Abstract
Background and aims:
The crosstalk between cancer stem cells (CSCs) and their niche is required for the maintenance of stem cell-like phenotypes of CSCs. Here, we identified ETS homologous factor (EHF) as a key molecule in decreasing the sensitivity of pancreatic cancer (PC) cells to CSCs’ niche stimulus. We also explored a therapeutic strategy to restore the expression of EHF.
Design:
We used a KPC mouse model and samples from patients with PC. Immunostaining, flowcytometry, sphere formation assays, anchorage-independent growth assay, in vivo tumorigenicity, RT-PCR, chromatin immunoprecipitation (ChIP) and luciferase analyses were conducted in this study.
Results:
CXCL12 derived from pancreatic stellate cells (PSCs) mediates the crosstalk between PC cells and PSCs to promote PC stemness. Tumoural EHF suppressed CSC stemness by decreasing the sensitivity of PC to CXCL12 stimulus and inhibiting the crosstalk between PC and CSC-supportive niches. Mechanically, EHF suppressed the transcription of the CXCL12 receptor CXCR4. EHF had a cell autonomous role in suppressing cancer stemness by inhibiting the transcription of Sox9, Sox2, Oct4 and Nanog. Rosiglitazone suppressed PC stemness and inhibited the crosstalk between PC and PSCs by upregulating EHF. Preclinical KPC mouse cohorts demonstrated that rosiglitazone sensitised PDAC to gemcitabine therapy.
Conclusions:
EHF decreased the sensitivity of PC to the stimulus from PSC-derived CSC-supportive niche by negatively regulating tumoural CXCR4. Rosiglitazone could be used to target PC stem cells and the crosstalk between CSCs and their niche by upregulating EHF.
Keywords: pancreatic cancer, stem cells
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal tumour with aggressive clinical courses, poor prognosis and limited treatment options. Chemotherapy resistance and tumour relapse are still two unresolved problems in PDAC treatment.1 2 Cancer stem cells (CSCs) contribute to PDAC recurrence and metastasis and cause resistance to chemotherapy.3–6
CSCs are regulated by the aberrant activation of cell-intrinsic signal pathways, including NOTCH, WNT and STAT3 pathways, and the overexpression of OCT4, SOX2, NANOG, KLF4, c-MYC amongst others.3 7 The recent insights into the complex nature of cancer stemness reveal that CSC phenotype is also regulated by cell-extrinsic factors derived from stromal cells.8–11 The major cell types of PDAC stroma are pancreatic stellate cells (PSCs).9 12 13 PSCs can secrete pro-stemness cytokines, such as IL-6, IL-8, TGFβ1 and CXCL12, which form the CSC niche and participate in the active crosstalk with cancer cells within the tumour microenvironment.14–16 The majority anti-CSC therapeutic strategies focus on targeting cell-intrinsic stemness-associated genes. However, most of these genes are shared between CSCs and normal stem cells. Therefore, the side effect of anti-CSC therapy remains a major concern that restricts its clinical application.17 Targeting the crosstalk between pancreatic cancer and its stemness-supporting niche may provide new therapeutic strategies for the prevention of pancreatic cancer progression.
Epithelium-specific ETS factor family member 3 or ESE3/ ETS homologous factor (EHF) is a member of the E26 transformation-specific (ETS) gene superfamily.18 Our previous work demonstrated EHF as a tumour suppressor in PDAC. In PDAC, EHF promotes E-cadherin expression and suppresses epithelial–mesenchymal transition.19 Furthermore, EHF deficiency induces the conversion and expansion of Treg cells and MDSCs by inhibiting TGFβ1 and GM-CSF secretion.20 In prostate cancer, EHF plays a vital role in the inhibition of cell-intrinsic CSCs signal by suppressing STAT3 and repressing the expression of TWIST1, ZEB2, BMI1 and POU5F1.21–23 However, the role of EHF in pancreatic CSC regulation is not fully understood. Although the critical function of EHF has been verified in different tumour types,19–25 no clinical and translational research involving EHF as a therapeutic target has been conducted.
In this study, we demonstrated that EHF could play a cell autonomous function and inhibit PDAC stemness by disrupting the crosstalk between CSCs and their PSC niche. Tumoural EHF decreased the sensitivity of PDAC to PSC-derived CXCL12 by repressing the CXCR4 expression. Moreover, we identified peroxisome proliferator–activated receptor γ (PPARγ) ligand rosiglitazone as a promising suppressor of PDAC stemness by upregulating EHF.
Results
Tumoural EHF is negatively correlated with stemness profiles in PDAC tissue
An immunohistochemical multiplex assay was conducted in archived tissues from a retrospective cohort of 93 patients with PDAC to examine the correlation between the expression of tumoural EHF and the proportion of PCSCs. The frequencies of CD133+ and ALDH1+ cells in the high-EHF group were significantly decreased compared with those in the low-EHF group (both P < 0.0001; Fig.1A–B and Fig.S1A). Furthermore, fresh PDAC tissues from a prospective cohort of 39 patients were collected and analysed (Fig.1C). As shown in Fig.1D–F and Fig.S1B, tumoural EHF IHC score was inversely correlated with the proportion of tumoural CD133+, ALDH+ and ESA+CD44+CD24+ cells in the prospective cohort. These results confirmed the findings from the archived PDAC tissues. Therefore, our results suggested that tumoural EHF negatively correlated with stemness profiles in PDAC tissues. Besides, the clinical significance of EHF expression and PCSCs is shown in Fig.1G–H, Fig.S1C–F and Tables S1–2.
Figure1. Tumoral EHF is negatively correlated with stemness profiles in PDAC tissue.
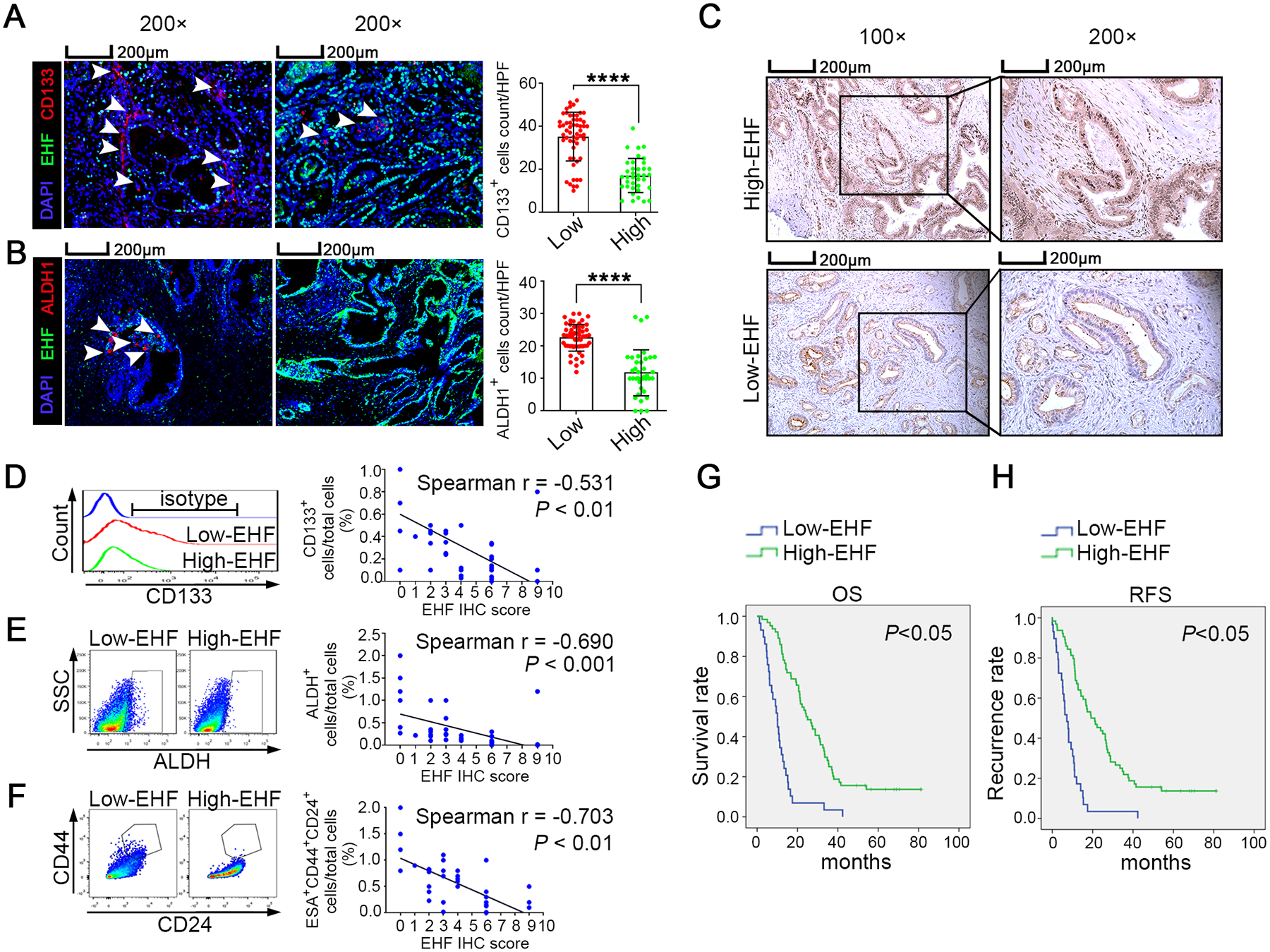
(A-B) The multiplex fluorescent IHC staining (left) of EHF expression and the accumulation of CD133+ cells (A) and ALDH1+ cells (B) in tumor tissues. The representative images from 93 pancreatic cancer cases were shown. The arrows indicated CD133+ cells and ALDH1+ cells. Bars, 200μM. Nonpaired Student’s t-test was used as statistical analysis; n=93, P<0.0001. (C-F) Single cell suspensions were prepared from 39 cases of fresh PDAC tissues and stained with ALDEFLUOR or specific antibodies against three CSC subsets (ALDH+ cells, CD133+ cells and CD44+ CD24+ cells). Representative IHC staining of EHF was shown(C). Bars, 200μm. Representative histogram and dot plots of CD133+ cells (D, left), ALDH+ cells (E, left) and CD44+CD24+ cells (gated on ESA+ epithelial cells; F, left). Spearman correlation analysis between EHF IHC score and the proportions of CD133+ cells (D, right), ALDH+ cells (E, right) and ESA+CD44+CD24+ cells (F, right); n=39. (G-H) Kaplan-Meier OS (G) and RFS (H) for different levels of EHF based on the log-rank statistic test (P<0.05). Patients were divided into EHF-low and EHF-high groups based on the multiplex fluorescent IHC results.
Tumoural EHF negatively regulates pancreatic cancer stemness
PDAC-EHF/shEHF cell lines were established (Fig.S2A) to determine whether tumoural EHF regulated PDAC stemness. The percentage of CD44+CD24+ cells in PDAC-EHF significantly decreased compared with that in the PDAC-vector control group (Fig.2A). By contrast, the percentage of CD44+CD24+ cells in PDAC-shEHF significantly increased compared with that of PDAC-scramble (Fig.2B). Similarly, EHF negatively regulated the ALDH activity (Fig. 2C–D) and the proportions of CD133+ cells (Fig.2E–F). In vitro sphere formation assay demonstrated that EHF negatively regulated the cellular sphere formation capacity of PDAC (Fig.2G–H). In in vivo limited dilution assay, the ectopic expression of EHF significantly reduced, whereas the knockdown of EHF increased the tumour incidence (Fig.2I). This result suggested that EHF suppressed CSC stemness in PDAC. Q-PCR and Western blot demonstrated that EHF negatively regulated stemness-related genes (Sox9, Sox2, Oct4 and Nanog) (Fig.2J–K) while increasing the expression of differentiation markers (Fig.S2B). These findings were further confirmed in the other PC cell lines and two PDX cell lines (Figs.S3–4). Ch-IP analyses revealed that EHF directly bound to the promoter region of Sox9, Sox2, Oct4 and Nanog (Fig.2L).
Figure2. Tumoral EHF negatively regulates pancreatic cancer stemness.
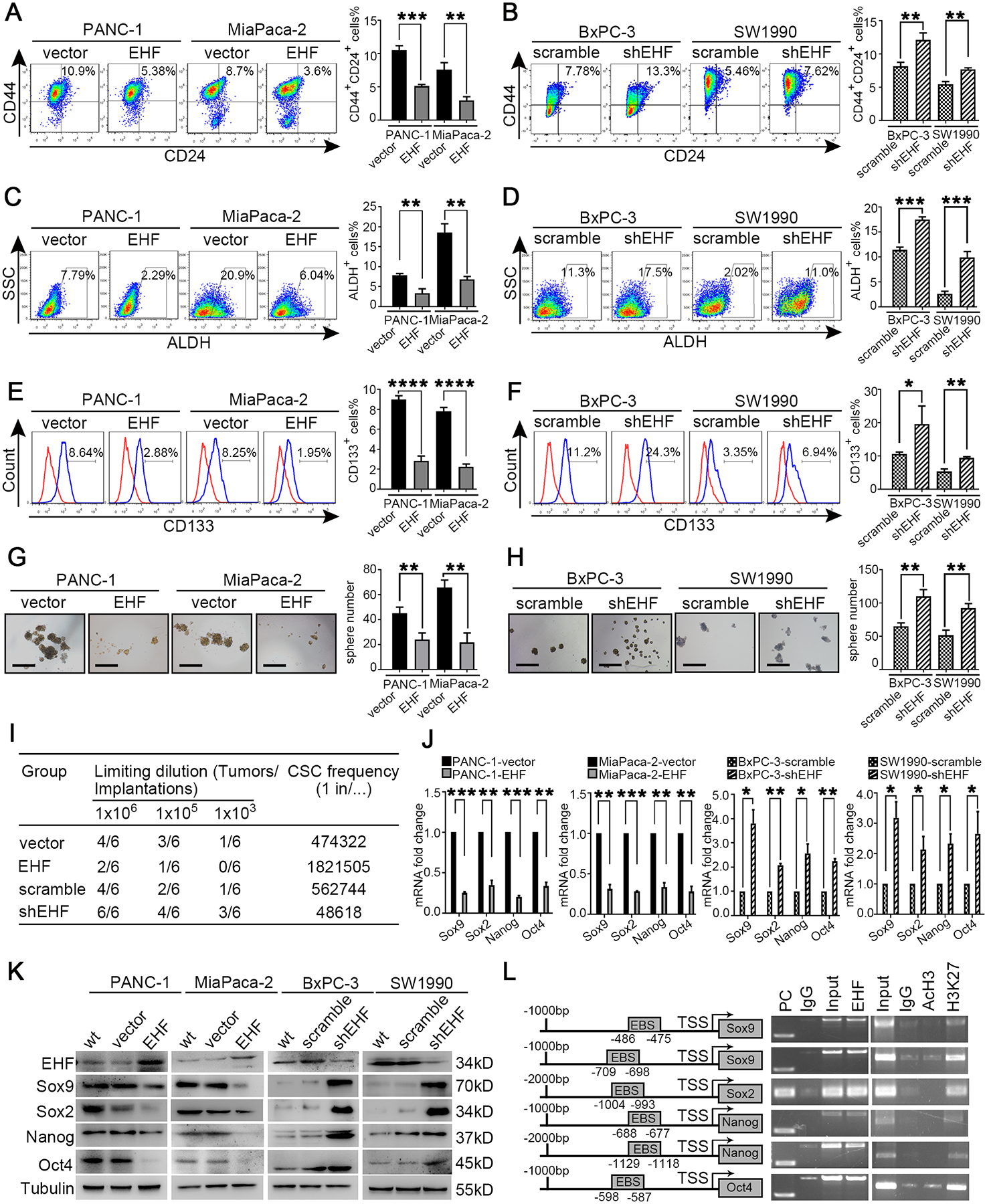
(A-B) The proportion of CD44+CD24+ cells in PANC-1-vector/EHF, MiaPaca-2-vector/EHF, BxPC-3-scramble/shEHF and SW1990-scramble/shEHF cells were analyzed using flow cytometry. Representative dot plots (A, left; B, left) and percentage of CD44+CD24+ cells (A, right; B, right) were shown. (C-D) The proportion of ALDH+ cells in indicated cells were analyzed using flow cytometry. Representative dot plots (C, left; D, left) and percentage of ALDH+ cells (C, right; D, right) were shown. (E-F) The proportion of CD133+ cells in indicated cells were analyzed using flow cytometry. Representative histograms (E, left; F, left) and percentage of CD133+ cells (E, right; F, right) were shown. (G-H) Sphere formation assays were performed in indicated cell lines. Representative images (G, left; H, left) and sphere number analysis (G, right; H, right) were shown. Bars:100μm. (I) In vivo limited dilution assays were performed to determine the effects of EHF overexpression or EHF depletion on CSC self-renewal of PANC-1 cells. Representative tumor incidence and CSC probabilities were shown. (J) Q-PCR on EHF and the stemness markers of Sox9, Sox2, Nanog and Oct4 were performed in indicated cells. Actin was used as internal control. (K) Western blot on EHF, Sox9, Sox2, Nanog and Oct4 were analyzed in indicated cell lines. β-tubulin was used as loading control. Representative results were shown. (L) Chromatin immunoprecipitation assay was performed to validate transcriptional regulation on Sox9, Sox2, Nanog and Oct4 by EHF. Predicted ETS binding sites (EBSs) in the promoters of human Sox9, Sox2, Nanog and Oct4(L, left).TSS, transcription start site. Binding of EHF to the promoters of the indicated genes in PANC-1 cells determined by chromatin immunoprecipitation (L, medium). IgG was used as negative control and anti-RNA polymeraseⅡwas used as positive control. PC, positive control. AcH3 and H3K27me3 occupancy on the identified EBSs in the promoters of the indicated genes in PANC-1 cells determined by chromatin immunoprecipitation (L, right). Representative results were shown. All experiments were repeated three times independently. Paired Student’s t-test was used as statistical analysis. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
Tumoural EHF suppresses the crosstalk between PDAC and PSCs
PSCs can support PDAC stemness through paracrine mechanisms.16 26 27 In vivo limited dilution assay was conducted to evaluate the role of tumoural EHF on the crosstalk between PDACs and PSCs. As shown in Fig.3A–B, the peritumoural injection of PSC-CM notably increased the tumour incidence in the PANC-1-vector group compared with that in the mice that received the control medium. However, the effect of PSC-CM was robustly suppressed in the PANC-1-EHF group.
Figure 3. Tumoral EHF suppresses the crosstalk between PDAC and PSCs.
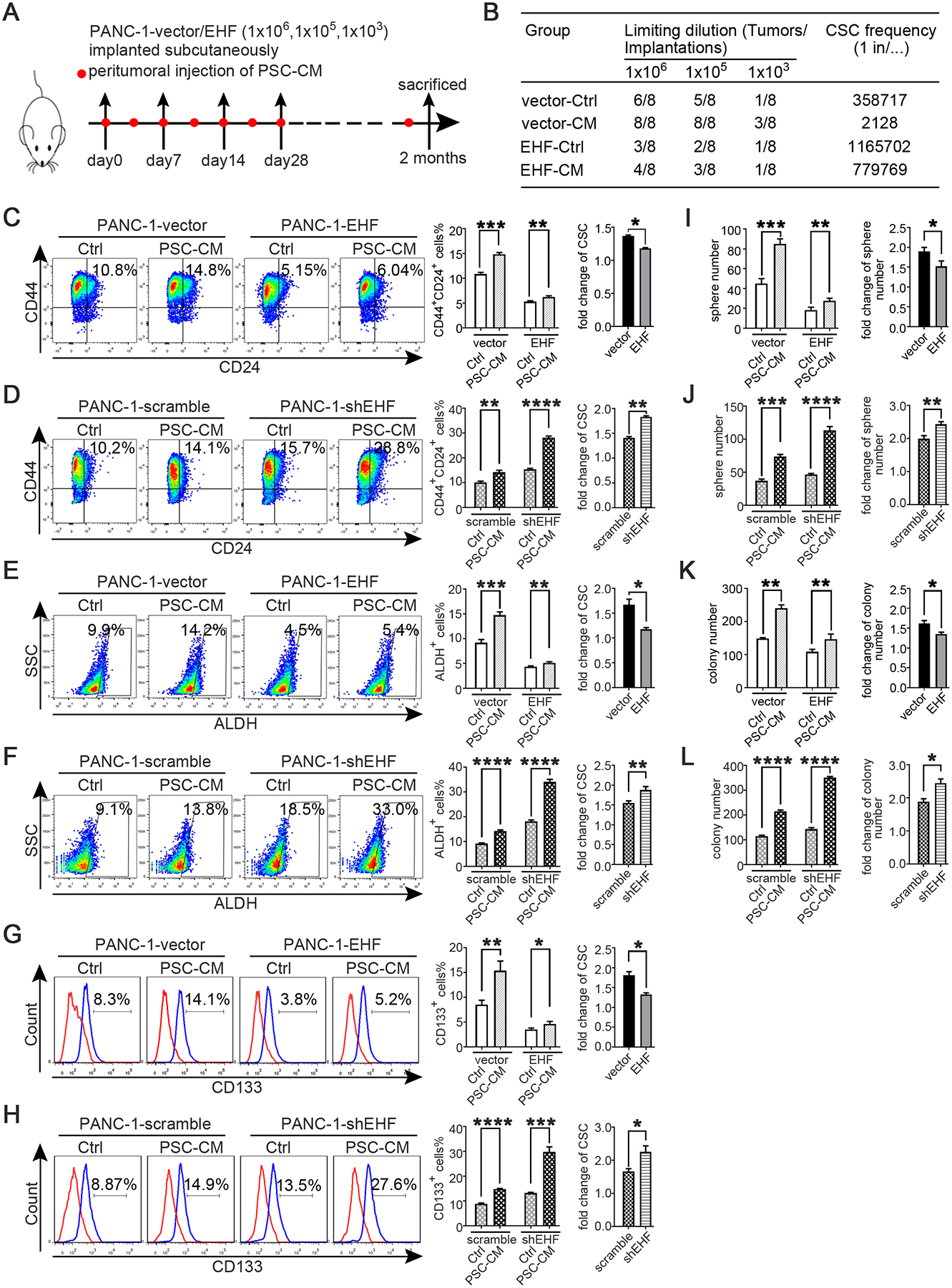
(A-B) In vivo limited dilution assay was performed to determine the effects of PSC-CM on CSC self-renewal of PANC-1-vector/EHF. Representative tumor incidence and CSCs probabilities were shown. All experiments were repeated three times independently. (C-H) PANC-1-vector, PANC-1-EHF, PANC-1-scramble and PANC-1-shEHF were cultured with PSC-CM or the control medium. The percentage of PCSCs in each cell line under each treatment were shown, the fold change of the percentage of PCSCs in each cell line after culturing with PSC-CM was calculated: (C-D) CD24+CD44+ cells, (E-F) ALDH+ cells, (G-H) CD133+ cells. Representative dot plots/ histogram (left), the statistical analysis of CSC percentage of each group (medium) and the statistical analysis of the fold change in each cell line (right) (I-J) Statistical analysis of the sphere number of each cell line under the treatment of serum-free medium and serum-free medium with PSC-CM added (left), statistical analysis of the fold change of sphere number after culturing with serum-free medium containing PSC-CM in each cell line(right) (K-L) Statistical analysis of the soft agar colony number of each cell line under the treatment of control medium and PSC-CM (left). Statistical analysis of the fold change of colony number after culturing with PSC-CM in each cell line (right) Paired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 and n.s. means non-significant.
PANC-1-vector, PANC-1-EHF, PANC-1-scramble and PANC-1-shEHF were incubated with the control medium or PSC-CM for 48 h to determine whether EHF might regulate the crosstalk between PDAC cells and PSCs, and the proportions of CSCs were determined through flow cytometry. A PSC-CM stimulus could increase the proportion of CSC populations compared with those treated with the control medium (Fig.3C–H). The abilities of PSC-CM to increase the CSC population were significantly enhanced by the shRNA knockdown of EHF and suppressed by the ectopic expression of EHF. Sphere formation and soft agar formation assays were also conducted. As shown in Fig.3I–J and Fig.3K–L, the promoting effects of PSC-CM on the self-renewal and anchorage-independent growth of CSCs were inhibited by the ectopic expression of EHF and remarkably increased by EHF depletion. These results were confirmed in multiple PDAC cell lines (Figs.S5–6). Therefore, our results indicated that tumoural EHF decreased the sensitivity of PDACs to PSC stimulus.
Tumoural EHF abrogates the sensitivity of PDAC to PSC-derived CXCL12 stimulus
Blocking antibodies for cytokines secreted by PSCs were added to PSC-CM to identify the mechanism by which EHF regulated the PDAC–PSC crosstalk. CXCL12 was found to be the potential cytokine that induced the different reaction of PDACs to PSC-derived stimulus depending on the EHF expression (Fig.S7).
In in vivo limited dilution assay, CXCL12 notably increased the tumour incidence of PANC-1-vector group compared with that in the control medium group. However, the effect of CXCL12 was robustly suppressed in the PANC-1-EHF group (Fig.4A–B). Then, the cell lines of PANC-1-vector, PANC-1-EHF, PANC-1-scramble and PANC-1-shEHF were treated with recombinant CXCL12 and the control medium. The CXCL12 treatment sharply increased the proportions of CSCs in low-EHF-expressing cell lines; the ectopic EHF overexpression remarkably suppressed the response to CXCL12 (Fig.4C–H). Sphere formation and soft agar formation assays were also conducted. As shown in Fig.4I–L, the CXCL12 treatment robustly increased tumour sphere formation and anchorage-independent growth, and the stimulating effects of CXCL12 were inhibited by the ectopic expression of EHF. Similar results were observed in other PDAC cell lines (Figs.S8–9). Therefore, tumoural EHF abrogated the sensitivity of PDAC to the CXCL12 stimulus.
Figure 4. Tumoral EHF abrogates the sensitivity of PDAC to PSC-derived CXCL12 stimulus.
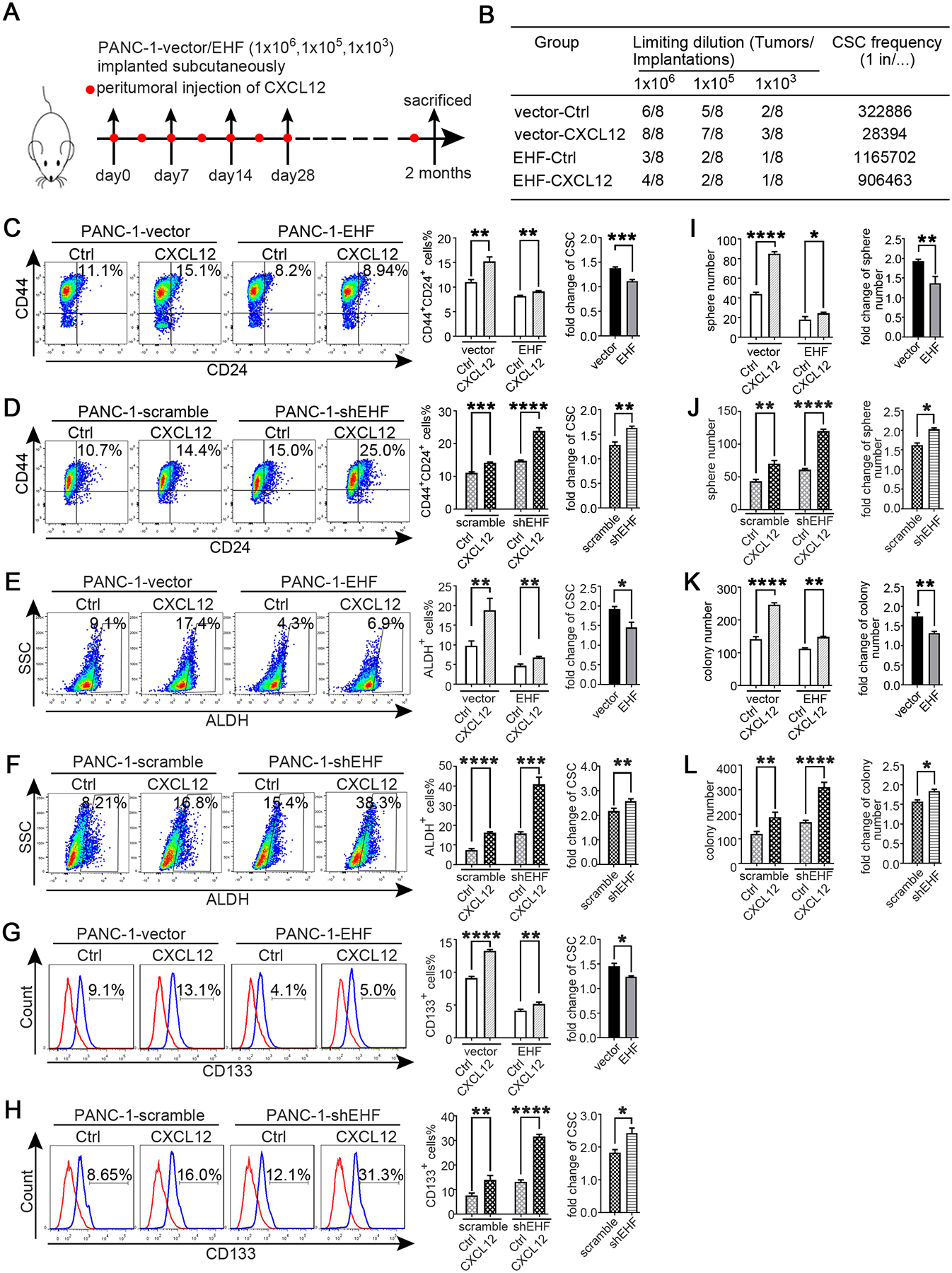
(A-B) In vivo limited dilution assay was performed to determine the effects of human recombinant CXCL12 on CSC self-renewal of PANC-1-vector/EHF. Control medium was used as the control of CXCL12.Tumor incidence and CSCs probabilities were shown.
(C-H) PANC-1-vector, PANC-1-EHF, PANC-1-scramble and PANC-1-shEHF were cultured with medium containing CXCL12 or the control medium. The percentage of PCSCs in each cell line under each treatment were shown, the fold change of the percentage of PCSCs in each cell line after culturing with medium containing CXCL12 was calculated: (C-D) CD24+CD44+ cells, (E-F) ALDH+ cells, (G-H) CD133+ cells. Representative dot plots/ histogram (left), the statistical analysis of CSC percentage of each group (medium) and the statistical analysis of the fold change in each cell line (right) (I-J) Statistical analysis of the sphere number of each cell line under the treatment of serum-free medium and serum-free medium with CXCL12 added (left), statistical analysis of the fold change of sphere number after culturing with serum-free medium containing CXCL12 in each cell line((right) (K-L) Statistical analysis of the soft agar colony number of each cell line under the treatment of control medium and medium containing CXCL12 (left), statistical analysis of the fold change of colony number after culturing with medium containing CXCL12 in each cell line((right). All experiments were repeated three times independently. Paired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 and n.s. means non-significant.
CXCR4 is transcriptionally repressed by EHF in PDAC
CXCR4 and CXCR7 are the receptors of CXCL12. Tumoural EHF did not regulate the secretion of CXCL12 in PSCs in coculture experiments (Fig.S10A–B), so we investigated whether EHF regulated the CXCR4/CXCR7 expression in PDACs. The expression of CXCR7 was not modulated by EHF (Fig.S10C). Q-PCR, Western blot and flow cytometry showed that the expression of CXCR4 was negatively regulated by EHF in the PDAC cell culture (Fig.5A–D and Fig.S11) and confirmed via western blot by using the harvested xenograft tumour tissues from the experiments in Fig. 2I (Fig.5E). Importantly, the EHF expression was negatively correlated with the CXCR4 expression in human PDAC tumour tissues (Fig.5F–I).
Figure 5. CXCR4 is transcriptionally repressed by EHF in PDAC.

(A) Q-PCR on CXCR4 mRNA were performed in indicated cell lines. Actin was used as internal control. (B) Western-blot on EHF and CXCR4 proteins in indicated cell lines were performed. Representative results were shown. (C-D) Percentage of CXCR4+ population in indicated cell lines were determined by flow cytometry. Representative histograms (C, left; D, left) and percentage of CXCR4+ population (C, right; D, right) were shown. (E) Western blot on EHF and CXCR4 proteins in harvested mice subcutaneous tumor tissues (tumor tissues were from Figure 2I). β-tubulin was used as loading control. Representative results were shown. (F-G) Representative IHC images of EHF and CXCR4 expression using human PDAC tissue sections (n=93) (F). Bars, 200μm. Spearman rank correlation analysis was used to evaluate the correlation between tumoral EHF and CXCR4 expression (n=93) (G). The number at the right side of the plots represented the case number. (H-I) Single-cell suspensions were made from 39 cases of fresh PDAC tissues and stained with antibodies against CXCR4. Tumoral CXCR4+ cells were determined by flow cytometry. Gated on EpCAM+ cells to exclude non epithelial cells. Representative histograms were shown (H). Spearman correlation analysis between EHF IHC score and the proportion of EpCAM+CXCR4+ population was shown (I); n=39, P<0.001. (J) EHF scanned motif logo. (K) Predicted EHF binding sites (EBSs) on the human CXCR4 promoter. Position relative to the transcription start site of CXCR4, EBS sequence and corresponding JASPAR score. (L) Binding of EHF to the promoter of CXCR4 was determined by chromatin immunoprecipitation. IgG was used as negative control. Anti-RNA PolymeraseⅡwas used as positive control. Representative results were shown. (M) PANC-1(left) and 293T cells (right) were transfected with either vector control or pCDH-EHF in conjunction with the luciferase reporter pGL3-empty vector, pGL3-CXCR4-EBS1-wt or pGL3-CXCR4-EBS1-mut. Results were expressed as fold induction relative to that of the corresponding cells transfected with the control vector after normalization of firefly luciferase activity according to Renilla luciferase activity. All experiments were repeated three times independently. Paired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 and ns means not significant.
In silico analysis showed one high-confidence EHF binding site (EBS) within the promoter region of CXCR4 in the JASPAR database (Fig.5J–K). Ch-IP was conducted in the PANC-1 cell line and revealed that EHF markedly bound to the promoter of human CXCR4 (Fig.5L). The luciferase analysis of PANC-1 and 293T showed that the EHF overexpression significantly decreased the transcription of CXCR4 (PANC-1, P=0.0065; 293T, P=0.0061) and the mutation of EBS1 substantially abrogated the EHF induced the trans-suppression of the CXCR4 promoter (PANC-1, P=0.3295; 293T, P=0.4918; Fig.5M).
EHF decreases the sensitivity of PDACs to PSCs derived CSC-supporting stimulus by suppressing CXCR4
BxPC-3-scramble-scramble, BxPC-3-scramble-shCXCR4, BxPC-3-shEHF-scramble and BxPC-3-shEHF-shCXCR4 were established (Fig.S12A–B) and treated with the control medium or PSC-CM to evaluate whether EHF decreased the sensitivity of PDACs to PSC-CM stimulus by suppressing CXCR4. As we previously observed, EHF depletion with shRNA increased the pro-CSC effects of PSC-CM (Fig.6A–C). However, knocking down CXCR4 in BxPC-3-shEHF cells almost abrogated the effects of PSC-CM treatment (Fig.6A–C). Similarly, the shRNA depletion of CXCR4 in PDAC cells abrogated the abilities of PSC-CM to promote the sphere formation of PDAC tumour (Fig.6D). In vivo limited dilution assay demonstrated that PSC-CM significantly increased the CSC frequency in PDAC cells (from 1/779769 to 1/245406), and the effects of PSC-CM were dramatically enhanced after EHF knockdown (CSC frequency increased from 1/210828 in the 1640 group to 1/1443 in the PSC-CM group; Fig.6E). Strikingly, CXCR4 knockdown abrogated the pro-CSC effects of the conditioned medium from PSC (Fig.6E). Collectively, our data supported that tumoural EHF decreased the sensitivity of PDACs to PSC-derived CSC stimulus by suppressing the CXCR4 expression.
Figure 6. EHF decreases the sensitivity of PDACs to PSCs derived CSC-supporting stimulus by suppressing CXCR4.

(A-C) BxPC-3-scramble/shEHF-scramble and BxPC-3-scramble/shEHF-shCXCR4 were cultured with PSC-CM or the control medium. The percentage of PCSCs in each cell line under each treatment were shown, the fold change of the percentage of PCSCs in each cell line after culturing with PSC-CM was calculated: (A) CD24+CD44+ cells, (B) ALDH+ cells, (C) CD133+ cells. Representative dot plots/ histogram (left), the statistical analysis of CSC percentage of each group (medium) and the statistical analysis of the fold change in each cell line (right). (D) Statistical analysis of the sphere number of each cell line under the treatment of serum-free medium and serum-free medium with PSC-CM added (left), statistical analysis of the fold change of sphere number after culturing with serum-free medium containing PSC-CM in each cell line((right). (E) In vivo limited dilution assay was performed to determine the effects of PSC-CM on CSC self-renewal of BxPC-3-scramble/shEHF-scramble and BxPC-3-scramble/shEHF-shCXCR4.Tumor incidence and CSCs probabilities were shown. All experiments were repeated three times independently. Paired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 and n.s. means non-significant.
Identification of compounds that induce EHF overexpression
EHF is a promising therapeutic target of PDAC, so 190 compounds from a drug library in our laboratory were screened to determine their effects on regulating the EHF expression (Table S3 and Fig.S13A). Amongst these 190 compounds, 14 could induce the EHF overexpression (Fig.S13B). Considering the efficacy on the EHF upregulation and the safety profile, we chose rosiglitazone as a candidate for further studies. As shown in Fig.7A–B, rosiglitazone significantly induced the mRNA and protein expression of EHF in PDAC cells and PDX-derived PDAC cells in a concentration-dependent manner.
Figure7. Identification of compounds that induce EHF overexpression.

(A-B). PANC-1, BxPC-3 and two primary cancer cell lines PDX1# and PDX2# were treated with rosiglitazone(5μM and 10μM,24h). DMSO was used as control. (A) Q-PCR was conducted to detect for EHF mRNA expression. (B) Western-blot for EHF expression were performed. Representative results were shown. (C) PPAR γ-scanned motif logo (D) Predicted PPAR γ response elements (PPREs) on the human EHF promoter. Position relative to the transcription start site of EHF, PPRE sequences and corresponding JASPAR scores. (E) Binding of PPAR γ to the promoter of EHF was determined by chromatin immunoprecipitation. IgG was used as negative control. Anti-RNA PolymeraseⅡwas used as positive control. Representative results were shown. (F) The promoter activity of EHF after treated with rosiglitazone. PANC-1 transfected with either luciferase reporter pGL3-empty vector or wild type pGL3-ESE3/EHF promoter were treated with rosiglitazone(10μM,24h). Forty-eight hours later, cells were collected for dual luciferase assay. Results were expressed as fold induction relative to those of the corresponding cells transfected with pGL3-empty vector after normalization of firefly luciferase activity according to Renilla luciferase activity. All experiments were repeated three times independently. Paired Student’s t-test was used as statistical analysis. *P<0.05 and **P<0.01.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated nuclear transcription factor. Rosiglitazone is a specific PPARγ agonist that improves glycemic control and insulin sensitivity in patients with diabetes by selectively activating PPARγ. Computational analysis showed two high-confidence PPAR γ response elements (PPREs) corresponding to the promoter regions of EHF in the JASPAR database (Fig.7C–D). Ch-IP primers were designed to investigate the binding site through the Ch-IP assay and evaluate whether PPAR γ directly bound to the promoter of EHF. As shown in Fig.7E, PPAR γ antibody could precipitate the PPRE sequence, which indicated the direct binding of PPAR γ to the EHF promoter. Luciferase analysis showed that rosiglitazone significantly increased the transcriptional activity of the EHF promoter (P=0.0423), suggesting that rosiglitazone induced the EHF overexpression through PPAR γ activation (Fig.7F).
Rosiglitazone inhibits PDAC stemness and suppresses the sensitivity to the stemness-promoting stimulus by upregulating the EHF expression
As shown in Fig.8A–B, rosiglitazone treatment inhibited the sphere formation capacity of PDACs and essentially blocked the CXCL12-mediated increase in the sphere formation of PDAC tumour. This finding suggested that rosiglitazone could be used to abrogate CXCL12 and PSC-mediated PDAC CSC self-renewal. To rigorously evaluate this hypothesis, we performed in vivo limited dilution tumorigenicity assay. As shown in Fig.8C, rosiglitazone significantly reduced tumour initiation and CSC frequency. Moreover, no significant difference in tumour incidence could be observed in PANC-1-CXCL12-rosiglitazone and PANC-1-ctrl-rosiglitazone groups. This result indicated that the stemness-promoting effect of CXCL12 was blocked by rosiglitazone. We also observed that rosiglitazone significantly deceased the percentage of the CXCR4+ population in PDAC cells (Fig.8D). In the orthotopic BALB/C tumour mouse model, the mice in the rosiglitazone group survived significantly longer than those in the DMSO control group (P=0.031; Fig.8E–F). The normalised BLI in the rosiglitazone group was notably lower than that in the DMSO control group, suggesting that orthotopic tumour growth was inhibited (Fig.8G–H). The harvested tumours from the orthotopic mouse model were analysed through flow cytometry. As shown in Fig.S14A, rosiglitazone significantly decreased the proportion of ALDH+ cells. Western blot and IHC indicated that rosiglitazone increased the expression of EHF and decreased the expression of stemness genes (Fig.S14B–C).
Figure 8. Rosiglitazone inhibits PDAC stemness and suppresses the sensitivity to the stemness-promoting stimulus by upregulating the EHF expression.
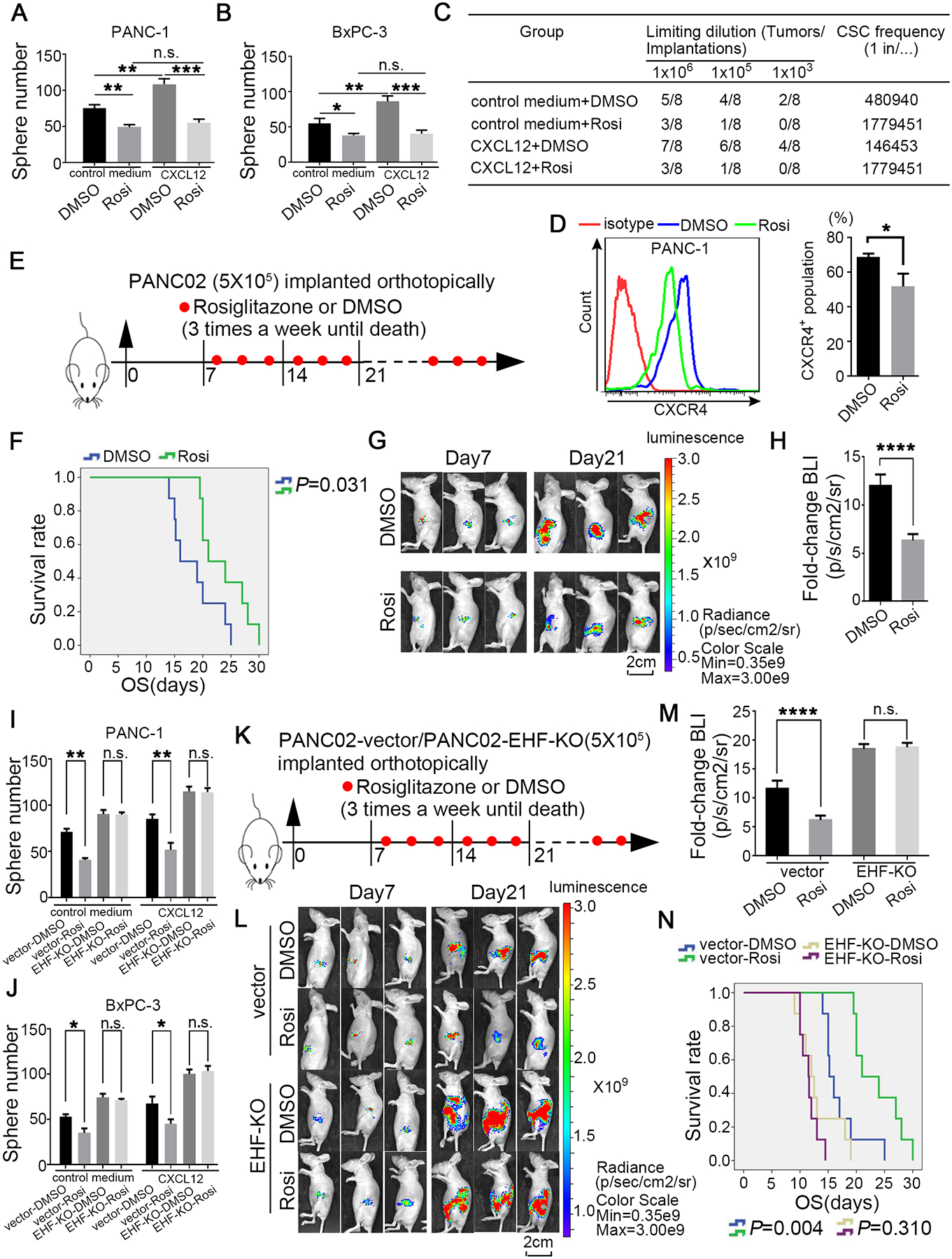
(A-B) Adherent cells were pre-treated with 5μM rosiglitazone for 48h; and then cells were collected and cultured with serum-free medium contained with 100ng/ml human recombinant CXCL12 in low-adherent 6-well plates. Representative results were shown (PANC-1, A; BxPC-3, B). (C) In vivo limiting dilution assays were performed to determine the effects of rosiglitazone on CSC self-renewal of PANC-1 cells with or without CXCL12 stimulus. Tumor incidences and CSCs probabilities were shown. (D) Rosiglitazone reduced the percentage of CXCR4+ population (5μM, 24h). Representative results were shown. (E) Schematic illustration for in vivo rosiglitazone therapeutic experiment in orthotopic mice model. (F) Kaplan-Meier survival curves with log-rank test (PANC02-DMSO vs. PANC02-rosiglitazone P=0.031). (G-H) Representative bioluminescent images of two groups on day 7 and day 21 after tumor implantation (G). Statistical analysis of the fold change of BLI after drug treatment (BLI on day 21 to BLI on day 7; H) (n=8 per group). (I-J) Adherent PDAC-vector/EHF-KO cells were pre-treated with 5μM rosiglitazone for 48h; and then cells were collected and cultured with serum-free medium contained with or without 100ng/ml human recombinant CXCL12 in low-adherent 6-well plates for the following sphere formation assays. Sphere number analysis were shown (PANC-1, I; BxPC-3, J). (K) Schematic illustration for in vivo rosiglitazone therapeutic experiment using PANC02-vector/EHF-KO cell lines in orthotopic mice model. (L-M) Representative bioluminescent images of the two groups on day 7 and day 21 after tumor implantation were shown (L). Statistical analysis of the fold change of BLI after drug administration (BLI on day 21 to BLI on day 7; M) (n=8 per group). (N) Kaplan-Meier survival curves with log-rank test were used to analyze the different effect after treating with DMSO and rosiglitazone. All experiments were repeated three times independently. Paired Student’s t-test was used for statistical analysis for in vitro experiments and unpaired Student’s t-test was used for animal experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.001 and n.s. means non-significance.
EHF was knocked out in PANC-1, BxPC-3 and PANC02 cells via the CRISPR/dCas9 system to determine whether rosiglitazone inhibited the stemness of PDACs via the PPARγ-EHF pathway (Fig.S15). As shown in Fig.8I–J, EHF-KO could abrogate the abilities of rosiglitazone to inhibit tumour sphere formation. In the orthotopic tumour mouse model (Fig.8K–M), rosiglitazone significantly reduced the tumour burden in the PANC02-vector group but not in the EHF-KO group. Importantly, rosiglitazone could improve survival in the vector control group but not in the EHF-KO group (Fig.8N).
Ibuprofen and allopurinol, two other compounds that upregulated the EHF expression, could similarly suppress CSC stemness in PDAC (Figs.S16–17).
Rosiglitazone sensitises PDAC to gemcitabine therapy in the KPC mouse model
Given the role of CSCs in chemotherapy resistance and the function of rosiglitazone on suppressing PDAC stemness, KPC mouse models were used to evaluate the therapeutic effects of gemcitabine plus rosiglitazone. When the tumour volumes reached 20–60 mm3, the KPC mice were randomised into four groups: vehicle, GEM, rosiglitazone and GEM plus rosiglitazone groups (Fig.9A and Fig.S18). Ultrasonic imaging showed that the tumour burdens significantly decreased in the GEM plus rosiglitazone group compared with that in the GEM group alone on day 30 (Fig.9B). Consistently, GEM plus rosiglitazone reduced the weight of pancreas compared with that of GEM monotherapy (Fig.9C). The proportion of ALDH+ cells significantly decreased when rosiglitazone was administered (Fig.9D–E). The IHC of ki-67 indicated that GEM plus rosiglitazone combination therapy more significantly reduced cancer cell proliferation compared with that with GEM monotherapy (Fig.9F–G). The Western blot of pancreatic tumour tissues from the KPC mice further confirmed that rosiglitazone could induce the expression of EHF and suppress the expression of stemness marker genes (Fig.9H). Finally, obvious survival benefits were observed in the GEM plus rosiglitazone group compared with those in the GEM group (Fig.9I). Therefore, rosiglitazone suppressed PC stemness and could be used as a new therapeutic method in the clinical practice of PDAC treatment (Fig.10).
Figure 9. Rosiglitazone sensitises PDAC to gemcitabine therapy in KPC mouse model.

(A) Experimental design program. (B) The representative ultrasound images of KPC mice treated with vehicle (n=6), gemcitabine (GEM; n=6), rosiglitazone (Rosi; n=6) and gemcitabine+rosiglitazone (GEM +Rosi; n=6) at day 30 after drug treatment (left). Pancreatic tumor (T), pancreas (P), spleen(S), kidney (K) and duodenum (D). Statistical analysis for the fold change of pancreatic tumor volumes measured by ultrasound system at day 30 after drug treatment (volumes in day 0 were used as baseline) (right). (C) The representative macroscopic images of pancreatic tumor in KPC mice treated with vehicle, gemcitabine, rosiglitazone and gemcitabine+ rosiglitazone after sacrifice (left). Statistical analysis for pancreas weight of KPC mice from different groups (right). (D) Rosiglitazone reduced the percentage of ALDH+ population in PDAC in KPC model. PI was used to exclude dead cells, CD45 was used to exclude leukocytes and DEAB was used as negative control. Representative dot plots (left) and statistical analysis (right) were shown. (E) The representative images of H&E slides from tumors of four groups. Scale bars: 200μm. (F) The representative images of KPC pancreatic tumor IHC for Ki-67 staining. Scale bars: 400μm. (G) Statistical analysis for percentage of Ki-67 positive cells in different groups. (H) Protein expression of EHF and stemness markers (Sox9, Sox2, Nanog and Oct4) were detected in pancreatic tumor tissues of KPC mice by western blot. Tubulin was used as loading control. Representative results were shown. (I) Kaplan-Meier survival curves with log-rank test for KPC mice treated with vehicle (n=8), gemcitabine (GEM; n=8), rosiglitazone (Rosi; n=8) and gemcitabine+ rosiglitazone (GEM +Rosi; n=8). The mouse experiments were repeated three times independently and non-paired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Figure 10. Schematic of the research.

ESE3/EHF regulated PDAC CSCs property through cell-intrinsic and extrinsic pathway. Rosiglitazone suppressed PC stemness and inhibited the cross-talk between PC and PSCs by upregulating ESE3/EHF.
Discussion
CSCs play a significant role in disease recurrence and treatment failure in pancreatic cancer. The crosstalk between CSCs and its niche is essential for stemness maintenance, cancer initiation and progression.3 28 Seino et al.29 revealed that cancer-associated fibroblasts (CAFs) transmit a pro-tumourigenic niche signal to PDACs through the juxtacrine production of stromal Wnt ligands. Daniel et al.30 reported that tumour organoids need PSC-secreted ligands for surviving. Our study supported that the cytokines IL6, IL8 and CXCL12 secreted by PSCs significantly increase the cancer stemness of PDAC.30–32 A PSC-derived CSCs niche shows potential for applications that promote cancer stemness, so targeting the crosstalk between CSCs and PSCs can be an efficient modality for the prevention of tumour recurrence.
EHF is a member of a highly diverse ETS superfamily. Our group first demonstrated EHF as a tumour suppressor that directly inhibits PDAC progression by upregulating E-cadherin while downregulating TGFβ1 and GM-CSF.19 20 The role of EHF in CSCs regulation was first identified in prostate cancer. Albino et al.33 reported that EHF directly controls the activity of the Lin28/let-7 axis, a key pathway involved in CSC expansion. EHF also represses the expression of the key CSCs genes TWIST1, ZEB2, BMI1 and POU5F1.23 Furthermore, the loss of EHF leads to the upregulation of IL-6 and the activation of the JAK/STAT3 pathway.21 Our data indicated that EHF not only plays a cell autonomous role in regulating CSC stemness but also has important functions in regulating the sensitivity to a pro-CSC stimulus from the PSC niche.
PSCs within the tumour microenvironment represent the principal source of CXCL12, which binds to its two receptors, CXCR4 and CXCR7 to activate a number of signalling pathways in pancreatic cancer cells, such as PKCα/NFκB, MAPK, PI3K-Akt-mTOR and JAK/STAT pathways.34 35 Moreover, Hermann et al.6 defined a subpopulation of migrating CSCs that are characterised by the expression of the CXCR4 receptor and critically involved in tumour metastasis. Mohammad et al.16 reported that CXCR4/CXCL12 and hedgehog signalling pathways mediate the chemoresistance of PC cells upon coculturing with PSCs. We found that tumoural EHF repressed the CXCR4 expression but not the CXCR7 expression. ChIP and dual-luciferase assays revealed that EHF directly bound to the promoter regions of CXCR4 to suppress its transcription. Our blocking experiment revealed that EHF decreased the sensitivity of cancer cells to the PSC stimulus by repressing the CXCR4 expression.
Our findings suggested that restoring the CSC-suppressing functions of EHF could be a promising approach in PDAC treatment. Here, we screened 190 compounds from our drug library on the basis of their effects on the EHF expression. These 190 compounds are commonly used drugs in clinical work and easily obtained. Rosiglitazone, a high-affinity synthetic agonist for nuclear PPAR γ, was identified as the most potent activator of the EHF expression with limited side effects. Upon activation with specific ligands, PPAR γ binds to PPAR γ-responsive elements (PPREs), which then mediate the target gene expression.36 Recently, studies have indicated that rosiglitazone and related thiazolidinediones may play inhibitory roles in various types of cancer cells, including pancreatic cancer, such as enhancing radiosensitivity,37 reducing immune suppression38 and inhibiting cell invasion and metastasis.39–41 In our current work, rosiglitazone-activated PPAR γ bound to the promoter region of EHF and upregulated its expression. In vivo and in vitro studies demonstrated that rosiglitazone decreased the sensitivity of PDAC to PSCs’ stimulus and inhibited tumour stemness properties by inducing tumoural EHF expression. Our results suggested that the effects of rosiglitazone on EHF upregulation could be translated into the development of targeted therapy against cancer stemness.
Our study first reported that EHF suppressed cancer stemness from intrinsic and extrinsic pathways. For the intrinsic pathway, EHF repressed the expression of SOX9, SOX2, OCT4 and Nanog. For the extrinsic pathway, EHF decreased the sensitivity of PDACs to the stimulus from the PSC-derived CSC-supportive niche by negatively regulating the tumoural CXCR4 expression. Conceivably, rosiglitazone could be used to target pancreatic stem cells and the crosstalk between CSCs and its niche by upregulating EHF.
Materials and methods
Patient and sample collection
A total of 93 sequential PDAC tissues were retrospectively collected from patients who received radical surgery R0 resection at the Tianjin Medical University Cancer Institute and Hospital from July 2011 to January 2015. The follow-up rate was 100% until the last follow-up on October 23, 2019. Then, 39 consecutive cases of fresh PDAC tissues were prospectively collected during operation from January 2018 to November 2019. All the patients provided written consent for the use of their specimens and disease information for future investigations according to the ethics committee of Tianjin Medical University Cancer Institute and Hospital, China, and in accordance with the recognised ethical guidelines of Helsinki (ID number of the ethics approval: Ek2017141).
Primary Human Pancreatic Cancer Cells
Human pancreatic tumours were obtained during surgery with written informed consent from all the patients. Low-passage (<10 passages) primary cancer cells were used for later experiments. Information of patients and cellular genomic background was listed in Tables S4–5.
Cell culture and transfection
The pancreatic cancer cell lines PANC-1, MiaPaca-2, BxPC-3, SW1990 and PANC02 were maintained as previously described.20 42 The human PSCs were set up as reported by Jesnowski et al.43 PDAC-vector, PDAC-EHF, PDAC-scramble and PDAC-shEHF were established as previously described and related sequence information was listed in Table S6.20 Mycoplasma contamination was excluded in these cell lines. The cells were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2 with DMEM and RPMI-1640 basic medium supplemented with 10% FBS as a medium.
Animals
Female NOD/SCID, BABL/Cnude and KPC mouse models (4–6 weeks old) were used. All the mice were maintained in specific pathogen-free conditions, and animal experiment procedures were approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital in compliance with the principles and procedures of the NIH Guide for the Care and Use of Laboratory Animals.
In vivo tumourigenicity assay
The cohorts of NOD/SCID mice were randomised into different groups. In each group, cancer cells at different dilutions were subcutaneously injected into the contralateral flanks of the NOD/SCID mice. Stem cell frequency was calculated via http://bioinf.wehi.edu.au/software/elda/.
Orthotopic mouse model
The cohorts of BALB/C nude mice were randomised into different groups. An orthotopic model was established using 5×105 luciferase-expressing PDAC tumour cells. Tumour growth was analysed through BLI.
KPC mouse model and preclinical animal cohorts
LSL-KrasG12D/+, LSL-Trp53R172H/+ and Pdx1-Cre mouse models were were generated in-house. Primers and PCR conditions for genotyping were listed in Tables S7–8. Preclinical studies were conducted with a KPC mouse model.
Immunohistochemistry (IHC) and multiplex fluorescent IHC
IHC score was determined by two independent pathologists who were blinded to the patients’ clinicopathological features and prognosis. For multiplex fluorescent IHC, stained tissues were scanned and captured using a Vectra Polaris system (PerkinElmer). Images captured were analysed using the inForm cell analysis software (PerkinElmer).
Flow cytometry
Primary pancreatic cells, pancreatic cancer cell lines and cells from tumour tissue digestions were stained with anti-hCD133, anti-hCD24, anti-hCD44, anti-ESA, anti-hCXCR4 or appropriate control antibodies. Detailed information of antibodies used was listed in Table S9. The ALDH activity were detected with ALDEFLUOR™ kits. Isotype controls were used as negative controls. Data were analysed using FlowJo 10.0.
Reverse transcription PCR (RT-PCR)
The total RNA of the cells was extracted with TRizol and converted to cDNAs by using a reverse transcription PCR (RT-PCR) system. Then, real-time fluorescent quantitative PCR was conducted to analyse the cDNA levels. Related primers were listed in Table S10.
Western blot
Target proteins were detected through Western blot with primary antibodies as follows: anti-EHF, anti-Sox9, anti-Sox2, anti-Nanog, anti-Oct4, anti-CXCR4, anti-E-cadherin, anti-CK19, anti-CAII and anti-tubulin.
Anchorage-independent growth assay
Each six-well plate was coated with 1 ml of bottom agar, and 5,000 cells were suspended in 1 ml of the top agar. Cells were incubated for 21 days, and colonies were analysed.
Sphere formation assay
Cells (5,000 cells/ml) were cultured in ultralow adhesion plates in a serum-free medium. After the cells were cultured for 2 weeks, tumour spheres with a diameter of >75 μm were counted.
Chromatin immunoprecipitation (Ch-IP) and luciferase analysis
Ch-IP assays were performed using a Ch-IP kit. The immunoprecipitated products were detected through PCR assays. Luciferase analysis was performed on the basis of the binding sites identified through Ch-IP analysis. Related sequences were listed in Table S11.
Preparation of PSC-CM
PSCs were grown to 70%–80% confluence in 10 cm dishes in complete culture media. Then, the medium was replaced with FBS-free DMEM/F12 (1:1), and the cells were cultured for additional 48 h.
Stimulation of PSC-CM/CXCL12
For in vitro studies, PSC-CM was added to the culture medium at a ratio of 1:1, and CXCL12 was added to the culture system at a final concentration of 100 ng/ml. For in vivo studies, 200 μl of PSC-CM or 200 μl of CXCL12 was intratumourally injected three times a week.
Treatment of Rosiglitazone
For in vitro studies, adherent PDAC cells were pre-treated with 5 μM rosiglitazone for 48 h and collected for further experiments. For In vivo tumourigenicity studies, rosiglitazone (100 mg/kg/day) was peritumourally injected three times a week. For orthotopic tumour models, rosiglitazone (100 mg/kg/day) was injected intraperitoneally three times a week.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 21.0. Each experiment was conducted in triplicate, and data were presented as mean ± SD unless otherwise stated. The variance between the groups was statistically compared. Student’s t test was conducted to compare the mean values. Kaplan–Meier curves were analysed for median survival. A log-rank test was carried out to analyse the differences in survival times amongst the patient subgroups. *P<0.05, **P<0.01 and ***P<0.001 indicated significant differences, and n.s. meant non-significant.
See Supplementary Methods for details
Supplementary Material
Significance of the subject.
- What is already known about this subject?
- PC is one of the leading causes of cancer-related death and is projected to become the second most lethal tumor by the year 2030.
- CSCs contributed to PC recurrence and metastasis.
- PC stemness is regulated by the aberrant activation of cell-intrinsic signal pathways and the crosstalk between CSCs and their niche.
- Patients with PC and low tumoural EHF expression gained poor overall and relapse-free survival.
- What are the new findings?
- Tumoural EHF decreased the sensitivity of PC to PSC-derived CXCL12 stimulus, which suppressed cancer stemness by inhibiting the crosstalk between PC and CSC-supportive niches.
- EHF transcriptionally suppressed CXCR4, which is the receptor of CXCL12.
- EHF also suppressed cancer stemness in a cell autonomous manner by transcriptionally suppressing Sox9, Sox2, Oct4 and Nanog.
- Rosiglitazone suppressed PC stemness and inhibited the crosstalk between PC and PSCs by upregulating EHF.
- How may it affect clinical practice in the foreseeable future?
- Our study identified that EHF suppressed cancer stemness from intrinsic and extrinsic pathways, which could be a promising target in PC therapy.
- Rosiglitazone could be used as a new therapeutic method in clinical practice to target pancreatic cancer stem cells and the crosstalk between CSCs and their niche.
Acknowledgments
We thank Prof. Xueli Bai and Prof. Qi Zhang (Department of Surgery, the Second Affiliated Hospital, Zhejiang University, China) for their technical assistance.
This work was supported by the National Natural Science Foundation of China (grants 82030092, 81720108028, 82072657, 81802432, 82072716, 81802433, 82072659, 81871968 and 81871978), the programs of Tianjin Prominent Talents, Tianjin Eminent Scholars, Tianjin Natural Science Fundation (20JCQNJC01330, 18JCJQJC47800, 19JCJQJC63100 and 19JCYBJC26200) and Tianjin Postgraduate Research and Innovation Project (2019YJSB104), Tianjin Research Innovation Project for Postgraduate Students (2019YJSB104).
Abbreviations used:
- Ch-IP
chromatin immunoprecipitation
- CSC
cell stem cells
- EBS
EHF binding site
- EHF
ETS homologous factor
- ESE3
epithelium-specific ETS factor family member 3
- GEM
gemcitabine
- HPF
high power field
- IHC
immunohistochemistry
- KPC
LSL-KrasG12D/+ mice, LSL-Trp53R172H/+ and Pdx1-Cre
- PC
pancreatic cancer
- PDAC
pancreatic ductal adenocarcinoma
- PDX
patient derived xenograft
- PPAR γ
peroxisome proliferators-activated receptor γ
- PSCs
pancreatic stellate cells
- Rosi
rosiglitazone
Footnotes
The authors declare no competing finical interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol 2020;21:e135–e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford HC, Pasca di Magliano M, Banerjee S. Signaling Networks That Control Cellular Plasticity in Pancreatic Tumorigenesis, Progression, and Metastasis. Gastroenterology 2019;156:2073–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Mayea Y, Mir C, Masson F, et al. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin Cancer Biol 2020;60:166–180. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030–7. [DOI] [PubMed] [Google Scholar]
- 6.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–23. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Aznar E, Wiesmuller L, Sainz B Jr., et al. EMT and Stemness-Key Players in Pancreatic Cancer Stem Cells. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabian A, Stegner S, Miarka L, et al. Metastasis of pancreatic cancer: An uninflamed liver micromilieu controls cell growth and cancer stem cell properties by oxidative phosphorylation in pancreatic ductal epithelial cells. Cancer Lett 2019;453:95–106. [DOI] [PubMed] [Google Scholar]
- 9.Mizutani Y, Kobayashi H, Iida T, et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res 2019;79:5367–5381. [DOI] [PubMed] [Google Scholar]
- 10.Chan TS, Shaked Y, Tsai KK. Targeting the Interplay Between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers. Front Oncol 2019;9:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begum A, McMillan RH, Chang YT, et al. Direct Interactions With Cancer-Associated Fibroblasts Lead to Enhanced Pancreatic Cancer Stem Cell Function. Pancreas 2019;48:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson JS, Pirola RC, Apte MV. Stars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progression. Front Physiol 2014;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farran B, Nagaraju GP. The dynamic interactions between the stroma, pancreatic stellate cells and pancreatic tumor development: Novel therapeutic targets. Cytokine Growth Factor Rev 2019;48:11–23. [DOI] [PubMed] [Google Scholar]
- 14.Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su S, Chen J, Yao H, et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018;172:841–856 e16. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Srivastava SK, Zubair H, et al. Co-targeting of CXCR4 and hedgehog pathways disrupts tumor-stromal crosstalk and improves chemotherapeutic efficacy in pancreatic cancer. J Biol Chem 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldaz P, Otaegi-Ugartemendia M, Saenz-Antonanzas A, et al. SOX9 promotes tumor progression through the axis BMI1-p21(CIP). Sci Rep 2020;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman R, Sementchenko V, Watson DJAR. The epithelial-specific Ets factors occupy a unique position in defining epithelial proliferation, differentiation and carcinogenesis. 2003;23:2125–31. [PubMed] [Google Scholar]
- 19.Zhao T, Jiang W, Wang X, et al. ESE3 Inhibits Pancreatic Cancer Metastasis by Upregulating E-Cadherin. Cancer Res 2017;77:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Jiang W, Zhao K, et al. Tumoral EHF predicts the efficacy of anti-PD1 therapy in pancreatic ductal adenocarcinoma. J Exp Med 2019;216:656–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albino D, Civenni G, Rossi S, et al. The ETS factor ESE3/EHF represses IL-6 preventing STAT3 activation and expansion of the prostate cancer stem-like compartment. Oncotarget 2016;7:76756–76768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dallavalle C, Albino D, Civenni G, et al. MicroRNA-424 impairs ubiquitination to activate STAT3 and promote prostate tumor progression. J Clin Invest 2016;126:4585–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albino D, Longoni N, Curti L, et al. ESE3/EHF controls epithelial cell differentiation and its loss leads to prostate tumors with mesenchymal and stem-like features. Cancer Res 2012;72:2889–900. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Xing J, Cheng R, et al. Abnormal Localization and Tumor Suppressor Function of Epithelial Tissue-Specific Transcription Factor ESE3 in Esophageal Squamous Cell Carcinoma. PLoS One 2015;10:e0126319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim JH, Cho JY, Park YB, et al. ESE-3 transcription factor is involved in the expression of death receptor (DR)-5 through putative Ets sites. Biochem Biophys Res Commun 2006;350:736–41. [DOI] [PubMed] [Google Scholar]
- 26.Hamada S, Masamune A, Takikawa T, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun 2012;421:349–54. [DOI] [PubMed] [Google Scholar]
- 27.Cave DD, Di Guida M, Costa V, et al. TGF-beta1 secreted by pancreatic stellate cells promotes stemness and tumourigenicity in pancreatic cancer cells through L1CAM downregulation. Oncogene 2020;39:4271–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbato L, Bocchetti M, Di Biase A, et al. Cancer Stem Cells and Targeting Strategies. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seino T, Kawasaki S, Shimokawa M, et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018;22:454–467 e6. [DOI] [PubMed] [Google Scholar]
- 30.Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T, Notta F, Navab R, et al. Senescent Carcinoma-Associated Fibroblasts Upregulate IL8 to Enhance Prometastatic Phenotypes. Mol Cancer Res 2017;15:3–14. [DOI] [PubMed] [Google Scholar]
- 32.Wei L, Ye H, Li G, et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis 2018;9:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albino D, Civenni G, Dallavalle C, et al. Activation of the Lin28/let-7 Axis by Loss of ESE3/EHF Promotes a Tumorigenic and Stem-like Phenotype in Prostate Cancer. Cancer Res 2016;76:3629–43. [DOI] [PubMed] [Google Scholar]
- 34.Righetti A, Giulietti M, Sabanovic B, et al. CXCL12 and Its Isoforms: Different Roles in Pancreatic Cancer? J Oncol 2019;2019:9681698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung Y, Cackowski FC, Yumoto K, et al. CXCL12gamma Promotes Metastatic Castration-Resistant Prostate Cancer by Inducing Cancer Stem Cell and Neuroendocrine Phenotypes. Cancer Res 2018;78:2026–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janani C, Ranjitha Kumari BD. PPAR gamma gene--a review. Diabetes Metab Syndr 2015;9:46–50. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Shen W, Li X, et al. The PPARgamma Agonist Rosiglitazone Enhances the Radiosensitivity of Human Pancreatic Cancer Cells. Drug Des Devel Ther 2020;14:3099–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bunt SK, Mohr AM, Bailey JM, et al. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother 2013;62:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhang DW, Lin DQ, et al. Peroxisome proliferator-activated receptor-gamma inhibits pancreatic cancer cell invasion and metastasis via regulating MMP-2 expression through PTEN. Mol Med Rep 2015;12:6255–60. [DOI] [PubMed] [Google Scholar]
- 40.Galli A, Ceni E, Crabb DW, et al. Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARgamma independent mechanisms. Gut 2004;53:1688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita M, Hasegawa A, Yamamori M, et al. In vitro and in vivo cytotoxicity of troglitazone in pancreatic cancer. J Exp Clin Cancer Res 2017;36:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao T, Jin F, Xiao D, et al. IL-37/ STAT3/ HIF-1alpha negative feedback signaling drives gemcitabine resistance in pancreatic cancer. Theranostics 2020;10:4088–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jesnowski R, Furst D, Ringel J, et al. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest 2005;85:1276–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


