Abstract
Sertoli-Leydig cell tumours (SLCTs) represent a rare cause of hyperandrogenic state. SLCTs are sex cord ovarian neoplasms, accounting for <0.2% of all ovarian tumours. Most of the sex cord-stromal tumours have a benign clinical course, with 10%–20% of them at risk of aggressive course. We report a case of a woman in her 30s who presented with androgenic alopecia, virilisation and secondary amenorrhoea. The evaluation revealed an extremely high testosterone level. Imaging for the localisation of source of excess testosterone with contrast-enhanced CT of the abdomen revealed a right ovarian mass. Hence, a diagnosis of testosterone-secreting ovarian tumour was considered. The patient underwent right salphingo-oophorectomy, and histopathology was reported as Sertoli cell tumour. Postoperatively, there was normalisation of serum testosterone levels with decrease in virilisation and resumption of spontaneous menstrual cycles. The patient conceived spontaneously after 2 months of surgery.
Keywords: Obstetrics, gynaecology and fertility; Endocrinology
Background
Women with hyperandrogenemia present with oligomenorrhoea, virilisation features like hirsutism, acne, androgenic alopecia, clitoromegaly and masculinisation. Virilisation in women can be seen with hyperandrogenemia secondary to ovarian hyperthecosis, simple virilising congenital adrenal hyperplasia (CAH), non-classic CAH, dehydroepiandrosterone sulfate (DHEAS)-secreting adrenal tumours and androgen-secreting ovarian tumours. Sertoli-Leydig cell tumours (SLCTs) are rare ovarian neoplasms which present in young women with oligomenorrhoea/amenorrhoea and virilisation. It is often mistaken for ovarian hyperthecosis/polycystic ovarian syndrome (PCOS) especially in obese women with insulin resistance features, PCOS being a more prevalent disorder. DHEAS-secreting adrenal tumour is the next more common entity.
Patients presenting with virilisation and biochemical hyperandrogenemia must be evaluated for androgen-secreting ovarian tumours, as most of these are intermediate adnexal tumours with good prognosis if detected and treated early. Detecting SLCTs in early stages gives the opportunity for fertility-sparing surgeries and management without chemotherapy, thus providing chances for spontaneous pregnancies and improved quality of life.
Case presentation
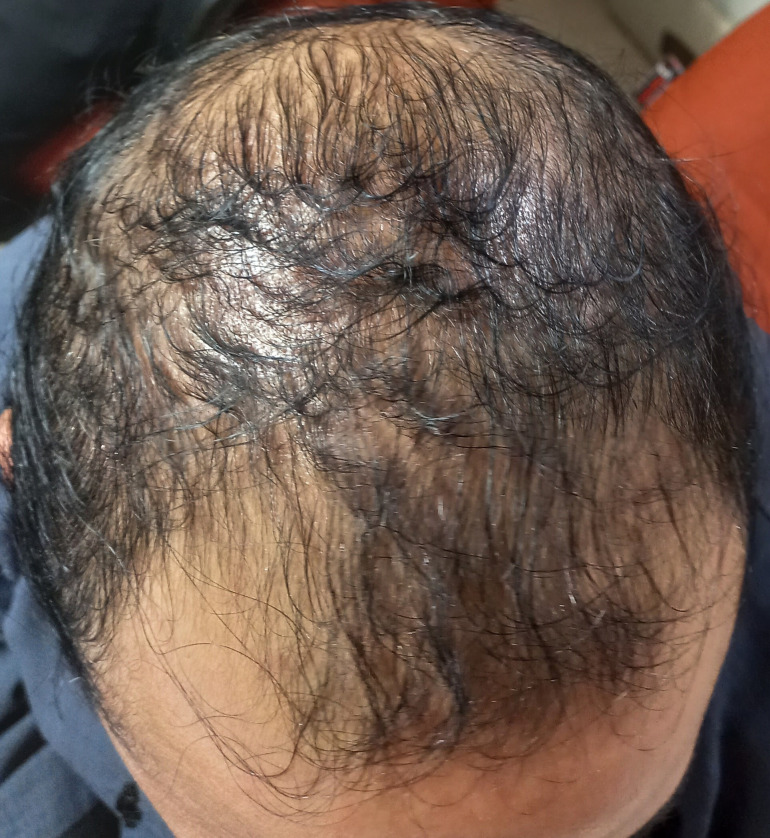
A woman in her 30s presented with amenorrhoea for 3 years. She also reported excessive growth of coarse facial hair since 1 year which required daily shaving and severe male pattern baldness (figure 1—androgenic alopecia). She was diagnosed with type 2 diabetes mellitus for the last 2 years and was on treatment for the same. She was also on thyroxine supplementation for primary hypothyroidism. There was no history of exposure of external androgens or steroids, salt-losing crisis, hyperpigmentation, abdominal striae or proximal myopathy. There were no discriminatory features of Cushing’s syndrome.
Figure 1.
Androgenic alopecia.
On examination, low-pitched voice, masculine body habitus, coarse terminal body hair with modified Ferriman Gallwey score of 32/36, oily skin and clitoromegaly with clitoral index (CI) of 50 mm2 were noticed. Blood pressure and systemic examinations were normal.
Investigations
Biochemical evaluation showed haemoglobin: 142 g/L (normal=120–150 g/L); thyroid-stimulating hormone: 2.140 µIU/mL (normal=0.27–4.2); triiodothyronine: 1.29 ng/mL (normal=0.8–2); thyroxine: 9.02 µg/mL (normal=5.1–14.1); testosterone: 4.4 ng/mL (normal=0.08–0.48); DHEAS: 171 µg/dL (normal=60.9–337); HbA1c (Glycosylated haemoglobin): 7.6% (normal=<5.7%); fasting blood sugar: 187 mg/dL (normal=70–100); creatinine: 0.7 mg/dL (normal=0.5–0.9); follicle-stimulating hormone: 2.7 mIU/mL (normal=3.5–12.5); luteinising hormone: 6.7 mIU/mL (normal=2.4–12.6); beta-human chorionic gonadotropin: <0.1 mIU/mL (normal=<1); 08:00 cortisol: 15.09 µg/dL (normal=4.82–19.5); alpha-fetoprotein: 1.2 ng/dL (normal=0–8.5); cancer antigen-125: 27.1 U/mL (normal=up to 35); carbohydrate antigen 19-9: 28.9 U/mL (normal=up to 39); lactate dehydrogenase: 211 U/mL (normal=125–220). Renal and liver function tests were normal. Serum electrolytes were normal.
Ultrasound of the abdomen revealed a solid right adnexal mass of 4.7×4.2 cm with normal left ovary and uterus.
Contrast-enhanced CT confirmed a relatively well-defined isohypodense soft tissue mass lesion showing post-contrast enhancement measuring 5.2×4.4×4.2 cm (Anteroposterior-Transverse-Craniocaudal) in the right adnexa with normal left ovary, Fallopian tubes and uterus (figure 2).
Figure 2.
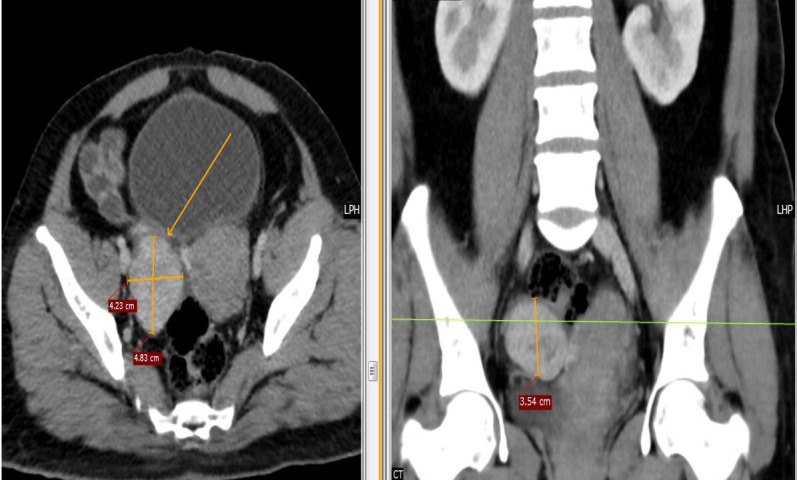
Contrast-enhanced CT shows a relatively well-defined isohypodense soft tissue mass lesion showing post-contrast enhancement measuring 5.2×4.4×4.2 cm (AP-TR-CC) in the right adnexa with normal left ovary, Fallopian tubes and uterus.
Based on age of presentation, clinical features, biochemical evaluation and imaging studies, a diagnosis of testosterone-secreting SLCT was considered.
Differential diagnosis
As the patient presented with secondary amenorrhoea, virilisation and androgenic alopecia, differential diagnoses of ovarian hyperthecosis, androgen-secreting ovarian tumours, DHEAS-secreting adrenal tumour and non-classic CAH were considered. As DHEAS levels were normal and testosterone levels were very high, differentials were narrowed down to ovarian SLCT and ovarian hyperthecosis.
Imaging studies revealed ovarian SLCT. She underwent explorative laparotomy with right salphingo-oopherectomy with peritoneal sampling and omentectomy. The histopathology confirmed the diagnosis of ovarian SLCT.
Treatment
The patient underwent explorative laparotomy with right salphingo-oopherectomy with peritoneal sampling and omentectomy.
Intraoperatively, her uterus and left ovary were normal in appearance; detailed abdominopelvic exploration did not reveal any tumour deposits or palpable node. Infracolic omentectomy and peritoneal biopsies were obtained.
Outcome and follow-up
Histopathology of excised right ovarian tissue shows polygonal cells having distinct cell borders, central nuclei with moderate eosinophilic cytoplasm, without necrosis or haemorrhage. Stroma is oedematous at places with loosely dispersed tumour cells with dilated thin-walled blood vessels—features suggestive of SLCT (figure 3). Surrounding tissues were free of malignant cells. Ascitic fluid cytology was negative for malignant cells. TNM (Tumoursize, Lymph node involvement, metastasis) stage pT1a was assigned. FIGO (Federation of International Gynaecologists and Obstetricians) staging was stage IA.
Figure 3.
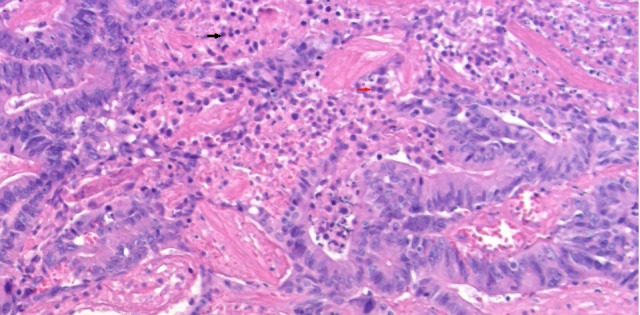
Histopathology of excised right ovarian tissue shows polygonal cells having distinct cell borders, central nuclei with moderate eosinophilic cytoplasm, without necrosis/haemorrhage. Stroma is oedematous at places with loosely dispersed tumour cells with dilated thin-walled blood vessels—features suggestive of Sertoli-Leydig cell ovarian tumour (Sertoli cell indicated by red arrow, Leydig cell marked by black arrow).
At 1-month follow-up postoperatively, the patient had significant improvement in virilising features. She noticed decreased growth of facial hair and resumed spontaneous menstrual cycle. Her serum testosterone levels normalised to 0.18 ng/mL (normal=0.08–0.48) after 1 month of surgery. She also conceived after 2 months of surgery.
Discussion
Androgen-secreting tumours may arise from ovary or adrenals. Most of the androgen-secreting tumours arise from ovaries. These include SLCTs, hilus cell tumours, lipoid cell tumours and rarely granulosa-theca tumours. SLCTs tend to occur during the second and fourth decades of life, whereas hilus cell tumours occur more frequently in post-menopausal women. Granulosa-theca tumours mostly produce oestradiol, but may rarely secrete testosterone. Virilising adrenal tumours secrete large amount of DHEAS, DHEA and androstenedione. Testosterone is usually produced by extraovarian conversion of these precursors. Testosterone-secreting adrenal tumours are very rare. Serum testosterone levels more than three times the upper limit of normal suggest the possibility of ovarian neoplasms.
SLCTs are rare ovarian tumours of the sex cord-stroma subset. Patients present with features of androgen excess like hirsutism, amenorrhoea, male pattern baldness, low-pitched voice, masculinisation, decrease in breast size, symptoms of mass effect like pelvic pain and clitoromegaly.1 The size of the glans clitoris is quantitated using the CI which is the product of the sagittal and transverse diameters of the glans clitoris. Clitoromegaly is defined as CI greater than 35 mm2 which is a clinical sign of excess androgenic stimulation.2 SLCTs are characterised by a varying proliferation of Sertoli and Leydig cells that secrete testosterone and cause virilisation in up to 85% of all patients.2 Most SLCTs are diagnosed in young patients at an early stage that are well differentiated and have an excellent prognosis.3 4
The WHO classification of SLCT includes three histological types of SLCTs, namely well-differentiated, moderately differentiated and poorly differentiated; heterologous elements and/or retiform patterns may be present in moderately and poorly differentiated neoplasms. Tumours of the retiform type or those presenting heterologous elements are rarely secretant.5
Surgery is the treatment of choice for stage I tumours. Fertility-sparing surgery may thus be offered. Fertility-sparing surgery with preservation of the uterus and contralateral ovary is recommended for stage IA.3 6 According to European Society of Medical Oncology guidelines (2018), fertility-sparing surgery may be an option for selected cases even in advanced stages. Some authors propose a completion of surgery after childbearing.7
Chemotherapy is offered for patients with spontaneous tumour rupture or malignant ascites (FIGO stage IC2, IC3), advanced disease (FIGO stages II–IV) and those with high mitotic activity tumours.
Relapse rates of 5%–8% were noted for stage IA tumours and of approximately 30% for stage IC. The mortality rate in case of relapse was around 70%. Risk factors for recurrence include advanced FIGO stage, poor differentiation, high mitotic activity, the presence of heterologous elements and a retiform pattern. One-fifth of all SLCT relapse cases and most recurrences appear within 3 years.3 Most patients with recurrence have systemic relapses at multiple locations, and peritoneum is the most commonly involved site.8
The SLCTs harbour hormone receptors and hence hormonal replacement therapy is contraindicated.9 Young patients undergoing radical surgery with bilateral salpingo-oophorectomy are consequently facing oestrogen depletion and menopausal symptoms with a strong impact on their quality of life. Whenever possible, conservation of the normal remaining ovary (endocrine-sparing surgery) might improve quality of life of very young women.9
Spontaneous pregnancies have been reported in women treated for SLCT.8–10
Learning points.
Sertoli-Leydig cell tumours (SLCTs) must be considered in women presenting with secondary amenorrhoea and severe virilisation features with elevated testosterone levels.
Early detection of tumours is crucial because tumours in early stages are curable and have good prognosis.
Removal of the source of excess testosterone by excision of SLCT leads to clinical and biochemical reversal of hyperandrogenic state.
Fertility-sparing surgery is a good option in early stages. This prevents women from becoming hormone deprived and helps them have spontaneous pregnancies.
Footnotes
Contributors: SM wrote the manuscript under the supervision of SS. SS supported in collecting review articles and management plans. SG operated the case. RK provided a histopathology report. SM and SS contributed to the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Seidler SJ, Huber A, Nef J, et al. Sertoli-Leydig cell ovarian tumors: is fertility or Endocrine-Sparing surgery an option upon relapse? Case Rep Oncol 2020;13:935–40. 10.1159/000508532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagatz GE, Kopher RA, Nagel TC, et al. The clitoral index: a bioassay of androgenic stimulation. Obstet Gynecol 1979;54:562–4. [PubMed] [Google Scholar]
- 3.Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol 1985;9:543–69. 10.1097/00000478-198508000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Morice P, Denschlag D, Rodolakis A, et al. Recommendations of the fertility task force of the European Society of gynecologic oncology about the conservative management of ovarian malignant tumors. Int J Gynecol Cancer 2011;21:951–63. 10.1097/IGC.0b013e31821bec6b [DOI] [PubMed] [Google Scholar]
- 5.Ray-Coquard I, Morice P, Lorusso D, et al. Non-epithelial ovarian cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018;29:iv1–18. 10.1093/annonc/mdy001 [DOI] [PubMed] [Google Scholar]
- 6.Nam SM, Kim JW, Eoh KJ, et al. A novel clinicopathological analysis of early stage ovarian Sertoli-Leydig cell tumors at a single institution. Obstet Gynecol Sci 2017;60:39–45. 10.5468/ogs.2017.60.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouy S, Arfi A, Maulard A, et al. Results from a monocentric long-term analysis of 23 patients with ovarian Sertoli-Leydig cell tumors. Oncologist 2019;24:702–9. 10.1634/theoncologist.2017-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang N, Chen R, Hua K, et al. A retrospective study of reproductive outcomes after fertility-sparing surgery and postoperative adjuvant chemotherapy in malignant ovarian germ cell tumors and sex cord-stromal tumors. J Ovarian Res 2017;10:52. 10.1186/s13048-017-0348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghalleb M, Bouzaiene H, Sghaier S, et al. Fertility sparing surgery for ovarian sex cord stromal tumors: a nine case series. Pan Afr Med J 2018;31:221. 10.11604/pamj.2018.31.221.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Kock L, Terzic T, McCluggage WG, et al. Dicer1 mutations are consistently present in moderately and poorly differentiated Sertoli-Leydig cell tumors. Am J Surg Pathol 2017;41:1178–87. 10.1097/PAS.0000000000000895 [DOI] [PubMed] [Google Scholar]