Abstract
Surface Plasmon Resonance (SPR) techniques are highly accurate in detecting biomolecular like blood group measurement, food adulteration, milk adulteration and recently developing as a rapid detection for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. In order to validate the clinical diagnosis, Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) of nasopharyngeal swabs has been utilized, which is time consuming and expensive. For fast and accurate detection of the SARS-CoV-2 virus, SPR based biosensing chips are described in this review article. SPR sensors have the potential to be employed for fast, accurate, and portable SARS-CoV-2 virus diagnosis. To combat the SARS-CoV-2 pandemic, there is considerable interest in creating innovative biosensors that are quick, reliable, and sensitive for COVID-19 diagnosis.
Keywords: Surface plasmon resonance, SARS-CoV-2, 2-D materials, Kretschmann configuration, biosensor
I. Introduction
According to the world health organization (WHO), globally, till 30 March 2022, there have been 464.80 million confirmed cases of COVID-19, including 6.06 million deaths. As of 27 March 2022, 10925.05 million vaccine doses have been administered. To spread this SARS-CoV-2 virus a rapid and accurate detection methods are required so that timely diagnosis must be start. Many of the techniques (like real-time reverse transcriptase polymerase chain reaction (RT-PCR), Antigen, Antibody etc. has been adopted but still the accurate and rapid detection techniques are yet to come, still the research is going on for the fast and accurate detection of SARS-CoV-2 virus. Optical sensing based on Lipid nanoparticles (LNPs) such as gold nanostructures, and iron oxide NPs has been examined, and their addition of nano materials allows for colorimetric, amperometric, and infra-emissive, as well as the development of the use of the sensors to support impedimetric and optametric biosensing, respectively. In order to meet the current demands of cost-effective, fast, and early detection of COVID-19 infection, new advancements in nanotechnology for the field of biosensing are urgently required to meet the challenges of the future.
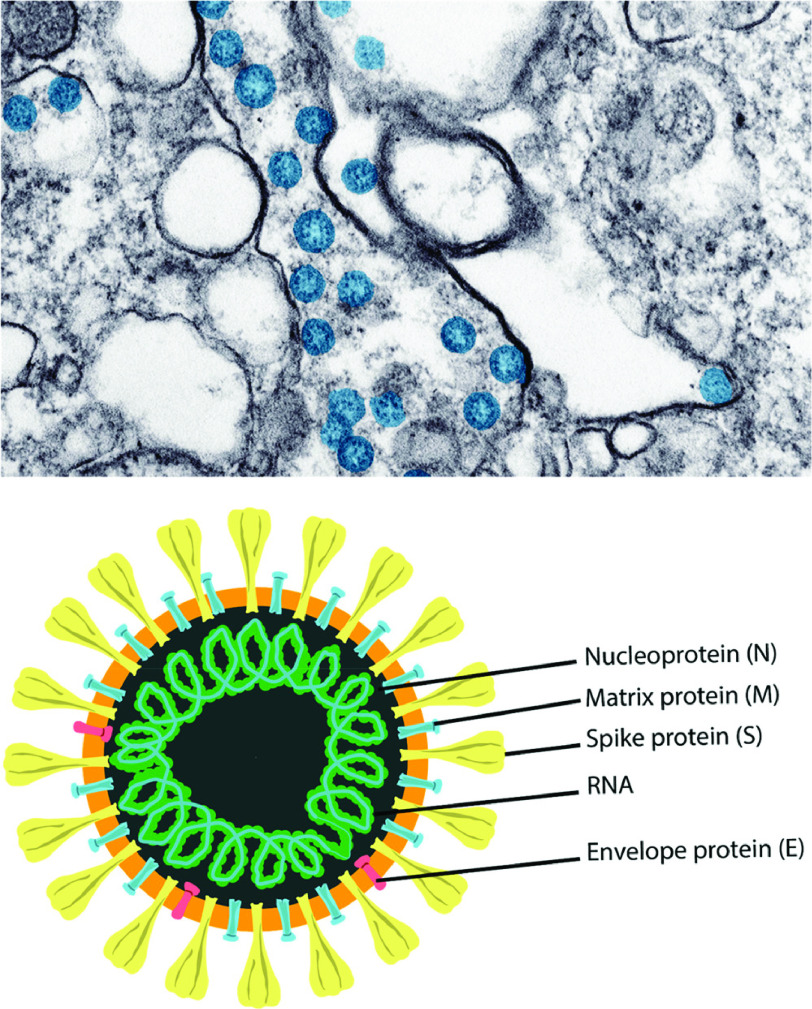
The virus has been colorized in blue, as shown in Fig.1 (adapted from the US Centers for Disease Control). The structural viral proteins that make up the virus structure are used to demonstrate how the virus is represented. SARS-CoV-2 was discovered for the first-time in-patient samples collected in Wuhan, China. Human airway epithelial cells were grown with a virus isolated from Bronchoalveolar Lavage (BAL) fluid collected from patients, and the results were promising. The supernatant from cells that had been injured or killed was collected and studied using negative-stained transmission electron microscopy [1].
Fig. 1.
Schematics form of SARS-CoV-2 and SARS-CoV-2 spherical virus particles in a cell [1].
Many biological samples may be examined for COVID-19 using a quantitative real-time polymerase chain reaction (qRT-PCR), which is labor-intensive and time-consuming, and may not be able to be implemented easily in remote or resource-constrained environments. As a result, it hampered the compilation of reliable data on SARS-CoV-2 infectivity and group distribution throughout the population. However, SPR is a powerful technique that can provide real-time, label-free information on the interaction between two binding partners. It can yield valuable information on the specificity of the interaction, association and dissociation kinetics, as well as binding affinity. The biosensor group at Pharmacia was the first to produce a commercially successful SPR instrument in 1990, resulting in a steady increase in its popularity over the last two decades. Today, it is used in pharmaceutical drug discovery, antibody characterization, proteomics, immunogenicity, food analysis and many other life science areas. Corona viruses infect humans and animals and are responsible for a wide variety of diseases, including respiratory, gastrointestinal, renal, and neurological [2]. Also, in some cases, it has been seen that the virus multiplied the earlier diseases in the body.
The very sensitive 2D materials based SPR sensor for detection SARS-CoV-2 has been discussed. SARS-CoV-2 virus detection using the SPR sensor has been reviewed for its sensitivity and other characteristics. Incorrectly handled quick test kits provide erroneous findings [3]–[6]. To identify SARS-CoV-2 in the future, a quick, precise, and sensitive SPR-derived biosensor is proposed. For more than 30 years, surface plasmon resonance (SPR) has been used to identify biomolecular interactions for therapeutic or scientific purposes, and it’s still going strong [7], [8]. Numerous SPR sensors have been created by industry and are being employed in biosensing applications. These include the Localized SPR (LSPR) biosensor, Optical sensing SPR (OSSPR) biosensor, and Compact SPR (CSPR) biosensor, to name a few examples [9]. Over the last few decades, researchers have become very excited about a new technology called surface plasmon resonance (SPR), which makes use of the interesting light-matter interaction that happens at a metal-dielectric interface. This technology is used to quickly and accurately measure a broad range of chemical, physical, and biochemical parameters. They can detect very low concentrations of chemical and biological species near the surface where they are being measured by monitoring the value of the refractive index in that area, this way real time monitoring can be done. Thus, any physical change at the surface that changes the refractive index will cause a reaction. There are no significant differences in the operating principles of the two types of biosensors. It is commonly known that the SPR sensor is a bio-analytical method that enables the ligand and analyte binding detection to the sensing area in real-time [8]. Because varying concentrations of the analyte bind to different types of glass, the refractive index (RI) fluctuates [10].
II. SARS-CoV-2 Virus Detection BY Surface Plasmon Resonance (SPR)
SPR is a strong technique for obtaining real-time, label-free data on the interaction of two binding partners. It can provide useful information on the interaction’s specificity, association and dissociation dynamics, as well as binding affinity. Today, it is also used in pharmaceutical drug discovery, antibody characterization, proteomics, immunogenicity, food analysis and many other life science areas. The SPR chip only improves its output signal when it has its target SARS-CoV-2 RNA in it. So, it’s very important to come up with simple, quick, but sensitive, and cost-effective ways to quickly detect the CORONA virus. COVID-19 quickly came to be known as the result of a corona virus that later became known as SARS-CoV-2 [11]. The large number of new human corona virus cases reported in recent years has demonstrated that there is a high demand for rapid diagnosis, rapid contact tracking, and intense containment, despite the fact that there is no proper vaccine or treatment available for the virus at this time. Attempts to accurately research the spread of SARS-2 illnesses and infections in the general public may be made more difficult as a result of this decision.
The SPR sensing chip will be one of the approaches that will be used to diagnose SARS-CoV-2 in the near future. In the current global financial crisis, it is necessary to develop more effective and precise diagnostic methods. It is typical practice to employ surface plasmon to detect the RI shift of a sensor layer after a chemical has been bound. It is the electromagnetic (EM) resonance of the free electron oscillations combined with the plasmon metal–dielectric semi-infinite interface [12], which would be made of silver and gold in the visible spectrum. This resonance generates a linked surface EM field at the interface of metal–dielectric layer, which decays exponentially in both medium as a result of the resonance. Due to the high sensitivity of this field to the Refractive Index (RI) shift of the dielectric layer, it has the potential to be employed as a detecting layer for SPR-based sensors [13], [14]. It is important to have a coupling medium in order to give the appropriate photon momentum along the interface in order for SPR to work.
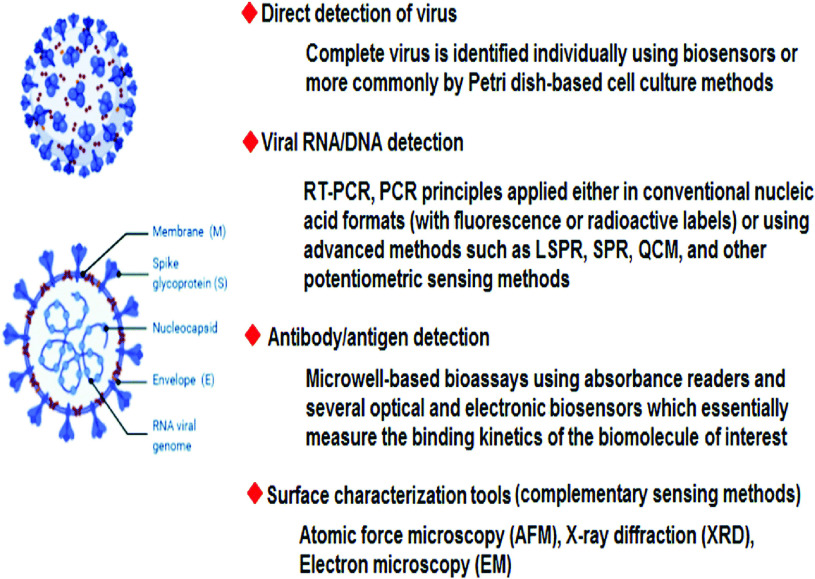
Additionally, as illustrated in Fig.2, significant effort has been undertaken to identify and treat the spread of disease in the community. SARS-CoV-2 has been detected using a variety of testing and diagnostic equipments, including point-of-care (POC) tests, and thermal screening guns, enzyme-linked immunosorbent assay (ELISA)-based immunoassays, and real-time reverse transcriptase polymerase chain reaction (PCR), chemiluminescence, and the characterization of antibody and cellular responses to viruses in the general population are currently being studied. These methods, on the other hand, have a number of disadvantages and constraints, including high prices, a lack of specificity, false-negative and false-positive results, and a lengthy testing period [1], [21]–[23].
Fig. 2.
Several significant biosensing and surface analysis techniques capable of detecting SARS-CoV-2 [15].
This can be performed by employing a grating waveguide, a high index prism, or an optical fibre, among other techniques [24]–[30]. SPR is traditionally achieved with the help prism coupling phenomenon (also known as the Kretschmann configuration) [31] by using a high-index prism when the incident light at one side of gold film’s interface flows via prism, allowing for absolute internal reflection at the prism/metal interface. The prism-based SPR sensors are easier to implement experimentally than to fabricate. It is simple to find a high refractive index prism with the necessary properties in the market. The sensing chip is created using a multi-layered structure that is then coated with the necessary materials with a high refractive index glass. The spin-coating mechanism is used to put the DBL layer onto the glass substrate. Utilizing this mechanism, it is possible to deposit 2D materials. The SPR chip is then attached to the prism’s flat surface using index matching gel. The SARS-CoV-2 protein was transported to the top flow cell on the sensor chip. Using a prism, light can be launched at one surface and collected at the other surface for detection. The sensor’s performance can be achieved at the detector. SPR’s sensitivity may be determined by observing how the angle changes as the refractive index changes [32]. SPR imaging [33] was used to demonstrate the possibilities of sensing technology in order to demonstrate its utility. In this particular instance, the sensor was constructed from DNA that is complementary to the complementary RNAs of the virus. The findings of the study proved the utility of RNA-binding agents in comparison to the gold sheet. One problem with the study was that it had two flaws: Because the created SPR array was not resistant to denaturing chemicals, the technique had to be maintained at a consistent rate as a result. It was discovered that the barley stripe mosaic virus-infected plants were not virus-free after using a “Phychip” sensor to detect their status [34]. The SARS-CoV-2 spike glycoprotein’s receptor-binding domain (RBD) was particularly utilized in the development of an aptasensor [35]. Whereas, the SARS-CoV-2 binding kinetics to RNA was investigated in P phase of SARS-protein [36]. Nonphorylated and phosphorylated N proteins revealed identical binding affinity for RNA; however, phosphorylated N protein had a greater binding affinity for RNA as compared to nonphorylated N protein. Not only did the virus’s critical portion bind to the N protein, but it may also have aided the binding of additional domains to the protein. In addition to the creation of nano-based detection techniques based on antigen-binding/colorimetric tests, the functionalization of nanomaterials with nucliec acids or antibodies allows the development of light/photothermal systems and platforms, as illustrated in Fig.3. There have been several research conducted in the past that have looked into different biosensors for the SARs-COV-2 virus detection, as shown in Table I. Aside from that, chimeric SARS-CoV-2, and SARS 2 RBDs were employed for ACE2 restraints, and the binding activity of the SARS-CoV-2 to the ACE2 was increased as a result of the integration of an N–O bridge into the chimeric SARS–CoV-2 [37]. A syringe containing 300
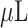 of running buffer or undiluted human serum from human plasma was used to inject the SARS-CoV-2 anti-nucleocapsid antibodies into the SPR sensor. The shift in SPR was computed using the response unit (RU) difference between the beginning and the end of the experiment. Regeneration of the sensor was possible three times using 10 mM of Glycine at pH 2.2 [38]. Other research developed an SPR sensor to identify the coronavirus by fusing gold-binding polypeptides (GBPs), and the GBP fusions were utilized to discover the coronavirus [39]. In addition, the affinity of chimeric SARS-CoV is larger than that of SARS-2 Corona antigen. There are numerous approaches for chemical sensing that have been developed on the basis of SPR sensors [40], [41].
of running buffer or undiluted human serum from human plasma was used to inject the SARS-CoV-2 anti-nucleocapsid antibodies into the SPR sensor. The shift in SPR was computed using the response unit (RU) difference between the beginning and the end of the experiment. Regeneration of the sensor was possible three times using 10 mM of Glycine at pH 2.2 [38]. Other research developed an SPR sensor to identify the coronavirus by fusing gold-binding polypeptides (GBPs), and the GBP fusions were utilized to discover the coronavirus [39]. In addition, the affinity of chimeric SARS-CoV is larger than that of SARS-2 Corona antigen. There are numerous approaches for chemical sensing that have been developed on the basis of SPR sensors [40], [41].
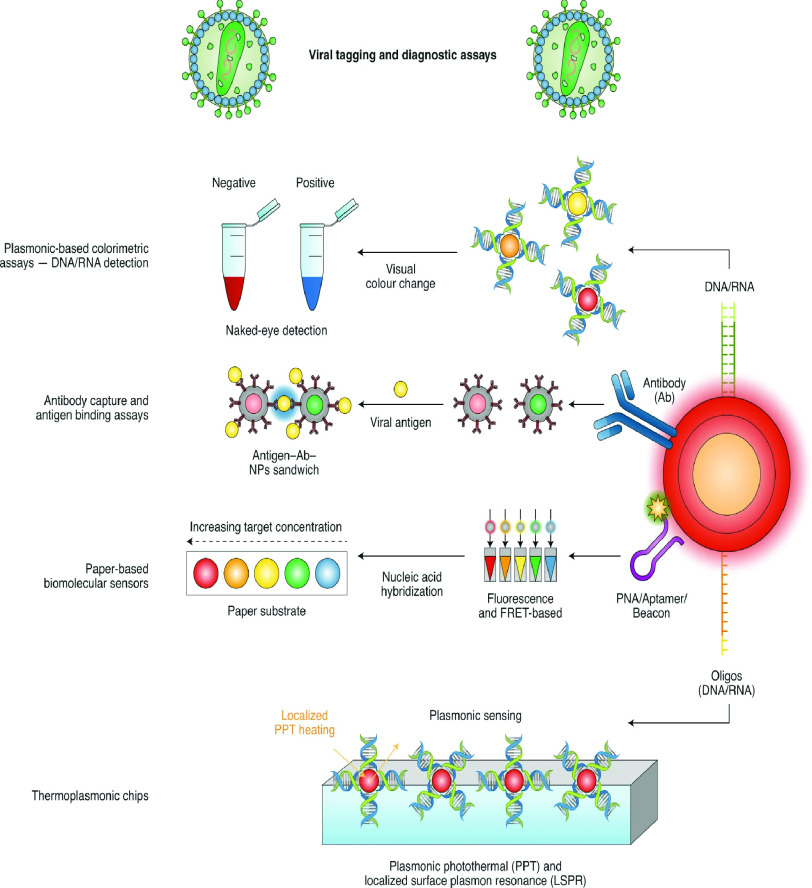
Fig. 3.
SARS-CoV-2 detection utilising nanoparticles based on viral labeling as well as diagnostic procedures: Photothermal treatment (PPT); Peptide nucleic acid (PNA); Localized surface plasmon resonance (LSPR); F‘orster resonance energy transfer (FRET); Nanoparticles (NPs); Antibody (Ab) [16].
TABLE I. SARS-COV-2 Detection Biosensing Methods and Their Characteristics [15].
| Type of Biosensor | Characteristics | Reference |
|---|---|---|
| Plasmonic biosensors | Many clinically relevant analytes may be detected using these biosensors, which are label-free and extremely sensitive. Human serum samples can be used directly for the detection of nucleocapsid antibodies (specific to SARS-CoV-2) using a SPR biosensor. | [3] |
| Field-effect transistor (FET)-based biosensing | A number of anticipated benefits of FET-based biosensors include the ability to be very sensitive and detect tiny amounts of target analyte immediately. Clinical analysis, point-of-care testing, and on-site diagnostics all have the ability to revolutionize from these biosensors. | [18] |
| Electro-chemical biosensors | For its high sensitivity/specificity, simplicity, low cost, ease of use and ability to be miniaturised and bulk produced in large quantities, electrochemical biosensors are popular among researchers. These biosensors can also be used in homes and clinics because of their point-of-care (POC) capabilities. | [19] |
| Surface-enhanced Raman scattering (SERS)-based biosensors | Scientists employ these biosensors due to their ability to detect analytes with remarkable sensitivity and precision utilising SERS-encoded nanoparticles (SERS tags) instead of colloidal gold. The adsorbed Raman reporter dyes, the gold/silver nanoparticle substrates, and the exact antibodies that attach to their respective targets—these are the main components of SERS tags. | [20] |
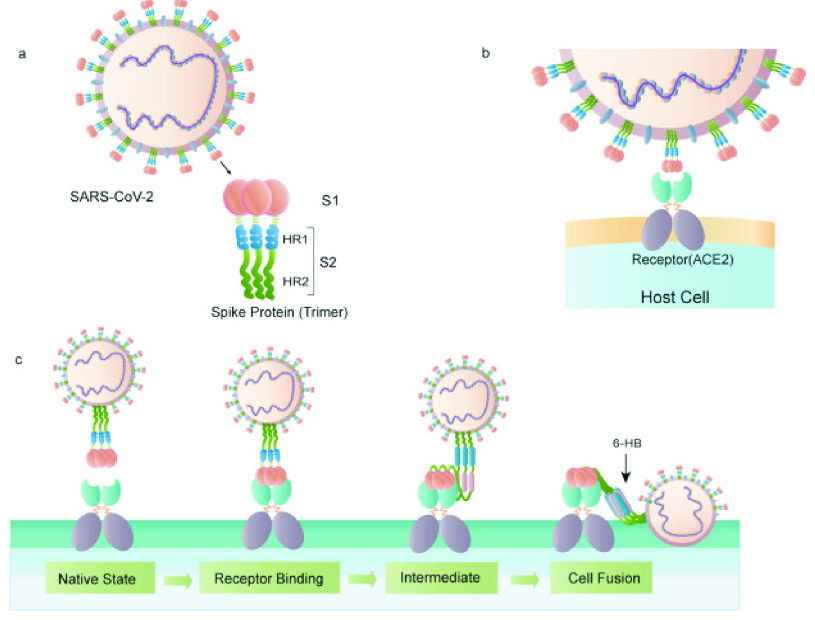
In Fig.4, trimers of the S protein form a unique bulbous, crown-like halo around the virus particle, indicating that the virus particle is infected. ACE2 is also a known SARS-CoV-2 receptor, as previously stated. As shown in Fig.4a and Fig.4b, the SARS-CoV S protein’s S1 sub unit interacts with ACE2 to promote the formation of endosomes, which is necessary for the initiation of viral fusion activity at low pH [42]. Cleavage of the S protein allows the SARS-CoV-2 FP to be revealed, which ultimately results in viral fusion. After being exposed to the action of some specific ligands, the fusion protein undergoes a conformational shift and inserts into the host cell membrane, as illustrated in Fig.4c [43]. Once the virus has entered the cell, it releases viral RNA, polyproteins are translated from the RNA genome, and the viral RNA genome is duplicated and transcriptionally transcribed by the assembly of the replicase–transcriptase complex, which is cleaved by proteins and assembled by the virus. Viral RNA is replicated in the host cell, and structural proteins are synthesized, assembled, and packed, followed by the release of viral particles [44]. Viral particles are released after they have been assembled and packed. SPR-based virus detection techniques that rely on the antigen–antibody response are summarised in Table II, with an emphasis on the materials utilizes to form the thin layers, as well as their thickness and virus detection sensitivity. Despite the fact that other publications employed a two-layer construction with unique metallic substrates, the overall thin film’s thickness was typically around 50 nm for viral detection devices. It is the invention of thin metal films that have resulted in numerous practical instances of high-sensitivity SPR detection of biological molecules [45]–[47]. Su et al. and Chang et al. [48], [49] have previously characterized the bilayer structural thin films of gold and silver, respectively. In the literature, there are only a few examples of how the thin film structure makes SPR-based virus detection more sensitive. The most interesting thing about this structure is that a gold film has been put over the silver film, which has an antioxidant impact on the structure. SPR signal response materials such as silver are excellent, however, they are easily oxidized. When ultrathin gold films with less than 10 nm thickness were made, however, the formation of nanoparticles and structural changes were observed [50]. It is also necessary to have long-term stability in order to identify viruses effectively.
Fig. 4.
Structure of the SARS-CoV-2 S protein. (a) The S protein’s schematic structure, (b) The S protein connects with the ACE2 receptor, and (c) The S protein promotes the binding and fusion process of virus and cell [17].
TABLE II. Comparing the Thickness and Material of Each Layer With Respect to the Detection of Limit (LOD) of the SPR Sensor Used to Detect Viruses [51].
| Layer Structure | Layer Thickness | Target Virus* | LOD | References |
|---|---|---|---|---|
| Gold thin Film | 50 nm | Influenza Virus | 193.3 ng/mL | [52] |
| Gold thin Film | 50 nm | Dengue Virus | 0.08 pM | [53] |
| Gold thin Film | 50 nm | Ebola Virus | 0.5 pg/mL | [54] |
| Gold/Silver thin Film | 8/37 nm | Influenza Virus | 30 PFU/mL | [48] |
| Gold/Silver thin Film | 10/35 nm | Influenza Virus | 144 copies/mL | [49] |
| Platium-di-selenide/Gold thin Film | 2/48 nm | COVID-19 | 1.95 nM | [55] |
III. Advancements in Prism Based SPR Sensors Performance Used for SARS-CoV-2 Detection
A. BK7/Au/PtSe2 /Graphene Film-Coated SPR Sensor
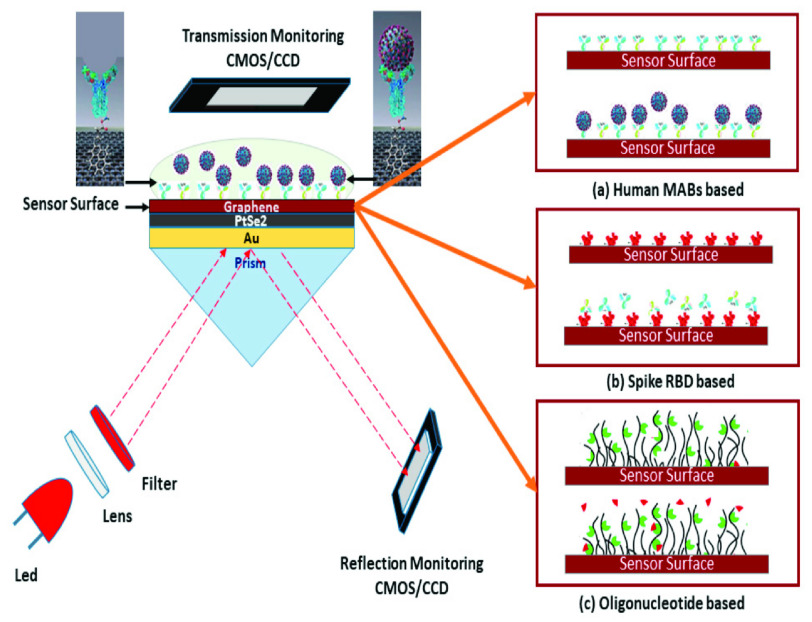
To detect the new Coronavirus, Aakib et al. [55] presented a highly sensitive SPR biosensor coated with graphene-based multiple-layer (BK7/Au/PtSe2/Graphene) materials. The proposed sensor uses total internal reflection (TIR) to detect ligand-analyte immobilisation in the detection area in real time. Changes in the ligand and analyte concentrations affect the RI of the sensing area, which has an effect on the excitation of surface plasmon polaritons (SPPs) of the multilayer sensor interface. The proposed sensor’s performance was investigated numerically using the transfer matrix method (TMM) and the finite-difference time domain (FDTD) approaches, respectively. The SPR biosensor that has been proposed allows for the rapid and exact detection of the COVID-19 virus at an early stage, which is important for preventing the spread of the pandemic. In addition, the outcomes of the proposed sensor was numerically studied using three distinct analytes and ligands, i.e., virus anti-spike proteins (IgM, IgG) as analytes along with virus spike RBD as ligand, the COVID-19 virus spike receptor-binding domain (RBD) as analyte along with the monoclonal antibodies (mAbs) as ligand and virus single-standard ribonucleic acid (RNA) as analyte along with the specific probe as ligand. Following an investigation, it was discovered that the proposed sensor has a sensitivity of 183.33°/refractive index unit (RIU) in SPR angle (SPR) and 833.33 THz/RIU in SPR frequency (SPRF) for detection of the SARS-COV virus spike RBD; 153.85°/RIU in SPR angle and 726.50 THz/RIU in SPRF for detection of the anti-spike protein; and finally, 140.35°/RIU in SPR angle and 500 THz/RIU in SPRF for viral RNA detection, it has been demonstrated that the total virus spike RBD detection method is more sensitive than the other two detection techniques. To greatly improve the sensitivity and plasmonic features of the GoosHänchen (GH) shift detection of a typical SPR sensor, highly sensitive two-dimensional (2D) nanomaterials were utilized in conjunction with each other [55].
The Kretschmann-Raether design is depicted in Fig. 5 as a five-layer detecting zone for the sensor. Using a CCD (charge-coupled device) as a monitoring device and incident light from monochromatic He–Ne laser at an acceptance angle onto a prism (Bk7), we can detect changes in the environment. The first and the second layer are made of a BK7 and a gold (Au) thin film where the calculated RI of Au (
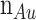 ) and the layer thickness (
) and the layer thickness (
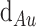 ) are
) are
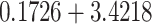 *i and 50 nm, respectively [7], [8]. By using the Kretschmann configuration, the first, second and third layers are organised of BK7 with
*i and 50 nm, respectively [7], [8]. By using the Kretschmann configuration, the first, second and third layers are organised of BK7 with
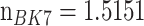 , Au thin film and lastly, made up of platinum-di-selenide (PtSe2), with a RI (
, Au thin film and lastly, made up of platinum-di-selenide (PtSe2), with a RI (
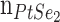 ) and a coating thickness are
) and a coating thickness are
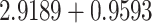 *i and 2 nm, respectively [56]. The fourth layer is graphene, and the RI of this layer is calculated as
*i and 2 nm, respectively [56]. The fourth layer is graphene, and the RI of this layer is calculated as
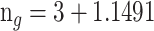 *i, and the coating thickness of the graphene layer is calculated as
*i, and the coating thickness of the graphene layer is calculated as
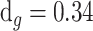 *L nm, where “L” is the number of graphene layers [7]. It is possible to utilise PBS (pH 7.4) as a VTM; the RI of PBS is calculated as
*L nm, where “L” is the number of graphene layers [7]. It is possible to utilise PBS (pH 7.4) as a VTM; the RI of PBS is calculated as
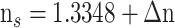 , where
, where
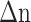 is a changing value due to the interaction of the ligand and analyte on the sensing surface. Authors presented a graphene-based multilayer coated SPR sensor for the COVID-19 virus detection in its early phases, which was developed by the authors. After being investigated for rapid diagnosis, the suggested sensor demonstrated the ability to distinguish between infected and uninfected persons with high accuracy and precision. With 183.3°/RIU sensitivity as compared to other conventional sensors, the SPR sensor made of BK7/Au/PtSe2/Graphene film-coated displayed superior performance.
is a changing value due to the interaction of the ligand and analyte on the sensing surface. Authors presented a graphene-based multilayer coated SPR sensor for the COVID-19 virus detection in its early phases, which was developed by the authors. After being investigated for rapid diagnosis, the suggested sensor demonstrated the ability to distinguish between infected and uninfected persons with high accuracy and precision. With 183.3°/RIU sensitivity as compared to other conventional sensors, the SPR sensor made of BK7/Au/PtSe2/Graphene film-coated displayed superior performance.
Fig. 5.
Schematic diagram of the five-layered (Bk7/Au/PtSe2/Graphene/ PBS) SPR biosensor for examination of SARS-CoV-2 produced virus; the SARS-CoV-2 virus can be detected using three modes of operation: (a) human mAbs immobilised on a viral spike RBD allow for fast detection of the whole virus spike (spike RBD as analyte and mAbs as ligand), (b) fast mAbs recognition using immobilized virus spike RBD (mAbs as analyte and spike RBD as ligand), and (c) viral RNA sequence detection on the graphene-implanted sensor surface with immobilised probe sequences [55].
B. BK7/Ag/Si/BaTiO3 Film-Coated SPR Sensor
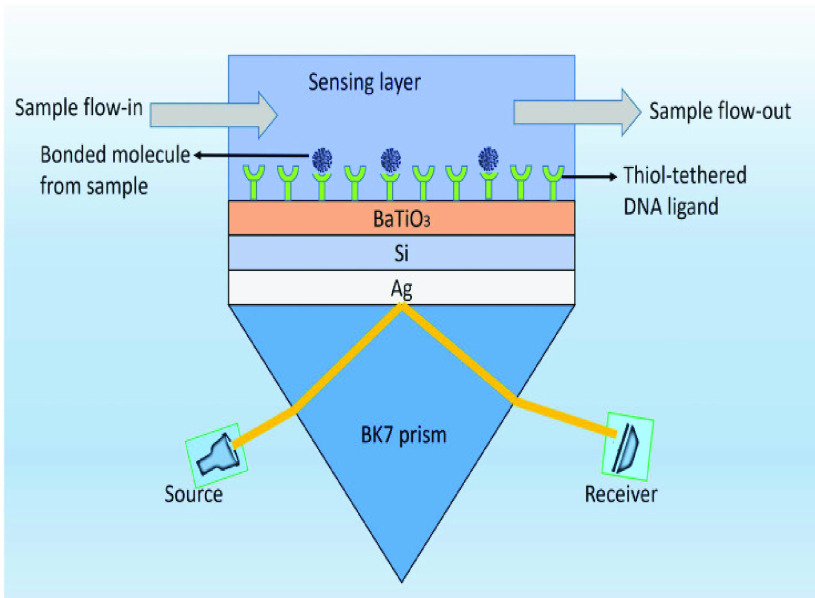
Uddin et al. [57] constructed a structure based on the Kretschmann configuration to detect coronavirus-2 in real time by layering silicon and BaTiO3 on top of Ag. In order to characterize the sensor response in terms of sensitivity, full width at half maximum, and minimum reflection, a comprehensive numerical analysis was performed utilizing the transfer matrix method (TMM) and the finite-difference time-domain (FDTD) technique. When the suggested design for SARS-CoV-2 detection is compared to the basic Kretschmann setup, it shows a 7.6-fold improvement in sensitivity. Additionally, with a figure of merit of 692.28°/RIU, the structure outperforms existing competitive SPR structures in both wavelength and angular interrogations, demonstrating persistent superiority over the competition.
Fig.6 depicts a schematic representation of the high sensitivity SPR biosensor setup that has been presented. This unique heterostructure, as depicted in the image, is made up of five separate layers that are interconnected. As a coupling prism, we employ BK7 glass, which is then followed by an Ag layer. Because of the greater SPR ratio of Ag, it has been demonstrated to have improved sensitivity when used as a substrate layer [58].
Fig. 6.
Five layered schematic structure of proposed SPR sensor [57].
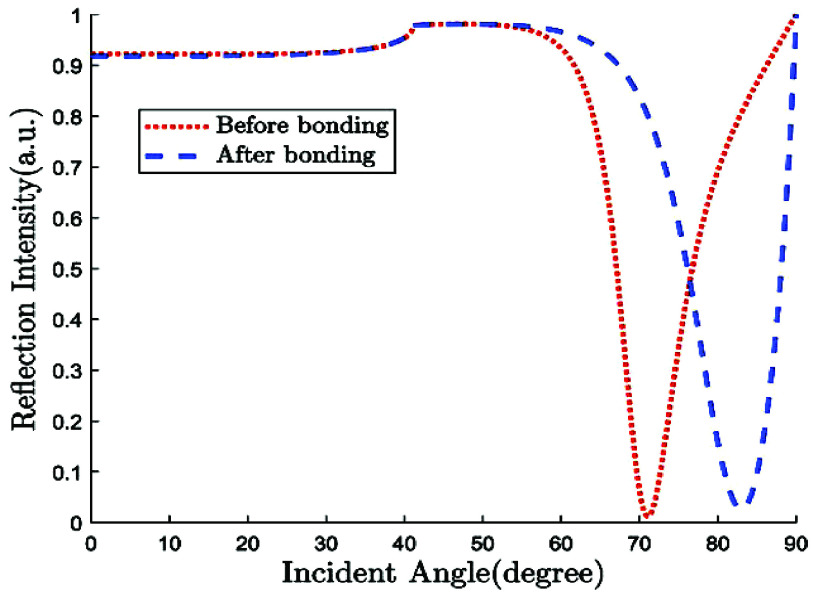
On top of Ag, the structure has three layers, each of which is composed of Si, BaTiO3, and thiol-tethered DNA, respectively. A design like this also contributes in the improvement of sensitivity. Recent research has demonstrated that Si has great potential for increasing sensitivity [59]. Because of Si’s capacity to improve the electric field strength of excitation light, this sensitivity increase has been shown to be a significant factor [60], [61]. Because of its high dielectric constant, BaTiO3 has also been shown to have beneficial impacts on the sensitivity. In conjunction with reduced dielectric loss, the proposed structure has the ability to make a considerable contribution to improving the sensitivity of the proposed structure [62]. SARS-CoV-2 receptor SARS-tethered DNA is employed as a ligand layer in the sensing medium since it has demonstrated outstanding capabilities as a SARS-CoV-2 receptor [3], [37]. For this suggested sensing method, samples from human nasopharyngeal swabs are sent via the sensing channel prior to analysis in a liquid medium. A considerable angle shift in SPR (as high as 12 degrees) is seen when SARS-CoV-2 RNA (RdRp-COVID sequence) from a sample hybridises with thiol-tethered DNA of receptor molecules, as illustrated in Fig. 7. In this study, it was determined that it was a novel SPR-based biosensor for the detection of SARS-CoV-2 in a label-free and real-time manner. In order to optimise the structure and analyse its performance, a large number of numerical simulations have been carried out. The following parameters have been reached for the suggested structure to detect the SARS-CoV-2 RNA: Sensitivity
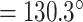 /RIU, FWHM
/RIU, FWHM
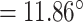 ,
,
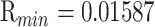 , and FOM
, and FOM
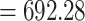 RIU
RIU
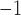 , respectively.
, respectively.
Fig. 7.
Reflectance curve for SARS-COV-2 detection [57].
C. Gold Nanospikes Film-Coated SPR Sensor
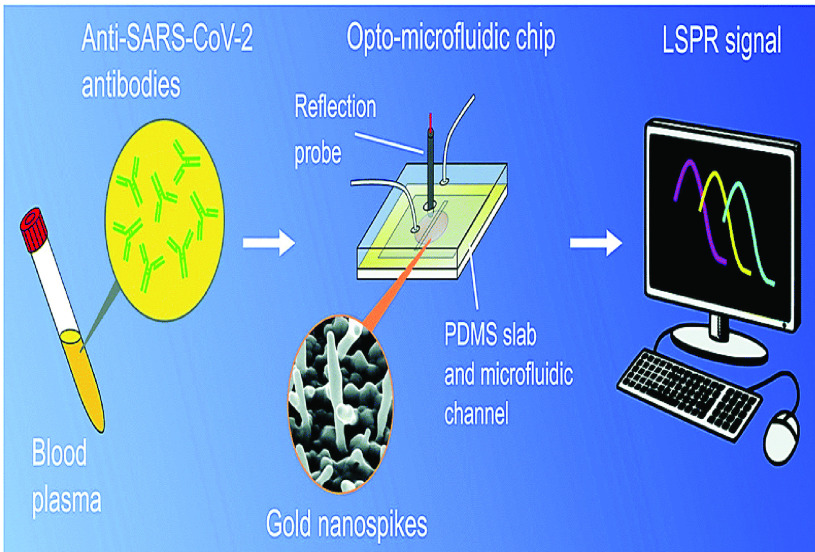
The use of gold nanospikes was used in one study to develop an opto-microfluidic sensing device that could detect the presence and concentration of antibodies specific to the SARS-CoV-2 spike protein in one litre of human plasma diluted in one millilitre of buffer solution [55]. According to the results of the antigen–antibody interaction, the concentration of the target antibody can be determined by the change in the wavelength of LSPR peak of the gold nanospikes created by this contact [64], [65]. It was established that the label-free microfluidic device has a limit of detection (LOD) of 0.08 ng/ mL, which is below the threshold of detection for clinically relevant concentrations [63]. It was discovered that this approach has potential as a point-of-care diagnostic tool to enhance current serological assays, and that it may make quantitative SARS-CoV-2 diagnoses easier to perform, cheaper to perform, and faster to perform. Validation of the sensor for COVID-19 antibody tests, as well as optimization of the electrode position process to manufacture gold nano-structures with reduced gap and a higher aspect ratio, is required in order for antibody–antigen binding to cause a larger shift in the LSPR peak and, consequently, enhance the sensor’s signal–to–noise ratio [63]. Antibodies towards the SARS-CoV-2 spike proteins have been detected in diluted human plasma using an opto-microfluidic sensing method which is very sensitive and rapid. Fig.8 depicts a schematic diagram of an LSPR-based sensing technology that incorporates an optical probe in conjunction with gold nanospikes embedded in a microfluidic device.
Fig. 8.
Schematics of the novel opto-microfluidic sensing device setup for the SARS-CoV-2 spike proteins detection in diluted human plasma [63].
D. Plasmonic Photothermal Biosensors
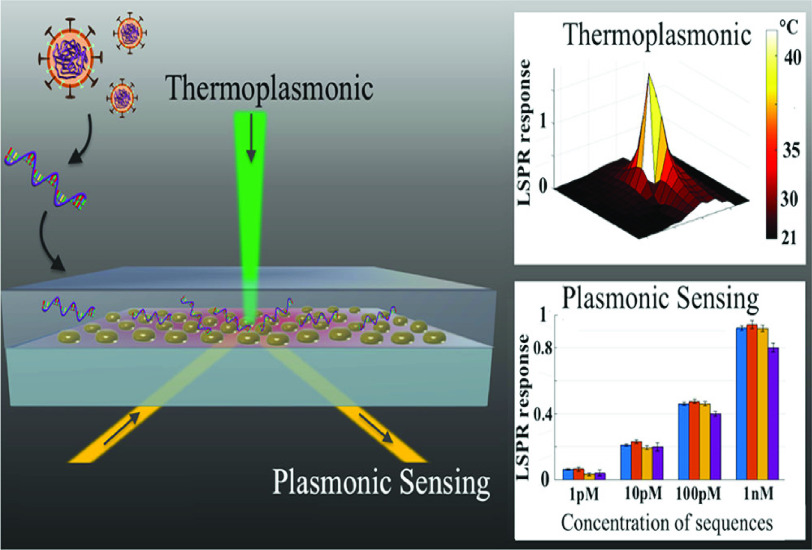
Qiu et al. [3] presented an alternative and effective strategy for the clinical COVID-19 diagnosis, which combines the plasmonic photothermal (PPT) effect with the localized surface plasmon resonance (LSPR) sensing transduction. Gold nanoislands (AuNIs) functionalized with complementary DNA receptors can detect the specific sequences from SARS-CoV-2 with high sensitivity. Thermoplasmonic heat is created on the same AuNIs chip for enhanced sensing capability by illuminating them at its plasmonic resonance frequency. The heat from a localised PPT can raise in situ hybridization temperatures, making it easier to distinguish between two gene sequences that are almost identical. When used in conjunction with an LSPR biosensor, the SARS-CoV-2 sequences may be detected at concentrations of just 0.22 pM, making it possible to accurately identify the target in a multigene mixture. An investigation on enhancement of the thermoplasmonic and its application to nucleic acid testing and highly contagious disease diagnostics is gained. Fig.9 shows the thermoplasmonic setup to detect the SARS-CoV-2 virus.
Fig. 9.
Schematic of thermoplasmonic setup to detect the SARS-COV-2 virus [3].
An LED source created a white light detecting beam that was then linearly polarized by a polarizer (P1). The birefringent crystal (BC) provided enough retardation to the s- and p-components of the linearly polarized light to form the spectral interferogram. The BK7 prism energized the local electromagnetic fields in the region of the AuNIs using the Kretschmann arrangement. For LSPR transduction, the plasmonic resonance wavelength is 580 nm. They were screened by an aperture-iris (I1/I2, Thorlabs) with a 0.5 mm hole diameter before being recorded by the spectrometer (AvaSpec, Avantes). A high-power 532 nm laser diode (LD, 532 nm peak wavelength, DJ532–40 Thorlabs) was utilised to heat the AuNI chips for PPT heating. A 552 nm long-wavelength pass filter (LPF) blocked the excitation signal prior to the spectrometer. For LSPR temperature calibration, digital temperature sensors (SHTC1, Sensirion) were used. To hybridise the target DNA with the immobilised probe,
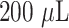 of nuclease-free water was injected into the AuNI microfluidic chamber for 800 seconds. The sensing beam reaching the spectrometer, which related to the ATR light from the PPT heat centre, was screened by a 0.5 mm aperture-iris. The dual-functional LSPR biosensors were used to conduct an investigation on misaligned nucleic acids and multisequence combinations. After hybridization, the buffer was cleansed with nuclease-free water. The spectrometer for plasmonic phase detection captured the entire testing process [3].
of nuclease-free water was injected into the AuNI microfluidic chamber for 800 seconds. The sensing beam reaching the spectrometer, which related to the ATR light from the PPT heat centre, was screened by a 0.5 mm aperture-iris. The dual-functional LSPR biosensors were used to conduct an investigation on misaligned nucleic acids and multisequence combinations. After hybridization, the buffer was cleansed with nuclease-free water. The spectrometer for plasmonic phase detection captured the entire testing process [3].
E. BK-7 Prism-Ag-Si
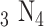 -BP-ssDNA and PBS Solution Based SPR Sensor
-BP-ssDNA and PBS Solution Based SPR Sensor
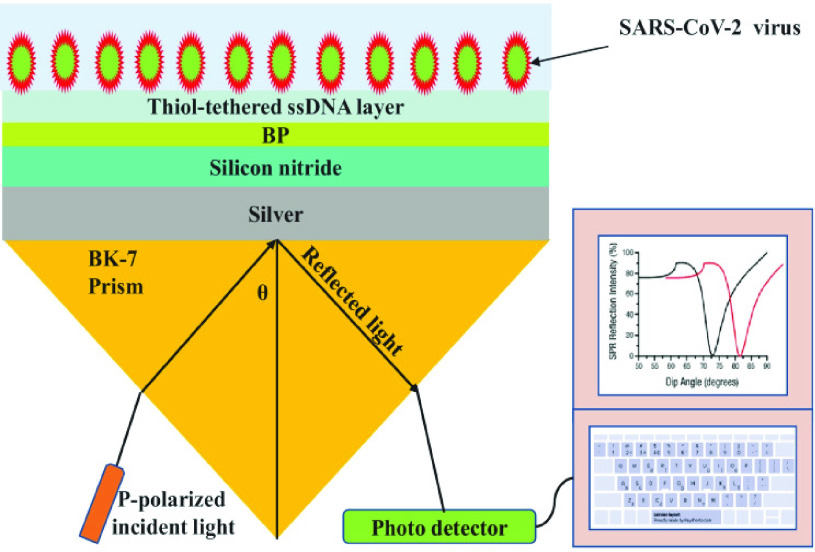
Similarly, Kumar et al.
[66] proposed a numerical analysis of four layer SPR biosensor for the rapid SARS-CoV-2 virus detection at first layer silver (
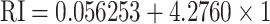 *i, thickness
*i, thickness
 nm) has been deposited on BK7 prism (RI
nm) has been deposited on BK7 prism (RI
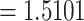 ), the second layer chosen Si3N4 (RI
), the second layer chosen Si3N4 (RI
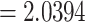 , thickness
, thickness
 5 nm), the third layer deposited of other 2D material BP (
5 nm), the third layer deposited of other 2D material BP (
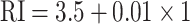 *i, thickness
*i, thickness
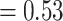 nm) for the sensing layer, phosphate-buffered solution (PBS) can be used as a RI of PBS that is deliberated as
nm) for the sensing layer, phosphate-buffered solution (PBS) can be used as a RI of PBS that is deliberated as
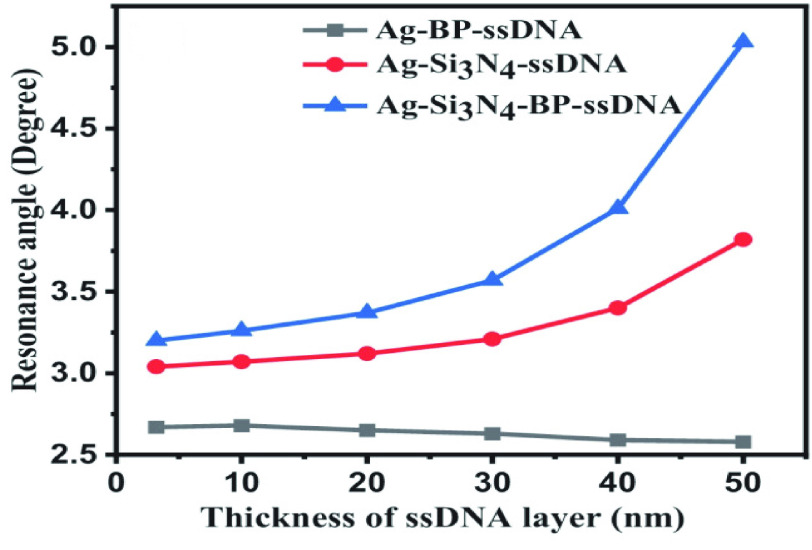
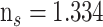 to 1.355 with thickness of 3.2 nm. The performances of the SPR sensor were investigated at the wavelength of 633 nm. The highest sensitivity obtained with the proposed structure, as shown in Fig.10 was 154°/RIU. The SARS-CoV-2 virus was selectively attached using the ssDNA layer as a bioreceptor sensing element. SARS-CoV-2 viruses were detected in this research using phosphate-buffered solution (PBS) solution. When SARS-CoV-2 viruses are added to a PBS solution, the solution’s refractive index changes from 1.334 to 1.355. One of the most interesting things about this experiment is the ability of the SARS-CoV-2 S glycoprotein to dissolve in a running buffer consisting of 10 mM hydroxy ethyl piperazine ethane sulfonic HEPES and NaCl solution at a concentration of 120 mM. Fig.11 shows different structures with a variation in ssDNA thickness and it is clearly noted the sensitivity increased with increasing in the ssDNA thickness.
to 1.355 with thickness of 3.2 nm. The performances of the SPR sensor were investigated at the wavelength of 633 nm. The highest sensitivity obtained with the proposed structure, as shown in Fig.10 was 154°/RIU. The SARS-CoV-2 virus was selectively attached using the ssDNA layer as a bioreceptor sensing element. SARS-CoV-2 viruses were detected in this research using phosphate-buffered solution (PBS) solution. When SARS-CoV-2 viruses are added to a PBS solution, the solution’s refractive index changes from 1.334 to 1.355. One of the most interesting things about this experiment is the ability of the SARS-CoV-2 S glycoprotein to dissolve in a running buffer consisting of 10 mM hydroxy ethyl piperazine ethane sulfonic HEPES and NaCl solution at a concentration of 120 mM. Fig.11 shows different structures with a variation in ssDNA thickness and it is clearly noted the sensitivity increased with increasing in the ssDNA thickness.
Fig. 10.
Schematic arrangement of BK-7 prism-Ag-Si{3}N{4}-BP-ssDNA and PBS solution with optimised thickness as sensing medium [66].
Fig. 11.
Sensitivity vs. change in thickness of ssDNA layer [66].
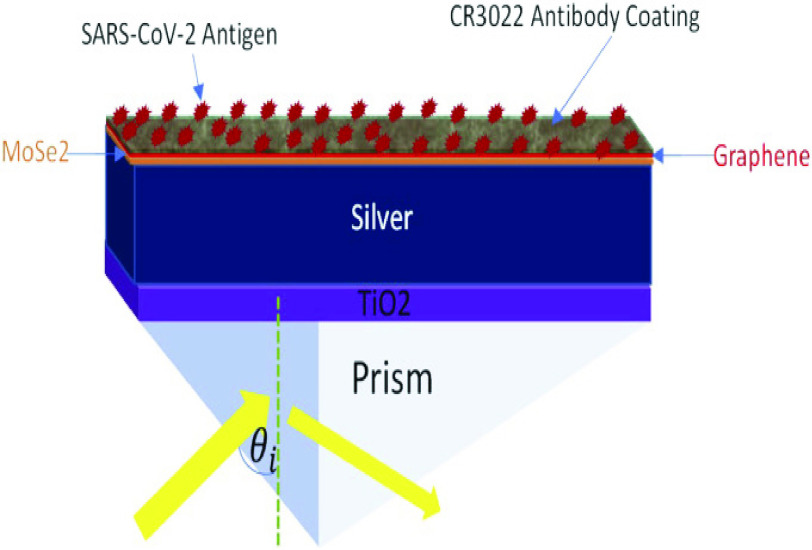
F. Prism Based Structure for SARS-COV-2 Detection Using TiOSi2 -Ag-Graphene Layers
As shown in Fig. 12, Moznuzzaman et al.
[67] presented a six-layer sensor structure. Silver is used in this sensor because of its plasmonic properties. In this setup, a silver layer is sandwiched between a MoSe2-graphene composite layer and a TiO2 thin sheet. Higher operating frequency increased optical nonlinearity and overall performance while minimising the Kerr effect [68]. Since all refractive index values of each material are examined at 633 nm wavelength. The proposed multilayered structure has shown better performance. An F.O.M. of 54.04 RIU
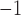 and a sensitivity of 194°/RIU were found, with a detection accuracy of about 0.2702 [67].
and a sensitivity of 194°/RIU were found, with a detection accuracy of about 0.2702 [67].
Fig. 12.
Prism based structure for SARS-COV-2 detection using TiO2-Ag-graphene layers [67].
IV. Future Perspectives
Rapid, easy, and large-scale diagnosis are required for the treatment and management of COVID-19 (especially in asymptomatic and/or early-stage patients) originating from SARS-CoV-2, as well as for the reduction and control of the virus’s transmission. As a rule, traditional methods for detecting respiratory viruses are expensive and time-consuming, requiring specialized laboratory equipment and highly-trained staff members. It is possible that biosensors will one day be used to detect diseases such as SARS-CoV-2 and associated viral infections, as well as other diseases, with excellent sensitivity and selectivity at a cheap cost. The identification of a focused sickness biomarker at low levels, according to this, SPR biosensor can be tremendously beneficial for enhancing the assessment of viral disease progression, disease management, and treatment options. New biosensors for viral samples are being developed using no labeling approaches like as surface plasmon resonance (SPR), Surface Enhanced Raman Scattering (SERS), and Quartz-Crystal Microbalance (QCM). SPR, SERS, and QCM technologies have all showed promise in the creation of new biosensors. The use of SPR technology in the creation of new biosensors for viral samples has also showed promise. At the moment, such biosensors are being utilized to identify RNA viruses such as influenza A/B, SARS-Corona, Ebola virus, MERS, Zika virus, and Dengue virus. A peptide monolayer was used to detect anti-SARS-CoV-2 antibodies, which were detected in the nM range, utilizing an SPR sensor covered with a peptide monolayer and functionalized with a recombinant protein from the SARS-CoV-2 nucleocapsid. It is now possible to diagnose bimolecular interactions in real time with the use of SPR, an optical sensing technology that was developed. The presence of a coupling medium that passes through the contact is required for the photon energy excitation to work properly. It is feasible to analyse single molecules by utilizing optical system shape structures such as grating structures, waveguide, localized surface plasmon (LSPR), prism coupling, and fiber optic structures.
TABLE III. The SPR Angle Shift for Various Doses of SARS-COV-2 S-Glycoprotein Solution in Contact With the Graphene/CR3022 Antibody [67].
| Concentration of SARS -CoV-2 S-glyco -protein solution (nM) | Refractive index of SARS -CoV-2 S-glyco -protein solution (RIU) | SPR angle,
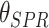 (deg) (deg) |
SPR angle shift,
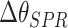 (deg) (deg) |
Cumulative shift of the resonance angle, C
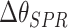 (deg) (deg) |
|---|---|---|---|---|
| 0.0000 | 0.0000 | 77.28 | 0.00 | 0.00 |
| 0.0001 | 0.0220 | 77.32 | 0.04 | 0.04 |
| 0.0010 | 0.0660 | 77.40 | 0.08 | 0.12 |
| 0.0100 | 0.1760 | 77.61 | 0.21 | 0.29 |
| 0.1000 | 0.3080 | 77.87 | 0.26 | 0.51 |
| 1.0000 | 0.4840 | 78.21 | 0.34 | 0.85 |
| 10.000 | 0.7040 | 78.64 | 0.43 | 1.28 |
V. Conclusion
The worldwide pandemic of COVID-19 has a significant impact on human existence. There has been a significant increase in the number of infections among people all around the world in recent years. In response to this international catastrophe, some countries, governments, and researchers are attempting to adjust their systems. It is the purpose of this review to provide an understanding of SARS-CoV-2 infection transmission as well as knowledge of the state-of-the-art diagnostic methodologies approach based on biosensor applications. According to the findings of this study, the SPR-based biosensor will be in high demand in the future for the detection of ongoing COVID-19 epidemic. As the RT-PCR test has been identified as the most effective method for detecting this virus, but has been also demonstrated to be inaccurate and time-consuming in a few instances. SPR-based sensors have been utilised to detect the SARS-CoV-2 virus in a short period of time. It was discovered that SARS-CoV-2 nucleoprotein phosphorylation causes the virus’ binding kinetics with RNA to be examined during the phosphorylation of SARS-CoV-2 nucleoprotein (N protein). A number of studies have been carried out using an SPR-based sensor to detect the SARS-CoV-2 virus, and it has been discovered that the sensor has extremely good performance in terms of sensitivity and figure of merit.
Acknowledgment
The authors would like to thank Co-PI Dr. Umesh Kumar Tiwari, a Principal Scientist, from the Advanced Materials and Sensors, Council of Scientific and Industrial Research–Central Scientific Instruments Organization (CSIR–CSIO), Chandigarh, India, of the CSIR funded Project titled “Design and Development of Deployable Thin Film-Based Evanescent Field Sensor to Check the Quality of Food From Adulteration” and Prof. Subramaniam Anantha Ramakrishna, the Director, of CSIR–CSIO.
Biographies

Purnendu Shekhar Pandey (Graduate Student Member, IEEE) received the B.Tech. degree in electronics and instrumentation engineering from Dr. A. P. J. Abdul Kalam Technical University, Uttar Pradesh, India, and the M.Tech. degree in digital communication from Uttarakhand Technical University, Dehradun, Uttarakhand, India, in 2013. He has ten years of teaching experience as an Assistant Professor with the Department of Electronics and Communication Engineering. He worked as a JRF under the Indian Space Research Organization (ISRO) sponsored project (Sanction No. ISRO/RES/3/775/18-19) at the Indian Institute of Technology (IIT) (Indian School of Mines) Dhanbad, India. He is currently a Research Scholar with IIT (Indian School of Mines) Dhanbad. He has authored or coauthored 28 research papers and a book. He holds eight patents published and one granted patent. His research interests include optical fiber-based surface plasmon resonance (SPR) sensor, prism-based SPR, nanomaterial-coated fiber Bragg grating (FBG) sensor for chemical and biochemical sensing, and biomedical instrumentation.

Sanjeev Kumar Raghuwanshi (Senior Member, IEEE) received the bachelor’s degree in electronic and instrumentation engineering from the Shri Govindram Seksaria Institute of Technology and Science, Indore, Madhya Pradesh, India, in August 1999, the master’s degree in solid state technology from IIT Kharagpur, Kharagpur, India, in January 2002, and the Ph.D. degree in the field of optics from the Department of Electrical Communication Engineering. He worked as a Postdoctoral Research Fellow with the Instrumentation and Sensor Division, School of Engineering and Mathematical Sciences, City, University of London, London, U.K., from 2014 to 2015. He was the Visiting Scientist with the National United University, Miaoli, Taipei, Taiwan, from June 2014 to July 2014. He was a Visiting Scientist (under INSA Visiting Scientist Fellowship Scheme 2020) with the Department of Electrical Communication Engineering, Indian Institute of Science (IISc), Bengaluru, India, from December 2021 to January 2022. He Works As An Associate Professor With The Indian Institute Of Technology (Iit) (Indian School Of Mines) Dhanbad’s Electronics Engineering Department. In The Previous Ten Years, He Has Published Over 100 Peer-Reviewed And Indexed International Sci Journal Articles. He Has Six Books In The Field Of Current Optical Fibers And Six Indian Patents Have Been Filed And Published In The Last Five Years. Several Central Governments Funding Agencies, Including The Department Of Atomic Energy, Government Of India (Goi); The Indian Space Research Organization (Isro), Goi; And The Council Of Scientific And Industrial Research (Csir), Goi, Have Sanctioned And Executed His Research And Development Projects. He Is A Life Member Of The Institution Of Electronics And Telecommunication Engineers (Iete) And The International Academy Of Physics Sciences, As Well As A Fellow Of The Optical Society Of India (Osi). For His Postdoctoral Degree, He Was Awarded The Erusmus Mundus Scholarship. He Has Contributed To Ieee Transactions on Instrumentation and Measurement, IEEE Sensors Journal, IEEE Photonics Technology Letters, and IEEE Journal of Quantum Electronics as a Reviewer. He serves on the editorial boards of various Indian publications and serves as a reviewer for them. He has been awarded with the “Best Research Award” (Canara Bank Publication Award) by the IIT (Indian School of Mines) Dhanbad from 2016 to 2018. He has been the receipt of International Travel Grants by the Department of Science and Technology, Science and Engineering Research Board (DST-SERB) to attend one international conferences on Photonics 2016, Berlin, Germany, in 2016; and Photonics West SPIE-2020, San Francisco, CA, USA, in 2020.

Azhar Shadab received the B.E. degree in electronics and communication engineering from Rajiv Gandhi Proudyogiki Vishwavidyalaya, Bhopal, India, in 2010, and the M.Tech. degree in electronics and communication engineering from the Jaypee University of Information Technology, Himachal Pradesh, India, in 2012. He is currently pursuing the Ph.D. degree with the Electronics Engineering Department, Indian Institute of Technology (Indian School of Mines) Dhanbad, Jharkhand, India. His current research interests include optical fiber sensors, fiber Bragg grating sensors for chemical, and gas sensing. He is a member of so many technical societies. He has worked with a project sponsored by the Department of Atomic Energy-Board of Research in Nuclear Sciences (DAE-BRNS), a subdivision of the Bhabha Atomic Research Center (BARC), in collaboration with the RRCAT, Indore; and presently working with a project sponsored by the Council of Scientific and Industrial Research (CSIR), India.

Md Tauseef Iqbal Ansari (Graduate Student Member, IEEE) received the B.Tech. degree in electronics and communication engineering (E.C.E.) from the Silicon Institute of Technology, Bhubaneswar, India, affiliated by the Biju Patnaik University of Technology, Rourkela, India, in 2018, and the M.Tech. degree in optoelectronics and optical communication engineering (O.O.C.E.) from the Electronics Department, Indian Institute of Technology (Indian School of Mines) Dhanbad, Jharkhand, India, in 2020. He is currently a Junior Research Fellow with a funded scholarship with the Indian Institute of Technology (Indian School of Mines) Dhanbad. He is also working on a project sponsored by the Council of Scientific & Industrial Research (CSIR), India. His research expertise includes the design and development of deployable thin film-based evanescent field sensors to check the quality of food from adulteration. His current research interests include optical fiber sensors, optoelectronics devices, biochemical, and gas sensing. He is also a member and engaged with various technical and professional societies, such as OSA and SPIE.

Umesh Kumar Tiwari received the Ph.D. degree in fiber optics and optical communication technology from the Indian Institute of Technology Delhi, New Delhi, India, in 2014. He is currently the Principal Scientist with the Advanced Materials and Sensors Division, Central Scientific Instruments Organisation (CSIR-CSIO), Chandigarh, India; and an Associate Professor of CSIR—Academy of Scientific and Innovative Research (AcSIR), New Delhi. He has authored/coauthored more than 100 research articles in journals and conference proceedings. He visited ITMO University, Saint Petersburg, Russia, under collaborative project. His current research interests include fiber Bragg grating (FBG) and long period grating (LPG)-based sensors, dielectric/plasmonic Metamaterials, optical tweezing/trapping using fiber nano tip, FBG-based accelerometer and acoustic sensing, and LPG-based biosensing (bacteria, pesticides, biomarker, and antigen). He has been a Fellow of the Institution of Electronics and Telecommunication Engineers, New Delhi, since 2015. He is a Life Member of the National Academy of Sciences India (NASI), Allahabad; and the Indian Science Congress Association, Kolkata. He is also a regular member of the International Society for Optics and Photonics (SPIE), USA. He was a recipient of the prestigious CSIR-Young Scientist Award in physical sciences by the CSIR, Government of India, in 2012; and IETE-CEOT (94) Award 2020 by the Institution of Electronics and Telecommunication Engineers, New Delhi. He was also a recipient of the CSIR-Raman Research Fellowship to work at Institut Néel, France.

Santosh Kumar (Senior Member, IEEE) received the Ph.D. degree from the Indian Institute of Technology (IIT) (Indian School of Mines) Dhanbad, Dhanbad, India. He is currently an Associate Professor with the School of Physics Science and Information Technology, Liaocheng University, Liaocheng, China. He has received an International Travel Grant from SERB-DST, Government of India, in 2016. He has guided seven M.Tech. dissertations and six Ph.D. candidates. He has published more than 190 research articles in national and international SCI journals and conferences. He has presented many articles at conferences held in Belgium and the USA. He has published two books titled Fibre Optic Communication: Optical Waveguides, Devices and Applications (University Press, 2017), India; and another book titled 2D Materials for Surface Plasmon Resonance-Based Sensors (CRC Press, Taylor and Francis Group, 2021). His current research interests include optical fiber sensors, nano and biophotonics, terahertz sensing and spectroscopy, and waveguide and interferometer. He is a Life Fellow Member of the Optical Society of India (OSI) and a Senior Member of OPTICA and SPIE. He is also a Traveling Lecturer of Optica. He is the Chair of the Optica Optical Biosensors Technical Group. He has delivered many invited talks and serves as the session chair for IEEE conferences. He has reviewed more than 950 SCI journals of IEEE, Elsevier, Springer, OPTICA, SPIE, and Nature. He is an Associate Editor of the IEEE Sensors Journal, IEEE Access, IEEE Transactions on NanoB ioscience, Frontiers in Physics, and Biomedical Optics Express.
Funding Statement
This work was supported by the Central Scientific Instruments Organisation (CSIR), Chandigarh, India, through the Project “Design and Development of Deployable Thin Film-Based Evanescent Field Sensor to Check the Quality of Food From Adulteration” under Sanction 70(0077)/19/EMR-II [CSIR(32)/2019-2020/663/ECE]. The work of Santosh Kumar was supported by the Double-Hundred Talent Plan of Shandong Province, China.
Contributor Information
Purnendu Shekhar Pandey, Email: purnendu12345@gmail.com.
Sanjeev Kumar Raghuwanshi, Email: sanjeevrus77@iitism.ac.in.
Azhar Shadab, Email: azharece1@gmail.com.
Md Tauseef Iqbal Ansari, Email: tauseefiqbal305@gmail.com.
Umesh Kumar Tiwari, Email: umeshtiwari@csio.res.in.
Santosh Kumar, Email: santosh@lcu.edu.cn.
References
- [1].Udugama B.et al. , “Diagnosing COVID-19: The disease and tools for detection,” ACS Nano, vol. 14, no. 4, pp. 3822–3835, Apr. 2020. [DOI] [PubMed] [Google Scholar]
- [2].Masters P. S.et al. , “Coronaviridae,” Fields Virol., vol. 1, pp. 825–858, May 2013. [Google Scholar]
- [3].Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G. A., and Wang J., “Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection,” ACS Nano, vol. 14, no. 5, pp. 5268–5277, May 2020. [DOI] [PubMed] [Google Scholar]
- [4].Shrivastav A. M., Cvelbar U., and Abdulhalim I., “A comprehensive review on plasmonic-based biosensors used in viral diagnostics,” Commun. Biol., vol. 4, no. 1, pp. 1–12, Dec. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Samson R., Navale G. R., and Dharne M. S., “Biosensors: Frontiers in rapid detection of COVID-19,” 3 Biotech, vol. 10, no. 9, pp. 1–9, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taha B. A., Al Mashhadany Y., Mokhtar M. H. H., Zan M. S. D. B., and Arsad N., “An analysis review of detection coronavirus disease 2019 (COVID-19) based on biosensor application,” Sensors, vol. 20, no. 23, p. 6764, Nov. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hossain M. B., Islam M. M., Abdulrazak L. F., Rana M. M., Akib T. B. A., and Hassan M., “Graphene-coated optical fiber SPR biosensor for BRCA1 and BRCA2 breast cancer biomarker detection: A numerical design-based analysis,” Photon. Sensors, vol. 10, no. 1, pp. 67–79, Mar. 2020. [Google Scholar]
- [8].Akib T. B. A., Nazmuschayadat M., and Hossain M. B., “Superior performance of surface plasmon resonance biosensor for recognizing of DNA hybridization,” in Proc. Int. Conf. Comput., Commun., Chem., Mater. Electron. Eng. (ICME), Jul. 2019, pp. 1–4. [Google Scholar]
- [9].Peng W.et al. , “Compact surface plasmon resonance imaging sensing system based on general optoelectronic components,” Opt. Exp., vol. 22, no. 5, pp. 6174–6185, 2014. [DOI] [PubMed] [Google Scholar]
- [10].Hossain M. B. and Rana M. M., “DNA hybridization detection based on resonance frequency readout in graphene on Au SPR biosensor,” J. Sensors, vol. 2016, pp. 1–7, Oct. 2016. [Google Scholar]
- [11].Zhu N.et al. , “A novel coronavirus from patients with pneumonia in China, 2019,” New England J. Med., 2020. [DOI] [PMC free article] [PubMed]
- [12].Roh S., Chung T., and Lee B., “Overview of the characteristics of micro- and nano-structured surface plasmon resonance sensors,” Sensors, vol. 11, no. 2, pp. 1565–1588, Jan. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abdulhalim I., Zourob M., and Lakhtakia A., “Surface plasmon resonance for biosensing: A mini-review,” Electromagnetics, vol. 28, no. 3, pp. 214–242, 2008. [Google Scholar]
- [14].Sharma A. K., Jha R., and Gupta B. D., “Fiber-optic sensors based on surface plasmon resonance: A comprehensive review,” IEEE Sensors J., vol. 7, no. 8, pp. 1118–1129, Aug. 2007. [Google Scholar]
- [15].Iravani S., “Nano- and biosensors for the detection of SARS-CoV-2: Challenges and opportunities,” Mater. Adv., vol. 1, no. 9, pp. 3092–3103, 2020. [Google Scholar]
- [16].Talebian S., Wallace G. G., Schroeder A., Stellacci F., and Conde J., “Nanotechnology-based disinfectants and sensors for SARS-CoV-2,” Nature Nanotechnol., vol. 15, no. 8, pp. 618–621, Aug. 2020. [DOI] [PubMed] [Google Scholar]
- [17].Huang Y., Yang C., Xu X.-F., Xu W., and Liu S.-W., “Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19,” Acta Pharmacol. Sinica, vol. 41, no. 9, pp. 1141–1149, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seo G.et al. , “Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor,” ACS Nano., vol. 14, no. 4, pp. 5135–5142, 2020. [DOI] [PubMed] [Google Scholar]
- [19].Mahari S., Roberts A., Shahdeo D., and Gandhi S., “eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCOVID-19 antigen, a spike protein domain 1 of SARS-CoV-2,” bioRxiv, 2020.
- [20].Wang C.et al. , “Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses,” ACS Appl. Mater. Interfaces, vol. 11, no. 21, pp. 19495–19505, May 2019. [DOI] [PubMed] [Google Scholar]
- [21].Soufi G. J.et al. , “SARS-CoV-2 (COVID-19): New discoveries and current challenges,” Appl. Sci., vol. 10, no. 10, p. 3641, May 2020. [Google Scholar]
- [22].Kaur B., Kumar S., and Kaushik B. K., “Recent advancements in optical biosensors for cancer detection,” Biosensors Bioelectron., vol. 197, Feb. 2022, Art. no. 113805. [DOI] [PubMed] [Google Scholar]
- [23].Li M., Singh R., Soares M. S., Marques C., Zhang B., and Kumar S., “Convex fiber-tapered seven core fiber-convex fiber (CTC) structure-based biosensor for creatinine detection in aquaculture,” Opt. Exp., vol. 30, no. 8, pp. 13898–13914, Apr. 2022. [DOI] [PubMed] [Google Scholar]
- [24].Soares M. S.et al. , “Immunosensing based on optical fiber technology: Recent advances,” Biosensors, vol. 11, no. 9, p. 305, Aug. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soares M. S.et al. , “Label-free plasmonic immunosensor for cortisol detection in a D-shaped optical fiber,” Biomed. Opt. Exp., vol. 13, no. 6, pp. 3259–3274, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kumar S.et al. , “MoS2 functionalized multicore fiber probes for selective detection of shigella bacteria based on localized plasmon,” J. Lightw. Technol., vol. 39, no. 12, pp. 4069–4081, Jun. 15, 2021. [Google Scholar]
- [27].Wang Y.et al. , “Water pollutants p-cresol detection based on Au-ZnO nanoparticles modified tapered optical fiber,” IEEE Trans. NanoBiosci., vol. 20, no. 3, pp. 377–384, Jul. 2021. [DOI] [PubMed] [Google Scholar]
- [28].Leitão C.et al. , “Cortisol AuPd plasmonic unclad POF biosensor,” Biotechnol. Rep., vol. 29, Mar. 2021, Art. no. e00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cennamo N.et al. , “(INVITED)quantitative detection of SARS-CoV-2 virions in aqueous mediums by IoT optical fiber sensors,” Results Opt., vol. 5, Dec. 2021, Art. no. 100177. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S266695012100122X [Google Scholar]
- [30].Cennamo N.et al. , “Proof of concept for a quick and highly sensitive on-site detection of SARS-CoV-2 by plasmonic optical fibers and molecularly imprinted polymers,” Sensors, vol. 21, no. 5, p. 1681, Mar. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kretschmann E. and Raether H., “Radiative decay of non radiative surface plasmons excited by light,” Zeitschrift Naturforschung A, vol. 23, no. 12, pp. 2135–2136, 1968. [Google Scholar]
- [32].Pandey P. S., Singh Y., and Raghuwanshi S. K., “Theoretical analysis of the LRSPR sensor with enhance FOM for low refractive index detection using MXene and fluorinated graphene,” IEEE Sensors J., vol. 21, no. 21, pp. 23979–23986, Nov. 2021. [Google Scholar]
- [33].B. H. Garcia, II, and Goodman R. M., “Use of surface plasmon resonance imaging to study viral RNA: Protein interactions,” J. Virol. Methods, vol. 147, no. 1, pp. 18–25, 2008. [DOI] [PubMed] [Google Scholar]
- [34].Florschütz K.et al. , “‘Phytochip’: On-chip detection of phytopathogenic RNA viruses by a new surface plasmon resonance platform,” J. Virol. Methods, vol. 189, no. 1, pp. 80–86, Apr. 2013. [DOI] [PubMed] [Google Scholar]
- [35].Cennamo N.et al. , “SARS-CoV-2 spike protein detection through a plasmonic D-shaped plastic optical fiber aptasensor,” Talanta, vol. 233, Oct. 2021, Art. no. 122532. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0039914021004537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen H.et al. , “Mass spectroscopic characterization of the coronavirus infectious bronchitis virus nucleoprotein and elucidation of the role of phosphorylation in RNA binding by using surface plasmon resonance,” J. Virol., vol. 79, no. 2, pp. 1164–1179, Jan. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shang J.et al. , “Structural basis of receptor recognition by SARS-CoV-2,” Nature, vol. 581, no. 7807, pp. 221–224, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Djaileb A.et al. , “A rapid and quantitative serum test for SARS-CoV-2 antibodies with portable surface plasmon resonance sensing,” Tech. Rep., 2020.
- [39].Park T. J., Hyun M. S., Lee H. J., Lee S. Y., and Ko S., “A self-assembled fusion protein-based surface plasmon resonance biosensor for rapid diagnosis of severe acute respiratory syndrome,” Talanta, vol. 79, no. 2, pp. 295–301, Jul. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Singh Y., Ansari M. T. I., and Raghuwanshi S. K., “Design and development of titanium dioxide (TiO2)-coated eFBG sensor for the detection of petrochemicals adulteration,” IEEE Trans. Instrum. Meas., vol. 70, pp. 1–8, 2021.33776080 [Google Scholar]
- [41].Pandey P. S., Raghuwanshi S. K., and Kumar S., “Recent advances in two-dimensional materials-based Kretschmann configuration for SPR sensors: A review,” IEEE Sensors J., vol. 22, no. 2, pp. 1069–1080, Jan. 2022. [Google Scholar]
- [42].Shang J.et al. , “Cell entry mechanisms of SARS-CoV-2,” Proc. Nat. Acad. Sci. USA, vol. 117, no. 21, pp. 11727–11734, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harrison S. C., “Viral membrane fusion,” Virology, vols. 479–480, pp. 498–507, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fehr A. R. and Perlman S., “Coronaviruses: An overview of their replication and pathogenesis,” Coronaviruses, pp. 1–23, 2015. [DOI] [PMC free article] [PubMed]
- [45].Chiu N.-F., Fan S.-Y., Yang C.-D., and Huang T.-Y., “Carboxyl-functionalized graphene oxide composites as SPR biosensors with enhanced sensitivity for immunoaffinity detection,” Biosensors Bioelectron., vol. 89, pp. 370–376, Mar. 2017. [DOI] [PubMed] [Google Scholar]
- [46].Wu L.et al. , “Sensitivity improved SPR biosensor based on the MoS2/graphene–aluminum hybrid structure,” J. Lightw. Technol., vol. 35, no. 1, pp. 82–87, Jan. 1, 2017. [Google Scholar]
- [47].Kumar R., Kushwaha A. S., Srivastava M., Mishra H., and Srivastava S. K., “Enhancement in sensitivity of graphene-based zinc oxide assisted bimetallic surface plasmon resonance (SPR) biosensor,” Appl. Phys. A, Solids Surf., vol. 124, no. 3, pp. 1–10, Mar. 2018. [Google Scholar]
- [48].Su L.-C.et al. , “Rapid and highly sensitive method for influenza a (H1N1) virus detection,” Anal. Chem., vol. 84, no. 9, pp. 3914–3920, May 2012. [DOI] [PubMed] [Google Scholar]
- [49].Chang Y.-F.et al. , “Simple strategy for rapid and sensitive detection of avian influenza a H7N9 virus based on intensity-modulated SPR biosensor and new generated antibody,” Anal. Chem., vol. 90, no. 3, pp. 1861–1869, Feb. 2018. [DOI] [PubMed] [Google Scholar]
- [50].Qi Z.-M., Xia S., and Zou H., “Slow spontaneous transformation of the morphology of ultrathin gold films characterized by localized surface plasmon resonance spectroscopy,” Nanotechnology, vol. 20, no. 25, Jun. 2009, Art. no. 255702. [DOI] [PubMed] [Google Scholar]
- [51].Takemura K., “Surface plasmon resonance (SPR)- and localized SPR (LSPR)-based virus sensing systems: Optical vibration of nano- and micro-metallic materials for the development of next-generation virus detection technology,” Biosensors, vol. 11, no. 8, p. 250, Jul. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wong C. L., Chua M., Mittman H., Choo L. X., Lim H. Q., and Olivo M., “A phase-intensity surface plasmon resonance biosensor for avian influenza A (H5N1) detection,” Sensors, vol. 17, no. 10, p. 2363, Oct. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Omar N. A. S.et al. , “Quantitative and selective surface plasmon resonance response based on a reduced graphene oxide–polyamidoamine nanocomposite for detection of dengue virus E-proteins,” Nanomaterials, vol. 10, no. 3, p. 569, Mar. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sharma P. K.et al. , “Surface plasmon resonance sensing of ebola virus: A biological threat,” Anal. Bioanal. Chem., vol. 412, no. 17, pp. 4101–4112, Jul. 2020. [DOI] [PubMed] [Google Scholar]
- [55].Akib T. B. A.et al. , “Design and numerical analysis of a graphene-coated SPR biosensor for rapid detection of the novel coronavirus,” Sensors, vol. 21, no. 10, p. 3491, May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rahman M. M., Rana M. M., Rahman M. S., Anower M. S., Mollah M. A., and Paul A. K., “Sensitivity enhancement of SPR biosensors employing heterostructure of PtSe2 and 2D materials,” Opt. Mater., vol. 107, Sep. 2020, Art. no. 110123. [Google Scholar]
- [57].Uddin S. M. A., Chowdhury S. S., and Kabir E., “Numerical analysis of a highly sensitive surface plasmon resonance sensor for SARS-CoV-2 detection,” Plasmonics, vol. 16, no. 6, pp. 2025–2037, Dec. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rahman M. S., Hasan M. R., Rikta K. A., and Anower M. S., “A novel graphene coated surface plasmon resonance biosensor with tungsten disulfide (WS2) for sensing DNA hybridization,” Opt. Mater., vol. 75, pp. 567–573, Jan. 2018. [Google Scholar]
- [59].Bhatia P. and Gupta B. D., “Surface-plasmon-resonance-based fiber-optic refractive index sensor: Sensitivity enhancement,” Appl. Opt., vol. 50, no. 14, pp. 2032–2036, 2011. [DOI] [PubMed] [Google Scholar]
- [60].Lahav A., Auslende M., and Abdulhalim I., “Sensitivity enhancement of guided-wave surface-plasmon resonance sensors,” Opt. Lett., vol. 33, no. 21, pp. 2539–2541, 2008. [DOI] [PubMed] [Google Scholar]
- [61].Verma R., Gupta B. D., and Jha R., “Sensitivity enhancement of a surface plasmon resonance based biomolecules sensor using graphene and silicon layers,” Sens. Actuators B, Chem., vol. 160, no. 1, pp. 623–631, 2011. [Google Scholar]
- [62].Wang Q., Niu L.-Y., Jing J.-Y., and Zhao W.-M., “Barium titanate film based fiber optic surface plasmon sensor with high sensitivity,” Opt. Laser Technol., vol. 124, Apr. 2020, Art. no. 105899. [Google Scholar]
- [63].Funari R., Chu K.-Y., and Shen A. Q., “Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip,” Biosensors Bioelectron., vol. 169, Dec. 2020, Art. no. 112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Li M., Singh R., Marques C., Zhang B., and Kumar S., “2D material assisted SMF-MCF-MMF-SMF based LSPR sensor for creatinine detection,” Opt. Exp., vol. 29, no. 23, pp. 38150–38167, 2021. [DOI] [PubMed] [Google Scholar]
- [65].Wang Z., Singh R., Marques C., Jha R., Zhang B., and Kumar S., “Taper-in-taper fiber structure-based LSPR sensor for alanine aminotransferase detection,” Opt. Exp., vol. 29, no. 26, pp. 43793–43810, Dec. 2021. [Google Scholar]
- [66].Kumar A., Kumar A., and Srivastava S., “Silicon nitride-BP-based surface plasmon resonance highly sensitive biosensor for virus SARS-CoV-2 detection,” Plasmonics, pp. 1–13, 2022. [DOI] [PMC free article] [PubMed]
- [67].Moznuzzaman M., Khan I., and Islam M. R., “Nano-layered surface plasmon resonance-based highly sensitive biosensor for virus detection: A theoretical approach to detect SARS-CoV-2,” AIP Adv., vol. 11, no. 6, Jun. 2021, Art. no. 065023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Maurya J. B., Prajapati Y. K., Singh V., Saini J. P., and Tripathi R., “Performance of graphene–MoS2 based surface plasmon resonance sensor using silicon layer,” Opt. Quantum Electron., vol. 47, no. 11, pp. 3599–3611, Nov. 2015. [Google Scholar]