Abstract
Sacculi of Bacillus sphaericus CCM 2177 contain a secondary cell wall polymer which was completely extracted with 48% hydrofluoric acid. Nuclear magnetic resonance analysis showed that the polymer is composed of repeating units, as follows: →3)-[4,6-O-(1-carboxyethylidene)]∼0.5-β-d-ManpNAc-(1→4)-β-d-GlcpNAc-(1→. The N-terminal part of the S-layer protein carrying S-layer homologous motifs recognizes this polymer as a binding site.
Crystalline bacterial cell surface layers (S-layers) represent the outermost cell envelope component of many bacteria and archaea (for reviews, see references 4, 23, 27, and 28). S-layers are composed of identical proteinaceous subunits, and they assemble into either oblique, square, or hexagonal lattice types. To answer the question of how S-layer proteins of gram-positive bacteria are anchored to the rigid cell wall layer, the whole cell envelope complexes of Bacillus stearothermophilus wild-type strains and an oxygen-induced variant strain were analyzed, and secondary cell wall polymers (SCWP) were found to function as binding sites for this class of secreted proteins (9, 21). Based on structure, teichoic acids, teichuronic acids, lipoteichoic acids, and lipoglycans are distinguished among SCWP (for reviews, see references 3, 10, 19, and 22). Most of the biological functions ascribed to SCWP, such as binding of cations, protecting the cell against toxic metals, keeping the peptidoglycan sacculus in an expanded state by charge repulsion, binding of protons to create an acidic cell wall during bacterial growth, and providing a biophysical barrier to prevent diffusion of substances, have been viewed in the context of their acidic nature (for a review, see reference 3).
In contrast to those of B. stearothermophilus wild-type strains (9, 11), the S-layer proteins of most gram-positive bacteria carry three typical S-layer homologous (SLH) motifs (16) at the N-terminal part, each of them consisting of approximately 50 to 60 amino acids. In addition to being present in S-layer proteins, SLH motifs were also identified at the C-terminal end of cell-associated exoenzymes or other exoproteins (14, 17). In several studies, SLH motifs were found to anchor the different types of cell-associated exoproteins to the rigid cell wall layer (6, 7, 14, 15, 17, 18, 20). However, only for a few organisms was it confirmed that an SCWP is involved in the binding process (6, 7, 18, 21). In the present study, the structure of the SCWP of Bacillus sphaericus CCM 2177 was characterized by nuclear magnetic resonance (NMR) analysis. Moreover, evidence was provided that the SCWP, not the peptidoglycan, recognizes the N-terminal part of the S-layer protein carrying at least one SLH motif.
Characterization of the S-layer of B. sphaericus CCM 2177.
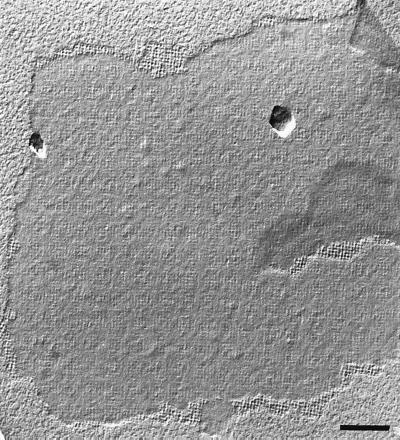
Freeze-etching of whole cells from B. sphaericus CCM 2177 grown in continuous culture in nutrient broth at 30°C at a dilution rate of 0.16 h−1 revealed that the cell surface was completely covered with a square S-layer lattice showing a rather smooth outer surface (data not shown). The S-layer protein could completely be extracted from cell wall fragments prepared as described previously (9, 21, 25) with 5 M guanidine hydrochloride (GHCl). During removal of GHCl by dialysis against 10 mM CaCl2 at 20°C, the S-layer subunits assembled into flat sheets with a maximum width of 2 μm. Negative staining and freeze-drying revealed that the self-assembly products represented double layers in which the individual S-layers were oriented to each other, with corrugated inner surfaces (Fig. 1). Upon dialysis of the GHCl-extracted S-layer protein against distilled water or 10 mM EDTA at 20°C, mainly amorphous aggregates were observed in negatively stained preparations (data not shown). The positive effect of calcium ions on the in vitro self-assembly was also seen by the degree of assembly (percentage of total S-layer protein assembled [21]). When dialysis was performed against distilled water or 10 mM EDTA, the degree of assembly was <5% after 4 h and increased to 25% 18 h after the dialysis procedure was started. For comparison, degrees of assembly of 55 and 80%, respectively, were achieved at the above-mentioned points of time when dialysis was performed against 10 mM CaCl2.
FIG. 1.
Electron micrograph of a freeze-dried S-layer self-assembly product of B. sphaericus CCM 2177. The outer S-layer surface is rather smooth, whereas the inner S-layer surface is much more corrugated. Bar, 200 nm.
Chemical analyses of native and HF-extracted peptidoglycan-containing sacculi.
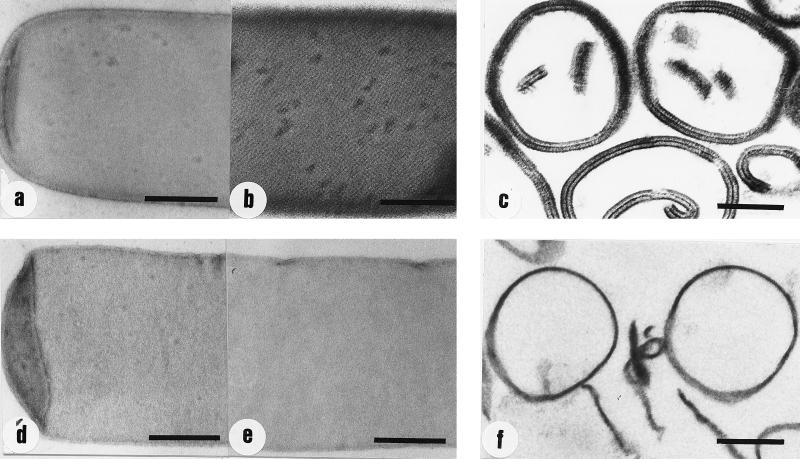
Native peptidoglycan-containing sacculi and those extracted with 48% HF were prepared as described previously (21). The results from amino acid and amino sugar analyses of hydrolyzed samples of native and HF-extracted sacculi are summarized in Table 1. For calculating the molar ratios between the individual components, glutamic acid (Glu) was set to a value of 1. With the exception of the excess glucosamine (GlcNH2) and the occurrence of substantial amounts of mannosamine (ManNH2), the molar ratios of all other components were typical of the A4α-chemotype (26). Extracting the native peptidoglycan-containing sacculi with 48% HF for 48 h at 4°C (21) led to the complete removal of the excess of GlcNH2 and of ManNH2, whereas the molar ratios for all other peptidoglycan constituents remained unchanged (Table 1). In comparison to native peptidoglycan-containing sacculi, those extracted with HF did not show any changes in size and morphology but appeared less electron dense in negatively stained preparations (Fig. 2a and d).
TABLE 1.
Composition of native and HF-extracted peptidoglycan-containing sacculi of B. sphaericus CCM 2177a
| Amino acid or sugar | Molar composition
|
||
|---|---|---|---|
| Native | HF-extracted (48 h) | HF-extracted (96 h) | |
| GlcNH2 | 2.76 | 1 | 0.50 |
| Glu | 1 | 1 | 1 |
| Ala | 1.33 | 1.29 | 1.36 |
| Asp | 0.51 | 0.52 | 0.53 |
| Lys | 0.53 | 0.54 | 0.54 |
| ManNH2 | 0.82 | 0 | 0 |
For liberation of the peptidoglycan constituents hydrolysis was performed with 6 N HCl for 6 h at 110°C (9, 21, 24). Molar composition is shown with glutamic acid (Glu) = 1. GlcNH2, glucosamine; Ala, alanine; Asp, aspartic acid; Lys, lysine; ManNH2, mannosamine. (Muramic acid could not quantitatively be determined by this analysis method.)
FIG. 2.
Electron micrographs of negatively stained and ultrathin-sectioned preparations of native and HF-extracted peptidoglycan-containing sacculi of B. sphaericus CCM 2177. (a and b) Negatively stained preparations of native peptidoglycan-containing sacculi before and after recrystallization of the S-layer protein, respectively; (c) ultrathin-sectioned preparation of native peptidoglycan-containing sacculi after recrystallization of the S-layer protein; (d and e) negatively stained preparations of HF-extracted peptidoglycan-containing sacculi (48% HF, 48 h at 4°C) before and after the addition of the GHCl-extracted S-layer protein and dialysis, respectively; (f) ultrathin-sectioned preparation of HF-extracted peptidoglycan-containing sacculi after the addition of the GHCl-extracted S-layer protein and dialysis. Bars, 250 nm.
Chemical and NMR analysis of the HF-extracted purified SCWP.
The HF-extracted SCWP, which represented about 50% of the native peptidoglycan-containing sacculi by weight, was purified by gel permeation chromatography according to a method described previously (24). Hydrolysis of the SCWP with 4 N HCl (6 h at 110°C) led to a GlcNH2-to-ManNH2 molar ratio of 1 to 1, whereas a molar ratio of 2.1 to 1 was obtained by using 6 N HCl (6 h at 110°C). For NMR analysis, 4.6 mg of the purified SCWP was dissolved in D2O (0.6 ml, 99.95%). Spectra were recorded at 300 and 330 K at 300.13 MHz for 1H and at 75.47 MHz for 13C with a Bruker AVANCE 300 spectrometer equipped with a 5-mm QNP probehead with z gradients. 1H spectra were referenced internally to sodium 3-trimethylsilyl-1-propane sulfonate (δ = 0); 13C spectra were referenced externally to 1,4-dioxane (δ = 67.40). COSY, TOCSY, HMQC, HMBC, and NOESY spectra were recorded with standard XWINNMR software (Bruker). 1H and 13C NMR spectra recorded at 300 K revealed the presence of pyruvate groups as indicated by a signal at 1.50 ppm in the proton domain and by signals at 24.79, ∼100.0, and 174.9 ppm in the HMBC spectrum. The signal intensity of the pyruvate methyl groups corresponded to ∼20 to 25% of the integral of the neighboring N-acetyl groups at δ 2.02 to 2.04. Pyruvate substituents were cleaved off, and a well-resolved spectrum was recorded at 330 K (Table 2). The 1H NMR spectrum displayed two major signals for anomeric protons at 4.82 ppm (J ∼ 1.0 Hz) and 4.59 ppm (J ∼ 7.7 Hz) as well as two minor signals at 4.88 and 5.21 ppm. Since the 13C NMR spectrum contained only two major signals for anomeric carbons (100.55 and 98.89 ppm), the SCWP had to be composed of disaccharide repeating units occurring in the β-anomeric configuration (JC1 = 164.2 and 165.1 Hz, respectively). The two minor anomeric 1H and 13C signals were assigned to reducing α-configured N-acetyl mannosamine (ManNAc; 5.21 and 91.8 ppm) and terminal 2-acetamido-2-deoxy-β-d-hexopyranosyl units (4.88 and 100.2 ppm). Comparison of the proton signal intensities indicated an average of 8 to 9 disaccharide residues in the polysaccharide sample. This finding was substantiated by matrix-assisted laser desorption ionization–time of flight data which revealed major signals at 2,073.4, 2,887.2, 3,294.2, 3,700.9 and 4,107.5 mass units, corresponding to 6 to 10 N-acetyl hexosamine disaccharide units [(HexNAc)2] units (M + Na + H2O).
TABLE 2.
NMR data of the pyruvate-free SCWP of B. sphaericus CCM 2177 recorded at 330 Ka
| Atom position | NMR analysis results for:
|
|||||
|---|---|---|---|---|---|---|
| →3)-β-ManNAc
|
→4)-β-GlcNAc
|
|||||
|
1H
|
13C chemical shift (ppm) |
1H
|
13C chemical shift (ppm) | |||
| Chemical shift (ppm) | J (Hz) | Chemical shift (ppm) | J (Hz) | |||
| 1 | 4.82 | 1.0 | 100.55 | 4.59 | 7.8 | 98.89 |
| 2 | 4.65 | 4.3 | 50.81 | 3.74 | ND | 56.14 |
| 3 | 4.03 | 9.8 | 77.99 | 3.70 | ND | 73.40 |
| 4 | 3.61 | 9.6 | 66.03 | 3.69 | ND | 80.06 |
| 5 | 3.46 | 2.4 | 77.25 | 3.52 | ND | 75.46 |
| 6 | 3.90, 3.78 | 12.2, 5.0 | 61.62 | 3.86, 3.80 | ND | 61.27 |
Other signals were as follows: 2.02 and 2.04 ppm (CH3), 23.38 and 23.07 ppm (CH3), and 175.31 and 175.41 ppm (CO). ND, not determined.
Homonuclear and heteronuclear correlation spectra allowed the straightforward assignment of two units of 2-acetamido-2-deoxy-mannopyranosyl and 2-acetamido-2-deoxy-glucopyranosyl residues. NOESY spectra revealed an interresidue Noe from H-1 of the N-acetyl glucosamine (GlcNAc) residues to H-3 of the ManNAc units, whereas irradiation of H-1 of the ManNAc moieties yielded signal enhancement of H-3 and -4 (having similar chemical shifts) of the GlcNAc units. Since substitution at C-3 would lead to an upfield shift of C-2, the observed shift value for C-2 (56.14 ppm) was only compatible with a 4-O-substitution of the GlcNAc unit. The observed value for the optical rotation [α]D20 − 16° (c 0.4, H2O) of the SCWP indicated the presence of two units each of β-d-configured residues of ManNAc and GlcNAc (12). Thus, the structure of the pyruvic acid-free SCWP may be proposed to be as follows: →3)-β-d-ManpNAc-(1→4)-β-d-GlcpNAc-(1→.
Recrystallization and affinity studies with whole S-layer protein and proteolytic cleavage fragments as well as native and HF-extracted peptidoglycan-containing sacculi.
The GHCl-extracted S-layer protein was mixed with native or HF-extracted sacculi under conditions described previously (21), and the suspensions were dialyzed against distilled water, 10 mM CaCl2, or 10 mM EDTA at 4°C for 18 h. Ultrathin sectioning revealed that complete outer and inner S-layers were formed on native peptidoglycan-containing sacculi when dialysis was performed against distilled water or 10 mM CaCl2 (Fig. 2c). Negative staining showed that the formation of an extensive square lattice structure was strongly dependent on the presence of calcium ions during dialysis (Fig. 2a and b). If dialysis was against distilled water, only small, randomly oriented patches with square lattice symmetry consisting of up to 10 morphological units were formed (data not shown). When dialysis was performed against 10 mM EDTA, neither the square S-layer lattice nor the outer or inner S-layer could be observed (data not shown). Thus, the results from electron microscopic investigations demonstrated that calcium ions are required for the correct binding of the S-layer protein to the rigid cell wall layer as well as for the formation of the square lattice structure. Independent of the dialysis conditions, the S-layer protein did not bind to HF-extracted (48% HF, 48 h, 4°C) sacculi (Fig. 2d to f), which, according to chemical analysis, represented pure peptidoglycan (Table 1).
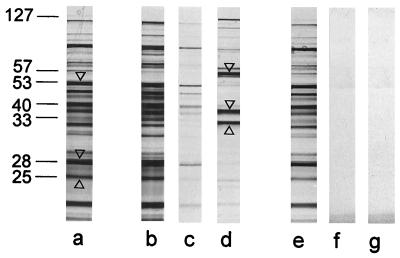
S-layer self-assembly products were dissolved in 2 M GHCl, and the S-layer protein was cleaved with endoproteinase Glu-C under conditions described previously (9, 21, 24). After removing GHCl by dialysis against 50 mM Tris-HCl buffer (pH 7.2), native peptidoglycan-containing sacculi were added. When the mixture was centrifuged, uncleaved S-layer protein and three major protein bands with estimated relative molecular masses of 57,000, 38,000, and 32,000 Da were enriched in the pellet (Fig. 3), whereas all other cleavage fragments remained unbound. The protein bands which showed affinity to native sacculi had N termini (AQVND) identical to that of the whole S-layer protein (AQVNDYNKISGYAKEAVQSLVDQGVIOGDTNGNFN) (SLH motif underlined) showing an estimated relative molecular mass of 127,000. Distinct protein bands of those remaining in the clear supernatant showing apparent relative molecular masses of 53,000, 28,000, and 25,000 Da were also subjected to N-terminal sequencing. Their N-terminal regions were TAPNG, DVKNT, and TAPNG, none of which could be identified within the N-terminal sequence of the whole S-layer protein. None of the proteolytic cleavage fragments could bind to HF-extracted sacculi (Fig. 3) representing pure peptidoglycan (Table 1).
FIG. 3.
Lanes: a, SDS-PAGE pattern obtained by proteolytic degradation of the whole S-layer protein of B. sphaericus CCM 2177 with endoproteinase Gluc-C; b and e, proteolytic cleavage fragments that remained in the clear supernatant after incubation with native and HF-extracted peptidoglycan-containing sacculi, respectively; c and f, proteolytic cleavage fragments which could be removed from the bound fraction by washing native and HF-extracted peptidoglycan-containing sacculi, respectively, with buffer; d and g, proteolytic cleavage fragments which remained attached to native and HF-extracted peptidoglycan-containing sacculi, respectively. Molecular masses (in thousands) are shown at the left. Protein bands subjected to N-terminal sequencing are indicated by arrowheads.
To conclude, the N-terminal part of the S-layer protein from B. sphaericus CCM 2177, carrying at least one SLH motif, recognizes a net negatively charged SCWP composed of GlcNAc, ManNAc, and pyruvic acid as the binding site. According to the chemical composition, this SCWP should be attributed to the teichuronic acids (3). The basic structure of the SCWP of B. sphaericus CCM 2177 is similar to those of polysaccharides from Bacillus polymyxa AHU 1385 (13), type e capsular polysaccharide of Haemophilus influenzae (29), and glycans copurified with the S-layer glycoproteins of Thermoanaerobacterium thermosaccharolyticum E207-71 and D120-70 (1, 2). The N-terminal 35 amino acids of the S-layer protein from B. sphaericus CCM 2177 showed 97.1 and 88.6% identity to the corresponding N-terminal parts of the S-layer proteins from B. sphaericus P-1 and 2362 (5, 8). The S-layer proteins from B. sphaericus P-1 and 2362 showed 80% identity for their N-terminal regions, while the sequence identity beyond the N-terminal 200 amino acids was less than 20%. Accordingly, the internal cleavage fragments of the S-layer protein of B. sphaericus CCM 2177 could not be mapped on the sequences of the S-layer proteins from B. sphaericus P-1 and 2362.
Acknowledgments
This work was supported by the Austrian Science Foundation (project P 12938), by the Ministry of Science and Transports, and by Hochschuljubiläumsstiftung der Stadt Wien (project H121/98).
We thank Christoph Hotzy and Aida Medovic for excellent technical assistance, Sonja Zayni for amino acid and sugar analyses, and Fritz Altmann for providing the matrix-assisted laser desorption ionization data.
REFERENCES
- 1.Altman E, Brisson J-R, Messner P, Sleytr U B. Chemical characterization of the regularly arranged surface layer glycoprotein of Clostridium thermosaccharolyticum D120-70. Eur J Biochem. 1990;188:73–82. doi: 10.1111/j.1432-1033.1990.tb15373.x. [DOI] [PubMed] [Google Scholar]
- 2.Altman E, Schäffer C, Brisson J-R, Messner P. Isolation and characterization of an amino sugar-rich glycopeptide from the surface layer glycoprotein of Clostridium thermosaccharolyticum D120-70. Carbohydr Res. 1996;295:245–253. doi: 10.1016/s0008-6215(96)90150-0. [DOI] [PubMed] [Google Scholar]
- 3.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Sonenshein A, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. New York, N.Y: Academic Press; 1993. pp. 381–410. [Google Scholar]
- 4.Beveridge T J. Bacterial S-layers. Curr Opin Struct Biol. 1994;4:204–212. [Google Scholar]
- 5.Bowditch R D, Baumann P, Yousten A A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989;171:4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brechtel E, Bahl H. In Thermoanaerobacterium thermosulfurigenes EM1 S-layer homology domains do not attach to peptidoglycan. J Bacteriol. 1999;181:5017–5023. doi: 10.1128/jb.181.16.5017-5023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauvaux S, Matuschek M, Béguin P. Distinct affinity of binding sites for S-layer homologous domains in Clostridium thermocellum and Bacillus anthracis cell envelopes. J Bacteriol. 1999;181:2455–2458. doi: 10.1128/jb.181.8.2455-2458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deblaere R. Expression of surface layer proteins. Patent WO 9519371-A. July 1995. [Google Scholar]
- 9.Egelseer E M, Leitner K, Jarosch M, Hotzy C, Zayni S, Sleytr U B, Sára M. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J Bacteriol. 1998;180:1488–1495. doi: 10.1128/jb.180.6.1488-1495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer W. Lipoteichioic acids and lipoglycans. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 199–216. [Google Scholar]
- 11.Jarosch, M., E. M. Egelseer, D. Mattanovich, U. B. Sleytr, and M. Sára. The S-layer gene sbsC of Bacillus stearothermophilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli. Microbiology, in press. [DOI] [PubMed]
- 12.Klyne W. The configuration of the anomeric carbon atoms in some cardiac glycosides. Biochem J. 1950;47:xli–xlii. [PubMed] [Google Scholar]
- 13.Kojima N, Kaya S, Araki Y, Ito E. Pyruvic-acid-containing polysaccharide in the cell wall of Bacillus polymyxa AHU 1385. Eur J Biochem. 1988;174:255–260. doi: 10.1111/j.1432-1033.1988.tb14091.x. [DOI] [PubMed] [Google Scholar]
- 14.Leibovitz E, Lemaire M, Miras I, Salamitou S, Béguin P, Ohayon H, Gounon P, Matuschek M, Sahm K, Bahl H. Occurrence and function of a common domain in S-layer and other exocellular proteins. FEMS Microbiol Rev. 1997;20:127–133. [Google Scholar]
- 15.Lemaire M, Miras I, Gounon P, Béguin P. Identification of a region responsible for binding to a cell wall within the S-layer protein of Clostridium thermocellum. Microbiology. 1998;144:211–217. doi: 10.1099/00221287-144-1-211. [DOI] [PubMed] [Google Scholar]
- 16.Lupas A. A circular permutation event in the evolution of the SLH domain? Mol Microbiol. 1996;20:897–898. doi: 10.1111/j.1365-2958.1996.tb02528.x. [DOI] [PubMed] [Google Scholar]
- 17.Matuschek M, Burchhardt G, Sahm K, Bahl H. Pullulanase of Thermoanaerobacterium thermosulfurigenes EM1 (Clostridium thermosulfurogenes): molecular analysis of the gene, composite structure of the enzyme, and a common model for its attachment to the cell surface. J Bacteriol. 1994;176:3295–3302. doi: 10.1128/jb.176.11.3295-3302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesnage S, Tosi-Couture E, Fouet A. Production and cell surface anchoring of functional fusion proteins between the SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol Microbiol. 1999;31:927–936. doi: 10.1046/j.1365-2958.1999.01232.x. [DOI] [PubMed] [Google Scholar]
- 19.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olabarria G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4772. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ries W C, Hotzy, Schocher I, Sleytr U B, Sára M. Evidence that a secondary cell wall polymer recognizes the N-terminal part of the S-layer protein from Bacillus stearothermophilus PV72/p2. J Bacteriol. 1997;179:3892–3898. doi: 10.1128/jb.179.12.3892-3898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salton M R J. The bacterial cell envelope—a historical perspective. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 1–22. [Google Scholar]
- 23.Sára M, Sleytr U B. Crystalline bacterial cell surface layers (S-layers): from cell structure to biomimetics. Prog Biophys Mol Biol. 1996;65:83–111. doi: 10.1016/s0079-6107(96)00007-7. [DOI] [PubMed] [Google Scholar]
- 24.Sára M, Dekitsch C, Mayer H F, Egelseer E M, Sleytr U B. Influence of the secondary cell wall polymer on the reassembly, recrystallization, and stability properties of the S-layer protein from Bacillus stearothermophilus PV72/p2. J Bacteriol. 1998;180:4146–4153. doi: 10.1128/jb.180.16.4146-4153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sára M, Kuen B, Mayer H F, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and its S-layer-deficient variant T5 in continuous culture and studies of the cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleifer K H, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sleytr U B, Beveridge T J. Bacterial S-layers. Trends Microbiol. 1999;7:253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- 28.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers (S-layers): from supramolecular cell structure to biomimetics and nanotechnology. Angew Chem Int Ed. 1999;38:1034–1054. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1034::AID-ANIE1034>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Tsui F-P, Schneerson R, Boykins R A, Karpas A B, Egan W. Structural and immunological studies of the Haemophilus influenzae type d capsular polysaccharide. Carbohydr Res. 1981;97:293–306. doi: 10.1016/s0008-6215(00)80675-8. [DOI] [PubMed] [Google Scholar]