Abstract
Traumatic encephalopathy syndrome (TES) criteria were developed to aid diagnosis of chronic traumatic encephalopathy (CTE) pathology during life. Interpreting clinical and biomarker findings in patients with TES during life necessitates autopsy-based determination of the neuropathological profile. We report a clinicopathological series of nine patients with previous repetitive head impacts (RHI) classified retrospectively using the recent TES research framework (100% male and white/Caucasian, age at death 49–84) who completed antemortem neuropsychological evaluations, T1-weighted magnetic resonance imaging, diffusion tensor imaging (n = 6), (18)F-fluorodeoxyglucose-positron emission tomography (n = 5), and plasma measurement of neurofilament light (NfL), glial fibrillary acidic protein (GFAP), and total tau (n = 8). Autopsies were performed on all patients. Cognitively, low test scores and longitudinal decline were relatively consistent for memory and executive function. Medial temporal lobe atrophy was observed in all nine patients. Poor white matter integrity was consistently found in the fornix. Glucose hypometabolism was most common in the medial temporal lobe and thalamus. Most patients had elevated plasma GFAP, NfL, and total tau at their initial visit and a subset showed longitudinally increasing concentrations. Neuropathologically, five of the nine patients had CTE pathology (n = 4 “High CTE”/McKee Stage III-IV, n = 1 “Low CTE”/McKee Stage I). Primary neuropathological diagnoses (i.e., the disease considered most responsible for observed symptoms) were frontotemporal lobar degeneration (n = 2 FTLD-TDP, n = 1 FTLD-tau), Alzheimer disease (n = 3), CTE (n = 2), and primary age-related tauopathy (n = 1). In addition, hippocampal sclerosis was a common neuropathological comorbidity (n = 5) and associated with limbic-predominant TDP-43 proteinopathy (n = 4) or FTLD-TDP (n = 1). Memory and executive function decline, limbic system brain changes (atrophy, decreased white matter integrity, hypometabolism), and plasma biomarker alterations are common in RHI and TES but may reflect multiple neuropathologies. In particular, the neuropathological differential for patients with RHI or TES presenting with medial temporal atrophy and memory loss should include limbic TDP-43. Researchers and clinicians should be cautious in attributing cognitive, neuroimaging, or other biomarker changes solely to CTE tau pathology based on previous RHI or a TES diagnosis alone.
Keywords: biomarker, chronic traumatic encephalopathy, concussion, hippocampal sclerosis, limbic-predominant age-related TDP-43 encephalopathy, traumatic encephalopathy syndrome
Introduction
Traumatic encephalopathy syndrome (TES) is a proposed research framework for the progressive cognitive and/or neurobehavioral symptoms in patients with substantial previous exposure to repetitive head impacts (RHI).1 Research diagnostic criteria for TES were developed to aid in antemortem detection of chronic traumatic encephalopathy (CTE), a neurodegenerative tauopathy.2 CTE-focused studies typically recruit aging adults with previous RHI because CTE occurs almost exclusively in these individuals.
Yet, RHI may also increase the risk for development of Alzheimer disease (AD), frontotemporal lobar degeneration (FTLD), Parkinson disease, or amyotrophic lateral sclerosis with or without co-occurring CTE pathology.3–6 The frequent occurrence of co-pathology in patients with CTE and the overlap in symptoms between TES (e.g., memory- or dysexecutive-predominant problems) and syndromes caused by other neurodegenerative diseases underscores the importance of linking clinical and biomarker data obtained during life with neuropathological data at autopsy.
One recent study evaluated antemortem structural magnetic resonance imaging (MRI) in patients with autopsy-confirmed CTE.7 Frontal and temporal lobe atrophy, based on visual ratings, was noted in patients with CTE compared with cognitively normal controls without known head trauma.7 Other neuroimaging and fluid biomarker studies rely on living individuals considered high-risk for CTE, such as symptomatic former professional collision sport athletes. These studies suggest that patients with RHI have lower brain volumes and compromised white matter integrity in limbic.8,9 frontal,8,10–12 and subcortical regions13–15 compared with unexposed controls.16,17 Fluid biomarkers of neuronal injury such as neurofilament light chain (NfL) and total tau inconsistently differentiate patients with RHI from controls and variably correlate with the extent of previous RHI.18–20
A major limitation of previous biomarker studies of RHI and TES is a lack of autopsy data, the gold standard for diagnostic confirmation of CTE pathology. Frontal and temporal brain regions susceptible to CTE pathology are vulnerable to other neurodegenerative pathologies observed in patients with TES (with or without CTE), such as AD21,22 or TAR DNA-binding protein of 43 kDa (TDP-43) proteinopathy.23,24 Common regions of neurodegeneration among patients with TES might reflect regional susceptibility to traumatic forces and heightened vulnerability to several neuropathological substrates. Closing the gap between group-level biomarker differences observed in high-risk populations during life and neurodegenerative disease pathology requires multi-modal biomarkers collected from patients with TES followed to autopsy.
We report a clinicopathological case series of nine patients with previous RHI and classified retrospectively using the recent TES research framework. Patients underwent detailed clinical evaluations including neuropsychological testing, neuroimaging, and plasma biomarker analyses (glial fibrillary acidic protein (GFAP), NfL, total tau) before death. Each patient underwent a comprehensive post-mortem neuropathological examination.
We first quantified whether there were shared regions of brain atrophy (structural T1-weighted MRI), affected white matter integrity (diffusion tensor imaging; DTI), or glucose hypometabolism (18F-fluorodeoxyglucose positron emission tomography; FDG-PET). We then separately investigated patients with autopsy-confirmed High CTE pathology.25 We characterize the neuropathological findings in areas of the brain most commonly affected. We additionally characterize neuropsychological testing and antemortem plasma biomarker data in the cohort.
Methods
Participants
We searched for patients within the University of California, San Francisco, Neurodegenerative Disease Brain Bank autopsy program. Selection criteria for this study were: (1) documented RHI during life from activities with high exposure risk (e.g., American football, boxing), (2) post-mortem neuropathological examination, (3) at least one antemortem neuropsychological evaluation, and (4) at least one antemortem structural MRI. We did not require that neuropsychological testing and structural MRI were obtained within a pre-specified time interval. Additional antemortem biomarkers were included in the study based on convenience of availability from the parent study protocols (see Supplementary Methods).
Details of type and duration of RHI during life were determined via review of available medical and research records from patients in the brain bank, including medical history and social histories ascertained through comprehensive patient and care partner interviews and neurological examinations. Activities considered high risk for RHI included American football, boxing, mixed martial arts, ice hockey, rugby, soccer, and wrestling.1 Additional sources of exposure were noted if the activity has a high risk for repeated head trauma (e.g., military service, motocross or competitive racecar driving).
For participants with documented evidence of RHI through collision sports, we also searched publicly available online records for additional details about duration or participation level if not explicitly mentioned elsewhere. Prior isolated traumatic brain injury was similarly determined through record review as well as data from the National Alzheimer's Coordinating Center Uniform Data Set (UDS) Health History forms26 but did not factor into RHI determination. Because of the retrospective nature of the study and stipulations of existing study protocols, additional details could not be readily obtained prospectively (e.g., family interview).
The search yielded nine patients (all male and white/Caucasian) who were followed prospectively through the UCSF Alzheimer's Disease Research Center or Program Project Grant on frontotemporal dementia. Initial enrollment in research occurred between 2009 and 2018. All patients provided informed consent to participate in Institutional Review Board (IRB)-approved research protocols during life and consented to brain donation.
Clinical diagnostic classifications
All patients initially received a diagnosis during life via multi-disciplinary consensus conference. Proposed criteria for symptoms associated with CTE (i.e., TES or similar terminology) have evolved significantly in the last decade (e.g.,1,27–29) with variable applications in research settings. Therefore, we retroactively applied the most recent National Institute of Neurological Disorders and Stroke (NINDS) TES consensus criteria framework to the nine patients meeting our selection criteria.1
Two investigators (BA, JT) reviewed available neurological and neuropsychological assessments (Supplementary Table S1), which included detailed histories and clinical interviews with patients and care partners. Clinicians are trained to assess for head trauma exposure, as well as other dementia risk factors, but standardized tools were not systematically applied. Each rater first independently determined whether the patient met TES criteria and, if so, assigned a level of diagnostic certainty: “Suggestive of CTE,” “Possible CTE,” or “Probable CTE.”1
Retroactive diagnoses were made blinded to neuropathological diagnosis. The TES determinations were made irrespective of clinical diagnoses made during life, neuroimaging, and other biomarkers (e.g., PET or cerebrospinal fluid [CSF]), although formal blinding to this information was not always feasible because of references made to existing or previous diagnostic formulations throughout clinical and research reports. Discrepancies were resolved by discussion and joint review of available records.
A core requirement of the TES framework is “substantial” RHI. In the case of American football, the framework operationalizes “substantial” as at least five years of participation needed to meet Possible CTE or higher level of diagnostic certainty. Other sources of exposure such as other collision sports or military blast exposure do not have explicit recommendations for duration. For the retroactive application of the TES framework in our case series, any patients with known RHI from a qualifying activity such as American football, but unknown number of years of participation, were considered “Uncertain TES” for this study.
In addition, we provide the clinical diagnoses reflecting multi-disciplinary consensus diagnosis for the patient at the time of the last research visit, because the evolving TES diagnostic criteria were not routinely applied across the time span of patient enrollment. These alternative consensus diagnoses importantly differ from the retrospective application of the current TES framework in that available neuroimaging and other biomarker data factored into the prospective consensus diagnoses.
Neuropathological assessment
All patients underwent autopsy between 2016 and 2020 using standardized sampling and staining protocols in the UCSF Neurodegenerative Disease Brain Bank as described elsewhere.30,31 Sampling procedures followed recommended guidelines for CTE, AD, FTLD, and synucleinopathies classification21,25,32 (phospho-tau antibody: CP-13, S202/T205, mouse, 1:250, courtesy of P. Davies; β-amyloid: 1-16, mouse, clone DE2, 1:500, Millipore; TDP-43 antibody: rabbit, 1:2000, Proteintech Group; alpha synuclein: LB509 mouse, 1:5000, courtesy of J. Trojanowski and V. Lee). AD burden (Aβ plaques and AD tau tangles) was defined as “None,” “Low,” “Moderate,” or “High” AD neuropathological changes (ADNC) based on current National Institute on Aging-Alzheimer's association (NIA-AA) criteria.21
Unless otherwise noted, all patients underwent immunohistochemical analysis of hyperphosphorylated tau and TDP-43 proteinopathy in the dentate gyrus (eight of nine with TDP-43 staining), CA1/subiculum, CA2, CA3/CA4, entorhinal cortex, and amygdala (eight of nine with tau staining). We report semi-quantitative ratings of regional lesion burden (e.g., “mild,” “moderate,” “severe”) made by the reviewing neuropathologist for different tau and TDP-43 inclusion types: neurofibrillary tangles, neuronal or glial cytoplasmic inclusions, neuropil threads, white or gray matter threads, dystrophic neurites.
CTE severity was defined according to McKee staging criteria2 and as “High” or “Low” based on recently proposed classification methods that account for the number of brain regions with CTE-tau deposition (regardless of burden/density).25 We first verified that participants with neuropathological diagnoses of CTE based on previous criteria also met recent consensus criteria (e.g., required neuronal tau inclusions). We also reevaluated participants who were considered free of CTE pathology during initial autopsy evaluation by sampling additional regions (orbitofrontal cortex, superior/middle temporal cortex) for CTE tau pathology, per recent consensus group recommendations.25
Neuropathologists were blinded to clinical diagnosis and medical history at the time of autopsy when documenting neuropathological findings. After autopsy, neuropathologists were unblinded to clinical history and final neuropathological diagnoses along with designations of primary, contributing, and incidental diagnoses were made by consensus among three neuropathologists (LTG, SS, WWS).
Primary neuropathological diagnosis was defined as the observed entity deemed responsible for the majority of the clinical cognitive and behavioral phenotype. This determination takes into account the severity and regional distribution of the findings. Contributing neuropathological diagnoses were defined as sufficiently developed to contribute to the clinical phenotype that is better explained by the primary neuropathological diagnosis. Contributing neuropathological diagnoses also explained additional clinical features that could not be attributed to the primary neuropathological diagnosis. Incidental neuropathological diagnoses were defined as coexistent neuropathological entities thought to contribute little, if at all, to the clinical presentation.
Neuropsychological testing
All nine patients underwent longitudinal neuropsychological evaluations (mean number of evaluations = 3.7, range 2–5; mean retest interval = 1.4 years, range 0.8–4.1 years; mean follow-up time = 3.7 years, range 1–7 years; mean time before death at last evaluation = 1.9 years, range 0–5 years) with testing protocols previously described.33
Cognitive composites were created for memory (California Verbal Learning Test short form, immediate and delayed recall; Benson figure recall), executive functioning (modified trail making test, lexical fluency, digit span backwards, Stroop inhibition, design fluency), and language (animal fluency, 15-item Boston Naming Test). Individual test raw scores were converted to z-scores based on a large sample of clinically normal older adults (n per test = 231–763, 65 ± 13 years old, 60% female, 16.8 ± 2.4 years of education) and then averaged within each domain to create the composite score.
Neuroimaging
Structural MRI
We report data from a total of 18 structural MRI scans obtained from the nine patients with TES (n = 5 with 2+ scans; first available scan: 4.1 ± 1.4 years before death, range 2–6 years; last available scan: mean 2.7 ± 2.6 years before death, range 0–6 years). Structural T1-weighted MRI was acquired at the UCSF Neuroimaging Center on one of two scanners (3T Siemens Tim Trio scanner [n = 12] or 3T Siemens MAGNETOM Prisma) as described previously.34 Before processing, all T1-weighted images were visually inspected for quality control and those with excessive motion or image artifact were excluded.
Tissue segmentation was performed using unified segmentation in SPM12.35 Each subject's gray matter segmentation was warped to create a study-specific template.36 Subject's native space gray and white matter segmentations were then normalized and modulated to study-specific template space using non-linear and rigid-body transformation. Each subject's segmentation was carefully inspected to ensure robustness of the process. Quantification of volumes in specific brain regions was accomplished by transforming a standard parcellation atlas (Desikan37) into International Consortium for Brain Mapping (ICBM) space and summing all modulated gray matter within each parcellated region of interest (ROI).37
Brain volume w-scores were then calculated voxel-wise and by ROIs based on the distribution of volumes obtained from 584 brain scans of clinically normal older adults enrolled in a longitudinal healthy aging study (Trio: N = 478, age 66.2 ± 11.8 years old, range 30–99 years old; 44% male; 17.5 ± 5.8 years of education; Prisma: N = 106, age 63.3 ± 13.1 years old, range 30–99 years old; 41% male, 16.6 ± 2.3 years of education).
W-scores represent the regression-based standardized brain volume adjusting for age, sex, years of education, and scanner and are interpreted similar to a z-score. W-score maps were created that demonstrated the number of patients (out of nine) with significantly low volume (W < -2.0) in a given voxel. Voxel clusters with a high frequency of patients with low volume were interpreted as shared regions of atrophy across the cohort.
We identified ROIs and extracted the mean W score across all voxels within the ROI for each patient. The ROIs were selected corresponding to general lobar volumes and specific subregions of interest based on visualization of the voxel-wise W-score frequency map: dorsal frontal, ventral frontal, temporal, parietal, occipital, thalamus, and the medial temporal subset of the overall temporal lobe.
Structural MRIs obtained closest to death for each patient were evaluated for presence or absence of cavum septum pellucidum (CSP) by the lead investigator (BA; neuropsychology fellow) and verified by a second investigator (JT; behavioral neurology fellow) using previously published methods.38 If present, we reported the grade and length of the CSP.
Additional structural MRI methodology is provided in the Supplementary Methods.
Diffusion tensor imaging
DTI was obtained in six participants using a 3T Siemens MAGNETOM Prisma (mean 3.7 ± 1.9 years before death, range 1–6 years). The b = 0 image was co-registered with the diffusion direction images, followed by gradient direction, eddy current and distortion correction using FSL.39,40 Diffusion tensors were calculated using a non-linear least-squares algorithm in Dipy.41 Registration of diffusion data was accomplished through the DTI-TK software package.42 An intersubject template was created through iterative linear and non-linear registration of DTIs. The DTIs in the group space were diagonalized into eigenvectors from which fractional anisotropy (FA) maps were calculated.
FA W scores (age- and sex-adjusted) were then calculated voxel-wise and by ROIs based on the mean and standard deviation of FA values obtained from 376 clinically normal older adults enrolled in a longitudinal healthy aging study (age 66.7 ± 12.2 years old, range 30–99 years old; 44% male; 17.5 ± 4.8 years of education). Voxel clusters with a high frequency of patients with low FA (W ≤ -2.0) were interpreted as shared regions of decreased white matter integrity across the cohort.
In addition, we identified ROIs from the ICBM-DTI-81 white matter labels and tract atlas and extracted the mean FA across all voxels within the ROI for each patient.43 We selected ROIs based on visualization of the voxel-wise W-score frequency map and previous research on DTI in RHI populations (superior longitudinal fasciculus, superior fronto-occipital fasciculus, genu of the corpus callosum, uncinate fasciculus, fornix, cingulum-hippocampal bundle) as well as a presumably less affected ROI for comparison (posterior corona radiata).17
Additional DTI methodology is provided in the Supplementary Methods.
FDG-PET acquisition and processing
Five participants underwent FDG-PET scans (mean 4.3 ± 1.2 years before death, range 3–6 years). Acquisition protocols FDG-PET have been described previously.44 PET scans were acquired at Lawrence Berkeley National Laboratory on a Siemens Biograph 6 Truepoint PET/CT scanner in three dimension acquisition mode. PET frames were realigned for motion correction, averaged, and co-registered onto the participant's MRI. Standardized Uptake Value Ratios (SUVR) maps were created at 30–60 min post-injection using the pons as the reference region (defined based on FreeSurfer-derived v5.3 Desikan-Killiany atlas).
FDG-PET hypometabolism W scores were then calculated based on the FDG-PET of cognitively unimpaired controls (N = 34, age 71.0 ± 11.8 years old, range 49–90 years old; 100% male, 17.0 ± 2.3 years of education). For this, PET images were warped to MNI space using the respective MRI-based transformation parameters and smoothed. Voxel-wise regressions were then performed adjusting for age in the control group, and used to compute W-score maps for each patient. W maps were then created that demonstrated the number of patients (out of 5) with significant hypometabolism (W ≤ -2.0) in a given voxel.
Additional FDG-PET methodology is provided in the Supplementary Methods.
Plasma GFAP, NfL, and total tau
Antemortem plasma GFAP, NfL, and total tau were available for eight of the nine patients with TES (mean time before death = 4.1 years, range 2–7 years) who were selected for analysis in a separate study of older adults from our center with documented previous RHI exposure and at least one available blood sample. Five patients had longitudinal GFAP and NfL data (15 total plasma samples from eight patients with TES; mean number of time points = 2.4, range 2–4; mean follow-up time = 2.8 years, range 1–4 years; mean time before death at last blood draw = 0.6 years, range 0–2 years) and two had longitudinal total tau data (11 total plasma samples from eight TES patients).
Venous blood was collected and stored at -80°C at UCSF until being packed with dry ice and sent to the University of Florida for analysis following standard shipping protocols (one thawing only). Plasma GFAP, NfL, and total tau were measured via multiplex single molecule arrays on an SR-X analyzer (Simoa, Quanterix Neurology 4-Plex B). All samples were analyzed in duplicate according to manufacturer's published protocols. We only included sample concentrations with coefficients of variance (CV) <20% (N = 4 total tau concentrations excluded). Ubiquitin carboxy-terminal hydrolase L1 measurement is included in this multiplex assay but was not reported because of a high rate of samples exceeding pre-specified CV thresholds.
Plasma GFAP, NfL, and total tau concentration W scores were then calculated based on mean concentrations of each protein in plasma samples obtained from 108 AD-biomarker negative (beta-amyloid PET), clinically normal (CDR = 0) healthy controls (age 73.2 ± 7.3 years old, range 52–91 years old; 50% female). These healthy control samples were analyzed at the same time as the patient samples. The W scores were age-adjusted so that values represent protein concentrations relative to the mean of age-matched healthy controls (i.e., the difference between actual and age-predicted protein concentrations, divided by the standard error in the control group). Higher W scores are interpreted as greater than expected protein concentrations for the patient's age.
Data availability
Qualified researchers from academic, not-for-profit institutions can request deidentified data associated with this study through the UCSF Memory and Aging Center after obtaining IRB approval from the UCSF Human Research Protection Program and completing a resource request.
Results
Clinical and neuropathological classifications
Eight of the nine patients had American football exposure, and several had multiple types of head trauma exposure (Table 1). Assessments of functional status closest to death and estimated causes/contributors to death are shown in Supplementary Table S2. Among the nine patients with TES, five were classified as Probable CTE and one was classified as Possible CTE. Three of the nine patients had documentation of RHI through collision sport and/or military service but either the duration of sport participation was unknown, or it was unclear whether military service involved blast exposure or combat training. These three patients therefore were classified as Uncertain TES. In addition, we questioned whether symptoms for one of the patients classified as Uncertain TES were potentially fully explained by another condition (behavioral variant frontotemporal dementia with motor neuron disease).
Table 1.
Neuroimaging-to-Autopsy Case Series of Nine Participants from the University of California, San Francisco Memory and Aging Center with Clinical Impairment and History of Repetitive Head Trauma
| P# | Exposure | Exposure duration (years) | Symptom duration (years) | Early symptoms | Late symptoms | TES diagnosisa | Consensus diagnosis during lifeb | Age at death | CTE stage/ level | ABC score | Primary and contributing neuropathological findings | Other incidental neuropathological findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Am. football | 17 | 13 | Irritability Explosivity Social withdrawal Depression Memory Executive dysfunction |
Parkinsonism Seizures |
Probable CTE | n/a | 72 | IV / High | A0B?C0 | CTE* Limbic TDP-43 with hippocampal sclerosis L subiculum infarcts |
Limbic AGD Mild arteriolosclerosis. ARTAG |
| 2 | Am. football | 24 | 9 | Mood |
Memory Executive dysfunction Seizures |
Probable CTE | Amnestic, multi-domain dementia due to AD and vascular | 74 | III / High | A1B1C0 | CTE* Limbic TDP-43 with hippocampal sclerosis |
Limbic AGD Mild arteriolosclerosis ARTAG Infiltrating glioma Low ADNC |
| 3 | Am. football | 23 | 8 |
Memory Visuospatial Word-finding Depression Anxiety |
n/a | Probable CTE | Amnestic, multi-domain dementia due to AD | 79 | I / Low |
A3B3C3 | High ADNC* Limbic TDP-43 with hippocampal sclerosis |
CTE Lymphocytic leukemia Mild arteriolosclerosis Limbic AGD |
| 4 | Am. football Motocross/ racecar driver |
8 (Am. football) +5 (racecar driving with ≥3 TBI) | 8 | Apathy Loss of empathy Disinhibition Compulsions |
Memory Language |
Probable CTE | bvFTD | 49 | None | A0B2C0 | FTLD-tau (CBD)* FTLD-TDP43 (U) with hippocampal sclerosis |
Limbic AGD |
| 5 | Am. football | 12 | 9 | Apathy Irritability Depression |
Visuospatial Memory |
Probable CTE | Non-amnestic, multi-domain dementia due to AD | 58 | None | A3B3C3 | High ADNC* | Limbic AGD Mild arteriolosclerosis Mild CAA LBD (amygdala) ARTAG |
| 6 | Boxing Military Bicycle crash with subdural hematoma |
≥12 professional boxing (≥100 bouts) | 7 |
Memory Inattention |
Worsening memory Word-finding Depression (mild) Irritability (mild) |
Possible CTE | Amnestic, multi-domain MCI (non-AD) | 81 | None | A0B2C0 | PART* | Vascular brain injury Limbic AGD ARTAG |
| 7 | Am. football Boxing Military |
≥20 military (unknown sport duration) | 13 |
Memory Executive dysfunction |
Parkinsonism | Uncertain TES (unknown exposure duration) | Amnestic, multi-domain dementia due to AD | 84 | III / High | A3B3C3 | High ADNC* CTE Limbic TDP-43 with hippocampal sclerosis |
Limbic AGD Moderate CAA LBD (Braak IV) Subdural hematoma White matter rarefaction |
| 8 | Am. football 2 late-life mTBIs (falls) |
N/A | 11 | Social withdrawal Loss of empathy Hyperorality Compulsions Delusions |
Loss of semantic knowledge | Uncertain TES (unknown exposure duration) | Right temporal variant of svPPA | 70 | Nonec | A1B2C1 | FTLD-TDP Type C* “Focal traumatic tau astrogliopathy” c |
Limbic AGD Thalamic infarcts Moderate arteriolosclerosis Low ADNC |
| 9 | Am. football Wrestling |
N/A | 12 | Disinhibition Loss of empathy Apathy Repetitive behaviors Sweet food preference |
Fasciculations Unsteadiness Breathing changes L > R hand tremor Dysphagia |
Uncertain TES (may be fully explained by other disorder) | bvFTD with MND | 59 | IV / High | A0B?C0 | FTLD-TDP Type B with MND* |
CTE Limbic AGD Mild arteriolosclerosis ARTAG ATAC |
All participants underwent comprehensive neurological and neuropsychological evaluations before death. Autopsies were performed through the University of California, San Francisco Neurodegenerative Disease Brain Bank. Head trauma exposure histories and symptom details were derived from antemortem evaluations that included detailed clinical histories and neurological examinations. “Early” and “Late” symptoms refer to participant- and informant-reported symptom trajectories or neurological examination findings (e.g., parkinsonism, fasciculations). Reported memory and/or executive dysfunction symptoms are bolded if objective neuropsychological testing in these domains was >1.5 standard deviations below the mean of healthy controls. Presence or absence of chronic traumatic encephalopathy and Alzheimer disease pathology along with staging were reported separately for each patient. “Primary and Contributing Neuropathological Findings” are neuropathological findings thought to contribute most to the patient's antemortem clinical symptoms. “Other Incidental Neuropathological Findings” were present but thought to have contributed minimally to clinical symptoms, per the reviewing neuropathologist, based on autopsy findings and available antemortem clinical data.
TES, traumatic encephalopathy syndrome; CTE, chronic traumatic encephalopathy; ABC, Amyloid phase/Braak stage of neurofibrillary tangles/CERAD neuritic plaque score (per NIA-AA guidelines for describing AD neuropathological changes); TDP, transactive response DNA-binding protein of 43 kDa; AGD, argyrophilic grain disease; ARTAG, age-related tau astrogliopathy; AD, Alzheimer disease; ADNC, Alzheimer disease neuropathological change21; TBI, traumatic brain injury; bvFTD, behavioral variant frontotemporal dementia; FTLD, frontotemporal lobar degeneration; CBD, corticobasal degeneration; CAA, cerebral amyloid angiopathy; LBD, Lewy body disease; MCI, mild cognitive impairment; svPPA, semantic variant of primary progressive aphasia; MND, motor neuron disease; ATAC, argyrophilic thorny astrocytes in clusters; U, unclassifiable.
Primary neuropathological diagnosis designated by the reviewing neuropathologist based on the expected role played by the disease in the patient's clinical syndrome.
TES Diagnosis was determined via investigator consensus (BA, JT) and represents the provisional level of certainty for chronic traumatic encephalopathy pathology through retroactive application of the most recently proposed traumatic encephalopathy syndrome (TES) diagnostic criteria.1 The TES criteria were applied blinded to neuropathological diagnosis and available neuroimaging or other biomarker data (e.g., Alzheimer disease biomarkers) because these data do not factor into the 2021 TES diagnostic criteria.
Formal application of evolving traumatic encephalopathy syndrome (TES) diagnostic criteria was not systematically applied by consensus conference throughout the time span that these patients were evaluated at our center. The “Consensus Diagnosis During Life” reflects consensus clinical diagnosis for the patient at the time of study visit and also takes into account available biomarkers collected through research for the patient (structural magnetic resonance imaging, positron emission tomography neuroimaging, and/or cerebrospinal fluid biomarkers).
Patient had a severe, focal tauopathy in fronto-temporal-limbic regions overlapping with features of ARTAG. Given the distribution of the astrogliopathy and the patient's relatively young age of symptom onset (59) and death (70), these findings were fthought by the reviewing neuropathologist to most likely have been associated with previous repetitive head trauma, but would not meet diagnostic criteria for chronic traumatic encephalopathy because of absence of clear neuronal involvement.
Five of the nine patients had CTE pathology at autopsy with four meeting the High CTE classification (McKee Stage III, n = 2; Stage IV, n = 2) and one meeting the Low CTE classification (McKee Stage I). Primary neuropathological diagnoses (i.e., the disease considered most responsible for observed symptoms) included CTE (n = 2), AD (n = 3), primary age-related tauopathy (n = 1), FTLD-TDP Type B with motor neuron disease (n = 1), FTLD-TDP Type C (n = 1), and FTLD-tau (corticobasal degeneration; n = 1).
Among the five patients with CTE pathology, three were classified clinically as Probable CTE (n = 2 High CTE, n = 1 Low CTE) and two as Uncertain TES because of unknown exposure duration and questions of whether symptoms were fully explained by another condition (both High CTE). Two patients clinically classified as Probable CTE had no CTE pathology at autopsy. Table 1 shows clinical diagnoses and all neuropathological findings for the nine patients.
Cognitive and mood symptoms
All patients underwent longitudinal neuropsychological evaluations. There was wide variability in cognitive scores at the initial evaluation (Supplementary Fig. S1). Annualized decline in memory z-score (mean 0.53 ± 0.50/year, median = 0.30/year) was nominally steeper than for executive function z-score (mean 0.34 ± 0.48/year, median = 0.22/year). Language z-scores also declined over time (mean 0.87 ± 1.32/year, median = 0.35/year); this likely was driven by three patients with TES in whom clinical phenotypes involving severe language difficulties (Patients #4, #8, and #9) developed. There were no consistent patterns of self-reported depression symptoms.
Cavum septum pellucidum and brain atrophy pattern
All patients underwent T1-weighted MRI. All nine patients had a CSP (Grade 3, n = 6; Grade 2, n = 3; mean length = 15.6 ± 4.9 mm) with no clear differences between patients with High CTE (Grade 3, n = 3; Grade 2, n = 1; mean length = 15.8 ± 4.8 mm) versus without (Grade 3, n = 3; Grade 2, n = 2; mean length = 15.4 ± 5.5 mm).
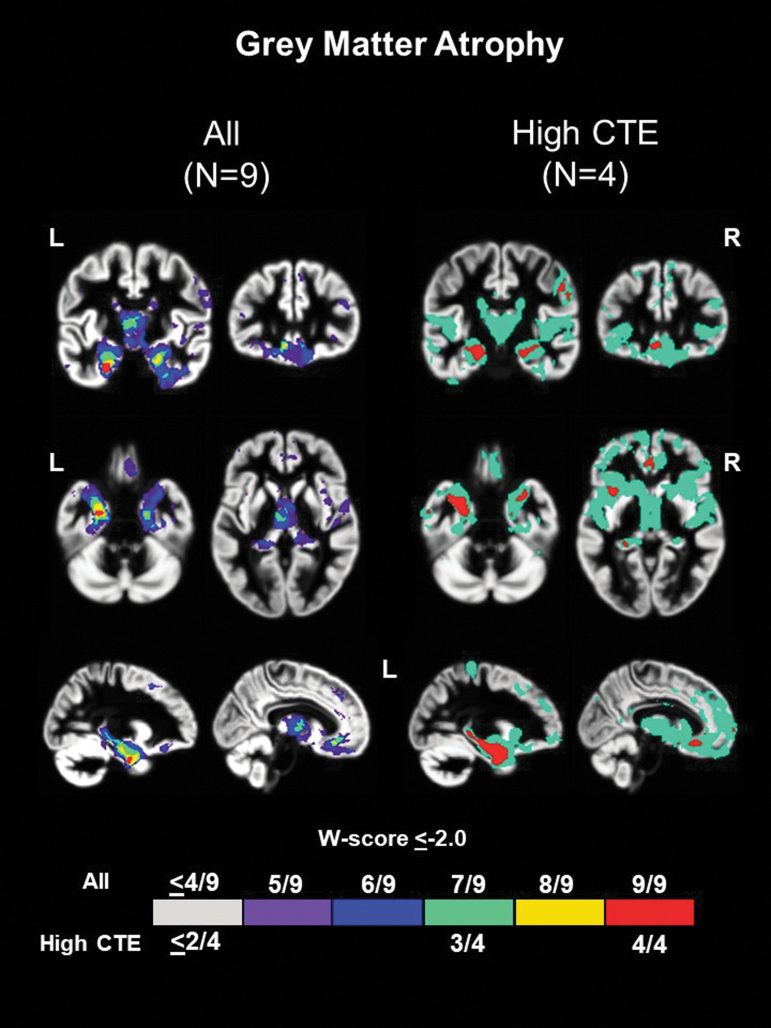
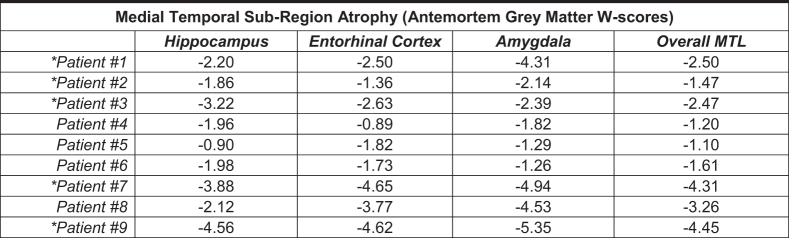
Significant medial temporal atrophy was noted on structural MRI closest to death among all nine patients irrespective of neuropathological diagnoses (Fig. 1 and Supplementary Fig. S2; medial temporal lobe (MTL) ROI median W score = -2.47). The thalamus was also a common area of atrophy (7/9 patients; thalamus ROI median W score = -1.82). Both the ventral frontal cortex (8/9 patients) and dorsal frontal cortex (7/9) had voxels with significant atrophy in most patients, although the common regions were relatively small within the larger ROIs (ventral frontal ROI median W score = -1.36; dorsal frontal ROI median W score = -0.85).
FIG. 1.
Common regions of grey matter atrophy in patients with repetitive head impacts (RHI) and traumatic encephalopathy syndrome (TES). W score frequency map showing common voxels with significantly low grey matter volume (W ≤ -2.0) among the nine patients irrespective of underlying neuropathologic diagnosis (left) and the subset of four patients with high chronic traumatic encephalopathy (High CTE) pathology.25 Warmer colors represent voxels with a higher frequency of patients with low volume (e.g., red voxels show regions where all nine patients had low volume).
When restricting to the four patients with High CTE at autopsy, significant and more widespread common areas of atrophy were noted for all patients in the MTL (extending further posteriorly along the hippocampus). Smaller regions where all four patients with High CTE had significant atrophy were seen in the ventral and orbitofrontal cortex, and right posterolateral frontal cortex (Fig. 1).
Five of the nine patients underwent longitudinal MRI scans (Supplementary Fig. S3). Medial temporal and thalamic regions consistently had the lowest volumes at the initial visit and seemed to show more rapid volume decline than other ROIs.
White matter integrity (DTI) and glucose hypometabolism (FDG-PET)
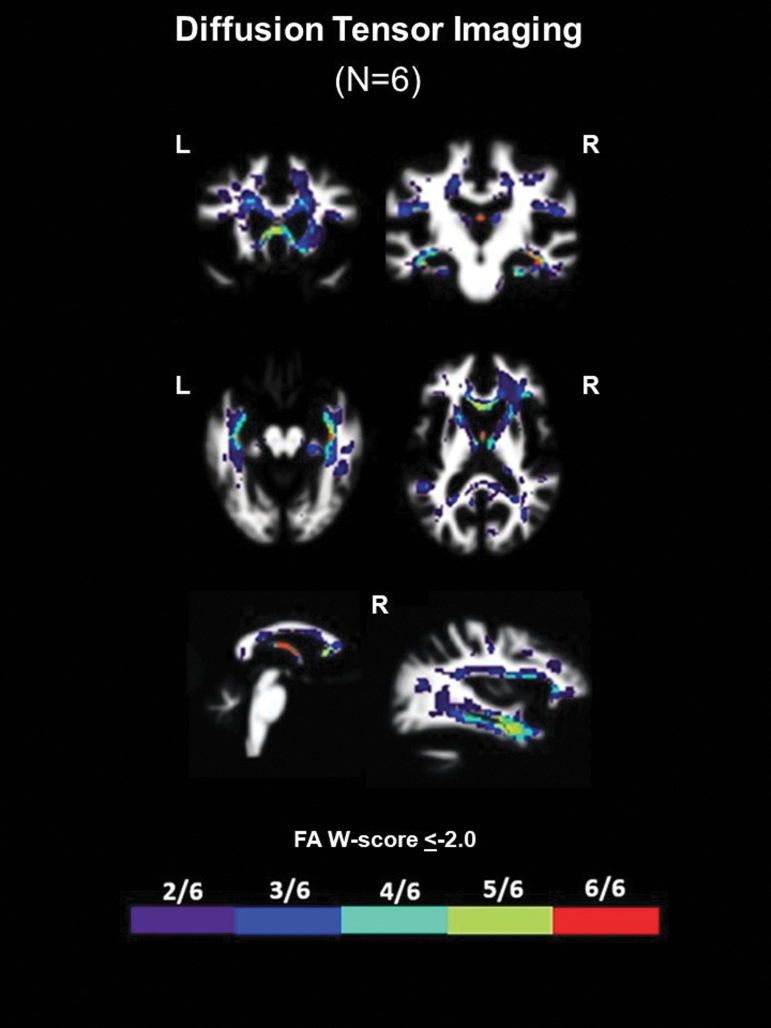
Six patients underwent antemortem DTI (N = 3 High CTE, N = 1 Low CTE, N = 2 no CTE). All six patients had significantly decreased FA along the fornix irrespective of neuropathological diagnoses (Fig. 2 and Supplementary Fig. 2; total fornix ROI median FA W score = -1.28). Five of the six patients had significantly decreased FA in the genu of the corpus callosum (genu CC ROI median W score = -1.24) and medial temporal white matter in the areas of the uncinate fasciculus (UF ROI median FA W score = -1.28) and cingulum-hippocampal bundle (C-H ROI median FA W score = -1.35).
FIG. 2.
Common regions of white matter injury in patients with repetitive head impacts (RHI) and traumatic encephalopathy syndrome (TES). W-score frequency map showing common voxels with significantly low fractional anisotropy (FA W score ≤ -2.0) among the six patients with diffusion tensor imaging irrespective of underlying neuropathologic diagnosis. Warmer colors represent voxels with a higher frequency of patients with low FA (e.g., red voxels show regions where all six patients had low FA; best visualized on slices showing the fornix).
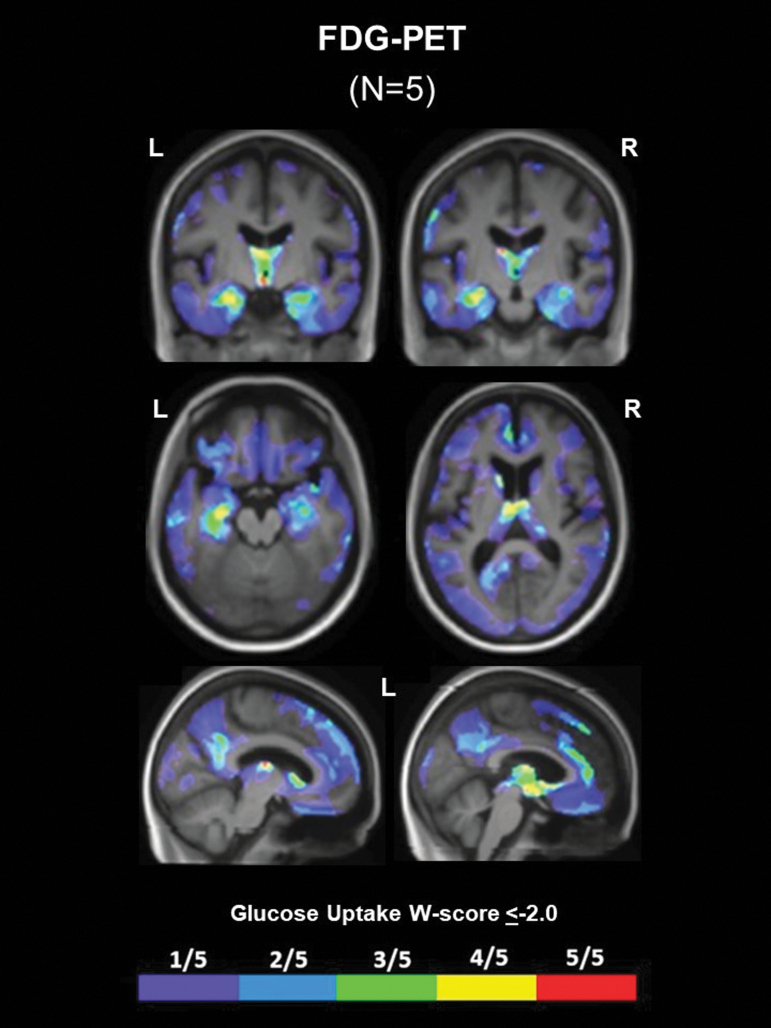
Five patients underwent antemortem FDG-PET (n = 2 High CTE, n = 1 Low CTE, n = 2 no CTE). Glucose hypometabolism generally corresponded to common areas of atrophy. Confluent areas of hypometabolism were observed in the thalamus of four of five patients (Fig. 3 and Supplementary Fig. 2). Four of five patients also showed hypometabolism in the MTL, and three of five patients had hypometabolism in the left dorsal frontal cortex. No other regions of hypometabolism were shared by more than two of the five patients with FDG-PET.
FIG. 3.
Common regions of glucose hypometabolism in patients with repetitive head impacts (RHI) and traumatic encephalopathy syndrome (TES). W-score frequency map showing common voxels with significantly low glucose metabolism (glucose uptake W score ≤ -2.0) on FDG-PET among five patients irrespective of underlying neuropathological diagnosis. Warmer colors represent voxels with a higher frequency of patients with low glucose metabolism (e.g., yellow and red voxels show regions where four or five patients had low glucose metabolism, respectively).
Multi-slice views of available images for all patients are provided in the Supplementary Figures.
Tau and TDP-43 neuropathology in the MTL
We examined neuropathological findings in MTL structures given that all nine patients had significant atrophy in this region (Table 2). All nine patients had tau pathology in the hippocampal formation, especially neuropil threads and neuronal inclusions. Co-morbid argyrophilic grain disease (AGD) was present in all patients. Seven patients had TDP-43 proteinopathy, of whom three were consistent with FTLD-TDP (1 type B, 1 type C, 1 type unclassifiable) and four were limbic-predominant TDP-43. Limbic-predominant TDP-43 pathology was more common and severe in patients with CTE diagnoses compared with those without CTE.
Table 2.
Neuropathological Evaluation of Tau (CP-13) and TDP-43 Proteinopathy Burden in Medial Temporal Lobe Regions
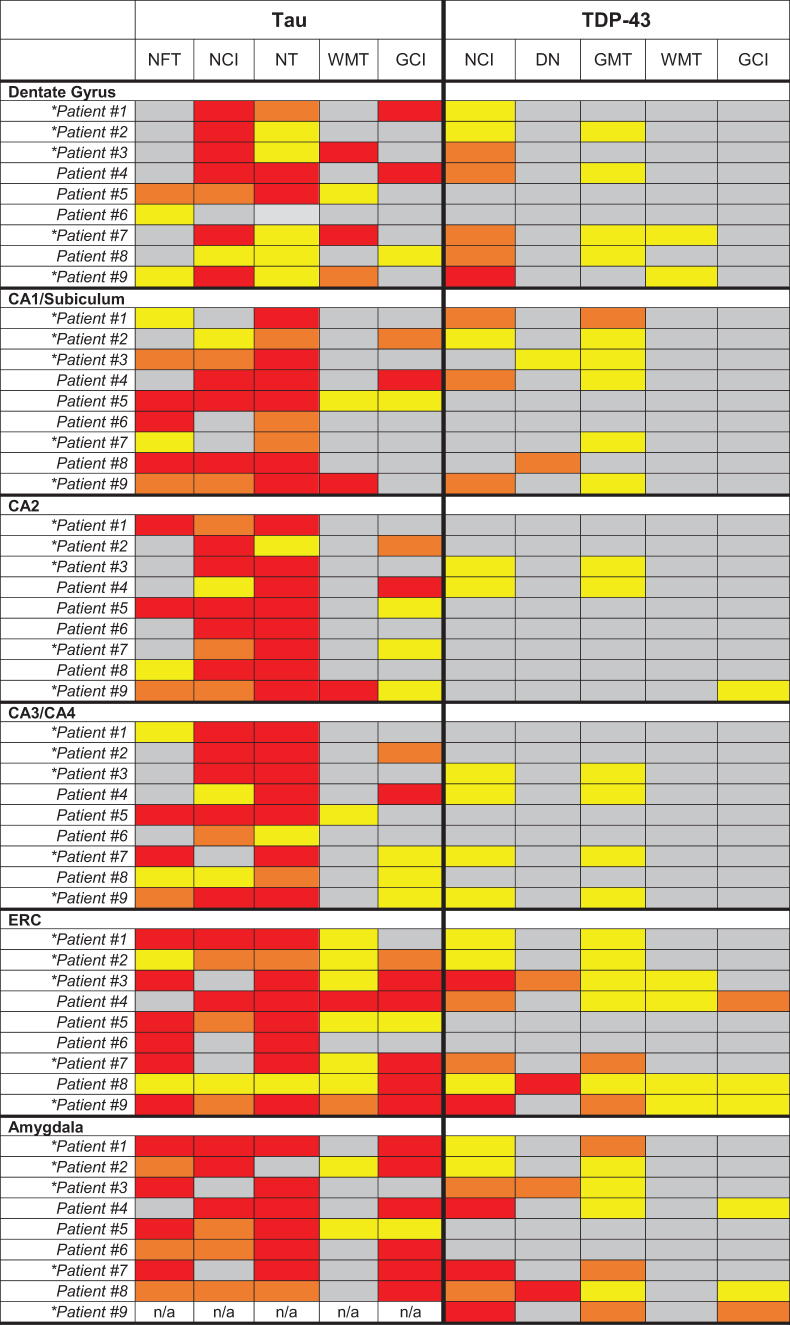
|
Each region was rated semi-quantitatively by a neuropathologist based on the type of cellular inclusion, morphology, and burden of pathology identified by immunohistochemistry (gray = Not Identified, yellow = mild/sparse, orange = moderate, red = severe/frequent). W scores reflecting volume loss based on antemortem T1-weighted magnetic resonance imaging are provided for the hippocampus, entorhinal cortex, amygdala, and a composite medial temporal lobe (MTL) region. Lower W scores represent lower gray matter volume in the region of interest compared with a large group of clinically normal, cognitively healthy controls.
NFT, neurofibrillary tangles; DN, dystrophic neuritis; GMT, gray matter threads/dots; WMT, white matter threads/dots; GCI, glial cytoplasmic inclusions; NCI, neuronal cytoplasmic inclusions (other than tangles or Pick bodies); NT – neuropil threads, ERC, entorhinal cortex.
Patients with chronic traumatic encephalopathy (CTE) pathology at autopsy (High CTE - #1, #2, #7, #9; Low CTE - #3).
*Correction added on June 22, 2022 after first online publication of June 6, 2022: The formatting of Table 2 was inadvertently reorganized, which incorrectly presented data. Table 2 has been corrected to reflect the correct formatting and presentation of data.
Five patients had hippocampal sclerosis, including all four with limbic-predominant TDP-43 proteinopathy and one with FTLD-TDP (type unclassifiable). Hippocampal sclerosis was not present in any patients without medial temporal TDP-43 proteinopathy. Medial temporal TDP-43 pathology was most commonly observed as neuronal cytoplasmic inclusions in the dentate gyrus, entorhinal cortex, and amygdala, and as gray matter thread pathology in the entorhinal cortex.
Plasma GFAP, NfL, and total tau
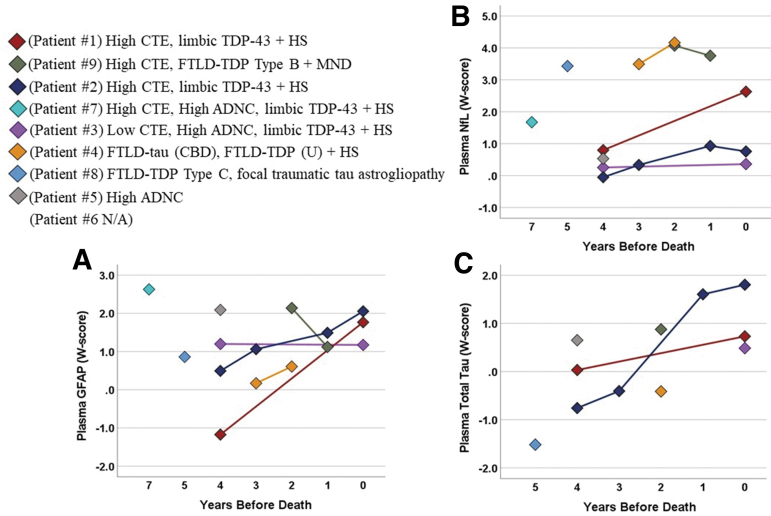
Most patients had higher plasma GFAP, NfL, and total tau at their initial visit than age-matched healthy controls (i.e., W scores >0; Fig. 4,). GFAP concentrations tended to be highest in patients with High ADNC among their neuropathological diagnoses. Among five patients with longitudinal GFAP and NfL data, three demonstrated variably increasing concentrations over time (GFAP: range = 8.7–72.8 pg/mL per year) and four had increasing NfL over time (range = 0.7–12.0 pg/mL per year). Two patients with longitudinal total tau data had High CTE and hippocampal sclerosis and also showed relative increases over time (0.12 pg/mL per year; 0.62 pg/mL per year).
FIG. 4.
Plasma biomarker changes in patients with repetitive head impacts (RHI) and traumatic encephalopathy syndrome (TES). Plasma concentrations of (A) glial fibrillary acidic protein (GFAP), (B) neurofilament light chain (NfL), and (C) total tau obtained during life from eight patients with plasma biomarker data. Data shown as W scores representing standardized protein concentrations relative to age-matched controls (N = 108; mean age = 73.2 ± 7.3 years old, range = 52–91 years old). Longitudinal evaluations were performed in five patients for GFAP and NfL and two patients for total tau. Neuropathological features for each patient are listed along with the specific “Patient #,” which corresponds with additional clinical history provided in Table 1. CTE, chronic traumatoc encephalopathy; FTLD, frontotemporal lobar degeneration; ADNC, Alzheimer disease neuropathological change; HS, hippocampal sclerosis; CBD, corticobasal degeneration; TDP43 – transactive response DNA-binding protein of 43 kDa; MND, motor neuron disease; U, unclassifiable.
Discussion
We report antemortem cognitive testing, neuroimaging, and plasma biomarker data in a series of nine patients with exposure to RHI that were followed to autopsy. Six met criteria for the recently proposed NINDS TES diagnostic framework (n = 2 High CTE, n = 1 Low CTE, n = 3 no CTE) and three were classified as Uncertain TES because of unknown duration of exposure (n = 2 High CTE, n = 1 no CTE). One patient classified as Uncertain TES had widespread CTE, but a diagnosis of frontotemporal dementia with motor neuron disease may have fully explained his symptoms.
Antemortem neuroimaging revealed universal presence of cavum septum pellucidum and significant atrophy in medial temporal/limbic regions. All six patients with antemortem DTI had compromised white matter integrity of the fornix. Most, but not all, of the patients also had regions within frontal cortex and the thalamus with significant atrophy along with compromised medial temporal white matter integrity (uncinate fasciculus, cingulum-hippocampal bundle). Atrophy patterns were similar when restricting to the four patients with widespread CTE pathology. FDG-PET imaging in a subset of five patients showed glucose hypometabolism in medial temporal/limbic regions.
These data extend previous findings in living patients with TES10 to include neuropathological data. All nine patients in our series had multiple neurodegenerative pathologies. The patients in this study were recruited from a brain bank that receives heterogeneous dementia referrals, which may explain the high rate of co-pathologies compared with other case series from banks targeting former professional collision sport athletes.3 Based on our small sample and acknowledging limitations of the retrospective study design, there is preliminary evidence that the new NINDS TES diagnostic framework has limited specificity to CTE pathology. Prospective applications of these criteria in larger samples are needed to adequately determine their clinical utility for differentiating patients with previous RHI and CTE from those without CTE.
Medial temporal/limbic brain changes observable on antemortem imaging in patients with previous RHI or TES could reflect multiple pathologies with or without CTE diagnosis. Immunohistochemical staining for tau revealed almost universal moderate-to-severe neuropil thread pathology in CA1/subiculum, CA2, CA3/CA4, entorhinal cortex, and the amygdala. Tau-containing neurofibrillary tangles were most common in the entorhinal cortex and the amygdala, and other types of neuronal tau cytoplasmic inclusions frequently were seen in the dentate gyrus, CA2, and CA3/CA4. The dentate gyrus, amygdala, and entorhinal cortex appeared most susceptible to TDP-43 pathology (neuronal cytoplasmic inclusions and/or gray matter threads/dots), especially in patients who also had CTE pathology.
Brain changes were also apparent in plasma biomarkers. Biomarkers reflecting astrocytic reactivity (GFAP) or neuronal injury (NfL, total tau) are not expected to be specific to CTE tau pathology but may be elevated in some patients with RHI or TES who have diverse neuropathological diagnoses. Non-specific plasma biomarkers may still be useful for early detection of or screening for inflammatory and/or neurodegenerative brain changes in high-risk populations such as older adults with previous RHI. In two patients with CTE and hippocampal sclerosis (no ADNC), both plasma GFAP and total tau appeared to increase over time.
In aging cohorts, plasma GFAP is tightly linked to AD-related Aβ plaques.45–47 Mechanisms underlying plasma GFAP changes in patients without AD are unclear, but could reflect astrocytic dysfunction and inflammation in non-AD disease pathogenesis.48,49 Incorporating AD-specific biomarkers such as Aβ-PET, CSF Aβ and phosphorylated tau, or plasma phosphorylated tau into studies of patients with previous RHI and TES may help identify biomarker signatures that are specific to CTE and minimize risk of misattributing biomarker changes to AD (co)pathology.
The most recent TES research framework aims to improve identification of CTE pathology in living patients based on a combination of RHI and observed symptoms.1 Memory difficulties are sufficient, but not necessary, to fulfill a core TES diagnostic feature and were consistently observed in our cohort. Abnormal TDP-43 deposition is among the most common co-pathologies with CTE.3 Limbic TDP-43 accumulation with or without hippocampal sclerosis is associated with medial temporal atrophy and memory loss in mixed etiology dementia.50–52
Of the five patients in our series with CTE at autopsy, four reported memory loss during life that was further supported by objective neuropsychological testing. All four of these patients also had limbic TDP-43 proteinopathy with hippocampal sclerosis. The patient with CTE and no limbic TDP-43 proteinopathy (and no hippocampal sclerosis) did not report memory difficulties during life, although objective testing revealed low memory scores potentially related to a severe behavioral/dysexecutive syndrome.
One previous study identified six patients with a diagnosis of co-occurring CTE and AD from the National Alzheimer's Coordinating Center database and compared their clinical profiles with 25 patients with AD only. There were no clear distinguishing cognitive or behavioral features between the two groups, although most of the CTE+AD patients were noted as having limbic TDP-43 accumulation and/or hippocampal sclerosis.53
The co-occurrence of limbic TDP-43 accumulation with CTE tau pathology may also be associated with RHI and likely contributes to medial temporal/limbic system dysfunction in patients with previous RHI and TES. The first study evaluating antemortem neuroimaging in autopsy-confirmed CTE cases also described frontal, anterior temporal, and medial temporal predominant atrophy (visual rating) compared with healthy controls, but temporal atrophy was not associated with CTE tau burden.7 Clinicians should consider limbic TDP-43 when medial temporal atrophy and memory loss are present in patients with RHI and TES, especially if there is biomarker evidence inconsistent with underlying AD.
Hippocampal sclerosis was observed in all patients with limbic TDP-43 in our cohort, but limbic TDP-43 may contribute to atrophy and cognitive changes even without the degree of neuronal loss associated with a diagnosis of hippocampal sclerosis.50 Two of our patients with High CTE and hippocampal sclerosis also developed seizures late in their disease course, although the seizure foci were unknown. Pre-clinical models support heightened susceptibility to seizures after RHI because of altered astrocyte reactivity despite no focal lesion.54
Future work should attempt to determine how strongly “pure” medial temporal CTE tau pathology translates to objective clinical changes such as memory loss or seizures in the absence of limbic-predominant TDP-43 proteinopathy with hippocampal sclerosis, and evaluate the direct association between RHI and limbic-predominant TDP-43. We showed that steady declines on tests of memory are common in patients with previous RHI and TES, with or without CTE pathology. Lack of “pure” CTE cases limits any conclusions about which features of longitudinal cognitive decline are attributable to CTE specifically.
Three of the four patients without evidence of CTE pathology had age-related neuropathological findings (e.g., aging-related tau astrogliopathy [ARTAG], argyrophilic grain disease (AGD), and/or hippocampal sclerosis) despite the relatively young ages of symptom onset and death (ages 49, 58, and 70). ARTAG is an umbrella term encompassing a spectrum of astrocytic tau pathologies with diverse morphologies and in varying locations. It is often observed in elderly patients, those with primary neurodegenerative tauopathies (e.g., FTLD-tau), and ∼40% of patients with early-onset AD.55
ARTAG occasionally poses a challenging and controversial neuropathological diagnosis to differentiate from CTE,25,48,56,57 the latter requiring neuronal tau inclusions.25 AGD is observed in ≤10% of brains of patients younger than 60 years old in community-based cohorts58,59 but, similar to ARTAG, was a co-pathology in up to 40% of patients with early-onset AD in a clinic-based brain bank.55 Conversely, limbic-predominant TDP-43 with hippocampal sclerosis is most common in the oldest old, especially as a co-pathology with late-onset AD,51,60 but is rare among patients with early-onset AD (<5%).55 Hippocampal sclerosis has been documented in other CTE case reports even at a young age.61–63 If or how these non-CTE neuropathologies are associated with the previous RHI requires further investigation.
Neuroimaging of living former National Football League players found an association between more head trauma exposure and higher frequency of white matter signal abnormalities,64 and a separate autopsy study showed moderate-to-severe white matter rarefaction and/or arteriolosclerosis occurred in ∼50% of patients with CTE.65 Our analysis of antemortem DTI and FDG-PET further implicated white matter changes as well as altered synaptic activity, specifically within medial limbic system regions like the fornix and anterior thalamus (i.e., Papez circuit). Medial limbic system dysfunction likely can lead to multiple cognitive (e.g., memory loss, executive dysfunction)66,67 and behavioral (e.g., aggression, anxiety)68,69 symptoms commonly described in patients with previous RHI and TES.
The neuropathology underlying limbic network dysfunction may be diverse. Neuroimaging changes seen in the fornix and thalamus are particularly interesting given their spatial proximity to the septum pellucidum.66 Lengthy separation of the septal leafs, which we saw in all nine of our patients, is common in former professional collision sport athletes38,70 and strongly implicates repetitive head trauma exposure.16 The direct clinical relevance of a CSP is unclear, but CSP may signal a higher likelihood of damage to surrounding medial limbic structures with well-established functional correlates.
Our study had notable strengths in the ability to link comprehensive clinical, neuroimaging, and plasma biomarker data to neuropathological diagnoses at autopsy. The clinical and neuropathological heterogeneity in patients with previous RHI and TES presents significant challenges to accurately diagnosing CTE pathology during life.
The patients included this case series represented a convenience sample identified through retrospective record review, limiting inferences that can be drawn but helping to generate hypotheses for future studies. Activities with high risk of RHI were not routinely documented for all patients within the UCSF Neurodegenerative Disease Brain Bank, so this study cohort likely does not represent all patients with potential head trauma exposure history warranting consideration of a TES diagnosis. We did not have a comparison group of patients without RHI to inform whether there were features of non-CTE pathology that may be from previous RHI.
Time points for clinical and biomarker data collection relative to time of death were variable. More detailed neuropathological descriptions were provided for MTL structures based on shared regions of brain volume loss across the nine patients and availability of tau and TDP-43 staining in this region. In-depth neuropathological characterization of other limbic circuit regions (e.g., thalamus) and more quantitative analysis in larger cohorts is warranted.
We could not prospectively apply the most recent TES research diagnostic framework to these patients, and our sample size was small, so we could not adequately evaluate the sensitivity and specificity of the new criteria to CTE pathology. We could not ensure blinding of other clinical diagnoses or biomarker data obtained during life when retroactively applying recent TES criteria, which may have introduced bias when, for example, determining whether alternate conditions could fully account for symptoms. Alternate test forms were not routinely employed for longitudinal neuropsychological testing, which could lower sensitivity to decline.
All but one patient had RHI exposure through American football, often many years. It is unclear how findings might translate to patients with different mechanisms of repetitive head injury or various degrees of head trauma exposure. The participants in this sample were all male and white/Caucasian, limiting generalizability. There is an urgent need to improve representation of females and underserved sociodemographic groups in studies investigating the links between head trauma and dementia.
Conclusions
Atrophy, decreased white matter integrity, and hypometabolism in medial temporal/limbic system regions, along with memory and executive function decline, are common in patients with RHI and TES, with and without CTE pathology. The neuropathological differential for patients with TES presenting with medial temporal atrophy and memory loss should include limbic TDP-43 accumulation with hippocampal sclerosis. Researchers and clinicians should be cautious in attributing neuroimaging, cognitive, or other biomarker changes solely to CTE tau pathology based on RHI or clinical TES diagnosis alone.
Supplementary Material
Acknowledgments
We are deeply grateful for the patients and their families for the willingness to participate in our research program.
Authors' Contributions
BMA: Conceptualization, Methodology, Formal analysis, Writing—Original Draft, Visualization. JAT: Conceptualization, Methodology, Writing—Review and Editing. LV: Conceptualization, Writing—Review and Editing, Visualization. KBC: Conceptualization, Methodology, Writing – Review and Editing. AMS: Conceptualization, Writing—Review and Editing. NM: Formal analysis, Data Curation, Visualization. CF: Formal analysis, Data Curation, Visualization. LI: Formal analysis, Data Curation, Visualization. RLJ: Formal analysis, Data Curation, Visualization. TT: Formal analysis, Data Curation, Visualization. MM: Conceptualization, Visualization. HG: Investigation, Data Curation, Project administration. RS: Investigation, Data Curation, Project administration. KKWW: Investigation, Resources, Data Curation, Writing—Review and Editing. HX: Investigation, Resources, Data Curation, Writing—Review and Editing. YC: Software, Resources, Data Curation, Supervision. HR: Software, Resources, Data Curation, Supervision. RCG: Writing—Review and Editing, Supervision. DCP: Writing—Review and Editing, Supervision. BLM: Resources, Writing—Review and Editing, Supervision, Funding acquisition. SS: Resources, Writing—Review and Editing. WWS: Conceptualization, Resources, Writing—Review and Editing. JHK: Conceptualization, Resources, Writing—Review and Editing, Supervision, Funding acquisition. LTG: Conceptualization, Resources, Writing—Review and Editing, Supervision. GDR: Conceptualization, Methodology, Resources, Writing—Review and Editing, Supervision, Funding acquisition.
Funding Information
We thank the following funding sources who have supported out work: NIH ADRC (P30AG062422) to GDR/BLM and PPG (P01AG019724) to BLM; NIH (R01AG045611, U01AG057195) and the Rainwater Charitable Foundation to GDR; NIH (R01(s) AG032289 and AG048234) and Larry L. Hillblom Network Grant (2014-A-004-NET) to JHK; NIH (K24AG043435, U54NS100717) to LTG; NIH (R01AG072475) to KBC; NIH (K23AG061253) to AMS; NIH (R01AG062758) to DCP; NIH (R01NS110944), American Federation for Aging Research, and Global Brain Health Institute to RCG; and NIH (K99AG065501) and Alzheimer's Association (AARF-16-443577) to RLJ.
Author Disclosure Statement
AMS has served as a consultant for Passage Bio and Takeda. JHK has provided consultation to Biogen. GDR has served as consultant for Eli Lilly, Eisai, Genentech, Roche, Johnson & Johnson , Merck, and Axon Neurosciences. LTG has received grant funding from Eli Lilly and consulted for CuraSen Inc. For the remaining authors, no competing financial interests exist.
Supplementary Material
References
- 1. Katz, D.I., Bernick, C., Dodick, D.W., Mez, J., Mariani, M.L., Adler, C.H., Alosco, M.L., Balcer, L.J., Banks, S.J., Barr, W.B., Brody, D.L., Cantu, R.C., Dams-O'Connor, K., Geda, Y.E., Jordan, B.D., McAllister, T.W., Peskind, E.R., Petersen, R.C., Wethe, J.V., Zafonte, R.D., Foley É, M., Babcock, D.J., Koroshetz, W.J., Tripodis, Y., McKee, A.C., Shenton, M.E., Cummings, J.L., Reiman, E.M., and Stern, R.A. (2021). National 'Institute of Neurological Disorders and Stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology 96, 848–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKee, A.C., Stern, R.A., Nowinski, C.J., Stein, T.D., Alvarez, V.E., Daneshvar, D.H., Lee, H.S., Wojtowicz, S.M., Hall, G., Baugh, C.M., Riley, D.O., Kubilus, C.A., Cormier, K.A., Jacobs, M.A., Martin, B.R., Abraham, C.R., Ikezu, T., Reichard, R.R., Wolozin, B.L., Budson, A.E., Goldstein, L.E., Kowall, N.W., and Cantu, R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mez, J., Daneshvar, D.H., Kiernan, P.T., Abdolmohammadi, B., Alvarez, V.E., Huber, B.R., Alosco, M.L., Solomon, T.M., Nowinski, C.J., McHale, L., Cormier, K.A., Kubilus, C.A., Martin, B.M., Murphy, L., Baugh, C.M., Montenigro, P.H., Chaisson, C.E., Tripodis, Y., Kowall, N.W., Weuve, J., McClean, M.D., Cantu, R.C., Goldstein, L.E., Katz, D.I., Stern, R.A., Stein, T.D., and McKee, A.C. (2017). Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackay, D.F., Russell, E.R., Stewart, K., MacLean, J.A., Pell, J.P., and Stewart, W. (2019). Neurodegenerative disease mortality among former professional soccer players. N. Engl. J. Med. 381, 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner, R.C., and Yaffe, K. (2015). Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 66, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehman, E.J., Hein, M.J., Baron, S.L., and Gersic, C.M. (2012). Neurodegenerative causes of death among retired National Football League players. Neurology 79, 1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alosco, M.L., Mian, A.Z., Buch, K., Farris, C.W., Uretsky, M., Tripodis, Y., Baucom, Z., Martin, B., Palmisano, J., Puzo, C., Ang, T.F.A., Joshi, P., Goldstein, L.E., Au, R., Katz, D.I., Dwyer, B., Daneshvar, D.H., Nowinski, C., Cantu, R.C., Kowall, N.W., Huber, B.R., Alvarez, V.E., Stern, R.A., Stein, T.D., Killiany, R.J., McKee, A.C., and Mez, J. (2021). Structural MRI profiles and tau correlates of atrophy in autopsy-confirmed CTE. Alzheimers Res. Ther. 13, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lepage, C., Muehlmann, M., Tripodis, Y., Hufschmidt, J., Stamm, J., Green, K., Wrobel, P., Schultz, V., Weir, I., Alosco, M.L., Baugh, C.M., Fritts, N.G., Martin, B.M., Chaisson, C., Coleman, M.J., Lin, A.P., Pasternak, O., Makris, N., Stern, R.A., Shenton, M.E., and Koerte, I.K. (2019). Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. 13, 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mills, B.D., Goubran, M., Parivash, S.N., Dennis, E.L., Rezaii, P., Akers, C., Bian, W., Mitchell, L.A., Boldt, B., Douglas, D., Sami, S., Mouchawar, N., Wilson, E.W., DiGiacomo, P., Parekh, M., Do, H., Lopez, J., Rosenberg, J., Camarillo, D., Grant, G., Wintermark, M., and Zeineh, M. (2020). Longitudinal alteration of cortical thickness and volume in high-impact sports. NeuroImage 217, 116864. [DOI] [PubMed] [Google Scholar]
- 10. Lesman-Segev, O.H., La Joie, R., Stephens, M.L., Sonni, I., Tsai, R., Bourakova, V., Visani, A., Edwards, L., O'Neil, J.P., and Baker, S.L. (2019). Tau pet and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. NeuroImage Clin. 102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hart, J., Kraut, M.A., Womack, K.B., Strain, J., Didehbani, N., Bartz, E., Conover, H., Mansinghani, S., Lu, H., and Cullum, C.M. (2013). Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: Aacross-sectional study. JAMA Neurol. 70, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tremblay, S., Henry, L.C., Bedetti, C., Larson-Dupuis, C., Gagnon, J.F., Evans, A.C., Théoret, H., Lassonde, M., and De Beaumont, L. (2014). Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain 137, 2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brett, B.L., Bobholz, S.A., España, L.Y., Huber, D.L., Mayer, A.R., Harezlak, J., Broglio, S.P., McAllister, T.W., McCrea, M.A., and Meier, T.B. (2020). Cumulative effects of prior concussion and primary sport participation on brain morphometry in collegiate athletes: a study from the NCAA-DoD CARE Consortium. Front. Neurol. 11, 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee, J.K., Wu, J., Bullen, J., Banks, S., Bernick, C., Modic, M.T., Ruggieri, P., Bennett, L., and Jones, S.E. (2020). Association of cavum septum pellucidum and cavum vergae with cognition, mood, and brain volumes in professional fighters. JAMA Neurol. 77, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schultz, V., Stern, R.A., Tripodis, Y., Stamm, J., Wrobel, P., Lepage, C., Weir, I., Guenette, J.P., Chua, A., Alosco, M.L., Baugh, C.M., Fritts, N.G., Martin, B.M., Chaisson, C.E., Coleman, M.J., Lin, A.P., Pasternak, O., Shenton, M.E., and Koerte, I.K. (2018). Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional American football players. J. Neurotrauma 35, 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asken, B.M., and Rabinovici, G.D. (2021). Identifying degenerative effects of repetitive head trauma with neuroimaging: a clinically-oriented review. Acta Neuropathol. Commun. 9, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asken, B.M., DeKosky, S.T., Clugston, J.R., Jaffee, M.S., and Bauer, R.M. (2018). Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): a systematic critical review. Brain Imaging Behav. 12, 585–612. [DOI] [PubMed] [Google Scholar]
- 18. Alosco, M.L., Tripodis, Y., Jarnagin, J., Baugh, C.M., Martin, B., Chaisson, C.E., Estochen, N., Song, L., Cantu, R.C., Jeromin, A., and Stern, R.A. (2016). Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement. (Amst) 7, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahim, P., Gill, J.M., Blennow, K., and Zetterberg, H. (2020). Fluid biomarkers for chronic traumatic encephalopathy. Semin. Neurol. 40, 411–419. [DOI] [PubMed] [Google Scholar]
- 20. Kochsiek, J., O'Donnell, L.J., Zhang, F., Bonke, E.M., Sollmann, N., Tripodis, Y., Wiegand, T., Kaufmann, D., Umminger, L., Di Biase, M.A., Kaufmann , E., Schultz, V., Alosco, M.L., Martin, B.M., Lin, A., Coleman, M.J., Rathi, Y., Pasternak, O., Bouix, S., Stern, R.A., Shenton, M.E., and Koerte, I.K. (2021). Exposure to repetitive head impacts is associated with corpus callosum microstructure and plasma total tau in former professional American football players. J. Magn. Reson. Imaging 54, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyman, B.T., Phelps, C.H., Beach, T.G., Bigio, E.H., Cairns, N.J., Carrillo, M.C., Dickson, D.W., Duyckaerts, C., Frosch, M.P., Masliah, E., Mirra, S.S., Nelson, P.T., Schneider, J.A., Thal, D.R., Thies, B., Trojanowski, J.Q., Vinters, H.V., and Montine, T.J. (2012). National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Theofilas, P., Ehrenberg, A.J., Nguy, A., Thackrey, J.M., Dunlop, S., Mejia, M.B., Alho, A.T., Paraizo Leite, R.E., Rodriguez, R.D., Suemoto, C.K., Nascimento, C.F., Chin, M., Medina-Cleghorn, D., Cuervo, A.M., Arkin, M., Seeley, W.W., Miller, B.L., Nitrini, R., Pasqualucci, C.A., Filho, W.J., Rueb, U., Neuhaus, J., Heinsen, H., and Grinberg, L.T. (2018). Probing the correlation of neuronal loss, neurofibrillary tangles, and cell death markers across the Alzheimer's disease Braak stages: a quantitative study in humans. Neurobiol. Aging 61, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nana, A.L., Sidhu, M., Gaus, S.E., Hwang, J.L., Li, L., Park, Y., Kim, E.J., Pasquini, L., Allen, I.E., Rankin, K.P., Toller, G., Kramer, J.H., Geschwind, D.H., Coppola, G., Huang, E.J., Grinberg, L.T., Miller, B.L., and Seeley, W.W. (2019). Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol. 137, 27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seeley, W.W. (2008). Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr. Opin. Neurol .21, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bieniek, K.F., Cairns, N.J., Crary, J.F., Dickson, D.W., Folkerth, R.D., Keene, C.D., Litvan, I., Perl, D.P., Stein, T.D., Vonsattel, J.P., Stewart, W., Dams-O'Connor, K., Gordon, W.A., Tripodis, Y., Alvarez, V.E., Mez, J., Alosco, M.L., and McKee, A.C. (2021). The second NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 80, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besser, L., Kukull, W., Knopman, D.S., Chui, H., Galasko, D., Weintraub, S., Jicha, G., Carlsson, C., Burns, J., Quinn, J., Sweet, R.A., Rascovsky, K., Teylan, M., Beekly, D., Thomas, G., Bollenbeck, M., Monsell, S., Mock, C., Zhou, X.H., Thomas, N., Robichaud, E., Dean, M., Hubbard, J., Jacka, M., Schwabe-Fry, K., Wu, J., Phelps, C., and Morris, J.C. (2018). Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis. Assoc. Disord. 32, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montenigro, P.H., Baugh, C.M., Daneshvar, D.H., Mez, J., Budson, A.E., Au, R., Katz, D.I., Cantu, R.C., and Stern, R.A. (2014). Clinical subtypes of chronic traumatic encephalopathy: Literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res. Ther. 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Victoroff, J. (2013). Traumatic encephalopathy: review and provisional research diagnostic criteria. NeuroRehabilitation 32, 211–224. [DOI] [PubMed] [Google Scholar]
- 29. Reams, N., Eckner, J.T., Almeida, A.A., Aagesen, A.L., Giordani, B., Paulson, H., Lorincz, M.T., and Kutcher, J.S. (2016). A clinical approach to the diagnosis of traumatic encephalopathy syndrome: a review. JAMA Neurol. 73, 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim, E.J., Brown, J.A., Deng, J., Hwang, J.L., Spina, S., Miller, Z.A., DeMay, M.G., Valcour, V., Karydas, A., Ramos, E.M., Coppola, G., Miller, B.L., Rosen, H.J., Seeley, W.W., and Grinberg, L.T. (2018). Mixed TDP-43 proteinopathy and tauopathy in frontotemporal lobar degeneration: nine case series. J. Neurol. 265, 2960-–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tartaglia, M.C., Sidhu, M., Laluz, V., Racine, C., Rabinovici, G.D., Creighton, K., Karydas, A., Rademakers, R., Huang, E.J., Miller, B.L., DeArmond, S.J., and Seeley, W.W. (2010). Sporadic corticobasal syndrome due to FTLD-TDP. Acta Neuropathol. 119, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mackenzie, I.R., Neumann, M., Bigio, E.H., Cairns, N.J., Alafuzoff, I., Kril, J., Kovacs, G.G., Ghetti, B., Halliday, G., Holm, I.E., Ince, P.G., Kamphorst, W., Revesz, T., Rozemuller, A.J., Kumar-Singh, S., Akiyama, H., Baborie, A., Spina, S., Dickson, D.W., Trojanowski, J.Q., and Mann, D.M. (2010). Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 119, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer, J.H., Jurik, J., Sha, S.J., Rankin, K.P., Rosen, H.J., Johnson, J.K., and Miller, B.L. (2003). Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and alzheimer disease. Cogn. Behav. Neurol. 16, 211–218. [DOI] [PubMed] [Google Scholar]
- 34. Ossenkoppele, R., Schonhaut, D.R., Schöll, M., Lockhart, S.N., Ayakta, N., Baker, S.L., O'Neil, J.P., Janabi, M., Lazaris, A., Cantwell, A., Vogel, J., Santos, M., Miller, Z.A., Bettcher, B.M., Vossel, K.A., Kramer, J.H., Gorno-Tempini, M.L., Miller, B.L., .Jagust, W.J., and Rabinovici, G.D. (2016). Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 139, 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashburner, J., and Friston, K.J. (2005). Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- 36. Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. [DOI] [PubMed] [Google Scholar]
- 37. Desikan, R.S., Ségonne, F., Fischl, B., Quinn, B.T., Dickerson, B.C., Blacker, D., Buckner, R.L., Dale, A.M., Maguire, R.P., Hyman, B.T., Albertk M.S., and Killiany, R.J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- 38. Gardner, R.C., Hess, C.P., Brus-Ramer, M., Possin, K.L., Cohn-Sheehy, B.I., Kramer, J.H., Berger, M.S., Yaffe, K., Miller, B., and Rabinovici, G.D. (2016). Cavum septum pellucidum in retired American pro-football players. J. Neurotrauma 33, 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson, J.L.R., and Sotiropoulos, S.N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jenkinson, M., Beckmann, C.F., Behrens, T.E., Woolrich, M.W., and Smith, S.M. (2012). FSL. Neuroimage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- 41. Garyfallidis, E., Brett, M., Amirbekian, B., Rokem, A., van der Walt, S., Descoteaux, M., and Nimmo-Smith, I., and Dipy Contributors. (2014). Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinform. 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keihaninejad, S., Zhang, H., Ryan, N.S., Malone, I.B., Modat, M., Cardoso, M.J., Cash, D.M., Fox, N.C., and Ourselin, S. (2013). An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer's disease. Neuroimage 72, 153–163. [DOI] [PubMed] [Google Scholar]
- 43. Staffaroni, A.M., Ljubenkov, P.A., Kornak, J., Cobigo, Y., Datta, S., Marx, G., Walters, S.M., Chiang, K., Olney, N., Elahi, F.M., Knopman, D.S., Dickerson, B.C., Boeve, B.F., Gorno-Tempini, M.L., Spina, S., Grinberg, L.T., Seeley, W.W., Miller, B.L., Kramer, J.H., Boxer, A.L., and Rosen, H.J. (2019). Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain 142, 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iaccarino, L., La Joie, R., Edwards, L., Strom, A., Schonhaut, D.R., Ossenkoppele, R., Pham, J., Mellinger, T., Janabi, M., Baker, S.L., Soleimani-Meigooni, D., Rosen, H.J., Miller, B.L., Jagust, W.J., and Rabinovici, G.D. (2021). Spatial relationships between molecular pathology and neurodegeneration in the Alzheimer's disease continuum. Cereb. Cortex 31, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asken, B.M., Elahi, F.M., La Joie, R., Strom, A., Staffaroni, A.M., Lindbergh, C.A., Apple, A.C., You, M., Weiner-Light, S., Brathaban, N., Fernandes, N., Karydas, A., Wang, P., Rojas, J.C., Boxer, A.L., Miller, B.L., Rabinovici, G.D., Kramer, J.H., Casaletto, K.B., and Mielke, M. (2020). Plasma glial fibrillary acidic protein levels differ along the spectra of amyloid burden and clinical disease stage. J. Alzheimers Dis. 78, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pereira, J.B., Janelidze, S., Smith, R., Mattsson-Carlgren, N., Palmqvist, S., Teunissen, C.E., Zetterberg, H., Stomrud, E., Ashton, N.J., Blennow, K., and Hansson, O. (2021). Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer's disease. Brain 144, 3505–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benedet, A.L., Milà-Alomà, M., Vrillon, A., Ashton, N.J., Pascoal, T.A., Lussier, F., Karikari, T.K., Hourregue, C., Cognat, E., Dumurgier, J., Stevenson, J., Rahmouni, N., Pallen, V., Poltronetti, N.M., Salvadó, G., Shekari, M., Operto, G., Gispert, J.D., Minguillon, C., Fauria, K., Kollmorgen, G., Suridjan, I., Zimmer, E.R., Zetterberg, H., Molinuevo, J.L., Paquet, C., Rosa-Neto, P., Blennow, K., and Suárez-Calvet, M. (2021). Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 78, 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arena, J.D., Johnson, V.E., Lee, E.B., Gibbons, G.S., Smith, D.H., Trojanowski, J.Q., and Stewart, W. (2020). Astroglial tau pathology alone preferentially concentrates at sulcal depths in chronic traumatic encephalopathy neuropathologic change. Brain Commun. 2, fcaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cherry, J.D., Kim, S.H., Stein, T.D., Pothast, M.J., Nicks, R., Meng, G., Huber, B.R., Mez, J., Alosco, M.L., Tripodis, Y., Farrell, K., Alvarez, V.E., McKee, A.C., and Crary, J.F. (2020). Evolution of neuronal and glial tau isoforms in chronic traumatic encephalopathy. Brain Pathol. 30, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nelson, P.T., Dickson, D.W., Trojanowski, J.Q., Jack, C.R., Boyle, P.A., Arfanakis, K., Rademakers, R., Alafuzoff, I., Attems, J., Brayne, C., Coyle-Gilchrist, I.T.S., Chui, H.C., Fardo, D.W., Flanagan, M.E., Halliday, G., Hokkanen, S.R.K., Hunter, S., Jicha, G.A., Katsumata, Y., Kawas, C.H., Keene, C.D., Kovacs, G.G., Kukull, W.A., Levey, A.I., Makkinejad, N., Montine, T.J., Murayama, S., Murray, M.E., Nag, S., Rissman, R.A., Seeley, W.W., Sperling, R.A., White III, C.L., Yu, L., and Schneider, J.A. (2019). Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 142, 1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jicha, G.A., and Nelson, P.T. (2019). Hippocampal sclerosis, argyrophilic grain disease, and primary age-related tauopathy. Continuum (Minneap Minn) 25, 208–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boyle, P.A., Wang, T., Yu, L., Wilson, R.S., Dawe, R., Arfanakis, K., Schneider, J.A., and Bennett, D.A. (2021). To what degree is late life cognitive decline driven by age-related neuropathologies? Brain 144, 2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. LoBue, C., Schaffert, J., Cullum, C. M., Peters, M.E., Didhbani, N., Hart, J., and White, C.L. (2020). Clinical and neuropsychological profile of patients with dementia and chronic traumatic encephalopathy. J. Neurol. Neurosurg. Psychiatry 91, 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shandra, O., Winemiller, A.R., Heithoff, B.P., Munoz-Ballester, C., George, K.K., Benko, M.J., Zuidhoek, I.A., Besser, M.N., Curley, D.E., Edwards, G.F., Mey, A., Harrington, A.N., Kitchen, J.P., and Robel, S. (2019). Repetitive diffuse mild traumatic brain injury causes an atypical astrocyte response and spontaneous recurrent seizures. J. Neurosci. 39, 1944–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Spina, S., La Joie, R., Petersen, C., Nolan, A.L., Cuevas, D., Cosme, C., Hepker, M., Hwang, J.H., Miller, Z.A., Huang, E.J., Karydas, A.M., Grant, H., Boxer, A.L., Gorno-Tempini, M.L., Rosen, H.J., Kramer, J.H., Miller, B.L., Seeley, W.W., Rabinovici, G.D., and Grinberg, L.T. (2021). Comorbid neuropathological diagnoses in early versus late-onset Alzheimer's disease. Brain 144, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kovacs, G.G., Ferrer, I., Grinberg, L.T., Alafuzoff, I., Attems, J., Budka, H., Cairns, N.J., Crary, J.F., Duyckaerts, C., Ghetti, B., Halliday, G.M., Ironside, J.W., Love, S., Mackenzie, I.R., Munoz, D.G., Murray, M.E., Nelson, P.T., Takahashi, H., Trojanowski, J.Q., Ansorge, O., Arzberger, T., Baborie, A., Beach, T.G., Bieniek, K.F., Bigio, E.H., Bodi, I., Dugger, B.N., Feany, M., Gelpi, E., Gentleman, S.M., Giaccone, G., Hatanpaa, K.J., Heale, R., Hof, P.R., Hofer, M., Hortobágyi, T., Jellinger, K., Jicha, G.A., Ince, P., Kofler, J., Kövari, E., Kril, J.J., Mann, D.M., Matej, R., McKee, A.C., McLean, C., Milenkovic, I., Montine, T.J., Murayama, S., Lee, E.B., Rahimi, J., Rodriguez, R.D., Rozemüller, A., Schneider, J.A., Schultz, C., Seeley, W., Seilhean, D., Smith, C., Tagliavini, F., Takao, M., Thal, D.R., Toledo, J.B., Tolnay, M., Troncoso, J.C., Vinters, H.V., Weis, S., Wharton, S.B., White, C.L., 3rd, Wisniewski, T., Woulfe, J.M., Yamada, M., and Dickson, D.W. (2016). Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 131, 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kovacs, G.G., Xie, S.X., Robinson, J.L., Lee, E.B., Smith, D.H., Schuck, T., Lee, V.M., and Trojanowski, J.Q. (2018). Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol. Commun. 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrer, I., Santpere, G., and van Leeuwen, F.W. (2008). Argyrophilic grain disease. Brain 131, 1416–1432. [DOI] [PubMed] [Google Scholar]
- 59. Rodriguez, R.D., Suemoto, C.K., Molina, M., Nascimento, C.F., Leite, R.E., de Lucena Ferretti-Rebustini, R.E., Farfel, J.M., Heinsen, H., Nitrini, R., Ueda, K., Pasqualucci, C.A., Jacob-Filho, W., Yaffe, K., and Grinberg, L.T. (2016). Argyrophilic grain disease: demographics, clinical, and neuropathological features from a large autopsy study. J. Neuropathol. Exp. Neurol. 75, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barnes, L.L., Lamar, M., and Schneider, J.A. (2019). Sex differences in mixed neuropathologies in community-dwelling older adults. Brain Res. 1719, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang, C., Nag, S., Xing, G., Aggarwal, N.T., and Schneider, J.A. (2020). A clinicopathological report of a 93-year-old former street boxer with coexistence of chronic traumatic encephalopathy, Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis with TDP-43 pathology. Front. Neurol. 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ling, H., Morris, H.R., Neal, J.W., Lees, A.J., Hardy, J., Holton, J.L., Revesz, T., and Williams, D.D. (2017). Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. 133, 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grinberg, L.T., Anghinah, R., Nascimento, C.F., Amaro, E., Leite, R.P., Martin Mdg, M., Naslavsky, M.S., Takada, L.T., Filho, W.J., Pasqualucci, C.A., and Nitrini, R. (2016). Chronic traumatic encephalopathy presenting as Alzheimer's disease in a retired soccer player. J. Alzheimers Dis. 54, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alosco, M.L., Koerte, I.K., Tripodis, Y., Mariani, M., Chua, A.S., Jarnagin, J., Rahimpour, Y., Puzo, C., Healy, R.C., Martin, B., Chaisson, C.E., Cantu, R.C., Au, R., McClean, M., McKee, A.C., Lin, A.P., Shenton, M.E., Killiany, R.J., and Stern, R.A. (2018). White matter signal abnormalities in former National Football League players. Alzheimers Dement. (Amst) 10, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alosco, M.L., Stein, T.D., Tripodis, Y., Chua, A.S., Kowall, N.W., Huber, B.R., Goldstein, L.E., Cantu, R.C., Katz, D.I., Palmisano, J.N., Martin, B., Cherry, J.D., Mahar, I., Killiany, R.J., McClean, M.D., Au, R., Alvarez, V., Stern, R.A., Mez, J., and McKee, A.C. (2019). Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol. 76, 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Senova, S., Fomenko, A., Gondard, E., and Lozano, A.M. (2020). Anatomy and function of the fornix in the context of its potential as a therapeutic target. J. Neurol. Neurosurg. Psychiatry 91, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Archer, D.B., Moore, E.E., Shashikumar, N., Dumitrescu, L., Pechman, K.R., Landman, B.A., Gifford, K.A., Jefferson, A.L., and Hohman, T.J. (2020). Free-water metrics in medial temporal lobe white matter tract projections relate to longitudinal cognitive decline. Neurobiol. Aging 94, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Siep, N., Tonnaer, F., van de Ven, V., Arntz, A., Raine, A., and Cima, M. (2019). Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging Behav. 13, 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kenwood, M.M., Kalin, N.H., and Barbas, H. (2022). The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 47, 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee, J.K., Wu, J., Banks, S., Bernick, C., Massand, M.G., Modic, M.T., Ruggieri, P., and Jones, S.E. (2017). Prevalence of traumatic findings on routine MRI in a large cohort of professional fighters. AJNR Am. J. Neuroradiol. 38, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers from academic, not-for-profit institutions can request deidentified data associated with this study through the UCSF Memory and Aging Center after obtaining IRB approval from the UCSF Human Research Protection Program and completing a resource request.