Abstract
Geobacter sulfurreducens contains a 9.6-kDa c-type cytochrome that was previously proposed to serve as an extracellular electron shuttle to insoluble Fe(III) oxides. However, when the cytochrome was added to washed-cell suspensions of G. sulfurreducens it did not enhance Fe(III) oxide reduction, whereas similar concentrations of the known electron shuttle, anthraquinone-2,6-disulfonate, greatly stimulated Fe(III) oxide reduction. Furthermore, analysis of the extracellular c-type cytochromes in cultures of G. sulfurreducens demonstrated that the dominant c-type cytochrome was not the 9.6-kDa cytochrome, but rather a 41-kDa cytochrome. These results and other considerations suggest that the 9.6-kDa cytochrome is not an important extracellular electron shuttle to Fe(III) oxides.
The mechanisms for Fe(III) oxide reduction by dissimilatory Fe(III)-reducing microorganisms are of interest because of the environmental and potential evolutionary significance of this type of respiration. Microbial reduction of Fe(III) to Fe(II) in aquatic sediments and aquifers impacts on the inorganic geochemistry of these systems and may also play an important role in the oxidation of natural and organic contaminants (9, 10, 12). Furthermore, microbiological and geological evidence suggests that dissimilatory Fe(III) reduction may have been one of the earliest forms of microbial respiration (25).
It has generally been considered that Fe(III)-reducing microorganisms must establish direct contact with insoluble Fe(III) oxides in order to reduce them (9, 10, 12). In soils and sediments, the need for direct contact can be alleviated by the presence of Fe(III) chelators or humic substances (humics) and other extracellular quinones. Solubilization of Fe(III) by Fe(III) chelators can greatly accelerate reduction of Fe(III) in aquifers (16, 17). Soluble humics and other extracellular quinones can accelerate Fe(III) oxide reduction because Fe(III) reducers can transfer electrons to the quinone moieties in humics and other compounds, and then the hydroquionones can abiotically transfer electrons to Fe(III) oxides with the regeneration of the quinone (11, 13, 22). Through such electron shuttling, low concentrations of extracellular quinones can greatly stimulate Fe(III) oxide reduction in soils (20).
Recently, it has been proposed that the dissimilatory Fe(III)-reducing microorganism, Geobacter sulfurreducens, releases a 9.6-kDa c-type cytochrome into the external environment which serves as an electron shuttle to promote reduction of insoluble Fe(III) oxides (23). If true, this would have an important impact on the understanding of Fe(III) reduction in sedimentary environments because both culturing and culture-independent studies of 16S ribosomal DNA sequences have indicated that Geobacter species are numerically significant organisms in the Fe(III) reduction zones of aquatic sediments and the subsurface (1, 3, 4, 21, 26).
Previous studies have demonstrated that Geobacter species contain c-type cytochromes that have been implicated in electron transport to Fe(III) (2, 6, 14, 18, 19). These include a small soluble c-type cytochrome in Geobacter metallireducens (2a). In those previous studies, it was considered that the c-type cytochromes were involved in Fe(III) reduction at or near the cell surface. Thus, the suggestion that G. sulfurreducens might release an extracellular c-type cytochrome to promote Fe(III) reduction is novel.
However, the hypothesis that the 9.6-kDa c-type cytochrome in G. sulfurreducens can serve as an extracellular electron shuttle to Fe(III) oxide was based on indirect evidence. For example, it was shown that the 9.6-kDa cytochrome that had been purified from cells (not from the external medium) could be oxidized with Fe(III) oxide, but no studies on the potential for the cytochrome to act as an external electron shuttle between G. sulfurreducens and Fe(III) oxides were conducted. Furthermore, although it was shown that culture supernatants had absorbance spectra consistent with the presence of c-type cytochromes, no data that directly demonstrated that this absorbance spectra were due to the presence of the 9.6-kDa cytochrome in the supernatant were presented. Since Geobacter species produce multiple c-type cytochromes (6, 14, 18), any of which could potentially account for the absorbance spectra observed in the supernatants, such data are crucial to support the electron-shuttling hypothesis.
Here we report that, in fact, the 9.6-kDa cytochrome is not an effective electron shuttle between G. sulfurreducens and Fe(III) oxide. Furthermore, under optimum culturing conditions for this organism, there was little, if any, release of the 9.6-kDa cytochrome into the extracellular medium. These results strongly suggest that G. sulfurreducens does not use the 9.6-kDa cytochrome as an electron shuttle for Fe(III) oxide reduction.
Fe(III) oxide reduction is not stimulated by the 9.6-kDa cytochrome.
G. sulfurreducens (ATCC 51573), as used in the previous study of Seeliger and coworkers (23), was obtained from our laboratory culture collection. In order to purify the 9.6-kDa c-type cytochrome, cells were grown in 10-liter glass carboys with acetate (20 mM) as the electron donor and fumarate (40 mM) as the electron acceptor, as previously described (18). Cells were harvested in late exponential phase by centrifugation and then resuspended for 45 min at 37°C in 50 mM HEPES buffer (pH 7.0) containing lysozyme (0.1 mg ml−1), DNase (40 U ml−1), and glycerol (10% [vol/vol]). Cells were broken in a French press at 12,500 lb/in2 (two passages). Cell debris was removed by centrifugation (5,000 × g; 20 min) and the supernatant was centrifuged (100,000 × g; 1 h). The 9.6-kDa protein was purified from this supernatant by fast protein liquid chromatography with gel filtration (Sephacryl) and cation exchange (SP-Sepharose) columns. The 9.6-kDa c-type cytochrome was about 90% pure (as determined by gel electrophoresis in combination with image analysis) after sequential passage of the soluble fraction over these two columns and was active against Fe(III) (23); spectrophotometric analysis confirmed that it was reduced rapidly by dithionite and oxidized by Fe(III) citrate. The protein was also localized to a periplasmic fraction prepared by osmotic shock, further identifying it as the periplasmic protein described by Seeliger and coworkers (23).
In order to determine whether the 9.6-kDa cytochrome could serve as an electron shuttle between G. sulfurreducens and Fe(III) oxide, washed-cell suspensions of G. sulfurreducens were prepared and resuspended in anaerobic bicarbonate buffer (30 mM; pH 6.7) containing acetate (10 mM) as an electron donor and amorphous Fe(III) oxide (10 mM) as the electron acceptor, as previously described for studies with the closely related G. metallireducens (11). Fe(II) was measured with a Ferrozine-based colorimetric assay (15) and results presented are the means of three incubations for each treatment. The cell protein concentration was 0.12 mg of protein ml−1.
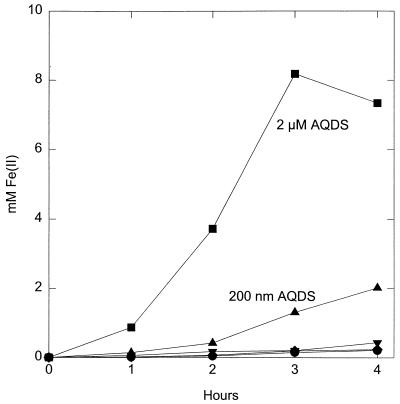
As has previously been reported for G. metallireducens (11, 13, 16), washed-cell suspensions of G. sulfurreducens only slowly reduced Fe(III) oxide (Fig. 1). This is consistent with the relatively slow growth of these organisms with insoluble Fe(III) oxide as the electron acceptor. The addition of a 200 nM final concentration of the 9.6-kDa cytochrome did not stimulate Fe(III) reduction (Fig. 1). This cytochrome concentration is ca. twofold higher than the highest concentration that Seeliger and coworkers estimated was released in cultures of G. sulfurreducens (23). In contrast, a 200 nM concentration of the humic analog anthraquinone-2,6-disulfonate (AQDS) stimulated Fe(III) reduction 10-fold. AQDS has previously been shown to serve as an external electron shuttle to promote Fe(III) oxide reduction by all Fe(III)-reducing microorganisms that have been evaluated (11, 13). Even increasing the concentration of the cytochrome to 2 μM (20-fold higher than that expected in culture supernatants) had no significant effect on Fe(III) reduction, whereas the same concentration of AQDS stimulated the rate of Fe(III) reduction 60-fold (Fig. 1). These results demonstrate that the 9.6-kDa cytochrome of G. sulfurreducens is not an effective electron shuttle between G. sulfurreducens and Fe(III) oxides.
FIG. 1.
Fe(III) oxide reduction by cell suspensions of G. sulfurreducens in the presence of 200 nM (▴) and 2 μM (■) AQDS and 200 nM (⧫) and 2 μM (▾) 9.6-kDa cytochrome. Control cultures (●) contained no added AQDS or cytochrome.
The 9.6-kDa cytochrome is not the dominant extracellular cytochrome.
As previously reported (23), supernatants of G. sulfurreducens cultures had a UV-visible spectrum characteristic of c-type cytochromes with absorbance maxima at 552, 522, and 417 nm when reduced with dithionite and an absorbance maximum at 407 nM in the oxidized state. Similar results were noted in this study. Redox difference absorption spectra of dithionite-reduced minus air-oxidized preparations of supernatant collected in late exponential phase corresponded to those of a 10 nM purified 9.6-kDa cytochrome. Several days into stationary phase, absorbance values were comparable to those obtained from a 50 nM concentration of the purified cytochrome.
However, when supernatants were concentrated 50-fold with centrifugal filters (Ultrafree 15; Millipore, Bedford, Mass.) and 20-μl aliquots were analyzed with sodium dodecyl sulfate (SDS) gel electrophoresis (with a 15% polyacrylamide gel buffered with tricine [24]), the 9.6-kDa c-type cytochrome could not be detected with either Coomassie blue stain or a more sensitive silver stain, even though the silver stain could detect less than a 1 nM concentration of the purified 9.6-kDa cytochrome treated in a similar manner. In studies in which a 100 nM concentration of the 9.6-kDa cytochrome (the concentration expected in supernatants according to the results outlined in reference 23) was added to growth medium or culture supernatant, the cytochrome was quantitatively recovered in solution after concentration in the centrifugal filters. This demonstrated that the failure to detect the 9.6-kDa cytochrome was not due to losses during the concentration procedure.
When the SDS gels of supernatant proteins were treated with the heme-staining method of Goodhew et al. (7), a single protein with an apparent molecular mass of 41 kDa was detected (Fig. 2). Further analysis of this protein has demonstrated that it is a c-type, membrane-bound cytochrome (8). None of the 9.6-kDa cytochrome was detected even though purified 9.6-kDa cytochrome added at a concentration equivalent to 10 nM in the culture supernatant stained for heme (Fig. 2). This result demonstrates that it is the 41-kDa cytochrome of G. sulfurreducens rather than the 9.6-kDa cytochrome that accounts for the c-type cytochrome spectrum that was observed in culture supernatants of G. sulfurreducens.
FIG. 2.
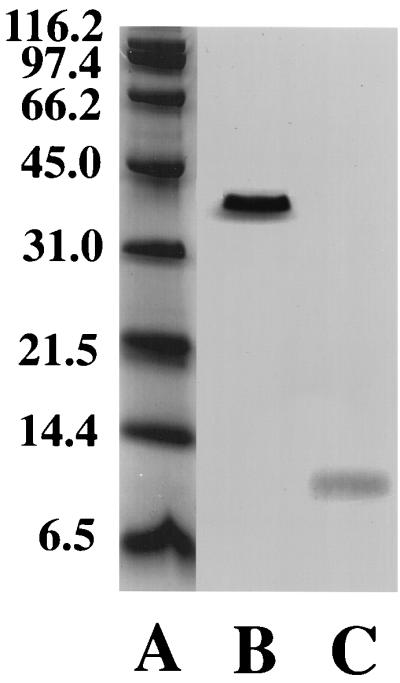
SDS-polyacrylamide gel electrophoresis analysis of 50× concentrated supernatant from late-exponential cultures of G. sulfurreducens (lane B) and purified 9.6-kDa c-type cytochrome (500 nM protein) (lane C). Lane A contains marker proteins of molecular mass of 6.5 to 116.4 kDa.
When studies were conducted to determine whether the 41-kDa cytochrome might act as an electron shuttle, it was found that this cytochrome, which is highly hydrophobic, rapidly adsorbed to Fe(III) oxide. These results indicate that the 41-kDa cytochrome is unable to serve as a soluble electron shuttle for Fe(III) oxide reduction.
Conclusions.
These results demonstrate that the previously proposed model (23), in which G. sulfurreducens releases the 9.6-kDa cytochrome into the external environment in order to serve as an electron shuttle to promote Fe(III) oxide reduction, is highly unlikely. Closer inspection has revealed that the 9.6-kDa cytochrome is, in fact, not released into the medium. Furthermore, even if the 9.6-kDa cytochrome were released, it would not be an effective electron shuttle between G. sulfurreducens and Fe(III) oxide. Independent studies have also demonstrated that the 9.6-kDa cytochrome is not an effective electron shuttle between G. sulfurreducens and other organisms (5). Thus, it is also unlikely that the 9.6-kDa cytochrome is involved in interspecies electron transfer as was also previously proposed (23). However, as previously suggested (2, 14, 18), it is likely that the c-type cytochromes of Geobacter species are involved in some aspect of electron transport to Fe(III) at or near the cell surface. The role of these cytochromes in Fe(III) reduction is currently under investigation.
Acknowledgments
We acknowledge the technical assistance of R. Allen and useful discussions with T. Magnuson.
This work was funded by the National Science Foundation (grant no. MCB-972 7840) and the Department of Energy NABIR program (grant no. DE-FG02-97ER62475).
REFERENCES
- 1.Anderson R T, Rooney-Varga J, Gaw C V, Lovley D R. Anaerobic benzene oxidation in the Fe(III)-reduction zone of petroleum-contaminated aquifers. Environ Sci Technol. 1998;32:1222–1229. [Google Scholar]
- 2.Caccavo F, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Champire J E. Physiology of the dissimilatory iron-reducing isolate GS-15; proposed name Geobacter metallireducens gen. nov., sp. nov. Ph.D. thesis. Amherst: University of Massachusetts; 1993. [Google Scholar]
- 3.Coates J D, Ellis D J, Blunt-Harris E L, Gaw C V, Roden E E, Lovley D R. Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates J D, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cord-Ruwisch, R. Personal communication.
- 6.Gaspard S, Vazquez F, Holliger C. Localization and solubilization of the iron(III) reductase of Geobacter sulfurreducens. Appl Environ Microbiol. 1998;64:3188–3194. doi: 10.1128/aem.64.9.3188-3194.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodhew C F, Brown K R, Pettigrew G W. Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta. 1986;852:288–294. [Google Scholar]
- 8.Lloyd, J. R. Unpublished data.
- 9.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley D R. Microbial reduction of iron, manganese, and other metals. Adv Agron. 1995;54:175–231. [Google Scholar]
- 11.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 12.Lovley D R, Coates J D, Saffarini D A, Lonergan D J. Dissimilatory iron reduction. In: Winkelman G, Carrano C J, editors. Iron and related transition metals in microbial metabolism. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 187–215. [Google Scholar]
- 13.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 14.Lovley D R, Giovannoni S J, White D C, Champine J E, Phillips E J P, Gorby Y A, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 15.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovley D R, Woodward J C. Mechanisms for chelator stimulation of microbial Fe(III)-oxide reduction. Chem Geol. 1996;132:19–24. [Google Scholar]
- 17.Lovley D R, Woodward J C, Chapelle F H. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature. 1994;370:128–131. doi: 10.1038/370128a0. [DOI] [PubMed] [Google Scholar]
- 18.Magnuson, T. S., A. L. Hodges-Myerson, and D. R. Lovley. Purification of the membrane-bound Fe(III) reductase complex from the dissimilatory Fe(III)-reducing bacterium Geobacter sulfurreducens. Submitted for publication. [DOI] [PubMed]
- 19.Naik R R, Murillo F M, Stolz J F. Evidence for a novel nitrate reductase in the dissimilatory iron-reducing bacterium Geobacter metallireducens. FEMS Microbiol Lett. 1993;106:53–58. [Google Scholar]
- 20.Nevin, K. P., and D. R. Lovley. Potential for nonenzymatic reduction of Fe(III) during microbial oxidation of organic matter coupled to Fe(III) reduction. Submitted for publication.
- 21.Rooney-Varga J N, Anderson R T, Fraga J L, Ringelberg D, Lovley D R. Microbial communities associated with anaerobic benzene mineralization in a petroleum-contaminated aquifer. Appl Environ Microbiol. 1999;65:3056–3063. doi: 10.1128/aem.65.7.3056-3063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott D T, McKnight D M, Blunt-Harris E L, Kolesar S E, Lovley D R. Quinone moieties act as electron acceptors in the reduction of humic substances by humic-reducing microorganisms. Environ Sci Technol. 1998;32:2984–2989. [Google Scholar]
- 23.Seeliger S, Cord-Ruwisch R, Schink B. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier to other acceptors or to partner bacteria. J Bacteriol. 1998;180:3686–3691. doi: 10.1128/jb.180.14.3686-3691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 25.Vargas M, Kashefi K, Blunt-Harris E L, Lovley D R. Microbiological evidence for Fe(III) reduction on early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 26.West, O., and D. R. Lovley. Growth of Geobacter species in response to stimulation of Fe(III) reduction in a sandy aquifer sediment. Submitted for publication. [DOI] [PubMed]