Abstract
Background:
Automated insulin delivery (AID) systems have proven effective in increasing time-in-range during both clinical trials and real-world use. Further improvements in outcomes for single-hormone (insulin only) AID may be limited by suboptimal insulin delivery settings.
Methods:
Adults (≥18 years of age) with type 1 diabetes were randomized to either sensor-augmented pump (SAP) (inclusive of predictive low-glucose suspend) or adaptive zone model predictive control AID for 13 weeks, then crossed over to the other arm. Each week, the AID insulin delivery settings were sequentially and automatically updated by an adaptation system running on the study phone. Primary outcome was sensor glucose time-in-range 70–180 mg/dL, with noninferiority in percent time below 54 mg/dL as a hierarchical outcome.
Results:
Thirty-five participants completed the trial (mean age 39 ± 16 years, HbA1c at enrollment 6.9% ± 1.0%). Mean time-in-range 70–180 mg/dL was 66% with SAP versus 69% with AID (mean adjusted difference +2% [95% confidence interval: −1% to +6%], P = 0.22). Median time <70 mg/dL improved from 3.0% with SAP to 1.6% with AID (−1.5% [−2.4% to −0.5%], P = 0.002). The adaptation system decreased initial basal rates by a median of 4% (−8%, 16%) and increased initial carbohydrate ratios by a median of 45% (32%, 59%) after 13 weeks.
Conclusions:
Automated adaptation of insulin delivery settings with AID use did not significantly improve time-in-range in this very well-controlled population. Additional study and further refinement of the adaptation system are needed, especially in populations with differing degrees of baseline glycemic control, who may show larger benefits from adaptation.
Keywords: Adaptation, Artificial pancreas, Automated insulin delivery, Glycemic control, Type 1 diabetes
Introduction
Automated insulin delivery (AID) systems have significantly improved outcomes in individuals with type 1 diabetes (T1D).1–9 Further improvements in glycemic outcomes with single-hormone (insulin only) AID systems may require additional features beyond basal rate modulation alone.10,11 Some systems now include automated correction boluses.12 Others adapt basal rates or other internal model parameters over time.4,13,14 These strategies came about because basal rate modulation of subcutaneously administered insulin by itself is not fast enough to prevent all instances of hyper- and hypoglycemia.13
We have previously reported on use of an adaptation system for AID that adjusted user profile basal rates and carbohydrate ratios, but these changes had to be manually implemented by study physicians.13 Neither this system nor any other adaptation system that explicitly adjusts user profile insulin delivery settings has been studied in randomized, crossover trials, so it is not clear how much benefit user profile adaptation is adding. To better understand the potential benefits of such features, we performed the first randomized, crossover trial of an AID system with weekly automatic adaptation of basal rates and carbohydrate ratios compared to sensor-augmented pump (SAP) in a 13-week randomized crossover comparison.
Methods
Study design
The study was conducted at five clinical sites in the United States. Design of the control algorithms and engineering of the AID device and adaptation system were done at the Harvard John A. Paulson School of Engineering of Applied Sciences, Harvard University, Cambridge, MA. The protocol was approved by the United States Food and Drug Administration and the Jaeb Center for Health Research Institutional Review Board and was registered at clinicaltrials.gov (NCT04436796). Informed consent was obtained before all study procedures.
Eligible participants were ≥18 years of age with T1D for at least 1 year and had been using an insulin pump for at least 3 months. Key exclusion criteria were pregnancy, or two or more episodes of hypoglycemia or hyperglycemia requiring an emergency room visit in the past 6 months.
Participants completed a 2-week run-in period with a Dexcom G6 continuous glucose monitor (CGM) if they were not current Dexcom CGM users. After the run-in, participants were randomized 1:1 to either continue SAP with their home pump and the study sensor, or AID with the study device. After 13 weeks, participants crossed over to the other arm. In the control arm of the study, participants were allowed to continue the use of the predictive low-glucose suspend (PLGS) feature of their home pump if they were already using this and it was compatible with the study CGM, otherwise they used SAP.
HbA1c processed at a central laboratory (Advanced Research and Diagnostic Laboratory, University of Minnesota) was collected at randomization and at 13 and 26 weeks (end of each study arm). Participants were contacted at 3 days, 2 weeks, 4 weeks, 6 weeks, and 9 weeks during each arm, with additional visits per investigator discretion.
AID and adaptation system
The design, development, and initial clinical evaluation of our smartphone-based AID application platform, the interoperable artificial pancreas system (iAPS), has been previously described15 and has been used in numerous prior investigations.15–17 In this study, the iAPS ran on a Google Pixel 3 smartphone and wirelessly paired with a Dexcom G6 sensor and a Tandem t:AP insulin pump.
Similar to these prior studies, the Zone Model Predictive Control (Zone-MPC) control algorithm18 used a target glucose range of 90–120 mg/dL during the day, and 100–120 mg/dL during the night (00:00–05:59), with glucose deviations above the zone weighted by a continuous function of glucose velocity and insulin-on-board. Participants were required to bolus for all meals based on each participant's personal carbohydrate ratio as previously described,13 although the iAPS allowed modification of these boluses by each user at their discretion. Users could also give correction boluses at their discretion. The iAPS included threshold-based hypoglycemia and hyperglycemia alarms, and a predictive low-glucose alarm for impending hypoglycemia that was used to monitor CGM glucose independent from the AID control algorithm.19
The adaptation system ran on the study phone each week, and automatically updated each subject's user profile insulin delivery settings in a predefined sequential manner. Basal rates were adapted once a week for 2 weeks, then carbohydrate ratios once a week for up to 5 weeks, then basal rates again once a week for 2 weeks, then back to carbohydrate ratios or the internal controller parameters once a week for the remaining weeks (Supplementary Fig. S1). Limits for each weekly automated adaptation were 0.2 U/h for basal rate and 15% for carbohydrate ratio. No specific limitation was imposed on the number of profile segments. The adaptations were not supervised, and overriding the system was only possible by resetting the adaptation back to baseline settings. The adaptation system was evaluated in-silico before clinical use.20
Outcome measures
The primary outcome was CGM-measured percent time-in-range 70–180 mg/dL in each 13-week period. The main secondary outcome, tested in a hierarchical manner to maintain the type 1 error at 5%, was noninferiority in percent time glucose <54 mg/dL. Other secondary outcomes included mean glucose, percent time glucose >180 mg/dL, percent time glucose >250 mg/dL, percent time glucose <70 mg/dL, glucose coefficient of variation, HbA1c, total insulin units per day, body mass index (BMI), and participant-reported questionnaires. Analyses excluded CGM data from the first week of system training during each one of the two 13-week crossover periods and included all available data during the remaining 12 weeks regardless of whether closed-loop was active. Safety outcomes included the frequency of severe hypoglycemia, diabetic ketoacidosis (DKA), and other serious adverse events.
Statistical methods
Sample size was computed to be 31 to have 90% power with a type 1 error rate (2-sided) of 5% for the following assumptions: two 13-week treatment periods (AID and SAP), crossover randomization, a true population value of 8% absolute increase in time-in-range in the treatment versus control periods, a standard deviation (SD) of 17%, and a correlation between the two periods of 0.70. The sample size was increased to 35 to account for any possible dropouts.
Primary outcomes were CGM-measured time-in-range 70–180 mg/dL (superiority) and time <54 mg/dL (noninferiority with a margin of 1%). Statistical analyses were performed on an intention-to-treat basis and all participants were included in the primary and all secondary analyses unless otherwise noted. Each outcome was compared between the two treatments using a repeated-measures linear regression model that included prerandomization baseline, the two crossover periods, and adjusted for period and site (as random effect).
Descriptive statistics include means with SDs and/or medians with interquartile ranges depending on the distribution of data. Except for noninferiority in percent glucose <54 mg/dL, all P-values are two-tailed. The type 1 error rate was controlled for the two primary outcomes using a hierarchical procedure where time <54 mg/dL can only be tested if time-in-range is statistically significant at alpha = 0.05. The false discovery rate was used for the secondary outcomes. Analyses were performed using SAS 9.4.
Results
Thirty-five participants (46% female) were randomly assigned to receive AID followed by SAP (n = 19) or SAP followed by AID (n = 16, Table 1). Age range was 19–72 years (mean 39) and diabetes duration was 2–55 years (median 18). Mean enrollment HbA1c was 6.9%, with 23 (66%) having HbA1c <7.0% and 4 (11%) having HbA1c >8.0%. During the SAP therapy period, 4 out of the 35 participants (11%) were using a personal pump with PLGS capabilities that was compatible with the study CGM. All 35 participants completed the trial (Supplementary Fig. S2).
Table 1.
Participant Characteristics at Enrollment or Randomization
| Overall (n = 35) | |
|---|---|
| Treatment assignment | |
| AID followed by SAP | 19 (54%) |
| SAP followed by AID | 16 (46%) |
| Age (years) | |
| Mean (SD) | 39 (16) |
| Range | 19–72 |
| Diabetes duration (years) | |
| Median (IQR) | 18 (12, 29) |
| Range | 2–55 |
| Current CGM users, n (%) | 29 (83) |
| Sex—female, n (%) | 16 (46) |
| Race | |
| White | 29 (83%) |
| Black/African American | 4 (11%) |
| Asian | 1 (3%) |
| More than one race | 1 (3%) |
| Education | |
| ≤Bachelor's degree | 29 (83%) |
| ≥Master's degree | 6 (17%) |
| Incomea | |
| <$100,000 | 12 (43%) |
| ≥$100,000 | 16 (57%) |
| Private health insurance | 32 (91%) |
| HbA1c at enrollment (%) | |
| Mean (SD) | 6.9 (1.0) |
| Range | 5.3–9.2 |
| HbA1c at randomization—central laboratory (%) | |
| Mean (SD) | 6.7 (1.0) |
| Range | 5.0–9.0 |
| C-peptide at randomization (nmol/L) | |
| <0.007 | 24 (69%) |
| ≥0.007 | 11 (31%) |
| BMI at enrollment (kg per m2) | |
| Mean (SD) | 28 (5) |
| Range | 21–45 |
| No. of SH's in the last 12 months | 2 (6%) |
| No. of DKA's in the last 12 months | None |
Seven participants did not report income.
AID, automated insulin delivery; BMI, body mass index; CGM, continuous glucose monitor; DKA, diabetic ketoacidosis; IQR, interquartile range; SAP, sensor-augmented pump; SD, standard deviation; SH, severe hypoglycemia.
Overall visit and phone contact completion rates were 100% and 99.6% during the AID and SAP periods. There were 195 unscheduled contacts during AID use (61 to review the automated setting changes that the adaptation system performed, 61 related to investigational device issues, which were primarily related to connectivity, 26 requiring additional system training, and so on) and 110 during SAP use.
Efficacy outcomes
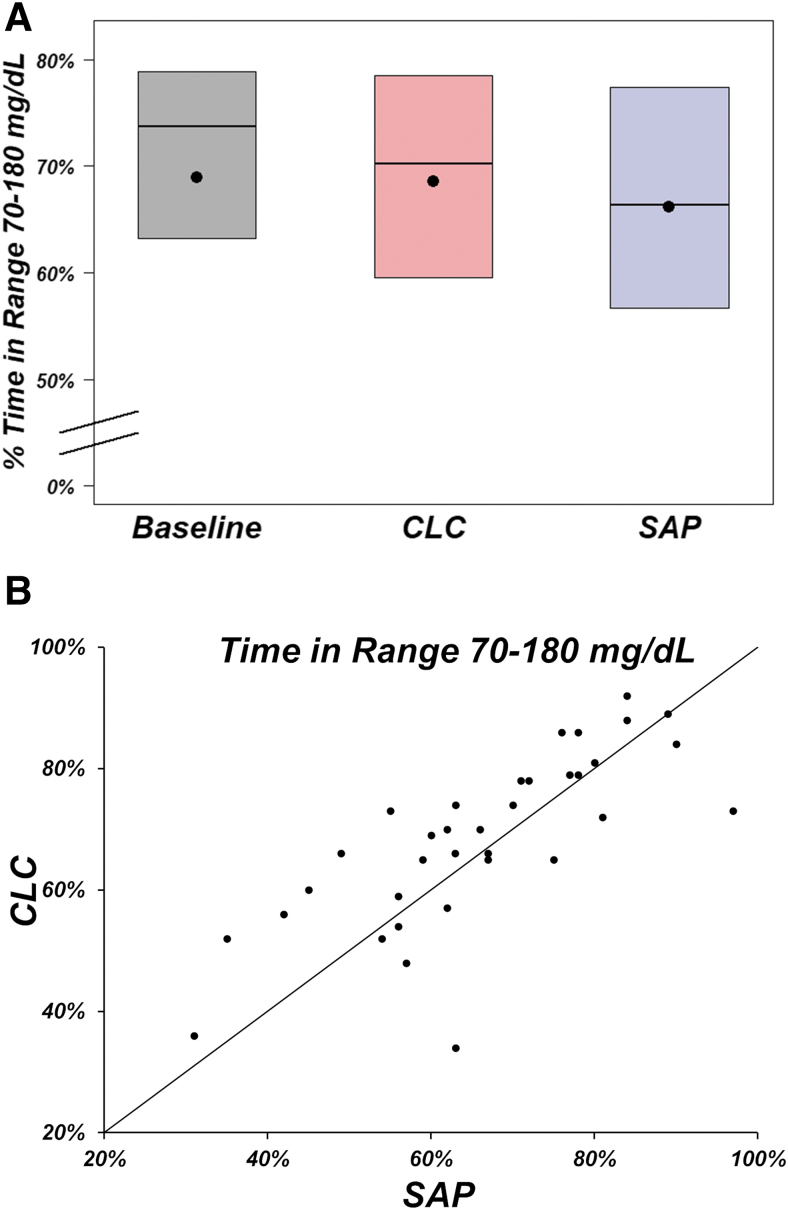
In the primary analysis, mean ± SD time 70–180 mg/dL was 69% ± 16% at baseline, 69% ± 14% during AID use, and 66% ± 15% during SAP use (mean adjusted difference [AID−SAP] = 2%, 95% confidence interval [CI]: −1% to 6%, P = 0.22; Table 2 and Fig. 1A, B). Mean time 70–180 mg/dL was 66% during the first AID period and 72% during the second AID period, and 69% during the first SAP period and 63% during the second SAP period. However, when including only participants with >80% CGM use in the SAP period and >80% closed-loop use in the AID period (n = 21), the mean adjusted difference for percent time-in-range 70–180 mg/dL was 5% (P = 0.002). Because the null hypothesis for the primary outcome was not rejected, statistical test for noninferiority in percent time below 54 mg/dL was not performed.
Table 2.
Primary and Hierarchical Outcomes by Treatment Periods
| Baseline n = 35 | AID use period n = 35 | SAP use period n = 35 | Mean adjusted difference [95% CI], P-value | |
|---|---|---|---|---|
| Hours of CGM data | ||||
| Median (IQR) | 309 (303, 329) | 1767 (1673, 1849) | 1892 (1780, 1945) | |
| Range | 253–357 | 1101–2032 | 1633–2053 | |
| Percentage of time-in-range 70–180 mg/dL | ||||
| Mean (SD) | 69% (16%) | 69% (14%) | 66% (15%) | 2% [−1% to 6%] P-value = 0.22a |
| Median (IQR) | 74% (63%, 79%) | 70% (59%, 79%) | 66% (56%, 78%) | |
| Percentage of time below 54 mg/dL | ||||
| Mean (SD) | 0.62% (0.89%) | 0.41% (0.39%) | 0.71% (0.82%) | −0.30% [−0.60% to −0.01%] P-value not calculatedb |
| Median (IQR) | 0.44% (0.00%, 0.81%) | 0.22% (0.09%, 0.72%) | 0.46% (0.17%, 0.88%) | |
Based on a repeated-measures least squares regression model adjusting for period as fixed effect and site as random effect. The model includes three time points: (1) baseline, (2) period 1 outcome, and (3) period 2 outcome. Carryover effect P-value was 0.07 for % time-in-range 70–180 mg/dL.
A hierarchical method was used to control the overall type I error for the two primary outcomes in this table. Since time-in-range was not statistically significant (P = 0.22), no P-value was calculated for time below 54 mg/dL.
CI, confidence interval.
FIG. 1.
(A) Percentage of time-in-range 70–180 mg/dL by period and treatment (n = 35 participants). Black dots denote mean values, horizontal lines in the boxes are medians, and the bottom and top of the boxes represent the 25th and 75th percentiles. In (B), the points above the line of identity denote participants who had better time-in-range 70–180 mg/dL during the CLC period compared with the SAP period. CLC, closed-loop control; SAP, sensor-augmented pump.
There were no significant differences between AID and SAP for mean glucose or hyperglycemia metrics, but hypoglycemia was less with AID than SAP (Table 3). Median (interquartile range [IQR]) for percent time below 54 mg/dL was 0.44% (0.00%, 0.81%) at baseline, 0.22% (0.09%, 0.72%) during AID use, and 0.46% (0.17%, 0.88%) during SAP use (mean difference = −0.30%, 95% CI: −0.60% to −0.01%, Table 2). Daytime (06:00–23:59) and nighttime (00:00–05:59) glycemic outcomes were similar to the 24 h glycemic outcomes (Supplementary Tables S1, S2). In addition, the overnight percent time in 70–180 mg/dL improved by 6% during AID versus SAP (95% CI: 0.6% to 12%, P = 0.03).
Table 3.
Secondary Continuous Glucose Monitor Metrics Over the Full 24 H of the Day by Treatment Period
| Baseline n = 35 | AID use period n = 35 | SAP use period n = 35 | Mean adjusted difference [95% CI], Pa | |
|---|---|---|---|---|
| Hours of data | ||||
| Median (IQR) | 309 (303, 329) | 1767 (1673, 1849) | 1892 (1780, 1945) | |
| Overall glucose control | ||||
| Percentage of time in the tight target range 70–140 mg/dL | ||||
| Mean (SD) | 44% (16%) | 44% (14%) | 43% (17%) | 1% [−4 to 7], 0.60 |
| Median (IQR) | 43% (33%, 54%) | 43% (36%, 55%) | 41% (32%, 57%) | |
| Mean glucose (mg/dL) | ||||
| Mean (SD) | 155 (29) | 158 (22) | 156 (27) | 2 [−6 to 10], 0.60 |
| Median (IQR) | 153 (136, 163) | 157 (143, 174) | 153 (135, 172) | |
| Coefficient of variation | ||||
| Mean (SD) | 33.6% (5.6%) | 34.5% (5.3%) | 34.9% (5.7%) | −0.5% [−2.1 to 1.1], 0.60 |
| Median (IQR) | 34.0% (31.4%, 37.7%) | 35.1% (31.9%, 37.5%) | 35.4% (30.8%, 40.0%) | |
| SD (mg/dL) | ||||
| Mean (SD) | 52.0 (13.7) | 54.9 (13.1) | 54.8 (13.6) | 0.03 [−3.3 to 3.4], 0.98 |
| Median (IQR) | 49.6 (44.0, 56.0) | 54.5 (46.4, 60.8) | 53.8 (47.2, 60.8) | |
| Percentage of time in target range 70–180 mg/dL >70% | ||||
| n (%) | 21 (60) | 18 (51) | 14 (40) | 11% [−5 to 27], 0.21 |
| Hypoglycemia | ||||
| Percentage of time <60 mg/dL | ||||
| Mean (SD) | 1.17% (1.52%) | 0.81% (0.73%) | 1.39% (1.45%) | −0.6% [−1.1 to −0.1], 0.01 |
| Median (IQR) | 0.77% (0.08%, 1.48%) | 0.58% (0.24%, 1.33%) | 1.00% (0.45%, 1.82%) | |
| Percentage of time <70 mg/dL | ||||
| Mean (SD) | 3.1% (3.5%) | 2.1% (1.7%) | 3.5% (3.1%) | −1.5% [−2.4 to −0.5], 0.002 |
| Median (IQR) | 2.3% (0.6%, 3.9%) | 1.6% (0.8%, 3.0%) | 3.0% (1.7%, 4.9%) | |
| LBGI | ||||
| Mean (SD) | 0.9 (0.8) | 0.6 (0.4) | 1.0 (0.7) | −0.3 [−0.5 to −0.1], 0.002 |
| Median (IQR) | 0.7 (0.2, 1.2) | 0.6 (0.3, 0.8) | 0.8 (0.5, 1.3) | |
| Hypoglycemic event rate per weekb | ||||
| Mean (SD) | 1.24 (1.60) | 0.84 (0.84) | 1.41 (1.61) | −0.5 [−1.1 to 0.3], 0.07 |
| Median (IQR) | 0.97 (0.00, 1.65) | 0.58 (0.10, 1.61) | 1.00 (0.29, 1.76) | |
| Percentage of time below 54 mg/dL <1% | ||||
| n (%) | 29 (83) | 31 (89) | 28 (80) | 9% [−6 to 26], 0.23 |
| Percentage of time in target range 70–180 mg/dL >70% and % of time below 54 mg/dL <1% | ||||
| n (%) | 16 (46%) | 15 (43%) | 10 (29%) | 14% [−3 to 30], 0.11 |
| Hyperglycemia | ||||
| Percentage of time >180 mg/dL | ||||
| Mean (SD) | 28% (17%) | 29% (15%) | 30% (16%) | −0.6% [−5 to 4], 0.75 |
| Median (IQR) | 24% (17%, 35%) | 28% (19%, 40%) | 29% (17%, 42%) | |
| Percentage of time >250 mg/dL | ||||
| Mean (SD) | 8% (10%) | 9% (8%) | 8% (8%) | 0.1% [−3 to 2], 0.95 |
| Median (IQR) | 4% (2%, 8%) | 7% (3%, 11%) | 6% (2%, 11%) | |
| Percentage of time >300 mg/dL | ||||
| Mean (SD) | 3% (5%) | 3% (4%) | 3% (4%) | −0.4% [−2 to 1], 0.60 |
| Median (IQR) | 1% (0%, 1%) | 1% (1%, 3%) | 1% (0%, 3%) | |
| HBGI | ||||
| Mean (SD) | 6.5 (4.7) | 6.8 (3.6) | 6.8 (4.1) | −0.07 [−1.3 to 1.1], 0.89 |
| Median (IQR) | 5.1 (3.9, 7.0) | 6.1 (4.3, 8.9) | 6.5 (3.8, 8.4) | |
| Hyperglycemic event rate per weekc | ||||
| Mean (SD) | 1.89 (2.69) | 2.39 (2.61) | 1.93 (1.91) | 0.3 [−0.5 to 1.1], 0.60 |
| Median (IQR) | 1.09 (0.47, 1.65) | 1.61 (0.58, 2.89) | 1.74 (0.36, 2.91) | |
Based on a repeated-measures least squares regression model adjusting for period as fixed effect and site as random effect. The model includes three time points: (1) baseline, (2) period 1 outcome, and (3) period 2 outcome. P-values and confidence intervals were adjusted for multiple comparisons using the Two Stage Benjamini-Hochberg adaptive false discovery rate procedure.
An hypoglycemic event is defined as at least 15 min <54 mg/dL. An event ends if at least 15 min above 70 mg/dL.
An hyperglycemic event is defined as at least 15 min >300 mg/dL. An event ends if at least 15 min below 250 mg/dL.
HBGI, high blood glucose index; LBGI, low blood glucose index.
Mean ± SD HbA1c was 6.7% ± 1.0% at randomization, 6.8% ± 0.8% at the end of the two 13-week AID periods, and 6.8% ± 0.9% at the end of the two 13-week SAP periods (P = 0.51, Supplementary Table S3). There was no difference between treatments in daily insulin amount (P = 0.95) or in BMI (P = 0.95, Supplementary Table S4).
Adaptations
During AID use, the system adapted insulin therapy settings automatically a median (IQR) of 7 days (7, 7 days). When compared with initial settings, adapted settings after 13 weeks slightly decreased the daily average basal rate by a median (IQR) of 4% (−8%, 16%) and increased the daily average carbohydrate-to-insulin ratio (leading to reduced insulin bolus) by a median (IQR) of 45% (32%, 59%), as shown in Supplementary Figure S3A and B, respectively.
The AID system was configured not to change the correction factor. The internal controller parameters were adapted for two participants. Mean ± SD time 70–180 mg/dL was 75% ± 15% during the first adaptation and decreased to 66% ± 15% during the 12th and last adaptation (Supplementary Fig. S3C) while median (IQR) time below 70 mg/dL was 1.2% (0.4%, 3.3%) during the first adaptation and 1.0% (0.0%, 2.2%) during the 12th and last adaptation (Supplementary Fig. S3D).
No manual adjustments of pump settings were performed by clinic staff during the closed-loop control (CLC) therapy period, as there were no safety concerns in response to automated adaptation-driven adjustments. During the SAP therapy period, there were 37 total such instances of manual pump parameter adjustments done in 21 out of the total of 35 participants (i.e., 14 participants received no adjustments). These adjustments were made in response to participant concerns about glycemic control and/or clinical site staff concerns about participant safety.
System use
Median percentage of CGM use was 93% (88%, 95%) during AID periods and 98% (94%, 98%) during SAP periods (Supplementary Table S5). During the AID periods, the median percentage of time the system was in closed-loop mode was 82% (61%, 91%). There were 60 reported device problems reported during AID use and 5 during SAP use. During AID use, 31 of the device problems were related to iAPS smartphone app malfunctions, generally related to screen freezing or other usability issues. Sixty-five percent of participants indicated in the System Usability Survey that the AID system was easy to use, while 24% disagreed. Similarly, 65% of participants reported that they would like to use the AID system more frequently, while 30% disagreed.
Adverse events
One severe hypoglycemic event occurred during the SAP period and no cases of diabetic ketoacidosis (DKA) occurred in either period. There were no other serious adverse events. Six other adverse events were reported for five participants during AID use (Supplementary Table S6). Out of the six adverse events during AID use, three cases of hyperglycemia related to infusion set failure, one case of unexplained hyperglycemia with ketosis, one case of site insertion bleeding, and one case of COVID-19.
Discussion
Clinician optimization of user profile insulin delivery settings (basal rates, carbohydrate ratios, and correction factors) is commonly performed in clinical practice. With the advent of hybrid AID systems, it has become less clear how to best optimize these parameters to achieve ideal results. Although it would be ideal for AID systems to not explicitly require user profile settings altogether and fully automate insulin delivery, limitations on the speed of insulin action and challenges such as exercise, large meals, and change in insulin requirements over time (especially during growth and development in pediatrics and adolescents)21 make this unlikely to succeed without some degree of manual intervention, especially for single-hormone systems.
To aid this process, some systems have built in short-term adaptation.18,22 Others continually optimize basal rates and/or other internal parameters, although these changes are not always visible to the user.1,4 The adaptation system evaluated in this study worked to optimize basal rates and carbohydrate ratios primarily, then near the end of the study examined changes to internal controller parameters (Supplementary Fig. S1). Basal rate is one of the inputs to the zone model predictive controller that is used to actively modulate insulin above and below baseline. The way that the Zone-MPC controller works is when the future glucose prediction is in the target zone, the system will receive user profile basal rate. If basal rate is not optimal, as it was not optimized before the study, adjustments of that basal rate would benefit the individuals to achieve higher time in the target range.
We found that the 2% mean adjusted improvement in time-in-range in the AID group did not reach statistical difference compared to SAP. This was, in part, due to the adaptations system being too heavily weighted against hypoglycemia, as shown by the median increase of 45% for average carbohydrate ratio (leading to reduced insulin bolus) over the course of the study. There was no systematic change in average basal rate over the course of the study. Another contributor to these results may be the level of baseline glycemic control for the study cohort, who had a baseline HbA1c <7%. It may be that there was little room for improvements in glycemic control from adaptation for AID systems using subcutaneous insulin in the selected study population.
Hypoglycemia rates (time <70 mg/dL) were reduced nearly 50% in the AID group. This reduction may benefit certain populations at higher risk of hypoglycemia, such as the elderly. Lack of clear change in average basal rates and in percent time <70 mg/dL over weekly iterations suggest that adaptations had limited effect on hypoglycemia, and the improvement during AID versus SAP may have been driven directly by the MPC controller. Similarly, the MPC controller had more leeway during overnight hours due to lack of effect of meals and the increased carbohydrate ratio.
Limitations of this study relate to use of a research AID system, which sometimes led to significant connectivity issues with the study pump and CGM that in some instances affected glycemia and may have affected the adaptation system. In addition, the mean baseline HbA1c for study participants was 6.9%. It is likely that improvements in time-in-range would have been more significant in those with suboptimal baseline glycemic control. Finally, the study compared adaptive AID therapy to the current standard of care, which was SAP therapy, as most individuals with T1D do not use an AID system today. Future comparison between AID with and without adaptation may serve to better differentiate the effect of adaptation.
In conclusion, we show the feasibility of performing unsupervised automated adaptation of insulin delivery settings with AID. However, the adaptations did not significantly improve time-in-range in this population. Despite increasing carbohydrate ratio and decreasing percent time in closed-loop mode, the clinical outcomes during AID versus SAP did not deteriorate highlighting the utility of AID systems.
To improve the adaptation system in the future, additional work will be performed on the sequence of insulin delivery settings to adapt, relative weighting of glycemic outcomes, effect of profiles segments of varying length and overlapping meals therein, and processing of invalid CGM points that could influence the profile settings recommendations. Additional study is needed, especially in populations with greater variations in baseline glycemic control, who may show larger benefits from adaptation.
Supplementary Material
Acknowledgments
The authors acknowledge Huipeng Zhang and Junfeng Zhang for contributions to algorithm and system verification, and Randy Tompot, Chris Rogers, and Judit Flo Gaya for contributions to the iAPS platform. The International Diabetes Closed-Loop (iDCL) Trial Study Group (site investigators noted): Joslin Diabetes Center, Harvard Medical School, Boston, MA: Lori Laffel (PI), Elvira Isganaitis (I), Louise Ambler-Osborn (I), Emily Freiner. Sansum Diabetes Research Institute, Santa Barbara, CA: Jordan Pinsker (PI), Mei Mei Church (I). Division of Endocrinology, Diabetes, Icahn School of Medicine at Mount Sinai, New York City, NY: Carol Levy (PI), Grenye O'Malley (I), Camilla Levister (I), Danielle Brooks, Selassie Ogyaadu, Mitchell Plesser. Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Department of Internal Medicine, Mayo Clinic, Rochester MN: Yogish C. Kudva (PI), Vinaya Simha (I), Donna DesJardins (I), Shelly McCrady-Spitzer, and Ravinder Jeet Kaur. Department of Pediatrics, Division of Pediatric Endocrinology and Diabetes, Stanford University School of Medicine: Bruce Buckingham (PI), Laya Ekhlaspour (I), Lisa Nolander (I), Liana Hsu. Jaeb Center for Health Research: John Lum, Roy Beck, Samantha Passman, Tiffany Campos, Dan Raghinaru, Craig Kollman, Carlos Murphy, Nandan Patibandla, Sarah Borgman. National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK): Guillermo Arreaza-Rubín (Project Scientist), Thomas Eggerman (Program Officer), Neal Green (Project Manager). DSMB: Steven H. Belle (Chair), Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, Thomas Eggerman (DSMB Executive Secretary for NIDDK).
Contributor Information
for the iDCL Trial Research Group:
Elvira Isganaitis, Louise Ambler-Osborn, Emily Freiner, Grenye O'Malley, Danielle Brooks, Selassie Ogyaadu, Mitchell Plesser, Vinaya Simha, Donna DesJardins, Shelly McCrady-Spitzer, Lisa Nolander, Liana Hsu, Roy Beck, Samantha Passman, Tiffany Campos, Carlos Murphy, Nandan Patibandla, Sarah Borgman, Guillermo Arreaza-Rubín, Thomas Eggerman, Neal Green, Steven H. Belle, Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, and Thomas Eggerman
Collaborators: for the iDCL Trial Research Group
Author Disclosure Statement
J.E.P. is currently an employee and shareholder of Tandem Diabetes Care, Inc., and has a significant financial interest in Tandem Diabetes Care. The work presented in the article was performed as part of his academic appointment at Sansum Diabetes Research Institute and is independent of his employment with Tandem Diabetes Care. E.D. reports receiving grants from JDRF, NIH, and Helmsley Charitable Trust, personal fees from Roche and Eli Lilly, patents on artificial pancreas technology, pending US Patent 62/686,931 and product support from Dexcom, Insulet, Tandem, and Roche. E.D. is currently an employee and shareholder of Eli Lilly and Company. The work presented in this manuscript was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. C.J.L. reports grants paid to her institution from the National Institutes of Health, Insulet, Dexcom, Abbott, the Juvenile Diabetes Research Foundation, and AECOM; receiving consultancy fees from Dexcom and Eli Lilly; and serving on the Data Safety Monitoring Board (DSMB) for the Juvenile Diabetes Research Foundation and National Institutes of Health.
H.D. reports receiving consultancy fees from Guidepoint Consulting. L.E. reports receiving consultancy fees from Tandem Diabetes Care and Ypsomed. F.J.D. reports receiving royalties or license fees from ModeAGC, Roche, Dexcom, and Insult, advisory board for ModeAGC, and pending US Patent 62/686,931. All other authors report no conflict of interest related to this article.
Funding Information
This study was funded by the National Institutes of Health (UC4 DK108483). Product support was provided by Dexcom, Inc., (research discount on CGM sensors, transmitters, and receivers) and Tandem Diabetes Care, Inc., (infusion sets and cartridges provided in-kind). Tandem t:AP insulin pumps were purchased from Tandem Diabetes Care, Inc., at full price. The funders and device manufacturers had no influence on the design or conduct of the trial and were not involved in data collection or analysis, the writing of the article, or the decision to submit it for publication.
Supplementary Material
References
- 1. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 2. Breton MD, Kanapka LG, Beck RW, et al. : A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breton MD, Kovatchev BP: One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown SA, Forlenza GP, Bode BW, et al. : Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nimri R, Bratina N, Kordonouri O, et al. : MD-Logic overnight type 1 diabetes control in home settings: a multicentre, multinational, single blind randomized trial. Diabetes Obes Metab 2017;19:553–561. [DOI] [PubMed] [Google Scholar]
- 8. Pinsker JE, Muller L, Constantin A, et al. : Real-world patient-reported outcomes and glycemic results with initiation of control-IQ technology. Diabetes Technol Ther 2021;23:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thabit H, Tauschmann M, Allen JM, et al. : Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R: Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015;3:17–26. [DOI] [PubMed] [Google Scholar]
- 11. Bally L, Thabit H, Kojzar H, et al. : Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 2017;5:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown S, Raghinaru D, Emory E, Kovatchev B: First look at control-IQ: a new-generation automated insulin delivery system. Diabetes Care 2018;41:2634–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dassau E, Pinsker JE, Kudva YC, et al. : Twelve-week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: effect on hemoglobin A1c and hypoglycemia. Diabetes Care 2017;40:1719–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palisaitis E, El Fathi A, Von Oettingen JE, et al. : The efficacy of basal rate and carbohydrate ratio learning algorithm for closed-loop insulin delivery (artificial pancreas) in youth with type 1 diabetes in a diabetes camp. Diabetes Technol Ther 2020;22:185–194. [DOI] [PubMed] [Google Scholar]
- 15. Deshpande S, Pinsker JE, Zavitsanou S, et al. : Design and clinical evaluation of the interoperable artificial pancreas system (iAPS) smartphone app: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol Ther 2019;21:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinsker JE, Deshpande S, McCrady-Spitzer S, et al. : Use of the interoperable artificial pancreas system for type 1 diabetes management during psychological stress. J Diabetes Sci Technol 2021;15:184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deshpande S, Pinsker JE, Church MM, et al. : Randomized crossover comparison of automated insulin delivery versus conventional therapy using an unlocked smartphone with scheduled pasta and rice meal challenges in the outpatient setting. Diabetes Technol Ther 2020;22:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gondhalekar R, Dassau E, Doyle III FJ: Velocity-weighting & velocity-penalty MPC of an artificial pancreas: improved safety & performance. Automatica (Oxf) 2018;91:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harvey RA, Dassau E, Zisser H, et al. : Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol 2012;6:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi D, Dassau E, Doyle III FJ: Multivariate learning framework for long-term adaptation in the artificial pancreas. Bioeng Transl Med 2019;4:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aiello EM, Deshpande S, Ozaslan B, et al. : Review of automated insulin delivery systems for individuals with type 1 diabetes: tailored solutions for subpopulations. Curr Opin Biomed Eng 2021;19:100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinsker JE, Laguna Sanz AJ, Lee JB, et al. : Evaluation of an artificial pancreas with enhanced model predictive control (eMPC) and a glucose prediction trust index with unannounced exercise. Diabetes Technol Ther 2018;20:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.