Abstract
Introduction
Prophylactic human papillomavirus (HPV) vaccines have been shown to be highly effective in protecting women against cervical infections, high-grade abnormalities and cancer caused by the targeted HPV types. However, the evidence for their effectiveness in women living with HIV (WLWH) is less clear.
Methods
WLWH and HIV-negative women who likely did (birth cohorts 1996 and later) and WLWH and HIV(−) negative who likely did not (birth cohorts before 1996) receive HPV vaccination (n=3028; 757 participants for each of the four groups). Between groups, we will compare cervicovaginal, anal and oral prevalent and 6–12 month persistent HPV6/11/16/18 infections as measured using a modified AmpFire HPV genotyping assay that tests for 15 high-risk or intermediate-risk HPV genotypes, HPV6 and HPV11. We will also compare the HPV immune response in HPV-vaccinated WLWH to HPV-vaccinated HIV-negative women using an anti-HPV16 and anti-HPV18 ELISA. Vaccination status will be confirmed through national vaccination records.
Analysis
We will calculate point prevalence and prevalence of 6–12 month persisting infections by individual HPV-type specific infections and groups of infections for each anatomic site and for each group of women. Results will be stratified by age at vaccination, age at enrolment and the number of doses (3 vs 2) as well as other factors possibly associated with HPV prevalence. Differences in endpoints between groups, overall and between subgroups, will be tested for statistical significance (p<0.05) using Fisher’s exact or Pearson χ2 test. Differences in geometric mean titres and seropositivity will be tested for statistical significance using the Mann-Whitney and Fisher’s exact tests, respectively.
Ethics and dissemination
The study was approved by the Albert Einstein College of Medicine Institutional Review Board and the Rwanda National Ethics Committee. Results will be disseminated through publication in peer-reviewed journals.
Keywords: public health, preventive medicine, public health, virology, HIV & AIDS, gynaecology
Strengths and limitations of this study.
The study is being conducted in Rwanda, a high cervical cancer burden country.
National introduction of human papillomavirus (HPV) vaccine in Rwanda in 2011 and relatively high prevalence of HIV makes a study of long-term HPV vaccination effectiveness in women living with HIV (WLWH) possible.
The study is not a randomised controlled trial but an observational study of populations selected from different groups of women.
A convenience sample of Rwandan WLWH living in and around Kigali will be enrolled, and therefore, the study population is not representative of all Rwanda or WLWH living elsewhere.
The quality of HPV vaccination status ascertainment is uncertain given the use of paper registers and the long-time period that has elapsed since vaccination.
Introduction
Current prophylactic vaccines against human papillomavirus (HPV), the necessary cause of virtually all cervical cancer,1 2 are based on the self-assembly of recombinantly expressed L1 protein in cell lines into virus-like particles (VLPs) that resemble native viral capsids but lack the viral genome required for viral replication and infectivity. The first generation of prophylactic HPV vaccines, Gardasil (Merck & Co, Kenilworth, New Jersey, USA)3 and Cervarix (GlaxoSmithKline, Wavre, Belgium),3 targeted HPV16 and HPV18 (HPV16/18), which cause approximately 70% of cervical cancers.4 Gardasil also targets HPV6 and HPV11 (HPV6/11), non-high-risk HPV types responsible for approximately 90% of anogenital warts (Condylomata acuminata).5
Several countries, including Australia,6–12 Scotland,13 Denmark14 and the USA,15–17 were early adopters of HPV vaccination and have documented reductions in infections, diseases and cervical abnormalities related to the HPV vaccine-targeted types. A meta-analysis on the impact of HPV vaccination found reductions in anogenital warts, HPV infections, cervical intraepithelial neoplasia (CIN) grade 2 (CIN2) or more severe diagnoses (CIN2+) among girls and women and on anogenital warts diagnoses among girls, women, boys and men.18 Recent reports from Finland,19 Sweden,20 England21 and Denmark22 provide real-world evidence that HPV vaccination prevents invasive cervical cancer.
Cervical cancer was included as an AIDS-defining disease in adolescents and adults in 1993.23 24 Women living with HIV (WLWH), the cause of AIDS, have a significantly elevated risk of cervical cancer,25 26 due to an impaired immune response to HPV, compared with HIV-uninfected (HIV(−)) women. Meta-analyses of HIV/AIDS cohorts reported a sixfold increased incidence of cervical cancer compared with the general female population/HIV(−) women.25–27 People living with HIV (PLWH) are also at an increased risk of other HPV-related malignancies, notably anal and oropharyngeal cancers.25 28 HIV coinfection has a profound impact on the natural history of HPV, thereby increasing the risk of invasive cervical cancer (ICC). HIV coinfection increases the: (1) likelihood of cervical and anal HPV persistence and (2) likelihood of cervical and anal HPV infections progressing to precancer.29 30
Data on HPV vaccine efficacy and effectiveness in WLWH are lacking.31 32 To date, most studies of HPV vaccine in WLWH focused on immunogenicity and safety. Generally, HPV vaccination in PLWH has been well tolerated, safe and resulted in good immune responses33–37; one study found lower seroconversion and anti-HPV antibody titres among PLWH compared with perinatally HIV-exposed, uninfected youth.37 Studies have noted an impact of HIV disease status (CD4 counts and viral suppression) on the immune responses to HPV vaccination.38–40 Studies have reported lower seroconversion and antibody titers in WLWH not taking (vs taking) antiretroviral therapy (ART) and with lower (vs higher) CD4 counts.34 39 Similarly, another study found peak antibody titres to be twofold to threefold higher in midadult WLWH with full HIV viral suppression compared with those not suppressed.40 Interestingly, higher anti-HPV18 titres but similar anti-HPV16 titres were reported in response to HPV vaccination by Cervarix compared with Gardasil in PLWH, but the differences in the immune responses were due to differences in women (WLWH) but not men.41
The few studies of HPV vaccine effectiveness in WLWH provided promising but inconclusive evidence. Immunogenicity studies in WLWH have been of insufficient sample size to address efficacy/effectiveness. Studies of HPV vaccination in select PLWH at high risk of anal cancer have been limited in sample size because of the high HPV anal exposure (and likely misclassification of exposure) to targeted HPV types prior to enrolment42 so that few truly incident events could be observed. Of those studies that did have endpoints, one recent trial of Gardasil in PLWH aged 27 years and older concluded that ‘This double-blind, randomised trial did not find a benefit from HPV vaccination to prevent anal HPV infection or anal high-grade squamous intraepithelial lesions’ and favourable but inconclusive benefit for protection against oral HPV.43
The paucity of HPV-vaccine effectiveness data in WLWH is especially a concern for the prevention of cervical cancer in sub-Saharan Africa (SSA), where cervical cancer is the most common cause of cancer death in women.44 Almost one-quarter of the global burden of cervical cancer occurs in SSA and an estimated 20% of cervical cancer in SSA is attributable to HIV coinfection.27 Evidence of the protective effects of HPV vaccination in SSA WLWH is needed, especially since most long-term public health planning to address cervical cancer in SSA depends on the unproven effectiveness of these vaccines.
Rwanda, a central/east African country that experiences a high burden of cervical cancer,45 is the ideal locale to answer questions about the long-term effectiveness and immunogenicity of Gardasil in WLWH for the following reasons. First, in 2011–2013, Rwanda, through a donation from Merck, launched a national HPV vaccination programme.46 In 2011, over 92 000 girls in primary school grade 6 (~12 years old) were vaccinated with three doses of Gardasil. During 2012 and 2013, a catch-up vaccination programme targeted girls in secondary school grade three (~15 years old).46 In 2014, HPV vaccination was supported by GAVI47 and reverted to vaccinating 12-year-old girls.48 In 2015, Rwanda switched from three doses to two doses, 6 months apart, for vaccinating 12-year-old girls. In all years, Rwanda achieved ≥90% annual coverage with the recommended number of HPV vaccine doses in the target population.46 47 49 50 Thus, Rwanda is one of the earliest and most successful adopters of HPV vaccination globally.
Second, the prevalence of HIV in Rwanda is 2.7% among 15–30 year olds.51 Approximately half of the Rwanda population of 13 million is female, and one-quarter of them are between the age of 15–30 years. Thus, there is approximately 450 000 WLWH aged 15–30 years living in Rwanda.52 The excellent HIV care programme in Rwanda allows for easy and efficient recruitment and following of WLWH in an observational study of HPV vaccination impact. Third, given Rwanda’s nearly complete national coverage with Gardasil, HPV vaccinated and unvaccinated WLWH are easily recruited based on birth year. Rwanda has excellent national vaccination records so that retrospectively the small number of participants misclassified by HPV vaccination status based on birth year subsequently can be correctly categorised.
The Einstein/Rwanda/DRC Consortium for Research in HIV/HPV/Malignancies therefore launched an observational cohort of HPV-vaccinated and HPV-unvaccinated WLWH and vaccinated and unvaccinated HIV(−) women to study the long-term effectiveness of HPV vaccination on cervicovaginal, anal and oral HPV carriage in Rwandan WLWH in late 2021. We will compare HPV-vaccinated WLWH to HPV-vaccinated HIV(−) women to measure the relative long-term effectiveness and immunogenicity of HPV vaccination in WLWH. We will compare HPV-vaccinated WLWH to HPV-unvaccinated WLWH to measure the reduction of HPV burden in WLWH attributable to HPV vaccination. As a secondary goal, we will conduct a natural history study to investigate determinants, including cervicovaginal microbiome, of short-term HPV persistence in young WLWH and HIV(−) women living in an SSA setting.
Methods
Study population and setting
At community health centres in Kigali, 3028 women aged 18–28 years will be enrolled: 757 WLWH (group 1) and 757 HIV(−) women (group 2) who may have received HPV vaccination (birth cohorts 1996 and later) and 757 WLWH (group 3) and 757 HIV(−) women (group 4) who were older and unlikely to have received HPV vaccination (birth cohorts before 1996). Most Rwandan women born in 2003 or later will have been vaccinated with two doses of Gardasil, women born between 1996 and 2002 will have been vaccinated with three doses of Gardasil and women born in 1995 or earlier will be unvaccinated. Within the HPV-vaccinated groups 1 and 2, the study will enrol at least 274 women who received three doses and 274 women who received two doses of the HPV vaccine, with the remaining 209 not selected for the number of doses and may have received three or two doses.
We will recruit by HIV status and age, which will serve as a useful and very good proxy for HPV vaccination status, which otherwise would be difficult to determine in real time at enrolment. HPV vaccination status then will be confirmed by retrospective review of the HPV vaccination records kept by the Rwanda Ministry of Health (MoH), who are collaborating on this project. The number of women recruited will therefore be stratified by age (as proxy for vaccine status) and HIV status. Because this study has age-specific enrolment goals for WLWH and HIV(−) women, once those enrolment goals are met for each study group, the respective groups will be closed and other eligible women for that study group will be excluded from participation.
In Kigali, the study staff will work with five public health facilities that participate in the Central Africa International Epidemiology Databases to Evaluate AIDS (IeDEA) programme (ca-iedea.org) and the WE-ACTx private health facility (table 1). If needed, the staff will work with up to four other public health clinics to recruit additional participants. We will recruit women attending collaborating health facilities and from the surrounding communities.
Table 1.
Study enrolment sites
| IeDEA sites | Non-IeDEA sites |
|
|
IeDEA, International Epidemiology Databases to Evaluate AIDS.
Study design
Eligibility criteria
Women are eligible to participate if they: live in Rwanda; are ages 18–28 years who are either known to be living with HIV or, if HIV status is unknown, consent to HIV testing to confirm HIV status; have had sex; are physically and mentally able and willing to participate in the study; and are willing to provide written and signed or thumb printed, informed consent. A summary of the target population by HIV and HPV vaccination statuses are shown in table 2.
Table 2.
Target population by HIV status and age
| HIV status | Approximate number of HPV vaccine doses | Birth year | Age at year of study enrolment (years) | N | Group | |
| In 2021 | In 2023 | |||||
| WLWH | Three doses | 1996–2002 | 19–25 | 21–26 | 274 | 1 |
| Two doses | 2003–2005 | 16–18* | 18–20 | 274 | ||
| Two or three doses | 1996–2005 | 19–25 | 18–26 | 209 | ||
| Subtotal | 757 | |||||
| 0 doses - unvaccinated | 1993–1995 | 26–30* | 28–31* | 757 | 3 | |
| HIV(−) | Three doses | 1996–2002 | 19–25 | 21–26 | 274 | 2 |
| Two doses | 2003–2005 | 16–18* | 18–20 | 274 | ||
| Two or three doses | 1996–2005 | 19–25 | 18–26 | 209 | ||
| subtotal | 757 | |||||
| 0 doses - unvaccinated | 1993–1995 | 26–30* | 28–31* | 757 | 4 | |
| Total | 3028 | |||||
*Only women aged 18–28 years are eligible for enrolment.
WLWH, women living with HIV.
Women are ineligible to participate if they: have positive pregnancy test or report to be pregnant at the time of visit or less than 6 weeks postpartum (will be asked to make an appointment six or more weeks postpartum); have a history of hysterectomy and no longer have a cervix; have a history of treatment for cervical abnormalities after cervical cancer screening; have a history of cervical cancer; and report no previous sexual activity. Women who report menstruating at the time of the visit will be asked to make a new appointment 2 weeks later.
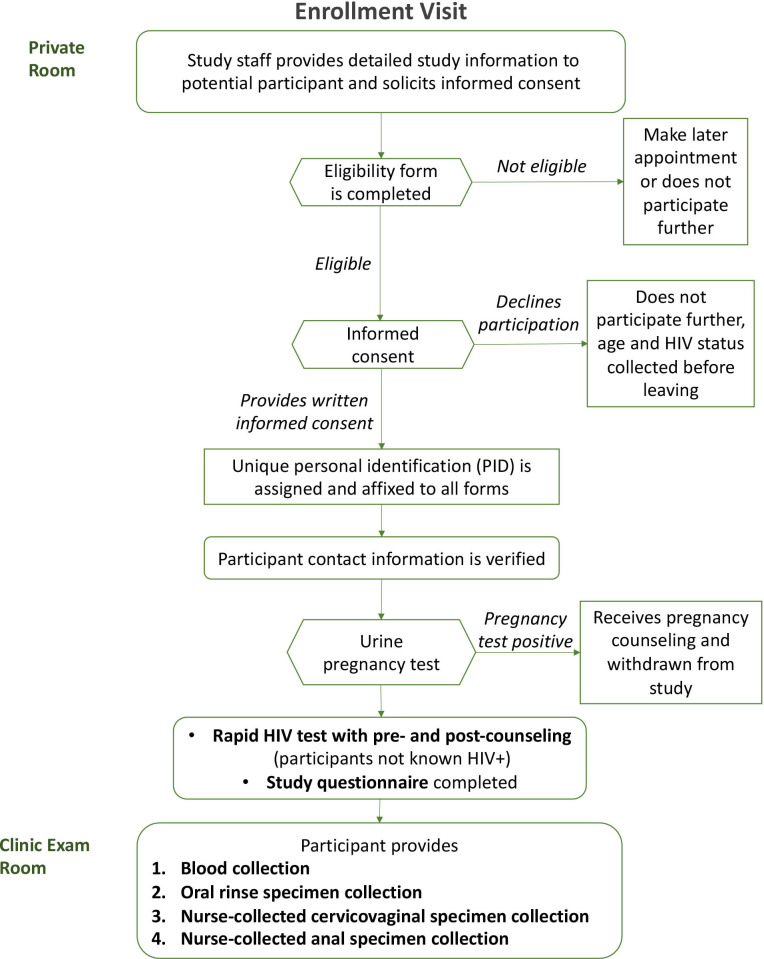
Enrolment visit
The enrolment visit is summarised in figure 1. After eligibility is confirmed and consent given, eligible, consenting women (participants) will provide a urine sample for pregnancy testing to confirm they are not pregnant. The consent form obtains the participants’ permission to extract from medical records information related to their HIV care including CD4 count, viral load, ART use and age of initiation of HIV care as well as HPV vaccination status.
Figure 1.
Enrolment visit procedures.
Participants recruited into groups 2 and 4, whose current HIV status is unknown, will have a rapid HIV test followed by a confirmatory test if the initial test result is invalid (inconclusive) or reactive (positive) according to the Rwanda National HIV Diagnostic Testing Guidelines.53 54 If the newly HIV-diagnosed participant chooses not to exit the study, she will be reclassified into either group 1 or 3, depending on HPV vaccination status.
Participants will complete a baseline questionnaire on sociodemographics and risk factors (eg, sexual behaviour) for HPV, undergo blood collection for HPV serology and finally cervicovaginal, anal and oral specimen collection for HPV testing.
Specimen collection
The study nurse first will collect one 5 mL tube of blood for EDTA plasma from all participants. For the oral specimen collection, participants will alternate between a 5 s squish and a 5 s gargle of 10 mL of saline for 30 s and then spit out the specimen into a sterile specimen container. The study nurse will then collect a cervicovaginal specimen using the AmpFire specimen collection brush (Atila BioSystems, Mountain View, California, USA), placing it into the posterior vagina without a speculum, turning the brush three turns left and right. Then the study nurse will withdraw the brush, place it into a collection tube, snap off the handle to break it and seal the collection tube. Finally, the study nurse will collect an anal specimen by inserting a water-moistened Dacron swab into the anal canal, turning the swab two to three turns left and right, before removing the swab. The study nurse will insert the swab into the collection tube, snap off the handle and seal the collection tube.
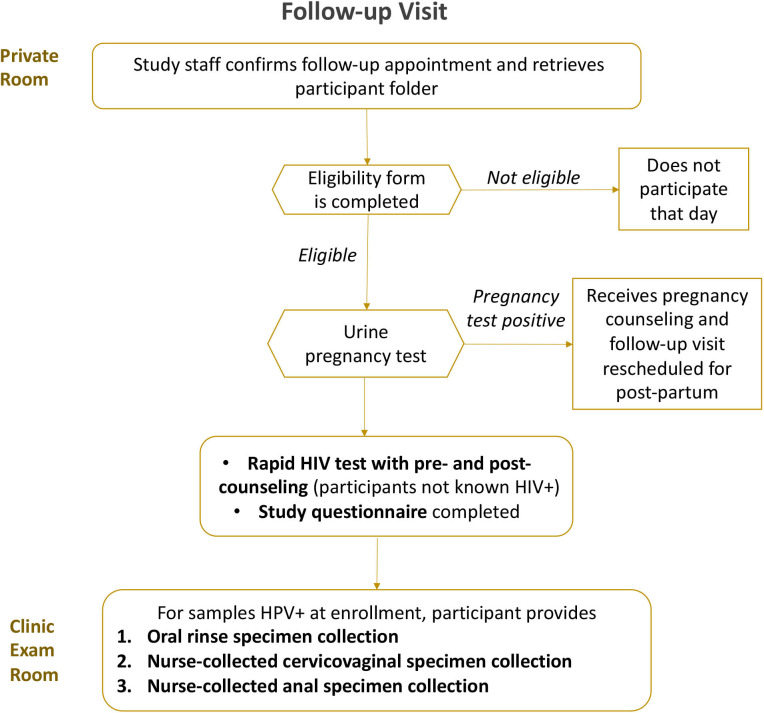
Six to 12-month follow-up visit of HPV-positive women
The follow-up visit is summarised in figure 2. Participants positive for HPV on any sample will have a 6–12 month follow-up visit to measure 6–12 month HPV type-specific persistence—a surrogate endpoint recommended by the WHO.55 At young ages, the risk of precancer is very low, but for safety purposes, participants will be offered colposcopy if they have a 6–12 month HPV type-specific persistent high-risk HPV cervical infection and anoscopy if they have a 6–12 month HPV type-specific persistent HPV16/18 anal infection. At this follow-up visit, participants will complete a brief sexual history questionnaire to identify likelihood of potential new exposure to HPV. If HIV negative at baseline, participants will also have a rapid HIV test. They then will have cervicovaginal, anal and/or oral specimens collected only from those tissue sites that were HPV positive at baseline for HPV testing as described.
Figure 2.
Follow-up visit procedures.
Clinical management
A summary of the management of HPV-positive results is shown in figure 3. Women with HPV type-specific persistent cervicovaginal HPV infection for one or more of the 13 high-risk HPV (hrHPV) types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), regardless of HIV status, will be referred for colposcopy and, as needed, treatment.
Figure 3.
Follow-up and clinical management of baseline HPV-positive results. HPV, human papillomavirus; WLWH, women living with HIV.
Following application of dilute acetic acid to the cervix, digital images of the cervix will be taken using a study-provided contemporary digital camera (eg, Samsung A21S or similar cellular phone camera). Then colposcopy-guided biopsies will be taken of all acetowhite, visible cervical abnormalities.56 Colposcopic impression and biopsy locations will be recorded.
Biopsies will be read by a local pathologist (and a second one if second opinion is required in order to guide care) and a US pathologist. All participants with a biopsy diagnosis of CIN grade 3 (CIN3) or more severe diagnoses (CIN3+) as rendered by any pathologist and WLWH with persistent HPV16 or HPV18 infection will undergo treatment by ablation or, if ineligible for ablative treatments, excision according to WHO guidelines.57–59 In accordance with US management guidelines,60 those diagnosed with CIN2 will not undergo immediate treatment due the likelihood of its regression and low risk of ICC following a CIN2 diagnosis especially in young women61 and the possible increased risk in negative reproductive outcomes such as preterm delivery following treatment.62 They will be advised to seek clinical follow-up in a year.
WLWH with persistent anal HPV16 or HPV18 infection will undergo anoscopy; HIV(−) participants with persistent HPV16 or HPV18 infection will not undergo anoscopy because only ~10% of anal cancer is due to HPV16 or HPV18 among HIV,63 the low absolute risk of anal cancer28 and the possibility of morbidity from treating anal abnormalities. A biopsy will be taken from acetowhite lesions. Anoscopy impression and location of biopsies will be recorded for clinical management files and saved in the participant record for study purposes. Those with a biopsy diagnosis of anal intraepithelial neoplasia (AIN) grade 3 (AIN3) or anal cancer will be treated or referred for anal cancer management, respectively. Those diagnosed with AIN grade 2 (AIN2) will not undergo immediate treatment due to the low risk of anal cancer following an AIN2 diagnosis and the morbidity associated with treatment. Those with untreated AIN2 or 6–12 month persistent anal hrHPV by non-HPV16/18 types will be advised to seek clinical follow-up in a year.
There are no management guidelines or evidence-based intervention for 6–12 month HPV type-specific persistent oral HPV infection. Therefore, participants will not receive any clinical intervention for HPV type-specific persistent oral infection.
Other participants, including hrHPV-negative WLWH and/or women with 6–12 month persistent low-risk HPV infection, will exit the study without further follow-up visits. Those diagnosed with cervical or anal cancer will be referred for cancer care.
Study outcomes
Main outcomes will be prevalence and 6–12 month type-specific persistence of cervicovaginal (as an excellent proxy for cervical sampling64), anal and/or oral infections by HPV6/11/16/18 as well as anti-HPV16 and anti-HPV18 geometric mean titres. Cervical and anal biopsy specimens diagnosed as CIN2 or more severe diagnoses (CIN2+) or AIN2 or more severe diagnoses (AIN2+), respectively, will also be tested for HPV for a secondary analysis to measure the effects of HPV vaccination on the prevalence of HPV type-specific precursors to anogenital cancer. In an exploratory aim, we will examine risk factors, including the cervicovaginal microbiome65 and current and past HIV status (positive vs negative and current ART, CD4 counts and HIV viral load), for HPV type-specific persistence in WLWH and HIV(−) women living in Rwanda.
HPV testing
We will use a modified version of the AmpFire HPV genotyping assay (Genotyping High Risk HPV Real Time Fluorescent Detection) for HPV genotyping of cervicovaginal, anal and oral specimens. AmpFire uses real-time PCR to detect 15 individual HPV genotypes including 13 hrHPV types and 2 intermediate-risk HPV types (HPV53 and HPV66). The modified AmpFire HPV genotyping assay will detect the 17 individual HPV genotypes, including vaccine targeted HPV6 and HPV11, in five assay reactions. The AmpFire testing platform was previously established at the study lab and validated against other commercially available assays at Rwanda Military Hospital.66–69 The assay will be run per the manufacturer’s instructions.
Anogenital samples, collected into a dry tube, will be processed directly according to the manufacturer’s instructions. Residual lysed anogenital specimens will be neutralised according to the manufacturer’s protocol and stored at −20°C for future use.
On arrival to the lab, the oral rinse specimens will be stored at 4°C for processing the next working lab day. Oral rinse specimens in saline will first be concentrated by centrifugation to enrich the sample before processing and then stored frozen at −20°C until tested for HPV. After testing, residual specimens will be discarded.
Formalin-fixed paraffin-embedded tissues diagnosed as CIN2+ or AIN2+will similarly have HPV genotyping using the AmpFire system according to the manufacturer’s protocol.
HPV serology
Anti-HPV16 and HPV18 IgG antibody geometric mean titers (GMTs) will be measured from plasma by an VLP-based ELISA using a previously described method.70–72 All serum from groups 1 and 2 (HPV-vaccinated groups) and a 10% sample from groups 3 and 4 (HPV-unvaccinated groups) (n=152, 76 from each group) will be tested. First, 20% of each group will be run at the HPV Serology Laboratory at the National Cancer Institute (NCI) Frederick National Laboratories, then the results will be replicated (≥90% correlation) at the study lab in Rwanda (masked to the original results), and then the remaining 80% of the testing will be finished at the study lab. As an additional quality control measure, a 10% random sample of specimens will be rerun at the HPV Serology Laboratory (masked to the original results) (interlaboratory reliability) and another 10% random sample of specimens at the study lab (intralaboratory reliability). Positive (eg, IS standards73 74) and negative controls (eg, negative plasma) will be included in some testing plates to monitor assay performance per WHO recommendations.75
Pathology
All histopathology slides will be scanned at the study lab and reviewed by a local pathologist, with a second local review if second opinion for clinical care is required, and then the Einstein study pathologist.76 If the diagnoses are concordant, no further review of the case will be performed. If the diagnoses rendered by the Rwanda and Einstein pathologists are discordant and at least one pathologist diagnoses CIN2+, the biopsy slide will be subjected to a joint review and consensus diagnosis. For negative/cervical intraepithelial neoplasia grade 1 pairs of diagnoses, which does not influence our analyses or the care of the participants, there will be no joint review.
Analysis and statistical power
Analyses
We will calculate point prevalence and the prevalence of persisting infection by individual HPV-type specific infections, with binomial 95% CIs, for each anatomic site and for the woman (all anatomic sites) for all four groups. We likewise will calculate point prevalence and the prevalence of persisting infection with 95% CI for all HPV types in aggregate and in subgroups of HPV types according to the protection afforded by Gardasil: Gardasil-targeted types (HPV6, 11, 16 and 18), Gardasil-untargeted types for which there might be cross-protection (HPV31, 33 and 45)18 77 78 and Gardasil-untargeted HPV types for which there is little or no evidence of cross-protection (HPV35, 39, 51, 52, 53, 56, 58, 59, 66 and 68). Results will be stratified by age and number (3 vs 2) of doses as well as other factors possibly related to HPV prevalence. Differences in prevalence of these HPV type subgroups (targeted, possible cross-protection and untargeted) between the four study groups of women will be tested using Fisher’s exact or Pearson χ2 test. Differences in prevalence of these HPV type subgroups by age, number of doses (3 vs 2) and other factors within the group will be tested for statistical significance (p<0.05) using a Fisher’s exact or Pearson χ2 test.
Notably, study groups of participants are fundamentally different populations (vs a randomised control trial that would recruit from the same population and, as result of randomisation, enrol similar, representative populations in each arm). Specifically, there are known differences in age and therefore possible differences in sexual activity between Gardasil-vaccinated (group 1) versus Gardasil-unvaccinated WLWH (group 2), and possible differences in sexual behaviours between Gardasil-vaccinated WLWH (group 1) versus HIV(−) women (group 3) (since HIV infection predominately is sexually transmitted in this population). Therefore, we will use a relative measure of effectiveness to account for the differences in age and possible differences in exposure to HPV. We will use logistic regression to calculate the OR of the point prevalence and the prevalence of 6–12 month persisting HPV infections of Gardasil-targeted HPV types, individually (HPV6, 11, 16 or 18) and in aggregate (HPV6, 11, 16 and 18), versus untargeted HPV genotypes for which there is no evidence of cross-protection, for each anatomic site individually (cervix, anus OR oral cavity) and combined (cervix, anus AND oral cavity). That is, we also will compare the ratio between study groups of participants as follows:
This will help us to account/adjust for differences, above what can be achieved statistically, in HPV exposure, prevalence and persistence due to differences in age (note: prevalence equals incidence times duration; prevalent infections tend to be more persistent with increasing age79) and sexual behaviours between groups. Additional logistic regression models may be used to adjust specifically on other factors, including age, number of doses and sexual behaviours, to account for population differences between study groups.
Differences in GMT and seropositivity will be tested for statistical significance using the Mann-Whitney and Fisher’s exact tests, respectively. Analysis of variance and logistic regression models will be used to adjust for/assess the association of other factors (eg, age at vaccination, age at enrolment into the study, number of doses, current and past HIV status, detection of HPV genotypes, etc) with GMT and seropositivity, respectively.
Sample size/power
We made the following assumptions: (1) at least 30% prevalence of HPV infection (of any anatomic site) at baseline, 25% of which will be Gardasil-targeted HPV types and 55% will be untargeted HPV types, (2) a 10% loss to follow-up in 6–12 months and (3) at least 70% of HPV infections persist for 6–12 months. We justify an HPV prevalence of at least 30% among WLWH less than 30 years of age in this study, based on 30% prevalence of high-risk HPV infection of the cervix alone in WLWH aged 30–34 years from our previous cervical cancer screening study in Rwanda.80 We expect that the HPV prevalence may be higher since the prevalence of HPV, like that of other sexually transmitted infections, tends to peak about 5–10 years after the median age of sexual initiation in a population,81 82 which in Rwanda is around 17 years of age.83 84 However, we conservatively used 30% prevalence to ensure adequate statistical power. We justify our assumption of a maximum of 10% loss to follow-up over 6–12 months, given our experience working in Rwanda over the last ~20 years. We justify 70% of prevalent HPV infections persisting for 6–12 months, given that 36%–50% of prevalent HPV infections persist 6 months in HIV(−) women,85 86 and WLWH have an impaired immunity to HPV compared with HIV(−) women.25 26
Sample size calculation relates to the relative effectiveness measure as discussed. Under the above assumptions and accounting for 10% misclassification of HPV vaccine status, a sample size of 757 vaccinated and 757 unvaccinated WLWH will provide ≥80% power (p=0.05) to detect an OR of ≤0.5 for persistent HPV6/11/16/18 infections relative to HPV35/39/51/52/53/56/58/59/66/68 (but not HPV31/33/45 because of possible cross protection), in Gardasil-vaccinated (group 1) versus Gardasil-unvaccinated WLWH (group 3). Consequently, because there will be more participants with a prevalent infection than persistent HPV infection (because there are no losses to follow-up or HPV viral clearance for which to account), there will be ≥80% power (p=0.05) to detect an OR of ≤0.75 in point prevalence of HPV6/11/16/18 infection relative to HPV35/39/51/52/53/56/58/59/66/68, in Gardasil-vaccinated (group 1) versus Gardasil-unvaccinated WLWH (group 3).
Using the variance of GMT for HPV antibodies from Einstein et al,87 88 a sample size of 274 will provide 80% power (p=0.05) to detect a 30% difference in GMT between HPV-vaccinated WLWH (group 1) and HIV(−) (group 2) women for the same number of doses. If there is no appreciable difference in the GMT between those who got three or two doses, we can combine those groups with different doses. In that case, with a sample size of 548, there will be 80% power (p=0.05) to detect a 22% difference between HPV-vaccinated WLWH (group 1) and HIV(−) women (group 2).
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research. The results of the study will be shared with interested parties including the Rwanda Ministry of Health, and we will establish a Community Advisory Board, both of which will help disseminate our findings to the general Rwandan public.
Discussion
Limitations
Several limitations in the proposed study are worth noting. First, our study is not a randomised controlled trial but an observational study of populations selected from different groups of women. We have proposed several approaches to account/adjust for those age differences but as with any observational study, these techniques may be unable to completely control for biases.
Populations recruited into this study are primarily from Kigali and therefore are not representative of all Rwanda, nor are they representative of other populations in SSA or elsewhere in the world. In 2018–2019, almost 80% of Rwandan PLWH had a suppressed HIV viral load,89 a higher percentage than for PLWH populations living in many SSA and other countries.90 Therefore, these results may not be generalisable to all WLWH populations, especially populations of WLWH who are severely immunocompromised.
We are using 6–12 month HPV type-specific persistence as a proxy for cancer risk, but ideally, we would want to measure the impact of Gardasil on high-grade cervical and anal abnormalities as a more proximal surrogate for cancer risk. The population will be still too young to have large numbers of these endpoints, although we will evaluate these endpoints in secondary analyses. This may warrant a follow-on, follow-up study to evaluate the longer term effectiveness and immunogenicity in the cohort, especially in the WLWH.
Strengths
Despite noted limitations, there are some important strengths of the study to highlight. First, the early introduction of HPV vaccination that is documented through a national registry, excellent HIV care including a national database and patient tracking and relatively high prevalence of HIV provides a unique opportunity to study the impact of HPV vaccination in WLWH, the population at the highest risk of cervical cancer25–27 that therefore would gain the greatest benefit from it, living in Rwanda. Although a comparative effectiveness randomised controlled trial of different HPV vaccines is probably still warranted to determine how best to protect this most vulnerable population,31 these data may provide some of the first evidence of long-term effectiveness of HPV vaccination in WLWH and inform such a trial. A second strength is that the study is being conducted in Rwanda, a high cervical-cancer burden country.45 The findings from this study, if positive, may encourage other high-burden countries to accelerate the introduction of HPV vaccination or at least target WLWH and PLWH populations. Finally, HPV DNA and serology testing are being done locally for this study could potentially be transferred and replicated in other LMICs that want to monitor and evaluate HPV vaccine impact in their populations.
Importantly, as a result of implementing this research protocol, we will continue to expand the local capacity to conduct state-of-the-art HPV and molecular epidemiological research by: (1) establishing next-generation sequencing technology to perform cervicovaginal, anal and oral microbiota characterisation for this study that will enable locally conducted studies of the human genome and genomic testing for personalised medicine in Rwanda; (2) building on current ELISA capabilities at the study lab to perform titration of plasma antibodies, a skill that can be applied to other studies of vaccine response; (3) enhancing data capture and management through increased capacity to use REDCap91–93; and (4) migrating current HPV vaccine records for participants living in Kigali into a common electronic database, which will allow us to conduct studies more easily on the impact of HPV vaccination on outcomes. Importantly, this will allow linkage of HPV vaccination status to both the Rwanda National HIV Registry and the Rwanda Cancer Registry, the latter of which we, in collaboration with the Rwanda MoH, helped re-establish.94 This will allow investigations of the long-term impact of HPV vaccination on cancer incidence in Rwandan WLWH and HIV(−) women and, as a result of herd protection, Rwandan men.
As building research capacity in Rwanda is a major goal of this project, all members of the research team will be asked and supported to lead at least one analysis and one manuscript preparation based on interests and expertise. Analytic and publication responsibilities will be divided equally and collaboratively among both Rwandan and US investigators.
Ethics and dissemination
The study was approved by the Albert Einstein College of Medicine Institutional Review Board (IRB#: 2021–13087) and the Rwanda National Ethics Committee.
All participant data collected will be entered or transferred to a secure REDCap database, with access to personal information restricted to the local staff. Study data will be stored in REDCap and maintained on password-protected study computers behind the institution firewall.
Copies of signed consents will be stored in locked file cabinets in a locked room, with access restricted to study personnel only. Data will be entered or transferred to a secure REDCap database, with access to personal information restricted to the local staff.
REDCap is a secure, web-based application designed to support data capture for research studies providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. REDCap is hosted on a secure server and has undergone a Governance Risk & Compliance Assessment and all REDCap electronic data files shared with Albert Einstein College of Medicine will be maintained by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)-compliant Epidemiology Study Management and Informatics Core Facility at Einstein. The Albert Einstein College of Medicine policy on use of REDCap can be found at: http://ric.einstein.yu.edu/ric_files/REDCapAppropriateUsePolicy.pdf.
Medical, screening and preventive services, all of which are minimally invasive, safe and outpatient, and have been done in millions of people, provided by the study are on par with or better than international standards of care, most of which have very low risks of even minor adverse events or harms. Positive test results and diagnoses may result in psychological distress and anxiety. Of note, pregnancy and HIV testing may cause pretest and post-test anxiety. Therefore, pretesting and post-testing counselling will be provided as needed. Any participant who tests HIV positive will be referred to the health facility’s HIV clinic for HIV management following the Rwanda MoH’s HIV management guidelines.
We will publish a series of reports in peer-review scientific journals to disseminate these results. We will share the results with the Rwanda MoH. Data from this study will be made available by request in accordance with NIH policies and Rwandan laws.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank the Rwandan women who participate in this study. We would also like to thank the research teams at Albert Einstein College of Medicine, the Einstein-Rwanda Research & Capacity Building Program and the US National Cancer Institute for their contributions in developing and implementing this research protocol.
Footnotes
Twitter: @GadMurenzi, @patrickfils
Contributors: GM, PEC and KA designed the study initially. GM, AP, PEC and KA refined and finalised the overall study design and wrote the grant proposal that is the basis for this protocol. GM, FS, NH, AM, JCG, AP, KA and PEC drafted and finalised the written protocol. All authors implemented it. PEC drafted this manuscript. All authors have read, revised and approved the final manuscript. GM and FS are cofirst authors; KA and PEC are cosenior authors.
Funding: This work is being supported by the National Institutes of Health (NIH) by grant numbers 5U54CA254568-02 and 2U01AI096299-13. This work is also being supported by the intramural research program of the National Cancer Institute (NCI)/NIH.
Competing interests: PEC is the Director of the Division of Cancer Prevention at the NCI, the NIH institute that funds this research. However, PEC has recused himself from any decisional or financial authority over this grant or any extramural HPV grants funded by the NCI. Other authors claim no competing interests. The study is receiving HPV genotyping tests at a reduce cost from Atila Biosystems.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907. 10.1016/S0140-6736(07)61416-0 [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer 2009;4:8. 10.1186/1750-9378-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010;102:325–39. 10.1093/jnci/djp534 [DOI] [PubMed] [Google Scholar]
- 4.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–56. 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AR, Tortolero-Luna G, Ferrer E, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine 2008;26 Suppl 10:K17–28. 10.1016/j.vaccine.2008.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis 2011;11:39–44. 10.1016/S1473-3099(10)70225-5 [DOI] [PubMed] [Google Scholar]
- 7.Gertig DM, Brotherton JML, Budd AC, et al. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med 2013;11:227. 10.1186/1741-7015-11-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Australian Government Department of Health . Historical data from the National HPV vaccination program register. Available: https://www.health.gov.au/resources/collections/historical-data-from-the-national-hpv-vaccination-program-register [Accessed 2 Jan 2022].
- 9.Tabrizi SN, Brotherton JML, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 2014;14:958–66. 10.1016/S1473-3099(14)70841-2 [DOI] [PubMed] [Google Scholar]
- 10.Crowe E, Pandeya N, Brotherton JML, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014;348:g1458. 10.1136/bmj.g1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brotherton JM, Gertig DM, May C, et al. HPV vaccine impact in Australian women: ready for an HPV-based screening program. Med J Aust 2016;204:184–e1. 10.5694/mja15.01038 [DOI] [PubMed] [Google Scholar]
- 12.Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis 2018;217:1590–600. 10.1093/infdis/jiy075 [DOI] [PubMed] [Google Scholar]
- 13.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017;17:1293–302. 10.1016/S1473-3099(17)30468-1 [DOI] [PubMed] [Google Scholar]
- 14.Baldur-Felskov B, Dehlendorff C, Munk C, et al. Early impact of human papillomavirus vaccination on cervical neoplasia-nationwide follow-up of young Danish women. J Natl Cancer Inst 2014;106:djt460. 10.1093/jnci/djt460 [DOI] [PubMed] [Google Scholar]
- 15.Markowitz LE, Liu G, Hariri S, et al. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016;137:e20151968. 10.1542/peds.2015-1968 [DOI] [PubMed] [Google Scholar]
- 16.Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction-national health and nutrition examination survey, United States, 2003-2014. J Infect Dis 2017;216:594–603. 10.1093/infdis/jix244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis 2013;208:385–93. 10.1093/infdis/jit192 [DOI] [PubMed] [Google Scholar]
- 18.Drolet M, Bénard Élodie, Pérez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394:497–509. 10.1016/S0140-6736(19)30298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer 2018;142:2186–7. 10.1002/ijc.31231 [DOI] [PubMed] [Google Scholar]
- 20.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med 2020;383:1340–8. 10.1056/NEJMoa1917338 [DOI] [PubMed] [Google Scholar]
- 21.Falcaro M, Castañon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 2021;398:2084–92. 10.1016/S0140-6736(21)02178-4 [DOI] [PubMed] [Google Scholar]
- 22.Kjaer SK, Dehlendorff C, Belmonte F, et al. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J Natl Cancer Inst 2021;113:1329–35. 10.1093/jnci/djab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention . From the centers for disease control and prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 1993;269:729–30. [PubMed] [Google Scholar]
- 24.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992;41:1–19. [PubMed] [Google Scholar]
- 25.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 26.Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med 2015;163:507–18. 10.7326/M14-2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 2021;9:e161–9. 10.1016/S2214-109X(20)30459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colón-López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018;36:68–75. 10.1200/JCO.2017.74.9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C-CJ, Palefsky JM. HPV-associated anal cancer in the HIV/AIDS patient. Cancer Treat Res 2019;177:183–209. 10.1007/978-3-030-03502-0_7 [DOI] [PubMed] [Google Scholar]
- 30.Liu G, Sharma M, Tan N, et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018;32:795–808. 10.1097/QAD.0000000000001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castle PE, Einstein MH, Sahasrabuddhe VV. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J Clin 2021;71:505–26. 10.3322/caac.21696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Res 2019;8:100174. 10.1016/j.pvr.2019.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis 2010;202:1246–53. 10.1086/656320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomet V, Penagini F, Trabattoni D, et al. Safety and immunogenicity of a quadrivalent human papillomavirus vaccine in HIV-infected and HIV-negative adolescents and young adults. Vaccine 2014;32:5657–61. 10.1016/j.vaccine.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 35.Levin MJ, Huang S, Moscicki A-B, et al. Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: effect of a fourth dose of vaccine. Vaccine 2017;35:1712–20. 10.1016/j.vaccine.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg A, Huang S, Moscicki A-B, et al. Persistence of memory B-cell and T-cell responses to the quadrivalent HPV vaccine in HIV-infected children. AIDS 2018;32:851–60. 10.1097/QAD.0000000000001773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscicki A-B, Karalius B, Tassiopoulos K, et al. Human papillomavirus antibody levels and quadrivalent vaccine clinical effectiveness in perinatally human immunodeficiency virus-infected and exposed, uninfected youth. Clin Infect Dis 2019;69:1183–91. 10.1093/cid/ciy1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahn JA, Xu J, Kapogiannis BG, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis 2013;57:735–44. 10.1093/cid/cit319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014;59:127–35. 10.1093/cid/ciu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Money DM, Moses E, Blitz S, et al. HIV viral suppression results in higher antibody responses in HIV-positive women vaccinated with the quadrivalent human papillomavirus vaccine. Vaccine 2016;34:4799–806. 10.1016/j.vaccine.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 41.Toft L, Tolstrup M, Müller M, et al. Comparison of the immunogenicity of Cervarix® and Gardasil® human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults. Hum Vaccin Immunother 2014;10:1147–54. 10.4161/hv.27925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cranston RD, Cespedes MS, Paczuski P, et al. High baseline anal human papillomavirus and abnormal anal cytology in a phase 3 trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected individuals older than 26 years: ACTG 5298. Sex Transm Dis 2018;45:266–71. 10.1097/OLQ.0000000000000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkin TJ, Chen H, Cespedes MS, et al. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS clinical trials group protocol A5298. Clin Infect Dis 2018;67:1339–46. 10.1093/cid/ciy274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 45.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 46.Binagwaho A, Wagner CM, Gatera M, et al. Achieving high coverage in Rwanda’s national human papillomavirus vaccination programme. Bull World Health Organ 2012;90:623–8. 10.2471/BLT.11.097253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallagher KE, Howard N, Kabakama S, et al. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low- and middle-income countries. PLoS One 2017;12:e0177773. 10.1371/journal.pone.0177773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngabo F, Franceschi S, Baussano I, et al. Human papillomavirus infection in Rwanda at the moment of implementation of a national HPV vaccination programme. BMC Infect Dis 2016;16:225. 10.1186/s12879-016-1539-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaMontagne DS, Bloem PJN, Brotherton JML, et al. Progress in HPV vaccination in low- and lower-middle-income countries. Int J Gynaecol Obstet 2017;138:7–14. 10.1002/ijgo.12186 [DOI] [PubMed] [Google Scholar]
- 50.Black E, Richmond R. Prevention of cervical cancer in sub-Saharan Africa: the advantages and challenges of HPV vaccination. Vaccines 2018;6:61. 10.3390/vaccines6030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rwanda population-based HIV impact assessment October 2019.
- 52.The World Bank . Rwanda, 2022. [Google Scholar]
- 53.Rwanda Ministry of Health . National guidelines for prevention and management of HIV, STIs & other blood borne infections, 2013. [Google Scholar]
- 54.Rwanda Ministry of Health . Circular of key changes in HIV prevention and management guidelines, 2018. [Google Scholar]
- 55.Lowy DR, Herrero R, Hildesheim A, et al. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol 2015;16:e226–33. 10.1016/S1470-2045(15)70075-6 [DOI] [PubMed] [Google Scholar]
- 56.Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-Based colposcopy practice. J Low Genit Tract Dis 2017;21:230–4. 10.1097/LGT.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization . WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. Available: https://apps.who.int/iris/bitstream/handle/10665/96735/WHO_RHR_13.21_eng.pdf [PubMed]
- 58.Santesso N, Mustafa RA, Schünemann HJ, et al. World Health organization guidelines for treatment of cervical intraepithelial neoplasia 2-3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynaecol Obstet 2016;132:252–8. 10.1016/j.ijgo.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization . WHO guidelines for the use of thermal ablation for cervical pre-cancer lesions. Geneva, Switzerland, 2019. [PubMed] [Google Scholar]
- 60.Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24:102–31. 10.1097/LGT.0000000000000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tainio K, Athanasiou A, Tikkinen KAO, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ 2018;360:k499. 10.1136/bmj.k499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ 2016;354:i3633. 10.1136/bmj.i3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis 2018;18:198–206. 10.1016/S1473-3099(17)30653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018;363:k4823. 10.1136/bmj.k4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown D, Müller M, Sehr P, et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine 2014;32:5880–7. 10.1016/j.vaccine.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 66.Murenzi G, Tuyisenge P, Kanyabwisha F, et al. Type-specific persistence, clearance and incidence of high-risk HPV among screen-positive Rwandan women living with HIV. Infect Agent Cancer 2021;16:16. 10.1186/s13027-021-00355-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Du H, Huang X, et al. Evaluation of an isothermal amplification HPV detection assay for primary cervical cancer screening. Infect Agent Cancer 2020;15:65. 10.1186/s13027-020-00328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang Y-W, Lozano L, Chen X, et al. An isothermal, multiplex amplification assay for detection and genotyping of human papillomaviruses in formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2020;22:419–28. 10.1016/j.jmoldx.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murenzi G, Kim H-Y, Munyaneza A, et al. Anogenital human papillomavirus and HIV infection in Rwandan men who have sex with men. J Acquir Immune Defic Syndr 2020;84:463–9. 10.1097/QAI.0000000000002376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kemp TJ, García-Piñeres A, Falk RT, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine 2008;26:3608–16. 10.1016/j.vaccine.2008.04.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrin DM, Coates EE, Costner PJ, et al. Comparison of adaptive and innate immune responses induced by licensed vaccines for human papillomavirus. Hum Vaccin Immunother 2014;10:3446–54. 10.4161/hv.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.HPV Serology Laboratory . HPV serology laboratory standard operating procedure. Available: https://frederick.cancer.gov/sites/default/files/2021-06/HSL%20LAB%20007%20HPV%20Antibody%20ELISA.pdf
- 73.National Institute for Biological Standards and Control . WHO international standard 05-134 HPV 16 antibodies. Available: https://www.nibsc.org/documents/ifu/05-134.pdf
- 74.National Institute for Biological Standards and Control . WHO international standard 1st who international standard for anti-human papillomavirus 18 serum. Available: https://www.nibsc.org/documents/ifu/10-140.pdf
- 75.World Health Organization . In vitro diagnostics and laboratory technology: ELISA tests. Available: https://www.who.int/diagnostics_laboratory/faq/elisa/en/
- 76.American Society for Clinical Pathology . Partners for cancer diagnosis and treatment in Africa: new telepathology system to serve all hospitals in Kigali, Rwanda. Available: https://www.ascp.org/content/docs/default-source/get-involved-pdfs/partners-in-cancer-diagnosis-newsletter/partners-newsletter-july-2017.pdf?sfvrsn=2
- 77.Barzon L, Squarzon L, Masiero S, et al. Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes. Vaccine 2014;32:5357–62. 10.1016/j.vaccine.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 78.Godi A, Panwar K, Haque M, et al. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine 2019;37:2455–62. 10.1016/j.vaccine.2019.03.052 [DOI] [PubMed] [Google Scholar]
- 79.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis 2005;191:1808–16. 10.1086/428779 [DOI] [PubMed] [Google Scholar]
- 80.Murenzi G, Kanyabwisha F, Murangwa A, et al. Twelve-year trend in the prevalence of high-risk human papillomavirus infection among Rwandan women living with HIV. J Infect Dis 2020;222:74–81. 10.1093/infdis/jiaa065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith JS, Melendy A, Rana RK, et al. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health 2008;43:S5.e1–S5.e62. 10.1016/j.jadohealth.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 82.de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007;7:453–9. 10.1016/S1473-3099(07)70158-5 [DOI] [PubMed] [Google Scholar]
- 83.Ntaganira J, Hass LJ, Hosner S, et al. Sexual risk behaviors among youth heads of household in Gikongoro, South Province of Rwanda. BMC Public Health 2012;12:225. 10.1186/1471-2458-12-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Umutesi J. Early sexual debut and subsequent risk factors among youth in Rwanda: a secondary data analysis of Rwanda demographic and health survey 2015. Kigali, Rwanda: University of Rwanda, 2016. [Google Scholar]
- 85.Rodríguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008;100:513–7. 10.1093/jnci/djn044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plummer M, Schiffman M, Castle PE, et al. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis 2007;195:1582–9. 10.1086/516784 [DOI] [PubMed] [Google Scholar]
- 87.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009;5:705–19. 10.4161/hv.5.10.9518 [DOI] [PubMed] [Google Scholar]
- 88.Einstein MH, Baron M, Levin MJ, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011;7:1343–58. 10.4161/hv.7.12.18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Government of Rwanda . Rwanda population-based HIV impact assessment (RPHIA): 2018–2019. Rwanda, 2019. [Google Scholar]
- 90.Han WM, Law MG, Egger M, et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV 2021;8:e766–75. 10.1016/S2352-3018(21)00265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.REDCap , 2021. Available: https://www.project-redcap.org/ [Accessed 29 Dec 2021].
- 94.National Institutes of Health . NIH reporter: building research capacity to address the challenge of non communicable diseases and injuries in Rwanda the GUKORANA research center. Available: https://reporter.nih.gov/search/h81sRNzkn0e71v5Y6Xo2WQ/project-details/9854425
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.