported questions is the best way to measure explicit biases.34 We asked if participants could recognize their own bias to evaluate a provider's conscious awareness or perception of their explicit bias.
Data analyses
The primary outcome is the frequency of provider implicit bias and provider characteristics that were associated with provider implicit bias. We performed a priori analysis using G*Power to determine minimum number of providers required to achieve sufficient level of power needed to avoid Type II error. A priori power calculations demonstrated 90 providers were needed to achieve a power of 0.8 for an effect size of 0.6. Our study includes 109 providers in the analysis. We evaluated which patient factors were most important for recommending diabetes technology through the ranking exercise. Provider characteristics and bias were analyzed with descriptive statistics and group comparisons (t-tests for continuous variables and chi-square/Fisher's exact for categorical variables). Informed by the findings of the univariate analysis, we built a multivariate logistic regression with provider bias as the dependent variable and provider characteristics (age, gender, race/ethnicity, practice years, clinic type, ability to recognize own bias) as independent variables to determine contributors to provider bias. All statistical analyses were conducted using RStudio 1.3 (Supplement B).
Results
A total of 109 providers participated in the study. The majority were pediatric providers (n = 75, 69%), female (n = 89, 82%), non-Hispanic White (n = 75, 69%), physicians (n = 61, 56%), with a mean age of 40.7 ± 10.5 years and mean years in practice of 7.7 ± 7.9 years (Table 1). As expected, there was no statistical difference in baseline provider demographics when evaluated by the randomization arm, consistent with successful randomization.
Table 1.
Participant Demographics
| Total (n) | % | |
|---|---|---|
| 109 | ||
| Mean age, years (SD) | 40.7 (10.5) | |
| Provider gender (female) | 89 | 82 |
| Provider race/ethnicity | ||
| NH White | 75 | 69 |
| NH Black | 7 | 7 |
| Hispanic | 6 | 5 |
| Other | 21 | 19 |
| Provider role | ||
| Physician | 61 | 56 |
| Nurse practitioner | 13 | 12 |
| CDCES | 24 | 22 |
| Other | 11 | 10 |
| Provider clinic type | ||
| Adult | 34 | 31 |
| Pediatric | 75 | 69 |
| Personal diagnosis of T1D | 16 | 15 |
| Mean practice, years (SD) | 7.7 (7.9) | |
| Geographic setting | ||
| Suburban | 10 | 9 |
| Urban | 99 | 91 |
| Received implicit bias training in the past (yes) | 60 | 55 |
| Bias is prevalent in diabetes care (agree/strongly agree) | 94 | 86 |
| I am able to recognize my own bias (agree/strongly agree) | 77 | 71 |
CDCES, Certified Diabetes Care and Education Specialist; SD, standard deviation; T1D, type 1 diabetes.
Racial–ethnic-mediated implicit bias
Racial–ethnic-mediated implicit bias was present in approximately one-third of our cohort (n = 37, 34%). Group comparison of provider demographic with the presence of implicit racial–ethnic bias is presented in Table 2. The presence of racial–ethnic-mediated implicit bias did not differ by the mean age, provider role (physician vs. APP vs. CDCES), provider type (pediatric vs. adult), practice setting, or the number of years in practice. Providers who agreed with the statement “I am able to recognize my own bias,” measuring explicit bias, had more bias than those who did not (89% vs. 61%, P-value = 0.001). This finding remained significant with a univariate analysis (odds ratio [OR] 5.25 [1.83, 19.01], P = 0.004; Table 3). Awareness of one's explicit bias remained significant in the multivariate analyses (adjusted OR 4.66 [1.60, 17.09], P = 0.009; Table 4).
Table 2.
Provider Characteristics for Race/Ethnicity-Mediated and Insurance-Mediated Bias
| |
Race/ethnicity-mediated bias |
Insurance-mediated bias |
||||
|---|---|---|---|---|---|---|
| Bias | No-bias | P | Bias | No bias | P | |
| N (%) | 37 (34) | 72 (66) | 0.001 # | 66 (61) | 43 (39) | 0.002 # |
| Mean age, years (SD) | 40.7 (10.3) | 40.7 (10.7) | 0.9 | 42.2 (11) | 39.3 (9.3) | 0.05 # |
| Provider gender (female), n (%) | 29 (78) | 60 (83) | 0.6 | 56 (85) | 33 (77) | 0.3 |
| Provider race/ethnicity | 0.5 | 0.8 | ||||
| NH White, n (%) | 24 (65) | 51 (71) | 46 (70) | 29 (67) | ||
| NH Black, n (%) | 5 (14) | 2 (3) | 4 (6) | 3 (7) | ||
| Hispanic, n (%) | 3 (8) | 3 (4) | 5 (8) | 1 (2) | ||
| Other, n (%) | 5 (14) | 16 (22) | 11 (17) | 10 (23) | ||
| Provider role | 0.7 | 0.9 | ||||
| Physician (MD/DO), n (%) | 19 (15) | 42 (58) | 35 (53) | 26 (60) | ||
| Nurse practitioner, n (%) | 6 (16) | 7 (10) | 8 (12) | 5 (12) | ||
| CDCES, n (%) | 9 (24) | 15 (21) | 19 (29) | 5 (12) | ||
| Other, n (%) | 3 (8) | 8 (11) | 4 (6) | 7 (16) | ||
| Provider clinic type | 0.8 | 0.6 | ||||
| Pediatric, n (%) | 25 (68) | 50 (69) | 44 (67) | 31 (72) | ||
| Adult, n (%) | 12 (32) | 22 (31) | 22 (33) | 12 (28) | ||
| Personal diagnosis of T1D, n (%) | 3 (8) | 13 (18) | 0.2 | 8 (12) | 8 (19) | |
| Practice years, mean (SD) | 8 (8.6) | 7.5 (7.6) | 0.8 | 9.3 (9) | 5.3 (5.3) | 0.006 # |
| Geographic settings | 1 | 0.6 | ||||
| Suburban, n (%) | 3 (8) | 7 (10) | 7 (11) | 3 (7) | ||
| Urban, n (%) | 34 (92) | 65 (90) | 59 (89) | 40 (93) | ||
| Have received implicit bias training in the past (Yes), n (%) | 23 (62) | 37 (51) | 0.3 | 37 (56) | 23 (53) | 0.3 |
| Bias is prevalent in diabetes care (agree/strongly agree), n (%) | 32 (86) | 62 (86) | 1 | 56 (85) | 38 (88) | 0.7 |
| I can recognize my own bias (agree/strongly agree), n (%) | 33 (89) | 44 (61) | 0.001 # | 49 (74) | 28 (65) | 0.4 |
P-value <0.05.
Bold values indicate statistical significance.
Table 3.
Unadjusted Odds Ratio for Race/Ethnicity-Mediated and Insurance-Mediated Provider Bias
| Insurance bias | P | Race/ethnicity bias | P | |
|---|---|---|---|---|
| Age | 1.03 (0.99, 1.08) | 0.06 | 0.99 (0.96, 1.04) | 0.9 |
| Race/ethnicity (NH White) | 1.11 (0.48, 2.52) | 0.8 | 0.76 (0.32, 1.79) | 0.5 |
| Clinic type (adult) | 1.29 (0.56, 3.05) | 0.5 | 1.09 (0.45, 2.53) | 0.8 |
| Practice years | 1.08 (1.02, 1.16) | 0.02 # | 1.00 (0.95, 1.06) | 0.8 |
| Recognize own bias (agree/strongly agree) | 1.54 (0.66, 3.57) | 0.3 | 5.25 (1.83. 19.01) | 0.004 # |
P-value <0.05.
Bold values indicate statistical significance.
Table 4.
Adjusted Odds Ratio for Race/Ethnicity-Mediated and Insurance-Mediated Provider Bias
| Insurance bias | P | Race/ethnicity bias | P | |
|---|---|---|---|---|
| Recognize own bias adjusted for age | 1.49 (0.6, 3.57) | 0.07 | 4.91 (1.70, 17.83) | 0.006# |
P-value <0.05.
Insurance-mediated bias
Implicit bias against public insurance was common in our cohort (n = 66, 61%). Group comparisons of provider characteristics stratified by insurance-mediated bias are presented in Table 2. The provider's gender, race/ethnicity, personal diagnosis of T1D, roles, workplace characteristics, or awareness of one's own explicit bias did not differ by the presence of insurance-mediated bias. Providers with insurance-mediated bias were older (42.2 ± 11 years vs. 38.3 ± 9.3 years, P = 0.05). When compared with those who had a bias, providers who did not have bias had fewer practice years (5.3 ± 5.3 years vs. 9.3 ± 9 years, P = 0.006). In the univariate logistic regression model, years in practice remained significantly associated with insurance-mediated bias (OR 1.08 [1.02, 1.16], P = 0.02; Table 3), however, age was not (OR 1.03 [0.99, 1.08], P = 0.06). In three multivariate regression models evaluated (Table 4), practice years were no longer significant.
Comparing pediatric and adult providers
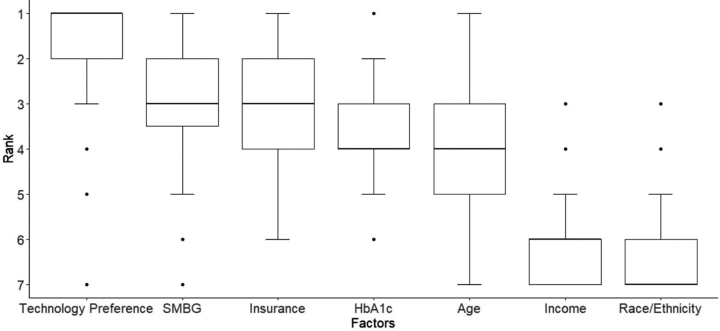
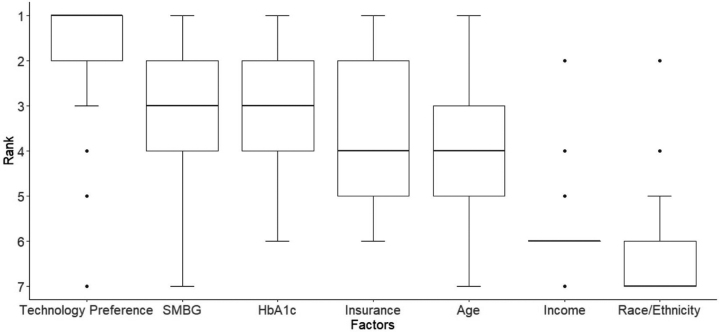
Pediatric providers' ranking preferences of patient factors to recommend CGM and insulin pump are presented in Figures 1 and 2, respectively. Technology preference was the most important factor for recommending CGM (mean rank 1.03 ± 1.56) and insulin pump therapy (mean rank 1.03 ± 1.56). Race/ethnicity was ranked to be the least important consideration in recommending insulin pump or CGM therapy (mean rank 5.86 ± 0.43 and 4.91 ± 0.39, respectively). Insurance coverage was the third most important factor in recommending CGM (mean rank 3.03 ± 1.56) and fourth most important factor in recommending insulin pump therapy (mean rank 4.03 ± 1.56).
FIG. 1.
Patient factors ranked in order of importance for pediatric providers to recommend CGM. CGM, continuous glucose monitoring.
FIG. 2.
Patient factors ranked in order of importance for pediatric providers to recommend insulin pump.
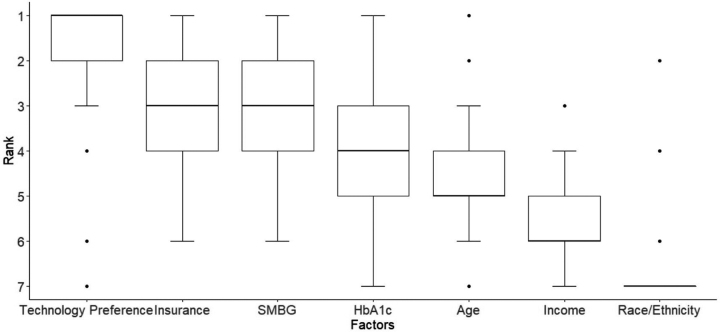
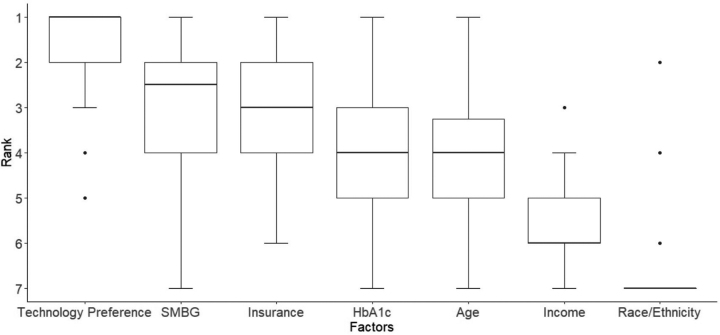
Adult providers ranking preferences of patient factors to recommend CGM and insulin pump therapy is presented as box plots in Figures 3 and 4, respectively. As seen with pediatric providers, technology preference was the most important factor for recommending CGM (mean rank 1.03 ± 1.56) and insulin pump (mean rank 1.03 ± 1.56). Race/ethnicity was ranked to be the least important consideration (mean rank for CGM 5.1 ± 0.39 and insulin pump 5.86 ± 0.43). Insurance coverage was the third most important factor in recommending CGM (mean rank 3.03 ± 1.56) and insulin pump therapy (mean rank 3.03 ± 1.56).
FIG. 3.
Patient factors ranked in order of importance for adult providers to recommend CGM.
FIG. 4.
Patient factors ranked in order of importance for adult providers to recommend insulin pump.
Discussion
To our knowledge, this is the first study measuring racial–ethnic-mediated implicit bias in pediatric and adult diabetes providers and the first to evaluate insurance-mediated bias in adult diabetes providers. Our study builds on earlier work and expands the evidence to include adult diabetes providers and the measurement of racial–ethnic-mediated implicit bias. These data shed light on the relationship between diabetes technology recommendations and the role of provider implicit bias against race/ethnicity and insurance status.
Historically excluded patients with T1D are more likely to be on public health insurance than private insurance.36 Therefore, insurance-mediated bias will directly and disproportionately impact patients of color. Importantly, it is established that historically excluded patients receive suboptimal care and have poorer outcomes irrespective of SES or insurance status.37,38 Thus, there is a dual and independent burden that public insurance, as a proxy for low SES, and race/ethnicity place on historically excluded individuals that must be addressed.
We report the presence of implicit racial–ethnic bias in one-third of our providers which likely impacts diabetes technology recommendations. These findings are consistent with multiple studies outlining the inequities associated with historically excluded race/ethnicity and diabetes technology from the patient's perspectives.37–40 DeSalvo et al. used data from the T1DX-QI Collaborative to demonstrate that non-Hispanic Black patients with private insurance had lower CGM use compared with non-Hispanic White patients that are publicly insured.37 Several studies suggested that racial–ethnic inequities in device use may be perpetuated by subconscious racial–ethnic bias.41,42 Taken together with the findings of this study, there is a need to address implicit racial–ethnic bias to address inequities in diabetes care.
Our work adds to the existing literature that training on antiracism alone does not sufficiently improve implicit racial–ethnic bias.43 We demonstrate that the providers' ability to recognize their explicit bias was not protective against implicit racial–ethnic bias. Most antiracism and equity-focused training are created to increase health care providers' awareness about their implicit bias.42–44 Training without actions can lead to high provider confidence in recognizing individual bias and we demonstrate that this is associated with a higher probability of being biased. We demonstrate that awareness and abhorrence of one's own explicit bias is not sufficient to protect against implicit bias. In a meta-analysis of 492 studies, Forscher et al. report that even when changes to implicit bias are seen, this does not necessarily translate to a change in explicit bias.43
To expand beyond the simple acknowledgment of bias, Chapman et al. suggested incorporating bias reducing strategies such as perspective taking to mitigate disparities resulting from implicit bias.21 Addressing implicit bias of providers to reduce disparities will involve a deliberate method to address structural and systemic racism and must be rooted in racial justice, economic equity, and equitable access to health care and education.
Consistent with the results of Addala et al.,22 we demonstrate the relationship between years in practice and the presence of insurance-mediated bias. Longer duration of practice is a driver for insurance bias, likely due to repeated encounters with limitations in diabetes technology coverage by public payers, the impact of past restrictive insurance coverage, and onerous paperwork required to secure coverage for individuals with public insurance.38,45 Interestingly, the relationship between provider age and insurance-mediated bias was not significant in the univariate analysis. We believe that this might be because of the limited number of older providers in the entire cohort given the mean age of the sample was 40.7 ± 10.5 years. These data are further supportive of the role and influence the U.S. medical care delivery system has on the medical provider's practice habits. This study reinforces the importance of communicating changes to diabetes providers and suggests adverse effects related to the complexity of rapidly changing and complicated insurance requirements.
Our study adds to the existing literature on providers' preference in recommending diabetes technology.20,36 Unsurprising, both pediatric and adult diabetes providers rate patient preference as the most important factor in recommending diabetes technology. This finding reinforces the importance of shared decision making as being part of the solution to addressing inequities in diabetes technology.45 Pediatric and adult diabetes providers rank the patients' SMBG as an important consideration in recommending diabetes technology, which may be related to earlier insurance requirements for diabetes technology.38,46 It will be important to establish a standardized means to assess readiness to start and continue diabetes technology to minimize the role of biases in the recommendation of diabetes technology.
The public insurance coverage policies were not identical between states, including SMBG requirement. Of the five states in the cohort only Alabama had a requirement of four times SMBG check per day for T1D patients on public insurance coverage. From our pediatric cohort, Ohio, Alabama, and Georgia public insurance provides CGM coverage for children with T1D. In our adult cohort, New York public insurance provides coverage for all patients with T1D.47
Our study has several limitations, including the small sample size with relatively young diabetes providers, which limits stratification by provider characteristics. Second, the participating providers were aware that this was a racial–ethnic bias study. Third, the providers participating in this survey were all from states with public payer coverage for diabetes technology, but the coverage policies were not identical between states, including SMBG requirements, thus this finding is not generalizable to diabetes providers in states with more restrictive diabetes device coverage. Also, like most surveys, there was no way to measure if the respondents were being honest and truthful in their answers.
These data suggest that addressing insurance-mediated bias with simplified coverage policies, increasing provider and patient education, and addressing implicit racial–ethnic bias through means other than increasing awareness alone are necessary steps to address disparities in diabetes care for individuals with T1D. Additional research is needed to measure effective strategies in mitigating biases and increasing equity for patients with diabetes. In addition, it will be important to elucidate the nuances and heterogeneity within the board racial–ethnic categories commonly used to capture and address the heterogeneity and unique lived experiences within a racial–ethnic subgroup. T1DX-QI had published practical approaches to advancing health equity using multilevel interventions, including shared decision making and quality improvement principles to reduce the impact of provider bias in recommending diabetes technologies and reducing inequities.26,28
This study of pediatric and adult diabetes providers in the United States underscores the need to address provider implicit bias with the presence of insurance-mediated bias in two-thirds of our cohort and implicit racial–ethnic bias in one-third of our cohort. Given the dual burden of race/ethnicity and low SES that is present in historically excluded individuals, it is necessary to address and ameliorate the role of implicit bias in diabetes care provision.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Sakinah C. Suttiratana and Dr. Sherita Hill Golden for their consultation in expanding the Diabetes Provider Implicit Bias Tool. The authors thank Helmsley Charitable Foundation for funding the QI Collaborative.
Authors' Contributions
O.E. conceptualized the study and is the Principal Investigator for the study. O.O., A.A., and O.E. wrote the first draft of the article. O.O. and A.A. are co-first authors and contributed equally to the study. All authors critically edited the article and approved the final version for submission.
Author Disclosure Statement
O.E. is a member of the Medtronic Diabetes Health Equity Advisory Board. He is compensated through his organization T1D Exchange.
Funding Information
Medtronic Diabetes funded this project. They had no role in the development of the D-PIB tool, data collection and analysis, the drafting of the article, or the decision to publish.
Supplementary Material
References
- 1. Kravarusic J, Aleppo G: Diabetes technology use in adults with type 1 and type 2 diabetes. Endocrinol Metab Clin 2020;49:37–55. [DOI] [PubMed] [Google Scholar]
- 2. Lyons SK, Ebekozien O, Garrity A, et al. : Increasing insulin pump use among 12-to 26-year-olds with type 1 diabetes: results from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes 2021;39:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dovc K, Battelino T: Evolution of diabetes technology. Endocrinol Metab Clin North Am 2020;49:1–18. [DOI] [PubMed] [Google Scholar]
- 4. Beck RW, Bergenstal RM, Laffel LM, Pickup JC: Advances in technology for management of type 1 diabetes. Lancet 2019;394:1265–1273. [DOI] [PubMed] [Google Scholar]
- 5. Addala A, Auzanneau M, Miller K, et al. : A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care 2021;44:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karter AJ, Parker MM, Moffet HH, et al. : Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA 2021;325:2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noor N, Ebekozien O, Levin L, et al. : Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance registry. Diabetes Care 44:e160–e162. [DOI] [PubMed] [Google Scholar]
- 8. Rodbard D: Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther 2017;19(S3):S25–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laffel LM, Kanapka LG, Beck RW, et al. : Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2388–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubin RR, Peyrot M: Treatment satisfaction and quality of life for an integrated continuous glucose monitoring/insulin pump system compared to self-monitoring plus an insulin pump. J Diabetes Sci Technol 2009;3:1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peyrot M, Rubin RR: Patient-reported outcomes for an integrated real-time continuous glucose monitoring/insulin pump system. Diabetes Technol Ther 2009;11:57–62. [DOI] [PubMed] [Google Scholar]
- 12. Grando MA, Bayuk M, Karway G, et al. : Patient perception and satisfaction with insulin pump system: pilot user experience survey. J Diabetes Sci Technol 2019;13:1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Majidi S, Ebekozien O, Noor N, et al. : Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes 2021;39:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agarwal S, Kanapka LG, Raymond JK, et al. : Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab 2020;105:e2960–e2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebekozien O, Agarwal S, Noor N, et al. : Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab 2021;106:e1755–e1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott A, O'Cathain A, Goyder E: Socioeconomic disparities in access to intensive insulin regimens for adults with type 1 diabetes: a qualitative study of patient and healthcare professional perspectives. Int J Equity Health 2019;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agarwal S, Crespo-Ramos G, Long JA, et al. : “I didn't really have a choice”: qualitative analysis of racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther 2021;23:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker AF, Hood KK, Gurka MJ, et al. : Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care 2021;44:1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. FitzGerald C, Hurst S: Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall WJ, Chapman MV, Lee KM, et al. : Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105:e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chapman EN, Kaatz A, Carnes M: Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013;28:1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Addala A, Hanes S, Naranjo D, et al. : Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the United States: findings from The Gatekeeper Study. J Diabetes Sci Technol 2021;15:1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanenbaum ML, Adams RN, Lanning MS, et al. : Using cluster analysis to understand clinician readiness to promote continuous glucose monitoring adoption. J Diabetes Sci Technol 2018;12:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hill-Briggs F, Adler NE, Berkowitz SA, et al. : Social determinants of health and diabetes: a scientific review. Diabetes Care 2021;44:258–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valenzuela JM, Seid M, Waitzfelder B, et al. : Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr 2014;164:1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebekozien O, Odugbesan O, Rioles N, et al. : Equitable post-COVID-19 care: a practical framework to integrate health equity in diabetes management. JCOM 2020;27:256–258. [Google Scholar]
- 27. Alonso GT, Corathers S, Shah A, et al. : Establishment of the T1D exchange quality improvement collaborative (T1DX-QI). Clin Diabetes 2020;38:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ebekozien O, Mungmode A, Odugbesan O, et al. : Addressing Type 1 Diabetes Health Inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes 2022;14:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shiyanbola OO, Bolt D, Tarfa A, et al. : A content validity and cognitive interview process to evaluate an Illness Perception Questionnaire for African Americans with type 2 diabetes. BMC Res Notes 2019;12:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willis G: Cognitive interviewing in survey design: state of the science and future directions. In: Vannette, DL, Krosnick, JA, eds. The Palgrave Handbook of Survey Research. New York: Springer International Publishing, 2018:103–107. DOI: 10.1007/978-3-319-54395-6_14 [DOI] [Google Scholar]
- 31. Bertrand M, Mullainathan S: Are Emily and Greg More employable than Lakisha and Jamal? A field experiment on labor market discrimination. Am Econ Rev 2004;94:991–1013. [Google Scholar]
- 32. Holbrook C, Fessler DMT, Navarrete CD: Looming large in others' eyes: racial stereotypes illuminate dual adaptations for representing threat versus prestige as physical size. Evol Hum Behav 2016;37:67–78. [Google Scholar]
- 33. Rice D, Rhodes JH, Nteta T: Racial bias in legal language. Res Politics 2019;6:2053168019848930. [Google Scholar]
- 34. Axt JR: The best way to measure explicit racial attitudes is to ask about them. Soc Psychol Personal Sci 2018;9:896–906. [Google Scholar]
- 35. Dehon E, Weiss N, Jones J, et al. : A systematic review of the impact of physician implicit racial bias on clinical decision making. Acad Emerg Med 2017;24:895–904. [DOI] [PubMed] [Google Scholar]
- 36. DeSalvo DJ, Noor N, Xie C, et al. : Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange Real-World Observational Study. J Diabetes Sci Technol 2021:19322968211049784. [Epub ahead of print]; DOI: 10.1177/19322968211049783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Addala A, Maahs DM, Scheinker D, et al. : Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes 2020;21:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smedley BD, Stith AY, Nelson AR, eds. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US), 2003. [PubMed] [Google Scholar]
- 39. Lipman TH, Hawkes CP: Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care 2021;44:14–16. [DOI] [PubMed] [Google Scholar]
- 40. Valenzuela JM, La Greca AM, Hsin O, et al. : Prescribed regimen intensity in diverse youth with type 1 diabetes: role of family and provider perceptions. Pediatr Diabetes 2011;12:696–703. [DOI] [PubMed] [Google Scholar]
- 41. Hagiwara N, Kron FW, Scerbo MW, et al. : A call for grounding implicit bias training in clinical and translational frameworks. Lancet 2020;395:1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hagiwara N, Lafata JE, Mezuk B, et al. : Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Counsel 2019;102:1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forscher PS, Lai CK, Axt JR, et al. : A meta-analysis of procedures to change implicit measures. J Pers Soc Psychol 2019;117:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson JE, Gavin JR, Kruger DF: Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Therap 2020;22:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elwyn G, Frosch D, Thomson R, et al. : Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wan W, Skandari MR, Minc A, et al. : Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care 2018;6:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howe G, Chavis J: Expanding Medicaid access to continuous glucose monitors. Center for Health Care Strategies. 2022. https://www.chcs.org/resource/expanding-medicaid-access-to-continuous-glucose-monitors/ (accessed February 16, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.