Abstract
We report the case of a school-aged boy who presented with clinical features suggesting acute appendicitis. However, further imaging which included CT, demonstrated an inflammatory mass involving the transverse colon raising the suspicion of lymphoma. He then developed intestinal obstruction, and in view of the rapid progression of the disease, he was thought to have non-Hodgkin’s lymphoma. He underwent an open excisional biopsy, which revealed a necroinflammatory process and no suggestion of lymphoma or an alternative malignancy or specific diagnosis. His steroid treatment was stopped, and he made a good recovery postoperatively. Positive COVID-19 antibodies, positive response to steroids, results and clinical features were consistent with paediatric inflammatory multisystem syndrome (PIMS-TS), with extensive investigation not offering an alternative diagnosis.
While PIMS-TS is a relatively new entity, we believe that this case highlights the importance of it being considered a differential diagnosis of a child presenting with an inflammatory mass.
Keywords: COVID-19, Paediatric oncology, Pathology, Paediatric Surgery, Gastroenterology
Background
Despite children having had milder clinical manifestations of COVID-19 infection compared with the adult population, there has been a group of paediatric patients affected by a multisystem inflammatory condition similar to Kawasaki disease and toxic shock syndrome. This syndrome associated with SARS-CoV-2 has been named paediatric multisystem inflammatory syndrome (PIMS-TS).
A total of 268 cases were identified in UK and Ireland by Public Health England initiated prospective national surveillance of PIMS-TS during the first wave.1 Interestingly, the same survey showed that only 14.8% of children had a positive PCR serology, whereas 63.6% had positive SARS-CoV-2 antibody, although only 44% were tested for antibodies.
Due to the multisystem involvement of PIMS-TS, it can be quite challenging to identify the condition and clinicians can be misled by a different pathology. Often these children require cardiovascular and respiratory support, with admission to a paediatric intensive care unit. A reasonable proportion has abdominal pain and diarrhoea.2 Some patients with PIMS-TS have abdominal concerns, which have prompted exploratory laparotomy and appendicectomy, especially early in our knowledge of PIMS-TS. Typically, there is an excellent response from non-specific immunosuppression, for example intravenous methylprednisolone and/or intravenous immunoglobulin treatment, alongside supportive care.
A multidisciplinary team approach involving various specialties is required in order to make the correct diagnosis of PIMS-TS and start the appropriate treatment. This case highlights how PIMS-TS affecting the gastrointestinal tract may mimic an intra-abdominal malignancy, which could deceive the clinical team and result in potentially unnecessary treatments for the patient. A child presenting with a multisystem non-specific illness during the current COVID-19 pandemic should prompt the clinician to consider PIMS-TS as differential diagnosis and involve the appropriate medical specialties. This is especially so, if there is clear evidence of COVID-19 infection in the child or household contacts 4–8 weeks beforehand.
Case presentation
A school-aged boy presented to a regional district general hospital with a 2-day history of fever and central/lower abdominal pain associated with reduced appetite and vomiting.
He was born at full term and 2 weeks via emergency caesarean section and had no postnatal complications. He had no previous medical history and his immunisations were up to date.
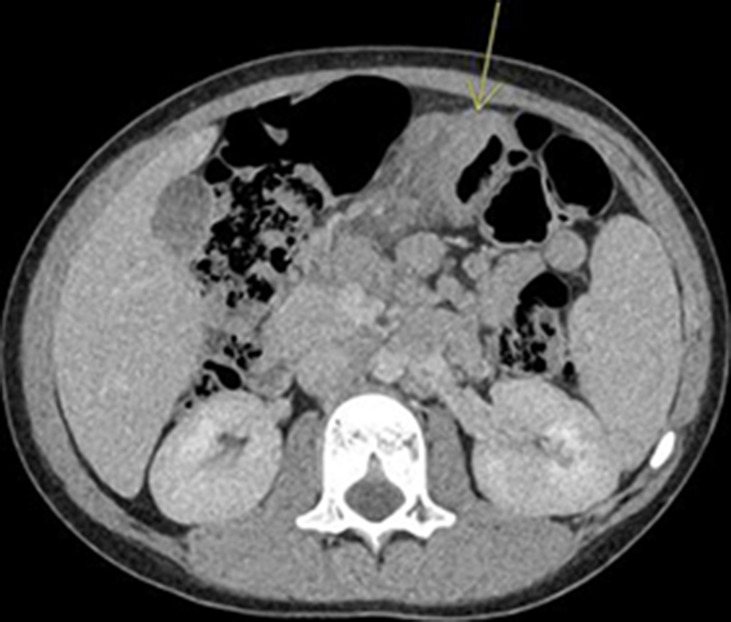
Under the care of the adult general surgical team, he was initially thought to have acute appendicitis and underwent an ultrasound scan, which showed inflammatory changes between his stomach and pancreas raising the suspicion of acute pancreatitis despite a serum amylase within normal range. He was subsequently commenced on intravenous fluids, antibiotics and underwent a CT scan, which revealed a mass-like thickening of the transverse colon involving the mesocolon, which raised the suspicion of a lymphoma (figure 1). He was, therefore, transferred to our tertiary centre under the care of the paediatric oncology team.
Figure 1.
Abdominal CT scan demonstrating the transverse colonic inflammatory mass.
On further clinical assessment, he was found to have a palpable epigastric fullness, which prompted further investigations of a possible intra-abdominal malignancy. He became increasingly unwell and his inflammatory markers continued to rise. His abdomen also became rapidly more distended and abdominal plain radiograph showed intestinal obstruction, which was initially treated conservatively with nasogastric and flatus tubes.
The rapid progression of symptoms supported the diagnosis of non-Hodgkin’s lymphoma and an ultrasound-guided percutaneous biopsy was planned. Unfortunately, sonographic assessment of the epigastric mass under general anaesthesia revealed that the mass was not amenable to the percutaneous approach. Therefore, a ‘mini’ laparotomy and an incisional biopsy of the nodal mass were performed. No obvious point of obstruction was seen at surgery, but an inflammatory nodal mass in transverse mesocolon was observed. In addition, he underwent a flexible sigmoidoscopy, which showed a normal appearance of the intestinal lumen. Postoperatively, the patient was started on oral methylprednisolone at 60 mg/m2/day as empirical volume-reducing treatment for lymphoma, which was discontinued after 4 days following the results of the biopsy.
The histopathology results of his biopsy did not show evidence of lymphoma but instead changes in both lymph nodes and mesenteric fat in keeping with a necroinflammatory process (figure 2).
Figure 2.
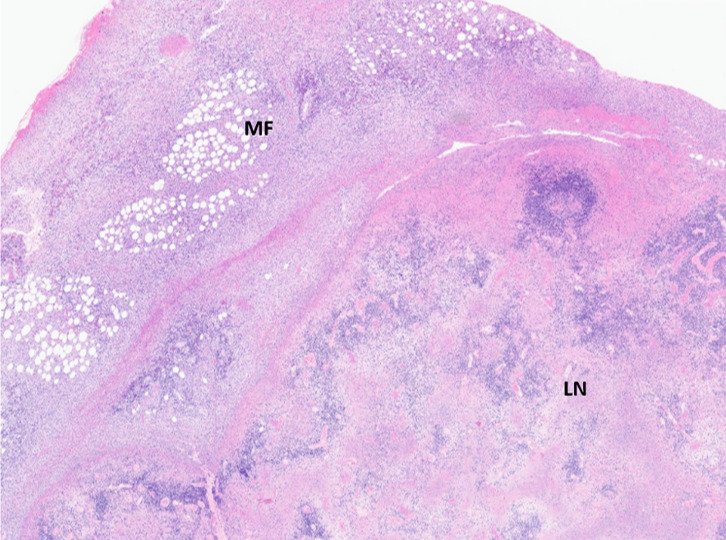
H&E stain (original magnification×2): low power overview of an extensively necrotic lymph node (LN) with marked inflammation of the surrounding mesenteric fat (MF).
The patient required a multidisciplinary team approach, which involved different paediatric specialities, namely oncology, paediatric surgery, rheumatology, cardiology, radiology and histopathology. Although he did not have a typical picture of PIMS-TS with SARS-CoV-2 (PIMS-TS) at the time of admission, it was concluded that his response to steroids, positive COVID-19 antibodies, clinical picture, echocardiography findings and results (including C-reactive protein up to 274 and raised D-dimer), in the absence of another cause, fit well with PIMS-TS.
He made a good postoperative recovery and was then discharged home with out-patient clinic follow-up arranged.
Investigations
Initial blood tests
Haemoglobin 112 g/L, platelet count 228×109/L, white blood cell 4.79×109 /L, lymph 0.96×109/L, amylase 46 IU/L, C-reactive protein 130 mg/L, bilirubin 11 umol/L, alanine aminotransferase 14 iu/L, alkaline phosphatase 177 IU/L, Na 137 mmol/L, K 3.8 mmol/L, urea 4.1 mmol/L, creatinine 50μmol/L.
PIMS-TS-specific blood tests
Prothrombin time 15 s (peak 18 s), activated partial thromboplastin time 32.5 s, fibrinogen >4.5 g/L (peak 6.1), D-dimer 1126 ng/mL, lactate dehydrogenase 236 IU/L, plasma N-terminal pro b-type natriuretic peptide 1523 ng/L, vitamin D 44 nmol/L, amylase 29 IU/L, ferritin 225 μg/L, troponin 16 ng/L.
Blood/urine tests to investigate other intra-abdominal tumours
Alpha fetoprotein, beta-human chorionic gonadotropin, urinary dopamine/ homovanillate/ vanillylmandelic acid - within normal range.
Relevant imaging investigations
X-ray chest: globular heart and prominent perihilar vasculature.
Ultrasound scan abdomen: echogenic ill-defined thickening with round echopoor lymph node gastrocolic mesentery/omentum±foci of free fluid.
Computed tomography abdomen: ill-defined inflammatory mass—smoky mesentery+enlarged lymph nodes gastrocolic mesentery and root of mesentery. Spleen mildly enlarged.
Staging computed tomography: no metastatic disease.
Echocardiogram: showing borderline low left ventricular function, at least mild mitral regurgitation and a small pericardial effusion.
Histology of mass biopsy
No evidence of lymphoma. Extensively necrotic fibrocollagenous tissue fragment showing mixed inflammation with predominance of neutrophils and eosinophils and collections of macrophages. Some evidence of vessel injury around the area of necrosis.
Prominent necrotising lymphadenitis with karyorrhoectic debris and some palisading histiocytes, but no true granuloma formation. The surrounding mesenteric fat extensively infiltrated by lymphocytes, eosinophils and abundant histiocytes (but with no Langerhans cells). Early fibroblastic proliferation and evidence of damage to the mesenteric vessels noted.
Microbiology tests to investigate different infectious causes
Nose and throat swabs COVID-19 PCR: not detected; SARS-CoV 2 antibody (IgG and IgM): positive.
Blood cultures: negative; faecal culture: insufficient; cytomegalovirus (CMV)/ Epstein-Barr virus (EBV)/adenovirus PCR: negative.
CMV IgM antibody: not detected; CMV IgG antibody: not detected; EBV IgM antibody: not detected; EBV IgG antibody: not detected; varicella-zoster virus IgG antibody: positive.
PCR to detect bacterial DNA: negative; DNA from Mycobacterium species: negative; DNA from Mycobacterium tuberculosis complex: negative.
Differential diagnosis
At initial presentation, the patient was thought to have possible appendicitis in view of his fever and abdominal pain. This was felt to be unlikely by the local surgical team and was excluded on ultrasound scan. However, the findings of ill-defined inflammatory changes on ultrasound and of focal thickening of the transverse colon on CT scan, led to the suspicion of lymphoma, and the patient was transferred to a tertiary centre for further investigation by the paediatric oncology team.
While awaiting biopsy, the patient’s abdominal distension worsened, and he developed signs of bowel obstruction. This was managed conservatively. However, this rapid deterioration was felt to be due to increase in size of the inflammatory mass, which further supported the suspected diagnosis of lymphoma. Due to the depth of the mass lesion, a percutaneous biopsy was not possible, and an open biopsy was performed. Following this, he underwent a CT neck/chest/abdomen/pelvis for full staging, which did not show lymphadenopathy aside from the abdominal changes already noted.
The biopsy showed marked inflammation with the presence of lymphocytes, eosinophils and histiocytes and large areas of necrosis. Atypical infections such as Mycobacterium tuberculosis can cause necroinflammatory lymph nodes, but there was no evidence of granuloma formation, no acid-fast bacilli or fungi were seen with tinctorial stains and no bacterial DNA was detected in the formalin-fixed paraffin-embedded sections.
Kikuchi-Fujimoto disease was the main differential diagnosis. This is a self-limiting condition, which presents with systemic symptoms such as fever. It is thought to be triggered by viral infection, but this is not proven. While mesenteric lymph node involvement has been described in children presenting with abdominal pain,3 it is rare. This, together with the extent of the necrosis and the prominent mesenteric fat involvement, prevented a definite diagnosis of Kikuchi’s disease.
Sclerosing mesenteritis was also considered; this is a chronic inflammatory disorder, which can be seen although rarely in children, but it is associated with a different morphology of fat necrosis to that seen in this case.
Once the biopsy result was back, the team started to consider alternative diagnoses. The patient had undergone echocardiogram as part of his prechemotherapy assessments, which was noted to have shown a small pericardial effusion and low normal systolic function, with a fractional shortening of 26%. Although the features were unusual, it was suggested that Paediatric Multisystem Inflammatory Syndrome temporally associated with SARS-CoV-2 (PIMS-TS) may have led to this presentation of significant lymphadenopathy with possible cardiac involvement. The patient was discussed with the rheumatology department, who felt that this was not a typical case of PIMS-TS, as there was no shock and no mucosal changes. However, as the patient had received steroids, this may have altered the course of the condition. Positive SARS-CoV-2 antibodies were then received, making the diagnosis of PIMS-TS significantly more likely.
Treatment
Due to the suspicions of lymphoma, the patient was initially commenced on hyperhydration, allopurinol and rasburicase as he was felt to be at high risk of tumour lysis syndrome. As he had ongoing fevers, he was initially commenced on ceftriaxone on presentation to his local hospital, and this was then switched to piperacillin-tazobactam at the tertiary centre. This was discontinued after 7 days.
Due to coagulopathy, the patient received fresh frozen plasma prior to his biopsy being performed as well as intravenous vitamin K. His clotting profile subsequently normalised.
Immediately following the patient’s biopsy, he was commenced on methylprednisolone as part of his planned chemotherapy regimen. Steroids were continued once the biopsy results were back, as the diagnosis was unclear but was felt to be inflammatory. These were discontinued after 5 days.
After review by the cardiology team, the patient was commenced on a course of aspirin to reduce the risk of coronary complications of PIMS-TS.
Outcome and follow-up
After discharge from the tertiary centre, he had a follow-up CT abdomen that showed ongoing resolution of the upper abdominal lymphadenopathy and splenomegaly, likely confirming the inflammatory/infective nature of the disease.
His 3-month oncology follow-up was reassuring and, therefore, he was discharged from clinic. Moreover, his 2-month cardiology follow-up was also unremarkable and in view of the normal echocardiogram, the aspirin was stopped. Further cardiology follow-up has been arranged in 1 year while his clinical progress will be monitored by the local paediatric medical team.
Discussion
PIMS-TS is a recently described condition, where patients develop ‘Kawasaki-like’ features following infection with the novel coronavirus SARS-CoV-2.2 Diagnostic criteria include:
Persistent fever, inflammation (neutrophilia, elevated CRP and lymphopaenia).
Evidence of single or multiorgan dysfunction, especially ‘warm shock’ from cardiac involvement.
Additional features (which may include abdominal pain and diarrhoea) and often red eyes, mucocutaneous inflammation and rash.
Absence of any other causes for symptoms, especially infection.
In this case, the absence of organ dysfunction made the diagnosis more challenging. In retrospect, the oxygen requirement our patient developed may have been part of PIMS-TS, but at the time it was thought to be due to pulmonary oedema secondary to hyperhydration. He also had deranged coagulation, which was felt to be explained by what at the time was thought to be lymphoma, but in retrospect, it was consistent with PIMS-TS.
While PIMS-TS is known to cause lymphadenopathy, such significant abdominal lymphadenopathy is unusual. We are aware of only a single case report of such marked mesenteric lymphadenopathy in an adult patient,4 and a small number in children. A small case series of children with PIMS-TS describes two children who developed signs of intestinal obstruction, one of whom required bowel resection; it is unclear from the manuscript whether the diagnosis of PIMS-TS was made before or after significant bowel involvement occurred.5 There are also several case reports of children who underwent appendicectomy for what was later diagnosed as PIMS-TS after presenting with fever and abdominal pain.6–8 There has also been a case report of a child who presented with possible appendicitis but rapidly deteriorated due to cardiac involvement of PIMS-TS.9
More subtle lymphadenopathy, comparable to that seen in mesenteric adenitis, is a common finding, occurring in 13–47% of children with PIMS-TS who undergo abdominal imaging and can be particularly seen in the right iliac fossa.10 It is, therefore, probable that some children with milder courses of PIMS-TS present with fever and abdominal pain, which are common presenting symptoms,1 and are discharged with a diagnosis of mesenteric adenitis, making the true prevalence of the condition difficult to estimate. However, as a previous case report has shown, these children are at risk of acute deterioration and should be monitored appropriately.8
Our report reminds clinicians that these children may also be at risk of bowel obstruction, and any family of child discharged from hospital should receive appropriate safety-netting advice on when to seek further medical attention.
Learning points.
Paediatric inflammatory multisystem syndrome (PIMS-TS) can cause significant intrabdominal lymphadenopathy and pain, which may mimic other pathologies.
Both lymphoma and PIMS-TS may respond rapidly to steroid therapies. Therefore, an urgent biopsy is crucial in leading to the correct diagnosis.
PIMS-TS is still a relatively new entity and should be considered in the differential diagnosis of any children presenting with unexplained inflammatory conditions.
Children with PIMS-TS are at risk of bowel obstruction and should be evaluated thoroughly should they present with or develop, severe abdominal pain.
A multidisciplinary team approach is essential in managing patients with PIMS-TS.
Footnotes
Contributors: ON, AF, BL, KT, MW, AD, DI have contributed in the following: conception—revising the article critically for important intellectual content; final approval of the version published; agreement to be accountable for the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
References
- 1.RCPCH . Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) - guidance for clinicians. Available: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance [Accessed 28 May 2021].
- 2.Sahn B, Eze OP, Edelman MC, et al. Features of intestinal disease associated with COVID-related multisystem inflammatory syndrome in children. J Pediatr Gastroenterol Nutr 2021;72:384-387. 10.1097/MPG.0000000000002953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hua C-Z, Chen Y-K, Chen S-Z, et al. Histiocytic necrotizing lymphadenitis mimicking acute appendicitis in a child: a case report. Front Pediatr 2021;9:916. 10.3389/fped.2021.682738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tantray H, DAc W, Pandith S, BUMSf T. Mesenteric lymphadenopathy in COVID-19 infection (Jammu and Kashmir sign) as a presenting sign in adult: case report; 2020. https://www.researchgate.net/profile/Imtiaz-Wani/publication/346884508_Mesenteric_lymphadenopathy_in_COVID-19_infection_Jammu_and_Kashmir_sign_as_a_presenting_sign_in_adult_case_report/links/5fe9ecca92851c13fecfa626/Mesenteric-lymphadenopathy-in-COVID-19-infection-Jammu-and-Kashmir-sign-as-a-presenting-sign-in-adult-case-report.pdf [Accessed 28 May 2021]. 10.1097/SR9.0000000000000010 [DOI]
- 5.Jurkiewicz B, Szymanek-Szwed M, Hartmann P, et al. Pediatric multisystem inflammatory syndrome in children as a challenging problem for pediatric surgeons in the COVID 19 pandemic—a case report. Front Pediatr 2021;9. 10.3389/fped.2021.677822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guanà R, Pagliara C, Delmonaco AG, et al. Multisystem inflammatory syndrome in SARS-CoV-2 infection mimicking acute appendicitis in children. Pediatr Neonatol 2021;62:122–4. 10.1016/j.pedneo.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang M, Wilson K, Wendt L, et al. The great gut Mimicker: a case report of MIS-C and appendicitis clinical presentation overlap in a teenage patient. BMC Pediatr 2021;21. 10.1186/s12887-021-02724-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan N, Chang HT, Leeman R, et al. Case of multisystem inflammatory syndrome in children presenting as fever and abdominal pain. BMJ Case Rep 2020;13:237306. 10.1136/bcr-2020-237306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Oro R, Fatahi Bandpey ML, García Martínez E, et al. Clinical and radiological findings for the new multisystem inflammatory syndrome in children associated with COVID-19. Radiología 2021;63:334–44. 10.1016/j.rxeng.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259–69. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]