Abstract
A woman in her 60s with a history of adult-onset Still’s disease (AOSD) in remission for 14 years received the ChAdOx1-S vaccine as a booster to her initial vaccination schedule (two doses of CoronaVac vaccine 6 months apart). Two weeks later, she consulted for symptoms suggestive of AOSD reactivation. This was confirmed during hospitalisation, where renal and cardiac involvement were also observed. Despite using high-dose corticosteroids, troponin T and N-terminal pro hormone B-type natriuretic peptide (NT-proBNP) were persistently elevated. Tocilizumab was used, with which the patient achieved complete remission of her symptoms and normalised her laboratory tests.
Keywords: COVID-19, Connective tissue disease
Background
Since the beginning of the COVID-19 vaccination campaign, there have been concerns regarding the safety of vaccines in patients with rheumatic diseases, especially considering that the adaptive immune system is activated.1
In this particular case, the clinical manifestations of the patient and the timing of the events support the possibility that the vaccine was the trigger for adult-onset Still’s disease (AOSD) reactivation.
Case presentation
We present the case of a woman in her seventh decade of life, with a history of glucose-6-phosphate dehydrogenase deficiency, who 15 years ago was hospitalised for a prolonged febrile syndrome characterised by a non-pruritic maculopapular rash on the extremities and trunk, arthralgias, dry cough, sore throat and night sweats. After an extensive study of infectious diseases without findings, and due to the persistence of symptoms and very high inflammatory parameters, an evaluation by rheumatology was requested. The autoimmunity study did not reveal abnormalities, but ferritin was high (2200 ng/mL) and an AOSD was diagnosed according to the Yamagushi criteria (fever, arthralgias, rash, leucocytosis, sore throat, abnormal liver function studies, and negative tests for antinuclear antibody and rheumatoid factor). She was treated with high-dose corticosteroids and was quickly discharged. After a year of follow-up, she achieved remission without continuing medications.
Now, 2 weeks after the administration of the COVID-19 ChAdOx1-S vaccine as a booster to her initial vaccination schedule (two doses of CoronaVac vaccine 6 months apart), the patient consulted for presenting with fever for 4 days, severe fatigue, arthralgia, exanthema in extremities, palpitations and darker than usual urine. She had no chest pain, dyspnoea, or other urinary or gastrointestinal symptoms. On physical examination, the presence of a salmon-coloured maculopapular skin rash over the extremities and arthritis in hands and ankles were observed.
Investigations
Laboratory tests on admission revealed elevated inflammatory parameters: C reactive protein 44 mg/dL; erythrocyte sedimentation rate 88 mm/hour; leucocytes 17×109/L; ferritin 15 457 ng/mL; troponin T 66 ng/L (without changes in the electrocardiogram); proteinuria in the subnephrotic range and haematuria with glomerular characteristics, without associated renal function impairment. In addition, there was a slight elevation of transaminases. An extensive autoimmunity study was normal (antinuclear antibody, anti-neutrophil cytoplasmic antibodies, extractable nuclear antigen antibodies, anti-dsDNA antibody, lupus anticoagulant, anticardiolipin antibodies, anti-beta-2 glycoprotein 1 antibodies).
Empirical antibiotic therapy with ceftriaxone was started; however, it was stopped early after obtaining negative results in the blood and urine cultures. An echocardiogram was performed that did not identify structural cardiac alterations.
Differential diagnosis
Systemic lupus erythematosus is an appropriate differential diagnosis, especially considering the renal involvement. It was ruled out with an extensive autoimmunity study. It is worth remembering that renal involvement and myocarditis have been described in AOSD.2 3
COVID-19 was also a possibility, but was quickly ruled out with a polymerase chain reaction test.
Other inflammatory conditions, such as sarcoidosis and immunoglobulin G4-related disease, were quickly dismissed due to the clinical picture and the results of the tests, including a normal chest computed tomography and abdominal ultrasound.
Treatment
In the context of reactivation of AOSD with renal involvement and possible myocarditis, it was decided to start intravenous methylprednisolone 80 mg daily, which was subsequently increased to 250 mg daily, for 3 days. Despite this, the elevation of troponin T reached 119 ng/L, and NT-proBNP up to 9783 pg/mL, indicating persistent and increased cardiac involvement. A heart nuclear magnetic resonance was performed that reported normal global and segmental function, with normal volumes, but with a focus of late enhancement, concordant with a zone of alteration on the T1 map, suggestive of myocarditis, in addition to minimal pericardial effusion and mild mitral regurgitation (figure 1). Given the persistence of symptoms and the presence of myocarditis, it was decided to use tocilizumab 500 mg (8 mg/kg) intravenously in a single dose. The patient had a rapid and significant improvement in her symptoms, with progressive decrease in the levels of serum markers of myocardial injury and inflammation, and normalisation of urinary abnormalities.
Figure 1.
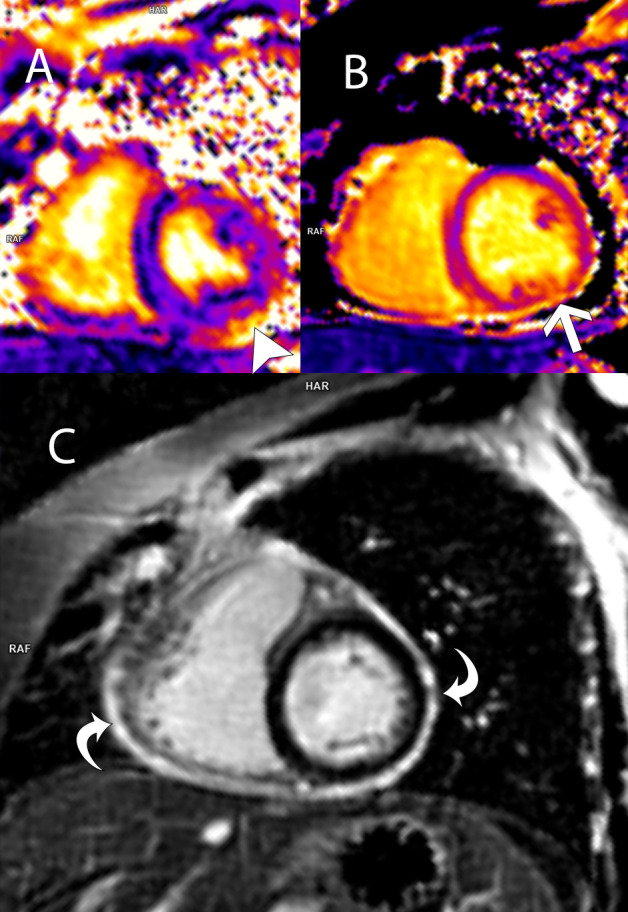
Cardiac 3 Tesla MRIs oriented in short-axis view at the mid-ventricular level. (A) T2 map shows an increase in myocardial T2 values at 50 ms (arrowhead); (B) T1 map shows elevated myocardial T1 times in the inferolateral segment at 1300 ms (arrow). Findings on T1 and T2 maps indicate myocardial oedema. (C) Phase-sensitive inversion recovery image 10 min after intravenous gadolinium shows diffuse enhancement of the pericardium, consistent with active pericarditis. There is no overt late myocardial enhancement.
Outcome and follow-up
She was discharged 2 days after receiving tocilizumab, with oral methylprednisolone, 32 mg daily. In the outpatient check-up, 2 months after discharge, the methylprednisolone dose had been lowered to 8 mg per day without the reappearance of symptoms and with normalisation of all laboratory values.
Discussion
The risk of post-vaccination autoimmune diseases has been discussed in the literature. For example, acute disseminated encephalomyelitis has been reported post-rabies vaccine, Guillain-Barre neuritis has been reported following swine influenza vaccine and autoimmune thrombocytopenia has been reported post-measles vaccine.4
Mechanisms postulated in the development of autoimmunity to vaccines include the following:
Molecular mimicry means similarity between vaccine molecules and the patient’s self-antigen.
Bystander activation.
By vaccine molecule-related signals triggering innate immunity and overcoming the regulatory mechanisms that prevent autoimmune diseases.5
The SARS-CoV-2 spike protein acts as a pathogen-associated molecular pattern. It causes overproduction of cytokines via Tool like receptor (TLR) mediated pathways and inflammasome pathways. TLR-7 and TLR-9 upregulate interferon-stimulated genes, and that leads to robust immune responses.6
Various autoimmune conditions have been reported post-ChAdOx1-S vaccine, including vaccine-related venous thrombosis, vaccine-induced thrombotic thrombocytopenia,7 post-vaccine neuromyelitis optica spectrum disorder8 and de novo Lofgren’s syndrome.9 In addition, AOSD cases have been reported after COVID-19 infection and influenza vaccinations.10
Other adverse events reported in the literature are mainly limited to thrombotic events,11 anaphylactic reactions,12 polymyositis,13 and mild local and systemic manifestations.14
Regarding reactivations of AOSD after the administration of vaccines against SARS-CoV-2, we could find a small number of case reports,15–19 published after our patient was discharged.
In this particular case, the clinical presentation and the timing of the events support the possibility that the vaccine was the trigger for this AOSD reactivation. However, there is still not enough information about vaccines’ safety in patients with AOSD to generate evidence-based recommendations.
Learning points.
Autoimmune and inflammatory disease reactivations are possible after COVID-19 vaccination.
A crisis of adult-onset Still’s disease can present with severe manifestations, including renal and cardiac involvement.
Timely treatment is essential to prevent damage in severe adult-onset Still’s disease.
Acknowledgments
Doctor Cristobal Ramos for image interpretation.
Footnotes
Twitter: @manos81cl
Contributors: SEIV—planning, conception and design. LM—acquisition and interpretation of data. MdIR—reporting and acquisition of data. RS—reporting and acquisition of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Benucci M, Infantino M, Marotto D, et al. Vaccination against SARS-CoV-2 in patients with rheumatic diseases: doubts and perspectives. Clin Exp Rheumatol 2021;39:196–202. 10.55563/clinexprheumatol/7afn90 [DOI] [PubMed] [Google Scholar]
- 2.Feist E, Mitrovic S, Fautrel B, Mechanisms FB. Mechanisms, biomarkers and targets for adult-onset still's disease. Nat Rev Rheumatol 2018;14:603–18. 10.1038/s41584-018-0081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Mezouar I, Abourazzak FZ, Ghani N, et al. An unusual manifestation in a patient with adult-onset still's disease: minimal glomerular lesion. Eur J Rheumatol 2014;1:123–4. 10.5152/eurjrheumatol.2014.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur KT, Epstein S, Bilski A, et al. Neurologic safety monitoring of COVID-19 vaccines. Neurology 2021;97:767–75. 10.1212/WNL.0000000000012703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int 2021;41:509–18. 10.1007/s00296-021-04792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manik M, Singh RK. Role of Toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J Med Virol 2022;94:869-877. 10.1002/jmv.27405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anamnart C, Tisavipat N, Owattanapanich W, et al. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: case report and systematic review. Mult Scler Relat Disord 2022;58:103414. 10.1016/j.msard.2021.103414 [DOI] [PubMed] [Google Scholar]
- 9.Rademacher J-G, Tampe B, Korsten P. First report of two cases of Löfgren's syndrome after SARS-CoV-2 Vaccination-Coincidence or causality? Vaccines 2021;9:1313. 10.3390/vaccines9111313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamidis AD, Koehler P, di Cristanziano V, et al. First manifestation of adult-onset still's disease after COVID-19. Lancet Rheumatol 2021;3:e319–21. 10.1016/S2665-9913(21)00072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbattista M, Martinelli I, Peyvandi F. Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: thrombosis at unusual sites. J Thromb Haemost 2021;19:2554–8. 10.1111/jth.15493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabanillas B, Novak N. Allergy to COVID-19 vaccines: a current update. Allergology International 2021;70:313–8. 10.1016/j.alit.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capassoni M, Ketabchi S, Cassisa A, et al. AstraZeneca (AZD1222) COVID-19 vaccine-associated adverse drug event: a case report. J Med Virol 2021;93:5718–20. 10.1002/jmv.27175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Khames Aga QA, Alkhaffaf WH, Hatem TH, et al. Safety of COVID-19 vaccines. J Med Virol 2021;93:6588–94. 10.1002/jmv.27214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magliulo D, Narayan S, Ue F, et al. Adult-Onset still's disease after mRNA COVID-19 vaccine. Lancet Rheumatol 2021;3:e680–2. 10.1016/S2665-9913(21)00219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone F, Cerasuolo PG, Bosello SL, et al. Adult-Onset still's disease following COVID-19 vaccination. Lancet Rheumatol 2021;3:e678–80. 10.1016/S2665-9913(21)00218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon YH, Lim D-H, Choi SW. A flare of Still’s disease following COVID-19 vaccination in a 34-year-old patient. Rheumatol Int 2021:1–6. 10.1007/s00296-021-05052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto S, Nishimura K, Yo K. Flare-up of adult-onset Still’s disease after receiving a second dose of BNT162b2 COVID-19 mRNA vaccine. Clin Exp Rheumatol 2021;132:139–40. 10.55563/clinexprheumatol/tvlpnc [DOI] [PubMed] [Google Scholar]
- 19.Sharabi A, Shiber S, Molad Y. Adult-Onset still's disease following mRNA COVID-19 vaccination. Clin Immunol 2021;233:108878. 10.1016/j.clim.2021.108878 [DOI] [PMC free article] [PubMed] [Google Scholar]


