Abstract
Objectives
Methylphenidate is a ‘prescription only’ drug against attention disorders which is increasingly used by adults. We investigated whether methylphenidate in adults was associated with an increased risk of psychiatric events such as depression, and suicide attempt and overall mortality.
Design
A population-based matched cohort design.
Setting
The Integrated Primary Care Information system, a general practitioners (GP) database in the Netherlands with a source population of 2.5 million inhabitants.
Participants
During the study period between 1 June 1996 and 1 January 2018, 8905 adults started methylphenidate and were matched to 10 non-users on sex, age, GP practice and ad prescription date. The total study population consisted of 97 198 participants.
Main outcome measures
Serious psychiatric events such as depression and suicide attempts, and overall mortality.
Analyses
Risks of development of each event during the use of methylphenidate were expressed as HR with 95% CI, adjusted for relevant confounders with methylphenidate as a time-dependent determinant. Additional adjustment was performed for the intervention (‘intention-to-treat’).
Results
Although during follow-up, the unadjusted risks of depression and suicide attempt were strongly increased in users, depression and psychosis became non-significant after adjustment for alcohol-abuse and substance-abuse and psychiatric disease in the medical history and after adjustment for ‘intention-to-treat’. However, the risk of suicide attempts remained significantly increased after full adjustment (HR 2.0; 95% CI 1.1 to 3.6), and was highest in women and in participants within the age-group of 18–40 years. The unadjusted risk of overall mortality was strongly increased, but this lowered to a significant 30% risk increase (HR 1.3; 95% CI 1.1 to 1.6) after full adjustment.
Conclusion
There is an increased risk of suicide attempts in adults up to 40 years of age after starting methylphenidate and this risk should be carefully considered before prescribing to this group.
Keywords: suicide & self-harm, adverse events, epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
It consists of a prospective population-based controlled cohort study with limited chance of selection-bias or information bias.
The use of complete general practitioner (GP) records in a healthcare system with a central role of the GP is a strength.
Limitations are that there are prescription, but not dispensing data and that there are no in-hospital drug data.
Introduction
Methylphenidate is a psychostimulant which is pharmacologically related to amphetamines and which was already registered more than 50 years ago for the treatment of children with a hyperkinetic syndrome, later named ‘attention deficit hyperactivity disorder’ (ADHD). Methylphenidate is increasingly used in children in many countries.1–3 ADHD is defined as a mental health disability, which usually begins before 12 years of age, and is characterised by three main symptoms: inattention, impulsivity and hyperactivity (without hyperactivity, the term ‘attention deficit disorder’ (ADD) may be used). The intensity of the symptoms tends to decrease with ageing, but in 40%–50% of people diagnosed with ADHD in childhood, symptoms may persist during adolescence and adulthood.4 5 Therefore, methylphenidate is also increasingly used in adults,4 which was considered as an ‘off-label’ group for several years. In patients with ADD or ADHD, methylphenidate improves the balance between concentration and distraction and decrease hyperactivity.6 Over the past years, these ‘prescription only’ drugs were increasingly used ‘off label’ in adults for a variety of indications. In 2018, methylphenidate was registered in the Netherlands for use in adults. Until April 2017, approximately 1200 reports of mostly serious adverse events attributed to methylphenidate were notified to the national Dutch Pharmacovigilance Centre, of which 542 (45.2%) in adults.7 Psychiatric adverse events were frequent among these reported events, but also cardiovascular events were reported.
Longitudinal studies have shown that ADHD in childhood is itself a risk factor for a diagnosis of psychosis in adult life.8–10 Research indicates that these disorders share common genetic11 and environmental aetiologies.8 12 A potential mediator of the association between ADHD and psychosis is the prescription of central stimulants for ADHD, which causes considerable concern for several clinicians.13 14 Central stimulants act as indirect dopamine agonists and are presumed to amplify neuronal signalling by prompting a marked increase in the extracellular concentration of neurotransmitters in the prefrontal cortex of the brain.14 Methylphenidate blocks the transporters of dopamine and norepinephrine, inhibits their presynaptic reuptake and has stimulant properties.6 Increased concentrations of synaptic dopamine have also been implicated in the generation of psychotic symptoms.15 Hence, the pharmacological mechanism of central stimulant medication can be viewed by clinicians as having the potential to induce psychotic symptoms and disorders.10 16
Therefore, the current study was performed to investigate whether the use of methylphenidate was associated with an increased risk of psychiatric adverse events such as depression, psychosis or suicide attempts in adults. We also investigated whether there was an increased risk of overall mortality in methylphenidate users.
Methods
Setting
The source population consisted of all patients who were registered with one of the general practitioners (GPs) who contribute information to the Integrated Primary Care Information (IPCI) database, which was established in 1992.17 IPCI is a longitudinal observational database with data from computer-based patient records retrieved from a selected group of GPs throughout the Netherlands, who voluntarily supply data to the database. In the Netherlands, the GP plays a central role in the healthcare system and acts as a gatekeeper by referring patients to other medical disciplines for outpatient or inpatient care and as a central receiver of information from secondary or tertiary care. Data from the GP computer system are downloaded on a monthly basis and sent to the IPCI gatekeeper who anonymises all information before further access is provided to the researchers. It is a dynamic cohort because over time, people may enter the population as new patients, or leave because of removal or death. Details of the database have been described elsewhere.17 18 Currently, more than 600 GPs are providing data to the database which has expanded to now more than 2 500 000 patients. The database is representative of the Dutch population regarding age and sex. The IPCI database complies with European Union guidelines on the use of medical data for medical research and has been proven valid for pharmacoepidemiological studies. For the use of IPCI for this study, permission has been granted by the IPCI review board (RvT 7/2017).
Source population
The source population consisted of 2 546 082 individuals with at least 1 year of medical history within the IPCI database with an average follow-up time of slightly more than 4 years. Start of the follow-up period was 1 June 1996 or 1 year after enrolment with the GP practice when starting after 1 June 1996 (most practices), and the end of follow-up was death, removal or end of the study on 1 January 2018, whichever came first.
Design and study population
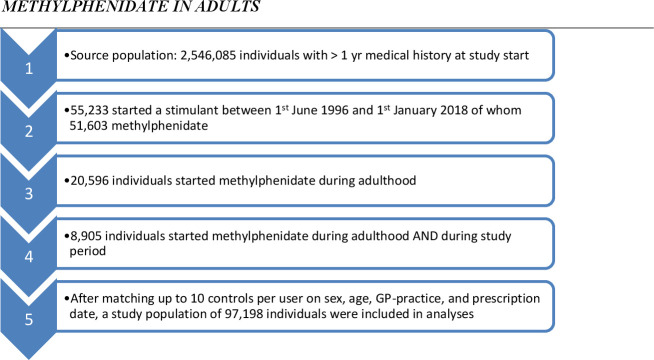
For this matched cohort design, we selected only starters with methylphenidate of 18 years or older. Out of 51 603 starters (‘first ever use’) with methylphenidate, 20 596 (40%) started during adulthood of whom 8905 during the study period (11 691 started as adult but before their practice participated in IPCI). Because methylphenidate was mainly prescribed to relatively young male adults, we sampled up to 10 non-users without history of methylphenidate, for each of these 8905 starters whom were matched on sex, age (less than 1-year difference) and GP-practice. This resulted in a total study population of 97 198 subjects (see flow diagram figure 1). For each matched set of 1 user and (mostly) 10 non-users during the study period, follow-up started at the day of first prescription of methylphenidate and this date was also allocated to the 10 non-users. All participants were eligible for GP healthcare during the study period.
Figure 1.
Flow scheme from source population to study population. GP, general practitioner.
Outcomes
All registered diagnoses and problem codes during the study period were gathered, as coded according to the International Classification of Primary Care (ICPC) coding thesaurus.19 Apart from overall mortality, the following psychiatric outcomes were studied: psychosis (ICPC P71-P73, P98); anxiety (P74); hypochondria (P75); depression (P76); suicide/suicidal attempt (P77); neurasthenia/burnout (P78, P79); personality disorder (P80) and other psychiatric illness (P99). For each diagnosis, those with a prevalent code before the index date were excluded from the follow-up analyses in order to study the association between methylphenidate and incident psychiatric outcomes. All mortality is registered in the IPCI database. For every deceased individual, month and year are registered. For the precise day of the month, the original medical record was studied.
Medication exposure
All medications prescribed by the GPs are automatically stored. For each prescription, the prescribed daily number, the strength, the total number of prescribed units (tablets and capsules) and route of administration (oral, parenteral, topical, etc.) are registered. Specialist medication is only included if the GP continues prescribing, for instance, if medication is chronic.
For each prescription of methylphenidate, we calculated the prescription length by dividing the total number of prescribed units by the prescribed daily number. For other drugs used in this study, such as antidepressants and antipsychotics, exposure was calculated in the same way.
Cofactors
As potential confounders of the association between methylphenidate and psychiatric events, we considered the following independent risk factors: sex, age, smoking, body mass index (BMI), alcohol abuse/intoxication (ICPC P15), medication abuse (P18), tobacco abuse (, drug abuse (P19), psychosis (P71-P73, P98), depression (P76), anxiety (P74) or neuroses in history (P78, P79), personality disorder (P80), other psychiatric disease (P99) and use of antidepressants (ATC code N06A), antipsychotics (N05A), anxiolytics (N05B) and sedatives (N05C). For the endpoint overall mortality, we considered independent risk factors, notably BMI, smoking, diabetes mellitus, hypertension, hypercholesterolemia and history of stroke, heart failure or arrhythmia. Smoking was distinguished into ‘current’, ‘past’ and ‘never’. Diabetes mellitus was defined as a diagnosis or problem code before the index date, if the patient had a prescription for a hypoglycaemic agent (ATC code ‘A10’), or if the patient had a fasting glucose level>7.0 mmol/L. Hypertension was considered as present if any of the ICPC codes K85, K86 or K87 was given as a diagnosis or problem code before the index date, or if the patient used an antihypertensive before the index date. As there were often multiple measurements for each cofactor, the one which was closest to the event date was chosen.
Statistics
Comparison between baseline variables in methylphenidate users and non-users were expressed as proportion ratios with 95% confidence limits to show the magnitude and significance of each variable. We calculated HRs with 95% CI in the cohort analysis in which users/non-users were followed for the occurrence of the above-mentioned psychiatric disease outcomes with a Cox proportional hazards model. In this model, methylphenidate was defined as a time-dependent risk factor and adjustment was performed for the cofactors listed above which were treated as confounders if they changed the point estimate by 10% or more. Because the percentage of missing data was sometimes large, that is, for smoking and BMI, we decided not to impute these values but rather to adjust with missing status as a separate dummy variable in a categorical set of values to investigate whether missing status was a confounder. Furthermore, we adjusted for the likelihood of being treated (adjustment for intention-to-treat). Finally, all 168 cases with a notification of suicide (attempt) were validated by reference to the medical patient records, as well as all 1027 deaths.
Patient and public involvement
There was no public or patient involvement. Only completely anonymised general practice data were used.
Results
General characteristics
On average, the 8905 starters on methylphenidate received 10 prescriptions with an average total duration of 370 days. The mean starting dose of the first prescription was 30 mg per day, while the mean dosage of the last prescription during the study period was 35 mg per day. Eighty-four percent of the prescribers of the first prescription was a GP. Further general characteristics of the study population are given in table 1. Fifty-four percent was male and 64% was in the age category of 18–40 years. Methylphenidate users had a significantly higher prevalence of tobacco, alcohol and other substance abuse, as well as of a history of psychiatric comorbidity.
Table 1.
Characteristics of the study population*
| Characteristic | Methylphenidate (n=8905) | No methylphenidate (n=88 293) |
Proportion ratio (95% CI) |
| Sex | |||
| Men | 4839 (54.3%) | 47 907 (54.3%) | 1.0 (reference) |
| Women | 4066 (45.7%) | 40 386 (45.7%) | 1.0 (0.9 to 1.1) |
| Age | 36.5 years | 36.3 years | |
| 18–40 years | 5737 (64.4%) | 56 888 (64.4%) | 1.0 (reference) |
| 41–60 years | 2584 (29.0%) | 25 847 (29.2%) | 1.0 (0.9 to 1.1) |
| 61–80 years | 476 (5.3%) | 4718 (5.3%) | 1.0 (0.9 to 1.1) |
| >80 years | 108 (1.3%) | 840 (0.01%) | 1.3 (1.1 to 1.6) |
| Follow-up (days) | 1943 days | 1944 days | |
| BMI* | |||
| <25 | 864 (9.7%) | 5617 (6.4%) | 1.0 (reference) |
| 25–30 | 717 (8.1%) | 5746 (6.5%) | 0.8 (0.7 to 0.9) |
| >30 | 629 (7.1%) | 4702 (5.3%) | 0.9 (0.8 to 1.0) |
| Smoking* | |||
| Never | 2111 (23.7%) | 21 629 (24.5%) | 1.0 (reference) |
| Past | 723 (8.1%) | 4634 (5.2%) | 1.6 (1.5 to 1.8) |
| Current | 2292 (25.7%) | 13 111 (14.8%) | 1.8 (1.7 to 1.9) |
| Alcohol abuse | 352 (3.9%) | 878 (1.0%) | 4.1 (3.6 to 4.7) |
| Acute alcohol intoxication | 105 (1.2%) | 398 (0.5%) | 2.6 (2.1 to 3.3) |
| Tobacco abuse | 810 (9.1%) | 3930 (4.5%) | 2.2 (2.0 to 2.3) |
| Medicines abuse | 87 (0.9%) | 230 (0.3%) | 3.8 (3.0 to 4.8) |
| Drug abuse | 525 (5.9%) | 859 (1.0%) | 6.4 (5.7 to 7.1) |
| History of: | |||
| Organic psychosis (delier) | 37 (0.4%) | 157 (0.2%) | 2.3 (1.6 to 3.3) |
| Schizophrenia | 36 (0.4%) | 282 (0.3%) | 1.3 (0.9 to 1.8) |
| Affective psychosis | 82 (0.9%) | 245 (0.3%) | 3.3 (2.6 to 4.3) |
| Anxiety | 922 (10.4%) | 3602 (4.1%) | 2.8 (2.6 to 3.0) |
| Hypochondria/hysteria | 55 (0.6%) | 392 (0.4%) | 1.4 (1.1 to 1.9) |
| Depression | 1772 (19.9%) | 5252 (5.9%) | 4.3 (4.0 to 4.6) |
| Suicide (attempt) | 161 (1.8%) | 410 (0.5%) | 4.0 (3.3 to 4.8) |
| Burnout/overstrain | 886 (9.9%) | 3962 (4.5%) | 2.6 (2.4 to 2.8) |
| Other neuroses | 169 (1.9%) | 674 (0.7%) | 2.5 (2.1 to 3.0) |
| Personality disorder | 479 (5.4%) | 822 (0.9%) | 6.3 (5.6 to 7.0) |
| Other non-specified psychotic disorder | 80 (0.9%) | 372 (0.4%) | 2.2 (1.7 to 2.8) |
| Other psychiatric disorder | 541 (6.1%) | 1100 (1.2%) | 5.3 (4.7 to 5.9) |
| Total psychiatric | 3729 (41.9%) | 13 549 (115.3%) | 4.5 (4.3 to 4.7) |
| Previous or current use of: | |||
| Antipsychotics | 835 (9.4%) | 1884 (2.1%) | 4.8 (4.4 to 5.2) |
| Antidepressants | 3037 (34.1%) | 10 213 (11.6%) | 4.0 (3.8 to 4.2) |
| Anxiolytics | 3338 (37.5%) | 16 390 (18.6%) | 2.6 (2.5 to 2.8) |
| Sedatives | 2498 (28.1%) | 10 201 (11.6%) | 3.0 (2.8 to 3.1) |
*Values were missing for BMI (n=78 923), smoking (n=52 698).
BMI, body mass index.
Incident psychiatric adverse events
There were significantly increased risks of incident organic psychosis (delier), affective psychosis, anxiety, depression, burnout, other neuroses, personality disorder, and other psychotic and other psychiatric disorders in methylphenidate users. There was a significantly increased risk of suicide attempts in users of methylphenidate. Increased risks were observed in smokers and people with a history of a variety of psychiatric diseases in the medical history. Further adjustment for smoking, BMI, alcohol abuse, acute alcohol intoxication, medicines abuse, tobacco abuse, drug abuse and psychiatric events in history reduced the risk estimates in table 2. However, adjustment for the intervention/intention-to-treat abolished almost all statistically significant relative risks in table 2. This means that with the exception of overall mortality and suicide (attempts), all significantly increased risks in table 2 were confounded by the intention-to-treat itself.
Table 2.
Number of cases and referents per psychiatric disease code/overall mortality occurring during follow-up and the risk (HR) to develop such a disease in users of methylphenidate in comparison to non-users
| Outcome | Case | Referents | HR (95% CI)* | HR (95% CI)† |
| Organic psychosis (delier) | 154 | 96 850 | 8.3 (5.2 to 13.0) | 1.7 (0.9 to 3.0) |
| Schizophrenia | 50 | 96 830 | 1.6 (0.4 to 6.7) | 0.9 (0.2 to 4.3) |
| Affective psychosis | 64 | 96 807 | 3.5 (1.4 to 6.9) | 1.2 (0.4 to 3.6) |
| Anxiety | 1374 | 91 300 | 1.8 (1.4 to 2.4) | 0.8 (0.6 to 1.1) |
| Hypochondria/hysteria | 109 | 96 642 | 1.5 (0.5 to 4.0) | 0.8 (0.3 to 2.6) |
| Depression | 1468 | 88 706 | 2.7 (2.1 to 3.3) | 1.0 (0.8 to 1.3) |
| Suicide/suicide attempt | 129 | 96 498 | 5.5 (3.5 to 8.6) | 2.0 (1.1 to 3.6) |
| Burnout/overstrain | 1583 | 90 767 | 1.4 (1.0 to 1.8) | 1.0 (0.7 to 1.4) |
| Other neuroses | 183 | 96 172 | 2.0 (1.0 to 3.9) | 0.7 (0.4 to 1.6) |
| Personality disorder | 412 | 95 485 | 6.0 (4.5 to 7.9) | 1.2 (0.8 to 1.6) |
| Other non-specified psychotic disorder | 162 | 96 584 | 3.2 (1.8 to 5.7) | 0.9 (0.5 to 1.8) |
| Other psychiatric disorder | 538 | 95 019 | 4.9 (3.8 to 6.5) | 1.4 (0.9 to 1.8) |
| Any psychosis | 347 | 95 727 | 4.8 (3.4 to 6.8) | 1.3 (0.9 to 2.0) |
| Death‡ | 946 | 96 252 | 7.5 (6.3 to 8.9) | 1.3 (1.1 to 1.6) |
*All HRs are adjusted for sex, and age by matching.
†All HRs are adjusted for sex, age, smoking, BMI, alcohol abuse, acute alcohol intoxication, medicines abuse, tobacco abuse, drug abuse, psychosis in history (for non-psychotic endpoints), depression in history (for non-depressive endpoints), anxiety in history (for non-anxiety endpoints), neuroses in history, personality disorder, other psychiatric disease and for ‘intention-to-treat’.
‡Additionally are adjusted for hypertension, diabetes mellitus, hypercholesterolemia and decreased renal function.
BMI, body mass index.
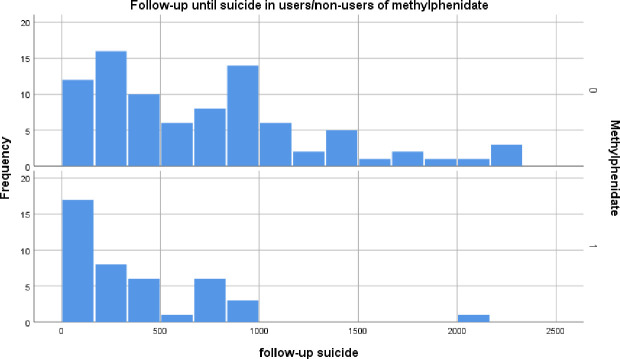
All 168 cases with a notification of suicide (attempt) were validated by reference to the medical patient records. Out of these 168 cases, 117 were suicide attempts of which 16 were successful, while the remaining 51 cases were notification of suicidal ideation, or phantasies/tendencies. A further 19 cases of successful suicide came from the validation of all 1027 cases of death. Restricting the analyses to these 136 cases of whom six were excluded because of a history of suicidal ideation/attempts before the index date, yielded an unadjusted relative risk of suicide in methylphenidate users of 5.5 (95% CI 3.5 to 8.6). After additional adjustment for the intervention, the relative risk went down to 2.0 (95% CI 1.1 to 3.6) for current use of methylphenidate but remained statistically significant. In table 3A, these risks were stratified according to sex and age-groups. The majority of suicide (attempts) were in the age group from 18 through 40 years. The risk of suicide (attempts) was highest in women. As one can see from figure 2, the suicide (attempt) occurs especially in the early period of follow-up in users, whereas it is spread over a longer period of follow-up in non-users.
Table 3.
Age-specific and sex-specific risks of suicide, suicidal attempts/ideation during use of methylphenidate
| Characteristic | Cases/controls (n) |
HR (95% CI) |
| Women | 64/44 043 | 3.9 (1.5 to 10.2) |
| Men | 65/52 294 | 1.1 (0.4 to 2.6) |
| 18–40 years of age | 84/62 196 | 2.4 (1.2 to 4.9) |
| 41–60 years of age | 38/28 108 | 1.3 (0.4 to 4.9) |
| 61–80 years of age | 4/4543 | 1.6 (0.0 to ∞) |
| >80 years of age | 3/836 | –* |
All hazard ratios (HR) are adjusted for sex, age, intervention, smoking, BMI, alcohol abuse, acute alcohol intoxication, medicines abuse, tobacco abuse, drug abuse, psychosis in history (for non-psychotic endpoints), depression in history (for non-depressive endpoints), anxiety in history (for non-anxiety endpoints), neuroses in history, personality disorder, other psychiatric disease and ‘intention-to-treat’.
*No cases exposed to methylphenidate.
BMI, body mass index.
Figure 2.
Time delay in follow-up days between first intake of methylphenidate and death. On the y-axis, the number of cases of death are given, while the x-axis represents the number of days of follow-up. In non-users with the same reference date as users (upper part of figure 2), this delay is spread over several years, whereas it is focused in the early weeks of intake in users (lower part of figure 2).
Overall mortality
Because the cause of death was not specifically coded in the GP database, we took overall mortality as an endpoint. Especially age, hypertension, diabetes mellitus and decreased renal function were associated with an increased risk of death.
We performed a validation of all 1027 cases of death to check the precise date of death, and to distinguish its causes—where possible—by going through the patient history. Thirty percent of all death occurred within 80 days after starting with methylphenidate. After restricting the analyses to cases in which the precise date of death could be verified (n=946), the unadjusted risk of all-cause mortality in users of methylphenidate was 7.5 (95% CI 6.3 to 8.9) but reduced to 1.3 (95% CI 1.1 to 1.6) after full adjustment. Apart from suicide, there was a large and significant risk increase in those who died during methylphenidate in palliative care with a risk of 12.7 (95% CI 9.5 to 16.9). As the pharmacologic effect of methylphenidate is immediate, we did not study duration-effect relationships. In extra analysis, we further adjusted all relative risks for dosage but this did not substantially change the risk estimates. Finally, we studied effect modification by sex-category and age category (table 4). The risk of mortality in the intervention group was significantly increased in women only. Furthermore, the risk was significantly increased by 60% in the age category 61–80 years of age. As one can see from figure 3, death occurred especially in the early period of follow-up in users, whereas it is spread over a longer period of follow-up in non-users.
Table 4.
Age-specific and sex-specific risks of al-cause mortality during the use of methylphenidate
| Characteristic | Cases/controls (n) | HR (95% CI) |
| Women | 375/44 077 | 1.7 (1.2 to 2.5) |
| Men | 571/52 154 | 1.1 (0.8 to 1.5) |
| 18–40 years of age | 67/62 478 | 1.2 (0.4 to 3.5) |
| 41–60 years of age | 240/28 191 | 1.1 (0.7 to 1.8) |
| 61–80 years of age | 412/4781 | 1.6 (1.2 to 2.2) |
| >80 years of age | 227/721 | 1.2 (0.7 to 2.0) |
All HRs are adjusted for sex, age, smoking, hypertension, diabetes mellitus, BMI, hypercholesterolemia, decreased renal function and ‘intention-to-treat’
BMI, body mass index.
Figure 3.
1 time delay in follow-up days between first intake of methylphenidate and suicide (attempt). On the y-axis, the number of cases of suicide (attempt) are given, while the x-axis represents the number of days of follow-up. In non-users with the same reference date as users (upper part of figure 3), this delay is spread over several years, whereas it is focused in the early weeks of intake in users (lower part of figure 3).
Discussion
From this study, we can conclude that although there was a strongly increased risk of psychiatric events in users of methylphenidate, most part of it was explained by confounding by intervention (‘intention-to-treat’). However, even after adjusting for the intervention, a significantly increased risk of suicide (attempts) after starting methylphenidate remained.
Especially the strong association with death in our study is striking. The risk increase of death was genuine but mainly explained by confounding by the indication palliative care because from validation of the medical records, it became clear that this risk increase was largely explained by starting methylphenidate in depressed or extremely tired patients in their latest phase of life. Because regular antidepressants take 6–8 weeks before they exert their therapeutic effects, they may be too late for treating depression in the last weeks of life and then psychostimulants may help. This is in line with British and Dutch guidelines.20
Similar to Dutch reports, data from the British Yellow Card scheme showed that, of 1335 adverse drug reaction reports regarding methylphenidate, 663 adverse reactions were psychiatric disorders, making these disorders the most frequently reported class of adverse drug reactions of methylphenidate (Vigilance and Intelligence Research Group; http://www.mhra.gov.uk/drug-analysis-prints/drug-analysis-prints-a-z/index.htm). Among these reports, 105 (15.8%) patients reported hallucinations, psychosis or psychotic disorders. Moreover, in an FDA review21 of data from 49 randomised controlled clinical trials investigating the effects of central stimulant medication in children, 11 adverse events related to psychosis or mania were observed during 743 person-years of follow-up in 5717 individuals, versus no events reported with placebo, giving a number needed to harm of 526 patients. Given these reports of treatment-emergent psychotic events with central stimulant medication, clinicians have been concerned that methylphenidate and other psychostimulants might provoke psychosis.10 13 In literature, the use of stimulant ADHD medication is considered as relatively contraindicated in patients with a history of psychosis.22 However, clinicians face a therapeutic dilemma without clear evidence to guide them when balancing the potential risk of psychotic events with the benefits of stimulants that are the first-line treatment for ADHD in adolescent and adult patients.23 Some observational studies22 24 that reported an increased risk of psychotic events associated with methylphenidate might be affected by confounding by indication; that is, patients who receive stimulant medication for ADHD are inherently different from those who do not and could have a greater risk of psychotic events independently of stimulant prescription. This type of confounding also played a role in our IPCI-study but by adjusting for independent risk factors and for the intervention, we were able to deal with it. In a study in 2016 that tried to adjust for confounding by indication, Man and colleagues25 used a within-individual case series design in a population of children and adolescents, and did not find an increased risk of psychotic events during methylphenidate treatment. In a direct comparison in the USA, methylphenidate was associated with a significantly lower risk of psychosis than amphetamine.26
Strengths and limitations
One of the major strengths is the population-based design of this study, as in the Netherlands everybody is designated to only one GP. Therefore, selection bias is highly unlikely. As the information on diseases is prospectively gathered and prescriptions are automatically and completely stored in the computer of the general practice, information bias is also highly unlikely. A limitation may be the fact that not every patient fills his prescription at the pharmacy. According to an earlier study, some 10% of prescriptions from the GP is not filled, and even if filled, patients may decide not to use their medicines.27 Also, we might have missed the most severe cases of ADHD/ADD, initially treated by the psychiatrist. However, in the majority of patients, the continuation of methylphenidate treatment goes through the GP. Therefore, we think that we will have enrolled also most of the severe cases, although somewhat later in their treatment course. And even if we miss some of the most severe cases, it is likely that this has led to conservative instead of inflated estimates.
However, chronic medication requires regular prescriptions and if patients get repeated prescriptions, it is more likely that these are really filled. But which product is ultimately filled, remains unknown in a GP database and this means that we could not adjust for regular or slow-release methylphenidate. Also, medication obtained via hospitals or outpatient clinics is missing, as well as illegal drug use. Usually, these types of exposure misclassification lead to an underestimation of the true risk because the group of non-exposed actually includes exposed individuals.28 Furthermore, although there is a specific guideline for GPs on the diagnosis and treatment of ADHD/ADD, it is possible that there is some misclassification of the diagnosis by GPs.
We adjusted for confounding by multivariable adjustment of the risk estimates by all known risk factors. Unfortunately, for some confounders, there were missing data (for instance, smoking and BMI). Because the percentage of missing data was sometimes large, we decided not to impute these values but rather to adjust with missing status as a separate dummy variable in a categorical set of values. In this way, we were able to investigate whether the missing status acted as a confounder but there was almost no confounding by missing status. An important potential confounder in any observation study in which the consequences of an intervention are studied, is the indication. Confounding by indication played a role in palliative care in which methylphenidate is used ‘off label’ as a psychostimulant. Also, as ADHD/ADD in childhood is a risk factor for a diagnosis of psychosis in adult life,6–8 confounding by indication will inevitably have played a role in the increased risk of psychosis which was found in this study although it disappeared after adjustment for the intervention. Although this might also partly explain the increased risk of suicide, an independent risk for methylphenidate remained and an adverse role of methylphenidate seems plausible because of its stimulant properties in a population of patients receiving methylphenidate despite psychiatric contraindications. Obviously, there is collinearity between the intervention at baseline and actual use during follow-up. People may decide not to fill a prescription for methylphenidate, fail to use it, stop it early or use it continuously. Although we adjusted for the intervention at baseline to adjust for non/registered mental comorbidity, changes during follow-up might have led to underestimation of the true risk, for instance, because patients with a lower vulnerability to suicidal thoughts stopped methylphenidate early during follow-up. Consequently, the risk of suicidal thoughts and attempts may have been underestimated. Therefore, it is important that our findings must be a starting point for further research.
In conclusion, in this large population-based study with data from GPs encompassing almost 100 000 people, methylphenidate was associated with a significantly increased risk of mortality, partly explained by ‘off label’ use as a psychostimulant in palliative care. There was a significantly increased risk of psychosis, and depression but this was probably confounded by intervention. However, there was also an increased risk of suicide in current users of methylphenidate, even after adjustment for the intervention. All in all, it seems that a cautious approach to prescribing methylphenidate in adults is warranted, especially in those with a history of suicidal ideation.
Supplementary Material
Footnotes
Contributors: BS and KC designed the study. Data gathering was performed by BS and KV. All contributed to analysis and writing of the manuscript. BS is responsible for the overall content as the guarantor.
Funding: This study was funded by the Health and Youth Care Inspectorate of the Netherlands.
Competing interests: KV works for a research department who received/receives unconditional research grants from Yamanouchi, Pfizer/Boehringer Ingelheim, Novartis, GSK, Chiesi, Amgen, UCB, Astra Zeneca and J&J.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Renoux C, Shin J-Y, Dell'Aniello S, et al. Prescribing trends of attention-deficit hyperactivity disorder (ADHD) medications in UK primary care, 1995-2015. Br J Clin Pharmacol 2016;82:858–68. 10.1111/bcp.13000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trip A-M, Visser ST, Kalverdijk LJ, et al. Large increase of the use of psycho-stimulants among youth in the Netherlands between 1996 and 2006. Br J Clin Pharmacol 2009;67:466–8. 10.1111/j.1365-2125.2009.03373.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry 2014;53:34–46. 10.1016/j.jaac.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cândido RCF, Menezes de Padua CA, Golder S, et al. Immediate-Release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2021;1:CD013011. 10.1002/14651858.CD013011.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry 2016;3:1157–65. 10.1016/S2215-0366(16)30190-0 [DOI] [PubMed] [Google Scholar]
- 6.Hennissen L, Bakker MJ, Banaschewski T, et al. Cardiovascular effects of stimulant and Non-Stimulant medication for children and adolescents with ADHD: a systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs 2017;31:199–215. 10.1007/s40263-017-0410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.lareb. Available: https://www.lareb.nl/nl/nieuwsoverzicht/betere-bewaking-veiligheid-methylfenidaat-voor-adhd-bij-volwassenen/
- 8.Dalsgaard S, Mortensen PB, Frydenberg M, et al. Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry 2014;29:259–63. 10.1016/j.eurpsy.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Kim-Cohen J, Caspi A, Moffitt TE. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry 2003;60:709–17. 10.1001/archpsyc.60.7.709 [DOI] [PubMed] [Google Scholar]
- 10.Hollis C, Chen Q, Chang Z, et al. Methylphenidate and the risk of psychosis in adolescents and young adults: a population-based cohort study. Lancet Psychiatry 2019;6:651–8. 10.1016/S2215-0366(19)30189-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thapar A, Martin J, Mick E, et al. Psychiatric gene discoveries shape evidence on ADHD’s biology. Mol Psychiatry 2016;21:1202–7. 10.1038/mp.2015.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peralta V, de Jalón EG, Campos MS, et al. The meaning of childhood attention-deficit hyperactivity symptoms in patients with a first-episode of schizophrenia-spectrum psychosis. Schizophr Res 2011;126:28–35. 10.1016/j.schres.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics 2016;137:peds.2015-2486. 10.1542/peds.2015-2486 [DOI] [PubMed] [Google Scholar]
- 14.Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 2006;31:2376–83. 10.1038/sj.npp.1301164 [DOI] [PubMed] [Google Scholar]
- 15.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 2012;69:776–86. 10.1001/archgenpsychiatry.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tost H, Alam T, Meyer-Lindenberg A. Dopamine and psychosis: theory, pathomechanisms and intermediate phenotypes. Neurosci Biobehav Rev 2010;34:689–700. 10.1016/j.neubiorev.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Lei J, et al. The introduction of computer-based patient records in the Netherlands. Ann Intern Med 1993;119:1036–41. 10.7326/0003-4819-119-10-199311150-00011 [DOI] [PubMed] [Google Scholar]
- 18.Vlug AE, van der Lei J, Mosseveld BM, et al. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med 1999;38:339–44. [PubMed] [Google Scholar]
- 19.Hofmans-Okkes IM, Lamberts H. The International classification of primary care (ICPC): new applications in research and computer-based patient records in family practice. Fam Pract 1996;13:294–302. 10.1093/fampra/13.3.294 [DOI] [PubMed] [Google Scholar]
- 20.Minton O, Stone P, Richardson A, et al. Drug therapy for the management of cancer related fatigue. Cochrane Database Syst Rev 2008:CD006704. 10.1002/14651858.CD006704.pub2 [DOI] [PubMed] [Google Scholar]
- 21.Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics 2009;123:611–6. 10.1542/peds.2008-0185 [DOI] [PubMed] [Google Scholar]
- 22.Cressman AM, Macdonald EM, Huang A, et al. Prescription stimulant use and hospitalization for psychosis or mania: a population-based study. J Clin Psychopharmacol 2015;35:667–71. 10.1097/JCP.0000000000000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018;5:727–38. 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shyu Y-C, Yuan S-S, Lee S-Y, et al. Attention-Deficit/Hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophr Res 2015;168:161–7. 10.1016/j.schres.2015.08.033 [DOI] [PubMed] [Google Scholar]
- 25.Man KKC, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry 2016;6:e956. 10.1038/tp.2016.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med 2019;380:1128–38. 10.1056/NEJMoa1813751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691–705. 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol 1977;105:488–95. 10.1093/oxfordjournals.aje.a112408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.