Abstract
Objectives
Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum (genital mycoplasmas) commonly colonise the urogenital tract in pregnant women. This systematic review aims to investigate their role in adverse pregnancy and birth outcomes, alone or in combination with bacterial vaginosis (BV).
Methods
We searched Embase, Medline and CINAHL databases from January 1971 to February 2021. Eligible studies tested for any of the three genital mycoplasmas during pregnancy and reported on the primary outcome, preterm birth (PTB) and/or secondary outcomes low birth weight (LBW), premature rupture of membranes (PROM), spontaneous abortion (SA) and/or perinatal or neonatal death (PND).
Two reviewers independently screened titles and abstracts, read potentially eligible full texts and extracted data. Two reviewers independently assessed risks of bias using published checklists. Random effects meta-analysis was used to estimate summary ORs (with 95% CIs and prediction intervals). Multivariable and stratified analyses were synthesised descriptively.
Results
Of 53/1194 included studies, 36 were from high-income countries. In meta-analysis of unadjusted ORs, M. hominis was associated with PTB (OR 1.87, 95% CI 1.49 to 2.34), PROM, LBW and PND but not SA. U. urealyticum was associated with PTB (OR 1.96, 95% CI 1.14 to 1.39), PROM, and SA. U. parvum was associated with PTB (1.79, 95% CI 1.28 to 2.52) and PROM. Seven of 53 studies reported any multivariable analysis. In two studies, analyses stratified by BV status showed that M. hominis and U. parvum were more strongly associated with PTB in the presence than in the absence of BV. The most frequent source of bias was a failure to control for confounding.
Conclusions
The currently available literature does not allow conclusions about the role of mycoplasmas in adverse pregnancy and birth outcomes, alone or with coexisting BV. Future studies that consider genital mycoplasmas in the context of the vaginal microbiome are needed.
PROSPERO registration number
CRD42016050962.
Keywords: GYNAECOLOGY, MICROBIOLOGY, OBSTETRICS, EPIDEMIOLOGY, Epidemiology
Strengths and limitations of this study.
We followed a published protocol with predefined outcomes and statistical analysis plan.
Two reviewers independently selected the studies, extracted data and performed risk of bias assessment.
Evidence for heterogeneity was examined and described both visually and statistically.
We triangulated findings across study designs.
Restriction to studies in English and German might have missed eligible articles.
Introduction
Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum, referred to together as genital mycoplasmas, commonly colonise the urogenital tract in women, and are often found together.1 2 These species do not appear to cause symptoms or harmful effects in non-pregnant women.2 3 Plummer et al found that M. hominis was associated with abnormal vaginal discharge only in non-pregnant women who also had bacterial vaginosis (BV).2 Colonisation with a genital mycoplasma has, however, been reported in many studies to be associated with several adverse pregnancy outcomes,4 5 including preterm birth (PTB); low birth weight (LBW); premature rupture of membranes (PROM) and preterm premature rupture of the membranes (PPROM), spontaneous abortion (SA) and perinatal or neonatal death (PND).1 6–12 Several research groups have suggested that M. hominis, while considered a part of the normal vaginal microbiota, might only be pathogenic in the presence of BV as part of a disturbed vaginal microbiota.4 5 13 There are, however, inconsistencies across studies, uncertainty about the interplay between specific organisms and the vaginal microbiota in general,14–16 and differences in recommendations for testing and treatment.13 17
Technological advances in the molecular detection of multiple vaginal and endocervical organisms in the same assay18 19 should make it easier to study the role of genital mycoplasmas in adverse pregnancy outcomes. Methods to distinguish between U. urealyticum and U. parvum were not widely available before 2000,20 21 and unspeciated Ureaplasma spp. detected by culture were reported together as U. urealyticum.18 Narrative reviews have not fully elucidated whether the apparent pathogenicity of genital mycoplasmas in pregnancy is associated with a particular organism, concurrent infection with multiple genital mycoplasmas and other lower genital tract organisms or confounding by other demographic, clinical and behavioural factors.4 5 13 A systematic and quantitative assessment of these questions is, therefore timely.
Objectives
The primary objective of this study was to investigate the associations between M. hominis, U. urealyticum and/or U. parvum and the risk of PTB, alone and in combination with BV. Secondary objectives were to investigate associations between each genital mycoplasma and LBW, PROM, SA and PND.
Methods
This systematic review followed a registered protocol,22 which covers multiple organisms, for which findings are reported elsewhere, including Neisseria gonorrhoeae23 and M. genitalium.24 We report our findings using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses25 (online supplemental file, A.1) and we also used methodological guidance about systematic reviews of observational studies.26 Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
bmjopen-2022-062990supp001.pdf (1.6MB, pdf)
Eligibility criteria, information sources and search strategy
Studies were eligible if they reported on pregnant women with and without M. hominis, U. urealyticum and/or U. parvum and included one or more of the outcomes: PTB, LBW, PROM (preterm or term), SA and PND. Standard definitions were used for all outcomes (PTB, delivery at <37 weeks gestation; LBW, birthweight <2.5 kg; PROM, rupture of membranes prior to onset of labour; PPROM, premature rupture at <37 weeks gestation; SA, delivery at <20 weeks gestation; stillbirth (death after >20 weeks in utero); perinatal or neonatal death (PND, stillbirths and death <28 days after birth), but we used author’s definitions if necessary.22 We excluded articles published before 2000 if they reported unspeciated U. urealyticum alone. If they reported on M. hominis and U. urealyticum, we included the study but did not extract results about U. urealyticum. We included cohort, cross-sectional and case–control studies, and randomised controlled trials.
A member of the team (MEJ) searched Medline, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL) for literature published from January 1971 to February 2021. We searched reference lists of included studies for additional potentially eligible studies but did not search grey literature sources. The searches did not include language restrictions, but we only read the full-text of articles in English and German (languages spoken by the review team). The full search strategy is in the online supplemental file (A.2). We used Endnote (V.7, Thomson Reuters) to import, deduplicate and manage retrieved records.
Study selection and data extraction
Two reviewers (MEJ, LMV) independently screened titles and abstracts and read the full text of potentially eligible papers. Disparities were resolved by discussion or by a third reviewer (DE-G). Where multiple reports presented data from the same study population, we identified a primary record with the most detailed information but included data from other publications. Two reviewers (MEJ, LMV) extracted data independently into an online database (Research Electronic Data Capture, REDCap, Vanderbilt University, Tennessee). Disparities were resolved by discussion or by a third reviewer (DE-G, NL or ELS).
Data extraction
Each reviewer extracted data about the study design, study setting and sociodemographic characteristics, specimen type and timing, laboratory tests, organisms tested for, outcomes reported, raw numbers of participants with and without each outcome and organism, where available, or author-reported effect size and 95% CIs. They extracted the adjusted OR (aOR, 95% CI) and recorded variables included in multivariable models, where possible. If results were described for more than one anatomical site, we used the following order of preference: vaginal or cervical swabs, amniotic fluid, placenta, urine, blood. Where there was more than one diagnostic method, we used data from nucleic acid amplification test (NAAT), then bacterial culture, followed by ELISA. The data underlying this article are available in the article and in its online supplementary material.
Risk of bias assessments
Two reviewers (MEJ, LMV) appraised each article independently, using checklists published by the UK National Institute for Health and Care Excellence (NICE).27 28 A qualitative judgement about internal and external validity was summarised as: all or most checklist criteria fulfilled (++), some criteria fulfilled (+) or few or no criteria fulfilled (-). We used funnel plots and the Egger test29 to investigate evidence for publication or small study biases across studies for outcomes reported by more than nine studies.
Data synthesis
We used Stata V.14.0 (StataCorp, College Station, Texas) for all analyses. We used the OR, with 95% CI as the measure of association for all study designs, since the OR and risk ratio are similar for rare outcomes, as it is the case for most of the outcomes of interest. This allowed us to analyse findings from different study designs together, where appropriate.30 We constructed 2×2 tables to calculate the OR or used the authors’ calculation when raw data were unavailable. We added 0.5 to each cell in the table if there were zero observations in one cell. For each exposure–outcome pair, we examined forest plots of univariable associations visually, displaying the OR (with 95% CI) and the I2 statistic, to examine between study heterogeneity. We used a random effects model to estimate a summary OR (95% CI), which is the average effect across all included studies.31 We stratified studies by study design in forest plots and, where the stratified estimates were compatible, we estimated the overall estimated OR with its 95% CI and a prediction interval, where there were three or more studies. The prediction interval takes into account all sources of between study variability to estimate a range of values—for the OR in a new study that is similar to the types of studies included in the meta-analysis.31 We then examined evidence from studies that also reported on BV. We described findings from analyses that were stratified by BV status, or in studies with a multivariable analysis, we reported the aOR, controlling for BV and other measured confounding variables.26
Results
Study selection
Our searches identified 1194 records and we screened 641, after exclusion of duplicates (online supplemental file, figure S1). Of 215 full-text articles, we included 53 studies. Articles excluded based on title and abstract mostly concerned neonatal respiratory outcomes, chorioamnionitis and infertility. Full-text articles were excluded for various reasons (online supplemental file, figure S1).
Study characteristics
Of the 53 studies, we identified 42 reporting on M. hominis (proportion detected <1%–70%), 19 reporting on U. urealyticum (proportion detected 0%–90%) and 14 reporting on U. parvum (2%–100%) and median total sample size 241, interquartile range (IQR) 145 to 688, range 6132 to 1039733 (table 1, online supplemental file, table S1). There were 26 cohort studies (online supplemental file, table 2.1),1 6 8 12 15 33–53 22 case–control studies (online supplemental file, table S2.2)7 9–11 32 54–70 and five cross-sectional studies (online supplemental file, table S2.3).71–75 Most studies were from high-income settings (36/53) (online supplemental file, tables S3.1-S3.3); ethnicity was reported in 23 studies, and maternal smoking in 14 (online supplemental file, table S4.1-S4.3). Most studies (50/53) stated the timing of specimen collection, and all described the laboratory tests used (online supplemental file, table S1): 27/53 bacterial culture only; 22/53 NAAT only (table 1, online supplemental file, table S1).
Table 1.
Summary of characteristics of studies included in the systematic review
| Characteristic | Total | M. hominis | U. urealyticum | U. parvum |
| Number of studies, n* | 53 | 42 | 19 | 14 |
| Study design, n | ||||
| Cohort | 26 | 22 | 10 | 7 |
| Case-control | 22 | 16 | 8 | 6 |
| Cross-sectional | 5 | 4 | 1 | 1 |
| Number of women, total (median; IQR) | 37 132 (241; 145–688) | 29 989 (250; 164–765) | 10 732 (214; 114–783) | 9890 (215; 145–972) |
| Study setting, income category, n | ||||
| High income | 36 | 27 | 14 | 12 |
| Upper-middle income | 9 | 8 | 3 | 2 |
| Lower middle-income or low | 2 | 2 | 0 | 0 |
| Not reported | 6 | 5 | 2 | 0 |
| Outcomes reported, n* | ||||
| Preterm birth | 40 | 30 | 16 | 13 |
| Low birth weight | 7 | 6 | 2 | 1 |
| Premature rupture of membranes | 13 | 11 | 3 | 2 |
| Spontaneous abortion | 10 | 10 | 4 | 2 |
| Perinatal death | 9 | 9 | 1 | 1 |
| Specimen type, n† | ||||
| Endocervical swab | 24 | 20 | 7 | 6 |
| Vaginal swab | 13 | 6 | 6 | 5 |
| Urine | 1 | 1 | 0 | 0 |
| Amnotic fluid | 8 | 5 | 4 | 1 |
| Placental membrane | 7 | 6 | 2 | 2 |
| Diagnostic method* | ||||
| NAAT | 22 | 13 | 16 | 11 |
| Culture | 28 | 28 | 0 | 0 |
| Culture and NAAT | 3 | 1 | 3 | 3 |
| Other‡ | 1 | 1 | 0 | 0 |
| Bacterial vaginosis assessed, n | 11 | 10 | 1 | 1 |
| Reported presence of STI, n | 21 | 22 | 7 | 7 |
| Reported on smoking status, n | 14 | 6 | 3 | 3 |
| Reported on multiple pregnancy, n | ||||
| Excluded | 26 | 20 | 11 | 8 |
| Included | 9 | 6 | 4 | 3 |
*The total number of studies included is 53. The totals for each organism and outcome sum to more than 53 because one study might have reported on more than one organism and outcome.
†One study used both urine and endocervical swab.
‡ELISA (with NAAT/Culture).
ELISA, Enzyme-linked immunosorbent assay; NAAT, nucleic acid amplification test; STI, sexually transmitted infection.
Of the 53 studies, 38 reported on a single microorganism (M. hominis, n=34; U. urealyticum, n=4); nine included two genital and seven reported on all three organisms (online supplemental file, figure S2). Only one study presented findings for combinations of more than one genital mycoplasma;47 the rest presented data separately, even if they had tested for more than one organism. Ten studies reported on the presence of BV; 33 36 43 44 47 51 53 57 58 69 we report the findings of these studies in the relevant section of the results for each genital mycoplasma. Twenty-one studies reported on other sexually transmitted infections (online supplemental file, tables S4.1-S4.3), including 2/21 reporting on syphilis, 9/21 gonorrhoea, 17/21 chlamydia, 6/21 M. genitalium, 7/21 trichomonas and 2/21 HIV.
Table 2 summarises the meta-analyses of each exposure–outcome pair and information about genital mycoplasmas in the presence or absence of BV (online supplemental file, table S5). In most meta-analyses, heterogeneity was low or moderate. Summary findings from different study designs were compatible, so we present summary measures across all study designs (figures 1–3, and online supplemental file, figures S3.1–S3.8).
Table 2.
Summary estimates, by outcome and organism, from random effects meta-analysis of unadjusted ORs, for associations between genital mycoplasmas and adverse birth outcomes, and summary of multivariable and analyses that stratify the main association by BV status
| Adverse outcome Organism |
Number of studies | Summary estimate* OR (95% CI) |
I2, % | Prediction interval | Any multivariable analysis† | Analyses of genital mycoplasmas and adverse birth outcomes in presence and absence of BV‡ |
| Preterm birth | ||||||
| M. hominis | 30 | 1.87 (1.49 to 2.34) | 29.2 | 0.98, 3.55 | 3 studies1 48,59 | MH+,BV+/PTB OR 1.58 (95% CI 0.94 to 2.77); MH+,BV-/PTB 1.18 (0.91, 1.52)33 |
| U. urealyticum | 16 | 1.96 (1.14 to 3.39) | 53.1 | 0.40, 9.73 | 3 studies1 41 47 | None reported |
| U. parvum | 13 | 1.79 (1.28 to 2.52) | 59.0 | 0.66, 4.85 | 2 studies1 47 | UP-,BV-/PTB; UP+,BV-/PTB Adjusted 1.6 (1.2 to 2.1); UP-,BV+/PTB aOR 1.6 (1.1 to 2.3); UP+,BV+/PTB aOR 2.6 (1.7 to 4.0)47 |
| Premature rupture of membrane | ||||||
| M. hominis | 11 | 1.94 (1.43 to 2.70) | 0.0 | 1.33, 2.83 | 1 study59 | None reported |
| U. urealyticum | 4 | 9.87 (1.81 to 53.72) | 49.0 | 0.02, 5757.86 | 0 studies | |
| U. parvum | 2 | 3.19 (1.25 to 8.15) | 0.0 | NC | 0 study | |
| Low birth weight | None reported | |||||
| M. hominis | 6 | 1.81 (1.29 to 2.52) | 0.0 | 1.12, 2.90 | 1 study34 | |
| U. urealyticum | 1 | 1.08 (0.08 to 14.41) | NA | NA | 0 study | |
| U. parvum | 0 | NA | NA | NA | 0 study | |
| Spontaneous abortion | None reported | |||||
| M. hominis | 10 | 0.93 (0.44 to 1.94) | 50.2 | 0.12, 7.14 | 0 study | |
| U. urealyticum | 3 | 2.43 (1.21 to 4.86) | 0.0 | 0.03, 217.73 | 0 study | |
| U. parvum | 2 | 1.65 (0.67 to 4.05) | 0.0 | NC | 0 study | |
| Perinatal or neonatal death | None reported | |||||
| M. hominis | 9 | 2.70 (1.31 to 5.57) | 30.4 | 0.52, 13.94 | 0 study | |
| U. urealyticum | 1 | 3.52 (0.14 to 87.08) | NA | NA | 0 study | |
| U. parvum | 1 | 2.78 (0.11 to 68.46) | 0 study | |||
*Meta-analysis of unadjusted ORs, using random effects model.
†Details for individual studies reported in online supplemental tables 2.1-2.3.
‡Further details of analyses based on exclusion of other infections, stratification, or multivariable analyses in online supplemental table S7.
.aOR, adjusted OR; BV, bacterial vaginosis; I2, heterogeneity; MH, Mycoplasma hominis; NA, not applicable; NC, could not be calculated; UP, Ureaplasma parvum; UU, Ureaplasma urealyticum.
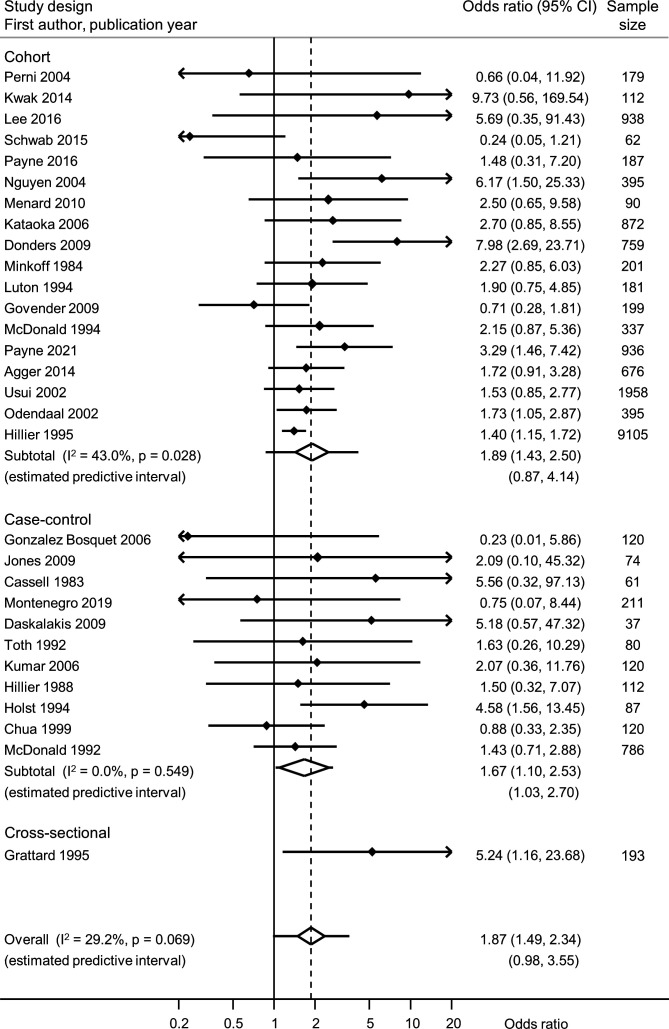
Figure 1.
Forest plot of univariable association between M. hominis and preterm birth, from random effects meta-analysis. Studies are in order of precision. Solid diamonds and lines either side are point estimates and 95% CIs for individual studies (arrows show where lower or upper confidence limits extend beyond the x-axis limits). Open diamond shows the point estimate and 95% CI for the summary OR and lines either side of the diamond show the prediction interval.
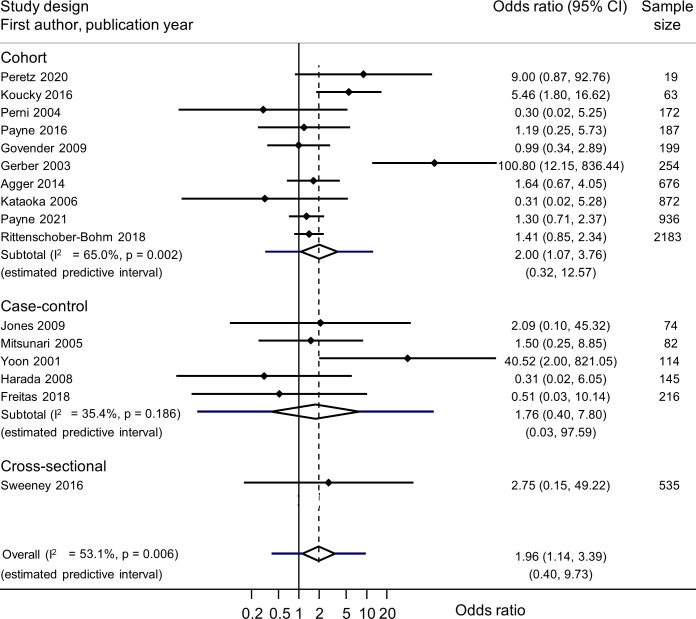
Figure 2.
Forest plot of univariable association between U. urealyticum and preterm birth, from random effects meta-analysis. Studies are in order of sample size. Solid diamonds and lines either side are point estimates and 95% CIs for individual studies (arrows show where lower or upper confidence limits extend beyond the x-axis limits). Open diamond shows the point estimate and 95% CI for the summary OR and lines either side of the diamond show the prediction interval.
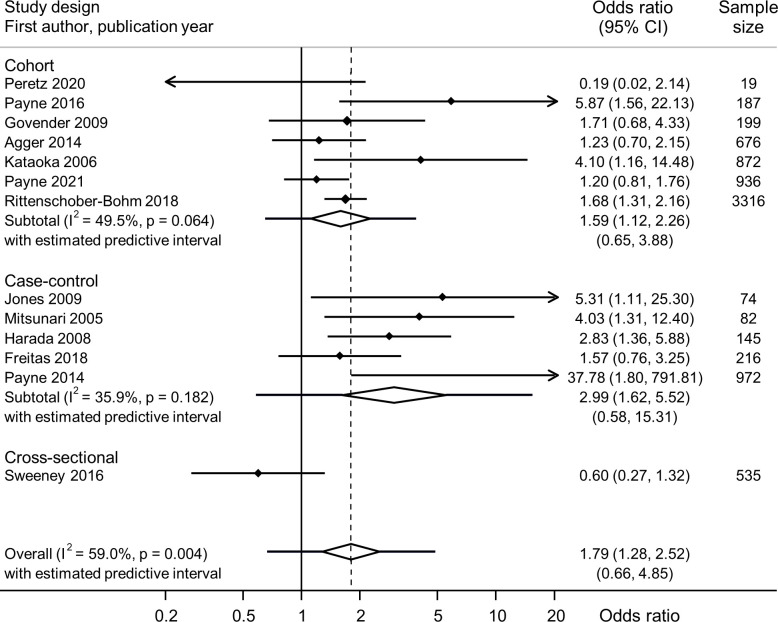
Figure 3.
Forest plot of univariable association between U. parvum and preterm birth, from random effects meta-analysis. Studies are in order of precision. Solid diamonds and lines either side are point estimates and 95% CIs for individual studies (arrows show where lower or upper confidence limits extend beyond the x-axis limits). Open diamond shows the point estimate and 95% CI for the summary OR and lines either side of the diamond show the prediction interval.
Risk of bias within and across studies
Based on the NICE checklists,27 28 none of the 53 studies met all or most (++/++) checklist criteria for internal and external validity, 23 studies met some (+/+)1 7 9 11 15 36 40 41 45–47 50 52 56 57 59 60 62 64–67 70 and 17 met few or no checklist criteria (−/−)6 8 10 12 33 38 39 42–44 49 53 54 63 68 69 75 (online supplemental tables S6.1- S6.3). Poor reporting of study methods meant that many items could not be assessed. In all study designs, control of confounding in most studies was poorly addressed or not addressed. Regression analysis for M. hominis (PTB, PROM, SA), U. urealyticum (PTB) and U. parvum (PTB) did not show evidence of small study effects (online supplemental file, table S7).
Associations between M. hominis and adverse pregnancy outcomes
There were 42 studies with data about M. hominis, reporting on 66 outcomes (online supplemental file, tables S2.1–S2.3,S3.1). Of these, 30 included data about PTB.1 6 8 10 15 32 33 36 38 40 42–46 48 50–53 57–59 63 65–67 69–71 M. hominis was associated with PTB in meta-analysis of unadjusted ORs (19 576 women, summary OR 1.87, 95% CI 1.49 to 2.34; I2 29.2%; prediction interval 0.98, 3.55) (figure 1). Three studies reporting a univariable association between M. hominis and PTB conducted multivariable analyses (table 2, online supplemental file, table S2.1-S2.2).1 48 59 The association was attenuated in one (aOR 1.1, 95% CI 0.5 to 2.5), after controlling for obstetric factors (previous PTB, miscarriage, multiple pregnancy and cervical incompetence).59 In two others, authors reported no association with PTB <37 weeks, but subgroup analyses showed associations with PTB <351 or <3348 weeks. In one study, no numerical results were reported (online supplemental file, table S2.1).34 In nine studies, authors also reported on BV (online supplemental file, table S5).33 36 43 44 51 53 57 58 69 In one study, the associations between M. hominis, BV and PTB could be examined in detail.33 M. hominis, in the absence of BV, was less strongly associated with PTB (OR 1.18, 95% CI 0.91 to 1.52) than in the presence of BV (OR 1.58, 95% CI 0.94 to 2.77).
Eleven studies included data about PROM.6 10 40 44 45 52 59 68 70 71 75 M. hominis was associated with PROM in meta-analysis of unadjusted ORs (4303 women, summary OR 1.94, 95% CI 1.40 to 2.70; I2 0.0%; prediction interval 1.33 to 2.83) (online supplemental file, figure S3.1). In one study with a multivariable analysis, the association was attenuated (aOR 1.1, 95% CI 0.3 to 3.7).59 Six studies included data about LBW.8 34 35 49 71 73 M. hominis was associated with LBW in meta-analysis of unadjusted ORs (2394 newborn, summary OR 1.81, 95% CI 1.29 to 2.52; I2 0.0%; prediction interval 1.12 to 2.90) (online supplemental file, figure S3.2). In one study, M hominis was associated with LBW in multivariable analysis, when considered as a continuous variable (reported p=0.01).34 In 9 studies with data about PND,8 32 35 40 45 51 54 72 73 meta-analysis of unadjusted ORs found an association with M. hominis (3696 women, summary OR 2.70, 95% CI 1.31 to 4.54; I2 30.4%; prediction interval 0.52 to 13.94) (online supplemental file, figure S3.3). In 10 studies with data about SA,6 7 11 35 36 39 40 51 54 61 there was no association with M. hominis in meta-analysis of unadjusted ORs (4531 women, summary OR 0.93, 95% CI 0.44 to 1.49; I2 50.2%; prediction interval 0.12 to 7.14) (online supplemental file, figure S3.4). No results of multivariable analyses were reported for PND or SA.
Associations between U. urealyticum and adverse pregnancy outcomes
Nineteen studies included data about U. urealyticum and 27 outcomes (online supplemental file, tables S2.1–S2.3,S3.2). There were 16 studies with data about PTB.1 10 12 15 37 38 40 41 46 47 52 55 56 60 64 74 In meta-analysis of unadjusted ORs, U. urealyticum was associated with PTB (6727 women, summary OR 1.96, 95% CI 1.14 to 3.39; I2 53.1%; prediction interval 0.40 to 9.73) (figure 2). Three studies reported multivariable analyses (table 2, online supplemental file, table S2.1).1 41 47 In one, multivariable and univariable associations were similar (aOR 1.4, 95% CI 0.8 to 2.2).47 In one, the adjusted OR was attenuated (3.4, 95% CI 1.3, 5.5).41 In the other, no numerical results were reported.1
For all other outcomes, data were only available for meta-analysis of unadjusted ORs. U. urealyticum was associated with: PROM in 4 studies10 37 40 52 (1372 participants, summary OR 9.87, 95% CI 1.81 to 53.72; I2 49.0%; prediction interval 0.02 to 5757.86) (online supplemental file, figure S3.5); LBW in one study12 (22 participants, OR 1.08, 95% CI 0.08 to 14.41; SA in three studies7 9 40 (1204 women, summary OR 2.43, 95% CI 1.21 to 4.86; I2 0.0%; prediction interval 0.03 to 217.73) (online supplemental file, figure S3.6) and PND in one study40 (872 participants, summary OR 3.52, 95% CI 0.14 to 87.08).
Associations between U. parvum and adverse pregnancy outcomes
Fourteen studies included data about associations between U. parvum and 19 outcomes (online supplemental file, tables 2.1-S2.33.1). Thirteen studies reported PTB.1 10 12 15 38 40 46 47 55 56 60 62 74 In meta-analysis of unadjusted ORs, U. parvum was associated with PTB (8229 women, summary OR 1.79, 95% CI 1.28 to 2.52; I2 59.0%; prediction interval 0.66 to 4.85) (figure 3). In one study,47 a multivariable analysis found a stronger association with PTB when both U. parvum and BV were present (aOR 2.6, 95% CI 1.7 to 4.0) than when U. parvum was present without BV (aOR 1.6, 95% CI 1.2 to 2.1), when compared with women colonised with neither (table 2, online supplemental file, table S5). In one, no numerical results were reported.1
For all other outcomes, data were only available for meta-analysis of unadjusted ORs. U. parvum was associated with PROM in two studies10 40 (946 participants, OR 3.19, 95% CI 1.25 to 8.15; I2 0.0%) (online supplemental file, figure S3.7) and with SA in two studies7 40 (986 participants, summary OR 1.65, 95% CI 0.67 to 4.05; I2 0.0%) (online supplemental file, figure S3.8). One study reported on LBW (22 participants, 1 event, OR 0.56, 95% CI 0.01 to 12.75)12 and one on PND (872 women, 1 event, OR 2.78, 95% CI 0.11 to 68.46).40
Discussion
Principal findings
This systematic review and meta-analysis included 53 studies about associations between M. hominis, U. urealyticum and U. parvum and five adverse pregnancy outcomes. Only 6/53 studies reported any multivariable analysis. In 51 studies, meta-analyses of unadjusted ORs found that M. hominis was associated with an increase in PTB, PROM, LBW and PND, U. urealyticum with an increase in PTB, PROM and SA, and U. parvum with an increase in PTB. In two studies from which data about both genital mycoplasmas and BV could be extracted, M. hominis and U. parvum were less strongly associated with PTB in the absence of BV than in the presence of BV.
Strengths and weaknesses of the study
The strengths of this systematic review and meta-analysis are first, that we followed a published protocol22 with predefined outcomes and statistical analysis plan. Study selection, data extraction and risk of bias assessment were undertaken independently by two reviewers, to reduce subjectivity. Second, we examined evidence for heterogeneity visually and statistically and calculated prediction intervals that take into account the variability in estimates from different studies and predict a range of values that could be expected in a new study.31 In several of the random effect models, the I2 value was zero, suggesting that the variability between the estimates is due to chance. This is consistent with meta-analyses in which the sampling error is high and CIs for estimates in individual studies all overlap (eg, online supplemental file, figures S3.1 and S3.2). Third, we triangulated findings across study designs;23 26 despite the different potential sources of bias, the summary estimates were compatible and we judged it reasonable to combine effect estimates.30 There were also limitations in the design of the review. Despite a predefined search strategy, with broad search terms, we might have missed relevant studies, particularly by restriction to languages not spoken fluently by the authors. There were too few studies to conduct all the planned sensitivity analyses by organism, but we described all studies that allowed stratification by BV status.
Comparison with the existing literature and interpretation
We found a systematic review about genital mycoplasmas that included studies published in English or Chinese up to March 2020.76 The focus of the review was on infertility, however, and limited search terms for studies about adverse pregnancy outcomes identified only nine of the 53 studies that we included, making comparison difficult.
The findings from this systematic review cannot be interpreted as showing causal associations between colonisation with M. hominis, U. urealyticum or U. parvum in pregnancy and some adverse pregnancy outcomes. We found associations in meta-analysis of unadjusted associations, but the confounder-adjusted estimates could not be summarised. Most studies in this systematic review did not control for confounding by either sociodemographic characteristics or co-occurrence with another organism or BV. We could not elucidate the role of co-occurrence with BV,4 5 because there were only two relevant studies, with imprecise estimates. Rittenschober-Böhm et al, studied more than 4000 women in Austria.47 They found univariable associations between both U. parvum (OR 1.7, 95% CI 1.3 to 2.2) and U. urealyticum (1.4, 95% CI 0.9 to 2.3) and spontaneous PTB. A strength of their study is the multivariable analysis, controlling for age, smoking, history of PTB and other infections. For U. parvum, the association with PTB was stronger when both BV and U. parvum were present than for U. parvum alone. The authors did not analyse the association with U. urealyticum further. Hillier et al investigated the association between M. hominis and PTB of LBW infants in more than 10 000 women in the USA.33 The association was stronger in the presence (1.58, 95% CI 0.94 to 2.77) than absence (1.18, 95% CI 0.91 to 1.52) of BV, but CIs for both estimates include the null value. Hillier et al also reported a stronger association with PTB when M. hominis was present with Bacteroides and BV (OR 2.1, 95% CI 1.5, 3.0). The authors did not, however, control for any other confounding factors.
Several of the limitations that we found in our review apply to systematic reviews of observational studies in general. Most included studies did not set out to study our review question and have small sample sizes. We extracted most data about genital mycoplasmas, our exposures of interest, from tables of covariates. Differences in the performance characteristics of diagnostic methods might have resulted in misclassification of colonisation status. Bacteriological culture has been considered the gold standard for the identification of genital mycoplasmas, but problems can arise from their fastidious growth requirements and a lack of reliable media. Commercialised kits for both culture and NAAT diagnosis are less laborious and have greater sensitivity and specificity compared with earlier in-house approaches.77 78 There were, however, confusions in nomenclature (eg, incorrect identification of biovar 1 as U. urealyticum rather than U. parvum)56 60 and misclassification of cultured ureaplasma as U. urealyticum. 6 50 53 65 68 70 Sample integrity is also important and greatly influenced by sample collection methods (eg, type of swab, transport medium), transportation (eg, cold chain maintenance) and storage (eg, duration and temperature at which kept in long-term storage). It was not possible to account for differences in anatomical sampling site that may have affected detection in individual studies, for example, M. hominis commonly colonises the lower genital tract while Ureaplasma spp. may colonise the upper genital tract.79 Other limitations include misclassification, for example, gestational age was assessed by obstetric ultrasound in only one third of studies and inconsistency in the timing during pregnancy of sampling for genital mycoplasmas.
The specificity of associations between different genital mycoplasmas and adverse pregnancy and their mechanisms of action remain unclear. Several studies included in this review postulate that subclinical Ureaplasma spp, ascending to the choriodecidual space cross the fetal membrane is followed by placental transfer into the amniotic cavity,7 72 74 80 81 leading to PROM, SA and PND in women with high bacterial load in the upper genital tract.81 82 The presence of genital mycoplasmas in the placental membranes and amniotic fluid might have a direct effect, but they also increase levels of a variety of cytokines and other inflammatory mediators, which might be the key drivers of adverse pregnancy outcomes.37 52 62 64 65 81 83 Gene sequencing methods show the diversity of the vaginal microbiota during pregnancy15 16 84 and genital mycoplasmas are often among the most plentiful of the many bacterial species identified. In our review, one study using 16 s rRNA sequencing found a group of bacteria, including U. parvum, that was associated with PTB,15 but another smaller study did not.55 Analysis of associations between microbial communities and PTB was beyond the scope of our systematic review. A better understanding of antimicrobial susceptibility is also needed. Genital mycoplasmas lack a rigid cell wall, which allows them to evade some antibiotics. Beta-lactam antibiotics and vancomycin are considered ineffective but macrolides, fluoroquinolones and tetracyclines are often effective.85 In pregnant women requiring antimicrobials, only macrolides should be used86 but high rates of antibiotic resistance are reported in many settings,4 87 88 and in the absence of definitive evidence of the benefits of treatment, cannot currently be recommended.
Implications
The findings of this systematic review show key areas for future research. First, there is a need for epidemiological studies that are designed specifically to investigate the pathogenicity of vaginal and cervical organisms alone and in the context of the vaginal microbiome. A holistic approach that includes gene sequencing and other molecular and culture methods to detect other endogenous and sexually transmitted organisms is required,14–16 taking into account the need for consistent strategies for specimen collection both in terms of the trimester(s) and the timing and types of specimens collected. These studies should also define potential causal pathways and address confounding from factors such as maternal age, smoking, obstetric history, co-occurrence and comorbidities. Second, there is a critical need to conduct research in low-income and middle-income settings where the prevalence of sexually transmitted infections, BV and genital mycoplasmas is high, and the burden of adverse pregnancy outcomes was greatest. If consistent and reproducible associations are found in observational studies, potential interventions need to be evaluated. Randomised controlled trials of screening and treatment for a range of vaginal and endocervical infections in pregnancy are underway.89 90 If these interventions prevent adverse pregnancy outcomes, further research will still be needed to understand the contributions of specific organisms or combinations thereof. Multiplex assays will facilitate these research studies but should not be used in routine clinical practice because of the risks of overdiagnosis and overtreatment.18 19
Conclusions
In this systematic review and meta-analysis, we found that genital mycoplasmas are associated with several different adverse pregnancy outcomes in univariable analysis only. The currently available literature does not allow conclusions about the role of genital mycoplasmas in adverse pregnancy and birth outcomes, alone or with coexisting BV. Future studies that consider genital mycoplasmas in the context of the vaginal microbiome are needed.
Supplementary Material
Footnotes
Twitter: @nicolamlow
Contributors: DE-G, NL, AJV, LMV conceived the idea for the review and DE-G, JK, NL, AJV, LMV, HW wrote the protocol. MEJ and LMV did the searches, screened, and selected studies and extracted data. DE-G, NL, ELS resolved disagreements. NL and HW did statistical analyses. MEJ wrote the first draft of the manuscript. MEJ, ELS, LMV and NL did the revision and editing. All authors commented on revisions of the manuscript and accept responsibility for its content. NL is guarantor for the overall content.
Funding: NL receives funding from the Swiss National Science Foundation, project numbers 197831, 160909; LMV is supported by an Australian National Health & Medical Research Council (NHMRC) Early Career Fellowship Grant (2018-2021); MEJ is a PhD research student supported through the Australian Award/UNSW UIPA scholarship and the Women And Newborns Trial of Antenatal Interventions and Management (WANTAIM) trial (ISRCTN No: ISRCTN37134032), funded by DFID/MRC/Wellcome Trust Joint Global Health Trials, Australian NHMRC Grant and Swiss National Science Foundation. DEG received salary support from r4d programme (Swiss Programme for Research on Global Issues for Development), grant number IZ07Z0-160909. AV receives salary support from the Australian NHMRC, through a Career Development Fellowship.
Competing interests: NL is on the advisory board of Sefunda AG, a start-up company that develops point-of-care tests for sexually transmitted infections.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Data relevant to the study are included in the article or the supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Agger WA, Siddiqui D, Lovrich SD, et al. Epidemiologic factors and urogenital infections associated with preterm birth in a midwestern U.S. population. Obstet Gynecol 2014;124:969–77. 10.1097/AOG.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plummer EL, Vodstrcil LA, Bodiyabadu K, et al. Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum associated with specific genital symptoms and clinical signs in nonpregnant women? Clin Infect Dis 2021;73:659–68. 10.1093/cid/ciab061 [DOI] [PubMed] [Google Scholar]
- 3.Horner P, Donders G, Cusini M, et al. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? - a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol 2018;32:1845–51. 10.1111/jdv.15146 [DOI] [PubMed] [Google Scholar]
- 4.Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 2013;26:231–40. 10.1097/QCO.0b013e328360db58 [DOI] [PubMed] [Google Scholar]
- 5.Taylor-Robinson D, Lamont RF. Mycoplasmas in pregnancy. BJOG 2011;118:164–74. 10.1111/j.1471-0528.2010.02766.x [DOI] [PubMed] [Google Scholar]
- 6.Lee MY, Kim MH, Lee WI, et al. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Yonsei Med J 2016;57:1271–5. 10.3349/ymj.2016.57.5.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira CNT, Oliveira MTS, Oliveira HBM, et al. Association of spontaneous abortion and Ureaplasma parvum detected in placental tissue. Epidemiol Infect 2020;148:e126. 10.1017/S0950268820001302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luton D, Ville Y, Luton-Sigy A, et al. Prevalence and influence of Mycoplasma hominis and Ureaplasma urealyticum in 218 African pregnant women and their infants. Eur J Obstet Gynecol Reprod Biol 1994;56:95–101. 10.1016/0028-2243(94)90263-1 [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi A, Khodabandehloo M, Ramazanzadeh R, et al. Association between Ureaplasma urealyticum endocervical infection and spontaneous abortion. Iran J Microbiol 2014;6:392–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One 2009;4:e8205. 10.1371/journal.pone.0008205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhadifar F, Khodabandehloo M, Ramazanzadeh R, et al. Survey on association between Mycoplasma hominis endocervical infection and spontaneous abortion using polymerase chain reaction. Int J Reprod Biomed 2016;14:181–6. 10.29252/ijrm.14.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peretz A, Tameri O, Azrad M, et al. Mycoplasma and Ureaplasma carriage in pregnant women: the prevalence of transmission from mother to newborn. BMC Pregnancy Childbirth 2020;20:456. 10.1186/s12884-020-03147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donders GGG, Ruban K, Bellen G, et al. Mycoplasma/Ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017;45:505–15. 10.1515/jpm-2016-0111 [DOI] [PubMed] [Google Scholar]
- 14.van de Wijgert JHHM. The vaginal microbiome and sexually transmitted infections are interlinked: consequences for treatment and prevention. PLoS Med 2017;14:e1002478. 10.1371/journal.pmed.1002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payne MS, Newnham JP, Doherty DA, et al. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the predict 1000 study). Am J Obstet Gynecol 2021;224:206.e1–23. 10.1016/j.ajog.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 16.Pace RM, Chu DM, Prince AL, et al. Complex species and strain ecology of the vaginal microbiome from pregnancy to postpartum and association with preterm birth. Med 2021;2:1027–49. 10.1016/j.medj.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vouga M, Greub G, Prod'hom G, et al. Treatment of genital Mycoplasma in colonized pregnant women in late pregnancy is associated with a lower rate of premature labour and neonatal complications. Clin Microbiol Infect 2014;20:1074–9. 10.1111/1469-0691.12686 [DOI] [PubMed] [Google Scholar]
- 18.Jensen JS. To test or not to test for Mycoplasma hominis and ureaplasmas: that's (not) the question. Clin Infect Dis 2021;73:669–71. 10.1093/cid/ciab065 [DOI] [PubMed] [Google Scholar]
- 19.Taylor-Robinson D, Horner P, Pallecaros A. Diagnosis of some genital-tract infections: Part 2. Molecular tests and the new challenges. Int J STD AIDS 2020;31:198–207. 10.1177/0956462419890526 [DOI] [PubMed] [Google Scholar]
- 20.Kong F, Ma Z, James G, et al. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol 2000;38:1175–9. 10.1128/JCM.38.3.1175-1179.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson JA, Stemke GW, Davis JW, et al. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol 2002;52:587–97. 10.1099/00207713-52-2-587 [DOI] [PubMed] [Google Scholar]
- 22.Vallely LM, Egli-Gany D, Pomat W, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: a systematic review and meta-analysis protocol. BMJ Open 2018;8:e024175. 10.1136/bmjopen-2018-024175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallely LM, Egli-Gany D, Wand H, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: systematic review and meta-analysis. Sex Transm Infect 2021;97:104–11. 10.1136/sextrans-2020-054653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenzer C, Egli-Gany D, Vallely LM, et al. Adverse pregnancy and perinatal outcomes associated with Mycoplasma Genitalium: systematic review and meta-analysis. Sex Transm Infect 2022;98:222–7. 10.1136/sextrans-2021-055352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekkers OM, Vandenbroucke JP, Cevallos M, et al. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 2019;16:e1002742. 10.1371/journal.pmed.1002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute for Health Care Excellence . The social care guidance manual. Great Britain: National Institute for Health and Care Excellence, 2016. [PubMed] [Google Scholar]
- 28.National Institute for Health Care Excellence . Methods for the development of NICE public health guidance. Great Britain: National Institute for Health and Care Excellence, 2012. [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low N. Chlamydia trachomatis and reproductive health: what can we learn from systematic reviews of observational studies? Sex Transm Infect 2020;96:315–7. 10.1136/sextrans-2019-054279 [DOI] [PubMed] [Google Scholar]
- 31.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 32.Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis 1983;10:294–302. [PubMed] [Google Scholar]
- 33.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 1995;333:1737–42. 10.1056/NEJM199512283332604 [DOI] [PubMed] [Google Scholar]
- 34.Berman SM, Harrison HR, Boyce WT, et al. Low birth weight, prematurity, and postpartum endometritis. association with prenatal cervical Mycoplasma hominis and Chlamydia trachomatis infections. JAMA 1987;257:1189–94. [PubMed] [Google Scholar]
- 35.Braun P, Lee YH, Klein JO, et al. Birth weight and genital mycoplasmas in pregnancy. N Engl J Med 1971;284:167–71. 10.1056/NEJM197101282840401 [DOI] [PubMed] [Google Scholar]
- 36.Donders GG, Van Calsteren K, Bellen G, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009;116:1315–24. 10.1111/j.1471-0528.2009.02237.x [DOI] [PubMed] [Google Scholar]
- 37.Gerber S, Vial Y, Hohlfeld P, et al. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis 2003;187:518–21. 10.1086/368205 [DOI] [PubMed] [Google Scholar]
- 38.Govender S, Theron GB, Odendaal HJ, et al. Prevalence of genital mycoplasmas, ureaplasmas and chlamydia in pregnancy. J Obstet Gynaecol 2009;29:698–701. 10.3109/01443610903184033 [DOI] [PubMed] [Google Scholar]
- 39.Harrison HR, Alexander ER, Weinstein L, et al. Cervical Chlamydia trachomatis and mycoplasmal infections in pregnancy. Epidemiology and outcomes. JAMA 1983;250:1721–7. [PubMed] [Google Scholar]
- 40.Kataoka S, Yamada T, Chou K, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 2006;44:51–5. 10.1128/JCM.44.1.51-55.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koucký M, Malíčková K, Cindrová-Davies T, et al. Prolonged progesterone administration is associated with less frequent cervicovaginal colonization by Ureaplasma urealyticum during pregnancy - Results of a pilot study. J Reprod Immunol 2016;116:35–41. 10.1016/j.jri.2016.04.285 [DOI] [PubMed] [Google Scholar]
- 42.McDonald HM, O'Loughlin JA, Jolley PT, et al. Changes in vaginal flora during pregnancy and association with preterm birth. J Infect Dis 1994;170:724–8. 10.1093/infdis/170.3.724 [DOI] [PubMed] [Google Scholar]
- 43.Menard JP, Mazouni C, Salem-Cherif I, et al. High vaginal concentrations of Atopobium vaginae and Gardnerella vaginalis in women undergoing preterm labor. Obstet Gynecol 2010;115:134–40. 10.1097/AOG.0b013e3181c391d7 [DOI] [PubMed] [Google Scholar]
- 44.Minkoff H, Grunebaum AN, Schwarz RH, et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol 1984;150:965–72. 10.1016/0002-9378(84)90392-2 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen DP, Gerber S, Hohlfeld P, et al. Mycoplasma hominis in mid-trimester amniotic fluid: relation to pregnancy outcome. J Perinat Med 2004;32:323–6. 10.1515/JPM.2004.060 [DOI] [PubMed] [Google Scholar]
- 46.Payne MS, Ireland DJ, Watts R, et al. Ureaplasma Parvum genotype, combined vaginal Colonisation with Candida albicans, and spontaneous Preterm birth in an Australian cohort of pregnant women. BMC Pregnancy Childbirth 2016;16:312. 10.1186/s12884-016-1110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rittenschober-Böhm J, Waldhoer T, Schulz SM, et al. First trimester vaginal Ureaplasma biovar colonization and preterm birth: results of a prospective multicenter study. Neonatology 2018;113:1–6. 10.1159/000480065 [DOI] [PubMed] [Google Scholar]
- 48.Usui R, Ohkuchi A, Matsubara S, et al. Vaginal lactobacilli and preterm birth. J Perinat Med 2002;30:458–66. 10.1515/JPM.2002.072 [DOI] [PubMed] [Google Scholar]
- 49.Sperling RS, Newton E, Gibbs RS. Intraamniotic infection in low-birth-weight infants. J Infect Dis 1988;157:113–7. 10.1093/infdis/157.1.113 [DOI] [PubMed] [Google Scholar]
- 50.Kwak D-W, Hwang H-S, Kwon J-Y, et al. Co-Infection with vaginal Ureaplasma urealyticum and Mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2014;27:333–7. 10.3109/14767058.2013.818124 [DOI] [PubMed] [Google Scholar]
- 51.Odendaal HJ, Popov I, Schoeman J, et al. Preterm labour-is Mycoplasma hominis involved? S Afr Med J 2002;92:235–7. [PubMed] [Google Scholar]
- 52.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol 2004;191:1382–6. 10.1016/j.ajog.2004.05.070 [DOI] [PubMed] [Google Scholar]
- 53.Schwab FD, Zettler EK, Moh A. Predictive factors for preterm delivery under rural conditions in post-tsunami Banda Aceh. J Perinat Med 2015;44:511–5. 10.1515/jpm-2015-0004 [DOI] [PubMed] [Google Scholar]
- 54.Embree JE, Krause VW, Embil JA, et al. Placental infection with Mycoplasma homonis and Ureaplasma urealyticum: clinical correlation. Obstet Gynecol 1980;56:475–81. [PubMed] [Google Scholar]
- 55.Freitas AC, Bocking A, Hill JE, et al. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018;6:117. 10.1186/s40168-018-0502-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harada K, Tanaka H, Komori S, et al. Vaginal infection with Ureaplasma urealyticum accounts for preterm delivery via induction of inflammatory responses. Microbiol Immunol 2008;52:297–304. 10.1111/j.1348-0421.2008.00039.x [DOI] [PubMed] [Google Scholar]
- 57.Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988;319:972–8. 10.1056/NEJM198810133191503 [DOI] [PubMed] [Google Scholar]
- 58.Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol 1994;32:176–86. 10.1128/jcm.32.1.176-186.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald HM, O'Loughlin JA, Jolley P, et al. Prenatal microbiological risk factors associated with preterm birth. Br J Obstet Gynaecol 1992;99:190–6. 10.1111/j.1471-0528.1992.tb14497.x [DOI] [PubMed] [Google Scholar]
- 60.Mitsunari M, Yoshida S, Deura I, et al. Cervical Ureaplasma urealyticum colonization might be associated with increased incidence of preterm delivery in pregnant women without prophlogistic microorganisms on routine examination. J Obstet Gynaecol Res 2005;31:16–21. 10.1111/j.1447-0756.2005.00246.x [DOI] [PubMed] [Google Scholar]
- 61.Munday PE, Porter R, Falder PF, et al. Spontaneous abortion--an infectious aetiology? Br J Obstet Gynaecol 1984;91:1177–80. 10.1111/j.1471-0528.1984.tb04733.x [DOI] [PubMed] [Google Scholar]
- 62.Payne MS, Feng Z, Li S, et al. Second trimester amniotic fluid cytokine concentrations, Ureaplasma sp. colonisation status and sexual activity as predictors of preterm birth in Chinese and Australian women. BMC Pregnancy Childbirth 2014;14:340. 10.1186/1471-2393-14-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toth KS, Letchworth AT, Noble AD, et al. The significance of infection in the aetiology of preterm labour. A prospective controlled study. J Obstet Gynaecol 1992;12:94–9. 10.3109/01443619209013603 [DOI] [Google Scholar]
- 64.Yoon BH, Oh SY, Romero R, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001;185:1162–7. 10.1067/mob.2001.117678 [DOI] [PubMed] [Google Scholar]
- 65.Daskalakis G, Thomakos N, Papapanagiotou A, et al. Amniotic fluid Interleukin-18 at mid-trimester genetic Amniocentesis: relationship to Intraamniotic microbial invasion and Preterm delivery. BJOG 2009;116:1743–8. 10.1111/j.1471-0528.2009.02364.x [DOI] [PubMed] [Google Scholar]
- 66.González Bosquet E, Gené A, Ferrer I, et al. Value of endocervical Ureaplasma species colonization as a marker of preterm delivery. Gynecol Obstet Invest 2006;61:119–23. 10.1159/000089457 [DOI] [PubMed] [Google Scholar]
- 67.Chua KB, Ngeow YF, Lim CT, et al. Colonization and transmission of Ureaplasma urealyticum and Mycoplasma hominis from mothers to full and preterm babies by normal vaginal delivery. Med J Malaysia 1999;54:242–6. [PubMed] [Google Scholar]
- 68.Kacerovský M, Pavlovský M, Tosner J. Preterm premature rupture of the membranes and genital mycoplasmas. Acta Medica 2009;52:117–20. 10.14712/18059694.2016.115 [DOI] [PubMed] [Google Scholar]
- 69.Kumar S, Suri V, Sharma M. Bacterial vaginosis in preterm labor. Int J Gynaecol Obstet 2006;95:40–1. 10.1016/j.ijgo.2006.05.022 [DOI] [PubMed] [Google Scholar]
- 70.Montenegro DA, Borda LF, Neuta Y, et al. Oral and uro-vaginal intra-amniotic infection in women with preterm delivery: a case-control study. J Investig Clin Dent 2019;10:e12396. 10.1111/jicd.12396 [DOI] [PubMed] [Google Scholar]
- 71.Grattard F, Soleihac B, De Barbeyrac B, et al. Epidemiologic and molecular investigations of genital mycoplasmas from women and neonates at delivery. Pediatr Infect Dis J 1995;14:853–8. 10.1097/00006454-199510000-00007 [DOI] [PubMed] [Google Scholar]
- 72.Kundsin RB, Driscoll SG, Monson RR, et al. Association of Ureaplasma urealyticum in the placenta with perinatal morbidity and mortality. N Engl J Med 1984;310:941–5. 10.1056/NEJM198404123101502 [DOI] [PubMed] [Google Scholar]
- 73.McCormack WM, Rosner B, Lee YH, et al. Isolation of genital mycoplasmas from blood obtained shortly after vaginal delivery. Lancet 1975;1:596–9. 10.1016/S0140-6736(75)91881-4 [DOI] [PubMed] [Google Scholar]
- 74.Sweeney EL, Kallapur SG, Gisslen T, et al. Placental infection with Ureaplasma species is associated with histologic chorioamnionitis and adverse outcomes in moderately preterm and late-preterm infants. J Infect Dis 2016;213:1340–7. 10.1093/infdis/jiv587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nasution TA, Cheong SF, Lim CT, et al. Multiplex PCR for the detection of urogenital pathogens in mothers and newborns. Malays J Pathol 2007;29:19–24. [PubMed] [Google Scholar]
- 76.Ma C, Du J, Dou Y, et al. The associations of genital mycoplasmas with female infertility and adverse pregnancy outcomes: a systematic review and meta-analysis. Reprod Sci 2021;28:3013–31. 10.1007/s43032-020-00399-w [DOI] [PubMed] [Google Scholar]
- 77.D'Inzeo T, De Angelis G, Fiori B, et al. Comparison of Mycoplasma IES, Mycofast revolution and Mycoplasma IST2 to detect genital mycoplasmas in clinical samples. J Infect Dev Ctries 2017;11:98–101. 10.3855/jidc.8039 [DOI] [PubMed] [Google Scholar]
- 78.Kusanovic JP, Vargas P, Ferrer F, et al. Comparison of two identification and susceptibility test kits for Ureaplasma spp and Mycoplasma hominis in amniotic fluid of patients at high risk for intra-amniotic infection. J Matern Fetal Neonatal Med 2020;33:3409–17. 10.1080/14767058.2019.1572742 [DOI] [PubMed] [Google Scholar]
- 79.Taylor-Robinson D. Infections due to species of Mycoplasma and Ureaplasma: an update. Clin Infect Dis 1996;23:671–84. 10.1093/clinids/23.4.671 [DOI] [PubMed] [Google Scholar]
- 80.Pavlidis I, Spiller OB, Sammut Demarco G, et al. Cervical epithelial damage promotes Ureaplasma Parvum ascending infection, Intrauterine inflammation and Preterm birth induction in mice. Nat Commun 2020;11:199. 10.1038/s41467-019-14089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasper DC, Mechtler TP, Reischer GH, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis 2010;67:117–21. 10.1016/j.diagmicrobio.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 82.Witt A, Berger A, Gruber CJ, et al. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am J Obstet Gynecol 2005;193:1663–9. 10.1016/j.ajog.2005.03.067 [DOI] [PubMed] [Google Scholar]
- 83.Li YH, Brauner A, Jonsson B, et al. Ureaplasma urealyticum-induced production of proinflammatory cytokines by macrophages. Pediatr Res 2000;48:114–9. 10.1203/00006450-200007000-00020 [DOI] [PubMed] [Google Scholar]
- 84.Doyle RM, Alber DG, Jones HE, et al. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta 2014;35:1099–101. 10.1016/j.placenta.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 85.Combaz-Söhnchen N, Kuhn A. A systematic review of Mycoplasma and Ureaplasma in Urogynaecology. Geburtshilfe Frauenheilkd 2017;77:1299–303. 10.1055/s-0043-119687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redelinghuys MJ, Ehlers MM, Dreyer AW, et al. Antimicrobial susceptibility patterns of Ureaplasma species and Mycoplasma hominis in pregnant women. BMC Infect Dis 2014;14:171. 10.1186/1471-2334-14-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bae I, Koh E, Kim S. Prevalence rate and antimicrobial susceptibilities of Ureaplasma urealyticum and Mycoplasma hominis in pregnant women residing in Jinju. Korean J Clin Microbiol 2010;2:S485. 10.5145/KJCM.2009.12.4.159 [DOI] [Google Scholar]
- 88.Bayraktar MR, Ozerol IH, Gucluer N, et al. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis 2010;14:e90–5. 10.1016/j.ijid.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 89.Vallely AJ, Pomat WS, Homer C, et al. Point-Of-Care testing and treatment of sexually transmitted infections to improve birth outcomes in high-burden, low-income settings: study protocol for a cluster randomized crossover trial (the WANTAIM trial, Papua New Guinea). Wellcome Open Res 2019;4:53. 10.12688/wellcomeopenres.15173.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grant JS, Chico RM, Lee AC, et al. Sexually transmitted infections in pregnancy: a narrative review of the global research gaps, challenges, and opportunities. Sex Transm Dis 2020;47:779–89. 10.1097/OLQ.0000000000001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062990supp001.pdf (1.6MB, pdf)
Data Availability Statement
No data are available. Data relevant to the study are included in the article or the supplementary information.