Abstract
Mesencephalic astrocyte-derived neurotrophic factor (MANF) and cerebral dopamine neurotrophic factor (CDNF) are endoplasmic reticulum (ER) luminal proteins that confer trophic activities in a wide range of tissues under diverse pathological conditions. Despite initially being classified as neurotrophic factors, neither protein structurally nor functionally resembles bona fide neurotrophic factors. Their highly homologous structures are comprised of a unique globular, saposin-like domain within the N-terminus joined by a flexible linker to a C-terminus containing a SAP-like domain, CXXC motif and an ER retention sequence. Neurotrophic factors exert effects by binding to cognate receptors in the plasma membrane, however no cell surface receptors have been identified for MANF and CDNF. Both can act as unfolded protein response (UPR) genes that modulate the UPR and inflammatory processes. The trophic activity of MANF and CDNF extends beyond the central nervous system with MANF being crucial for the development of pancreatic β-cells and both have trophic effects in a variety of diseases related to the liver, heart, skeletal tissue, kidney, and peripheral nervous system. In this article, the unique features of MANF and CDNF, such as their structure and mechanisms of action related to ER stress and inflammation will be reviewed. Recently identified interactions with lipids and membrane trafficking will also be described. Lastly, their function and therapeutic potential in different diseases including a recent clinical trial using CDNF to treat Parkinson’s disease will be discussed. Collectively, this review will highlight MANF and CDNF as broad-acting trophic factors that regulate functions of the endoplasmic reticulum.
Keywords: MANF, CDNF, UPR, ER stress, inflammation
Discovery of MANF and CDNF as trophic factors
Neurotrophic factors are secreted proteins that regulate neuronal development, plasticity and survival. The first neurotrophic factor nerve growth factor (NGF) has been studied since the 1940’s (reviewed in: (Levi-Montalcini, 1987) and was sequenced in 1971 (Angeletti and Bradshaw, 1971). Other neurotrophic factors include brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and neurotrophins (NT3–7). All these neurotrophic factors are secreted from a cell and bind to receptors on the plasma membrane such as Tropomyosin receptor kinase (Trk) family receptors, p75NTR, and GDNF family receptor α (GFR α) (Huang and Reichardt, 2001; Airaksinen and Saarma, 2002). Most of these receptors act as tyrosine kinases or activate one (e.g. GFRα activates Ret) to trigger downstream signaling pathways such as Ras/ERK (extracellular signal-regulated kinase) or phosphatidyl inositol-3-kinase PI3K/Akt pathways (Huang and Reichardt, 2001; Airaksinen and Saarma, 2002). The consequences of these signaling cascades promote synapse formation, neuronal maturation, differentiation and, survival.
Over the past two decades, mesencephalic astrocyte-derived neurotrophic factor (MANF) and cerebral dopamine neurotrophic factor (CDNF) were discovered as secreted proteins with trophic activity (Petrova et al., 2003; Lindholm et al., 2007). MANF was first identified using a screen for neurotrophic proteins in conditioned media from a rat-derived, ventral mesencephalic cell line. MANF was purified from the conditioned media and the sequence revealed 100 % homology to a portion of the predicted human protein (arginine rich protein, hARP), however, the N-terminal arginine-rich sequence of hARP is absent from MANF protein (Petrova et al., 2003). Four years later, a MANF homolog, CDNF, was identified using bioinformatic tools, cloned into an expression vector and purified from conditioned media of cells (Lindholm et al., 2007). Recombinant CDNF protein protected midbrain dopaminergic neurons in two Parkinson’s disease (PD) models, using 6-hydroxydopamine (6-OHDA) lesioned rats (Lindholm et al., 2007; Voutilainen et al., 2011) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated mice (Airavaara et al., 2012). Exogenous MANF has been shown to protect and restore midbrain dopaminergic neurons after 6-OHDA lesioning in rats (Voutilainen et al., 2009). The observed neurotrophic effects of MANF and CDNF on the nigrostriatal dopaminergic neurons has led to recent clinical trials using CDNF for the treatment of Parkinson’s disease (Huttunen and Saarma, 2019).
Although first identified and named for their neurotrophic activity, both MANF and CDNF are also expressed outside the central nervous system and have activity on non-neuronal cells, particularly in tissues with high metabolic activity (Lindholm et al., 2007; Mizobuchi et al., 2007; Lindholm et al., 2008; Danilova et al., 2019). For example, MANF is highly expressed in neurons and tissues with a secretory function such as the pituitary gland, pancreas, and testis. MANF has been shown to act as a trophic factor in the heart especially after ischemia, and is crucial for the development of pancreatic β cells (Tadimalla et al., 2008; Glembotski et al., 2012; Lindahl et al., 2014; Danilova et al., 2019). In MANF knock out mice, the most severe phenotype occurs in the periphery where the loss of pancreatic β cells results in diabetes (Lindahl et al., 2014). CDNF is most prominently expressed in tissues like heart, muscle, and brown adipose tissue (Danilova et al., 2019).
Both MANF and CDNF proteins reside in the endoplasmic reticulum (ER) lumen and show increased secretion from the cell under stress conditions. They confer trophic activity to many tissue and cell types as secreted proteins but can also convey cellular protection from within the ER. Although both proteins are categorized as neurotrophic factors by virtue of their neuroprotective and restorative effects, they do not structurally resemble any other neurotrophic factors such NGF, BDNF or GDNF. Additionally, no cell surface receptor has been identified for either protein. MANF and CDNF are thought to exert their functions from inside the cell by regulating ER stress. This review will discuss the currently proposed mechanisms by which CDNF and MANF promote cell survival across a diverse collection of pathological states.
Structural features of CDNF and MANF
The structures of MANF and CDNF are unique among neurotrophic factors. They have 59% amino acid identity with one another but do not have sequence homology with the other extensively characterized neurotrophic factors (e.g. BDNF, GDNF, NGF); instead, they constitute a unique protein family (Parkash et al., 2009). The genes encoding MANF and CDNF are named ARMET (arginine rich, mutated in early stage tumors) and ARMETL1 (ARMET like 1), respectively. The human Manf gene contains an amino-terminal arginine rich sequence; however, this sequence is now known to be in the 5’ UTR and not translated and therefore the nomenclature is somewhat misleading (Petrova et al., 2003; Lindholm et al., 2007). Manf and Cdnf are conserved across vertebrates (such as zebrafish, Danio rerio) that have orthologous genes of both Manf and Cdnf whereas invertebrates, such as fruitfly Drosophila melanogaster, roundworm Caenorhabditis elegans, and sponge Suberites domungula only have one orthologous Manf/Cdnf gene (Lindholm et al., 2007; Sereno et al., 2017).
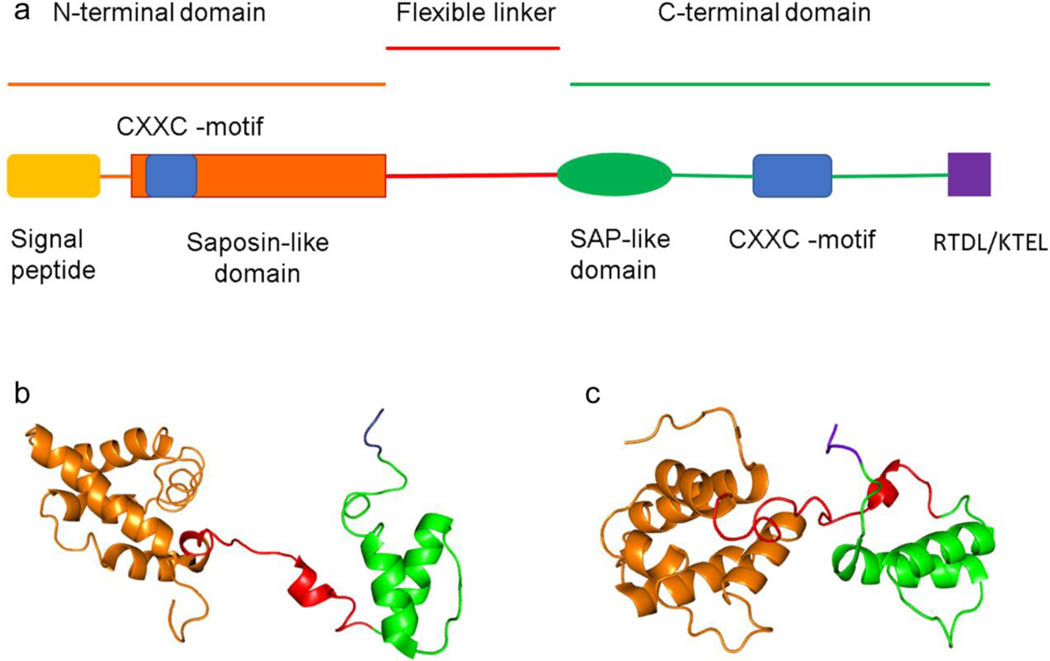
MANF and CDNF are relatively small, soluble proteins (18–20 kDa). Using X-ray crystallography, the structure of the CDNF (proteolytic fragment lacking C-terminus) and MANF (full length) were resolved to 1.9 and 2.8 Å, respectively (Parkash et al., 2009). Additional structural information was provided on the C-terminus of CDNF using NMR spectroscopy (Hellman et al., 2010; Latgé et al., 2013). MANF and CDNF have similar structures with a globular N-terminus and an unstructured C-terminus connected by a flexible linker (Figure 1). The N-terminus of MANF and CDNF have a signal peptide sequence necessary for translation into the ER where the peptide is cleaved (Mizobuchi et al., 2007). In addition to the signal peptide, the N-terminus of MANF and CDNF contains a saposin-like protein (SAPLIP) domain. SAPLIP proteins such as granulysin and NK-lysin are known for their interaction with lipid membranes. Recently, MANF was shown to interact with the lipid sulfatide and this interaction in the extracellular space increased the uptake of MANF into the cells (Bai et al., 2018).
Figure 1. Structure of human (Homo sapiens) MANF and CDNF.
The different domains and motifs of MANF and CDNF structure. “RTDL” and “KTEL” are the ER retention sequences for MANF and CDNF, respectively (A). The structures of MANF (B) and CDNF (C) created from PDB codes 2KVD (residues 25–182) and 4BIT (residues 27–187), respectively, using PyMOL 2.3.4 software (Parkash et al., 2009; Latge et al., 2015).
MANF and CDNF have two CXXC motifs with one located in N-terminus and the other in the C-terminus (Mizobuchi et al., 2007; Parkash et al., 2009). The CXXC motif is present in many proteins with antioxidant properties, such as protein disulfide isomerases (PDIs), which are ER resident proteins critical for protein folding (Appenzeller-Herzog and Ellgaard, 2008). The cysteines in the CXXC motifs of PDIs catalyze exchange of disulfide bonds in substrate proteins, therefore promoting their correct folding (Appenzeller-Herzog and Ellgaard, 2008). Although MANF does not seem to have oxidoreductase activity, its C-terminal CXXC motif was shown to be essential for its cytoprotective and anti-apoptotic effects both in Drosophila (Lindström et al., 2013) as well as in mouse primary neurons, a rat cerebral ischemia model and a human cell line (Mätlik et al., 2015; Božok et al., 2018). Additionally, a recent study shows that the conserved cysteines of CXXC motifs are necessary for MANFs function as a chaperone, a protein that assists the correct folding of another protein, when cell undergoes reductive ER stress (Arrieta et al., 2020).
The C-terminus of both proteins contains a SAP-like (SAF-A/B, Acinus and PIAS) domain which is a putative DNA/RNA binding domain. The SAP-like domain of MANF showed binding to the pro-inflammatory transcription factor NFκB subunit p65 (Chen et al., 2015). The interaction of MANF with the NFκB subunit p65 correlated with an attenuation of TNFα-induced NFκB activation. MANF lacking the C-terminal SAP-like domain was unable to attenuate NFκB activation by TNFα. The SAP domain of MANF has also been shown to interact with BiP/GRP78 in its ADP-bound state and prevent nucleotide exchange, thus altering the activity of BiP/GRP78 (Yan et al., 2019). The function of the SAP domain in CDNF is not yet known.
The C-termini of MANF and CDNF are partially unstructured and contain an ER retention sequence (ERS). Luminal ER resident proteins are maintained within the ER under homeostatic conditions by C-terminal ERS tails that are thought to interact with KDEL receptors (Munro and Pelham, 1987; Mei et al., 2017). The canonical ERS is the 4 amino acid sequence lysine-aspartic acid-glutamic acid-leucine or “KDEL” in the single letter code. In humans, the C-terminal amino acids for MANF and CDNF are RTDL and KTEL, respectively. These sequences are both necessary and sufficient for ER retention (Henderson et al., 2013). The ERS of both MANF and CDNF also confers secretion of a reporter protein in response to ER calcium depletion (Trychta et al., 2018). The RTDL sequence of MANF’s ERS was the basis for a reporter of ER calcium depletion termed SERCaMP or secreted ER calcium monitoring protein (Henderson et al., 2014). Both MANF and CDNF are secreted in response to ER calcium depletion (Apostolou et al., 2008; Glembotski et al., 2012; Trychta et al., 2018). The ERS is important for regulating secretion of MANF and CDNF in response to ER stress associated with ER calcium depletion.
Collectively, these data support that MANF and CDNF are structurally similar ER resident proteins with several unique properties that set them apart from other well-established trophic factors.
MANF and CDNF in the unfolded protein response (UPR)
MANF is a UPR gene
The endoplasmic reticulum is the intracellular organelle responsible for many critical functions such as lipid synthesis, calcium storage and protein maturation. The cell experiences “ER stress” when these functions are disrupted and activates mechanisms to restore homeostasis. In the case of protein misfolding in the ER, a series of signaling cascades that comprise the unfolded protein response (UPR) are activated (Walter and Ron, 2011). There are three principal pathways of the UPR and these are regulated by signal transducing transmembrane proteins: ATF6 (activating transcription factor 6), IRE1 (inositol requiring enzyme 1) and PERK (double-stranded RNA-activated protein kinase–like ER kinase). Each of the pathways transduces signals to subsets of transcription factors that orchestrate the upregulation of genes directed towards slowing translation, folding proteins properly and removing misfolded proteins (Okada et al., 2002; Shoulders et al., 2013). Manf was first identified as a UPR gene in a microarray screen for UPR genes triggered by tunicamycin, a drug that causes protein misfolding in the ER (Lee et al., 2003). The promoter of Manf contains a functional ER stress element II (ERSE-II) supporting its regulation as a UPR gene (Mizobuchi et al., 2007). In 2007, Mizobuchi et al. found that the mRNA and protein expression of the murine homologue of MANF was upregulated by pharmacological inducers of the UPR (Mizobuchi et al., 2007). They also showed that MANF protein is localized to the ER and contains both a signal peptide and a CXXC motif (Mizobuchi et al., 2007). Drosophila Manf (DmManf) was subsequently shown to have genetic interactions with Grp78, Perk and Xbp1 further supporting its connection to the UPR (Lindström et al., 2013). Tunicamycin was one of the pharmacological agents used to show DmManf behaves like a UPR gene and subsequent screening studies using tunicamycin found MANF was upregulated in the developing rodent brain (Wang et al., 2015) and cardiomyocytes (Liu et al., 2018a). Using the expression of folding-defective polypetides in the ER as an alternative method to induce ER stress, the results are similar to those seen with tunicamycin with transcriptional and translational increases in MANF, but not in CDNF, that are proportional to other core UPR protein/genes BiP/Grp78/Hspa5, Grp94/Hsp90B1, ERp72/Pdia4, Sdf2L1/Sdf2L1, Grp170/Hyou1, and ERdj3/DNAjb11 (Bergmann et al., 2018). One study has reportered that tunicamycin caused an increase in CDNF expression in primary neurons (Zhang et al., 2018a), but currently the data supporting CDNF acts a UPR gene is limited. Collectively, the current literature supports that Manf is a UPR gene, but through which of the UPR pathways?
MANF and CDNF expression are regulated by ATF6 and IRE1 pathways of the UPR
In cells lacking both ATF6 and XBP1, the expression of MANF both basally and following tunicamycin treatment was minimal and near background (Lee et al., 2003). A subsequent study implicated ATF6 as a primary regulator of MANF expression in cardiomyocytes and other cells of the heart (Tadimalla et al., 2008). Genetic interactions studies conducted in both Drosphila and C. elegans, where there is only one homolog representing both Manf and Cdnf, suggest that the IRE1/XBP1 pathway is involved in transcriptional regulation of Manf and Cdnf (Lindström et al., 2013; Hartman et al., 2019). In a mouse model of spinocerebellar ataxia, decreased XBP1 expression correlated with decreased levels of MANF, which implicates the IRE1 pathway as a regulator of MANF (Yang et al., 2014). In 2018, Wang et al. showed that expression of the spliced form of XBP1 (XBP1s) increased MANF protein levels and chromatin immunoprecipitation confirmed that XBP1s binds to the ER stress-response elements located in the Manf promoter region (Wang et al., 2018). Taken together, these data support that Manf and to lesser extent Cdnf are activated through the IRE1/XBP1 pathway. However, the ATF6 pathway also implicated in the regulation of Manf expression. The type of cell and ER stressor appear to determine the relative contribution from each of these pathways in activating Manf and Cdnf.
MANF and CDNF are negative regulators of the UPR
A year after Mizobuchi identified Manf as a putative UPR gene, Apostolou et al confirmed Manf as a UPR gene that was able to attenuate ER stress-induced cell death (Apostolou et al., 2008). When MANF was knocked down using RNAi, an increase in other UPR genes was observed, suggesting that MANF may serve to negatively regulate the UPR (Apostolou et al., 2008). Transcriptomic analysis of Drosophila showed that overexpression of DmManf resulted in a downregulation of the UPR, while conversely, absence of MANF caused UPR activation (Palgi et al., 2012). The ability of MANF to negatively regulate the UPR was also observed in C. elegans (Richman et al., 2018; Hartman et al., 2019). For example, the loss of C. elegans Manf-1 caused an elevated basal expression of UPR genes which correlated with an increased resistance to tunicamycin-induced larval growth arrest (Hartman et al., 2019). Similarly, in developing neurons derived from MANF deficient mice, the UPR is constitutively active and neurite outgrowth is impaired (Tseng et al., 2017). MANF-deficient mice also have chronic activation of UPR in the brain (Pakarinen et al., 2020) and pancreatic islet cells (Lindahl et al., 2014). In a rodent cerebral ischemia model, MANF attenuated ischemia-induced UPR as indicated by decreases in BiP/Grp78, phosphorylated IRE1, and spliced XBP1 (Yang et al., 2014). In rat models of Alzheimer’s disease and Parkinson’s disease, respectively, CDNF reduced β-amyloid induced UPR activation and toxicity in hippocampal neurons (Zhou et al., 2016) and 6-OHDA lesioning in dopaminergic neurons (Voutilainen et al., 2017). More recent studies showed similar results in that, MANF overexpression reduced UPR activation caused by β-amyloid in human neuroblastoma cells (SH-SY5Y) which corresponded to decreased cellular toxicity whereas knocking down MANF increased UPR activation and toxicity associated with β-amyloid (Xu et al., 2019). Most studies have found that MANF and CDNF decrease UPR activation, however, three studies have found an increase in UPR activation (Sun et al., 2017; Arancibia et al., 2018; Lindahl et al., 2020). For example, a CDNF expressing plasmid transfected into HEK293 cells caused an increase in UPR gene expression compared to mock transfected cells. The expression of CDNF also reduced thapsigargin-induced cell death. Removal of the C-terminal ERS from CDNF caused CDNF to be secreted and no longer conferred protection against thapsigargin (Arancibia et al., 2018). The effect of the CDNF without an ERS was not examined for UPR effects. Interestingly, Cdnf knock-out mice did not show increased expression of UPR genes in their midbrain (Lindahl et al., 2020). A third study showed that recombinant MANF increased UPR genes in SH-SY5Y neuroblastoma cells which correlated with reduced 6-OHDA related toxicity (Sun et al., 2017). Collectively, the literature supports that MANF and CDNF regulate the UPR, mostly to reduce UPR activation, and maintain cell viability under conditions of ER stress. The question remains: how do they accomplish this?
While the exact mechanism by which MANF and CDNF regulate the UPR remains to be elucidated, some studies suggest a connection to BiP/GRP78. Overexpression of the ER resident chaperone BiP/GRP78 increases intracellular MANF levels (Oh-Hashi et al., 2012). MANF coimmunoprecipitates with BiP/GRP78 in a calcium-dependent manner and decreasing ER calcium causes MANF to be secreted from the cell (Glembotski et al., 2012). Both studies support a model whereby MANF interacts with BiP/GRP78 in the ER and is released to the secretory pathway if ER calcium decreases. Subsequently, MANF was shown to interact with the nucleotide binding domain of GRP78. Specifically, MANF inhibits release of ADP from GRP78 and prevents ATP from binding. The consequence of this interaction stabilizes GRP78-client complexes which has been speculated to promote transfer to downstream quality control effectors (Yan et al., 2019). This may include the ability of BiP/GRP78 to interact with IRE1, ATF6 and PERK (Carrara et al., 2013). By removing MANF, the stability of this complex may be reduced, and cells are more likely to initiate UPR signaling, likewise, overexpressing MANF may help to favor stabilization and reduce the ability to activate UPR signaling. Future studies with MANF as well as with CDNF are needed to fully understand how they serve to negatively regulate the UPR under different forms of ER stress.
Anti-inflammatory effects of MANF and CDNF
In addition to modulating the UPR, MANF and CDNF display anti-inflammatory properties. In C. elegans, loss of MANF caused an increase in basal UPR and a resistance to tunicamycin-induced larval growth arrest as described above. Pathway analysis of mRNA changes, from a microarray experiment, revealed that when compared to wildtype, Manf-1-deficient worms are enriched for genes involved in innate immunity, a form of inflammation in the worm. The findings indicated that Manf-1 mutants fail to repress components of the innate immune system during tunicamycin induced ER stress. Manf-1 mutant worms placed on a pathogenic strain of bacteria known to activate the innate immune response showed impaired growth compared to wild-type worms (Hartman et al., 2019). These data suggest that MANF modulates the innate immune response and UPR in C. elegans.
More evidence for a role of MANF and CDNF as modulators of inflammation comes from studies in mammalian systems. In rat primary astrocytes undergoing oxygen glucose deprivation (OGD), recombinant MANF reduces BiP/GRP78 as well as NFκB p65, a key transcription factor in regulating proinflammatory cytokines. The decreased levels of NFκB p65 corresponded with the decreased levels of secreted IL1β, TNFα and IL-6 (Zhao et al., 2013). Similarly, tunicamycin treatment of rat primary astrocytes leads to increased mRNA expression and secretion of inflammatory cytokines (i.e. IL-1β, TNFα and IL-6), but CDNF overexpression in astrocytes prior to tunicamycin treatment reduced this inflammatory cytokine response (Cheng et al., 2013). Further evidence of anti-inflammatory effects is the upregulation of CDNF in rat primary microglia treated with Lipopolysaccharide (LPS) (Zhao et al., 2014). LPS is a bacterial endotoxin that binds the toll-like receptor 4 (TLR4) on the cell surface and increases expression of pro-inflammatory cytokines such as PGE2, IL-1β and TNFα. Recombinant CDNF protein reduced LPS-mediated production of PGE2 and IL-1β as well as the associated toxicity in rat microglial cultures. The antagonistic effects of CDNF correlated with a decreased phosphorylation of c-Jun N-terminal Kinase (JNK), one of the primary kinase pathways activated by LPS. In LPS treated microglia, CDNF was also shown to decrease activation of the AKT/Fox01/mTor pathway with a concomitant decrease in extracellular TNFα (Zhang et al., 2019), which provides further support to the ability of CDNF to decrease cytokine secretion. Furthermore, neural stem cells (NSCs) secrete proinflammatory cytokines (IL-1β, TNFα and IFNy) when exposed to LPS and MANF protein antagonized LPS-induced secretion of proinflammatory cytokines and reduced the level of phosphorylated p38 - mitogen-activated kinase (p38 MAPK) in mouse NSCs (Zhu et al., 2016). Neuroinflammation is associated with the degenerating nigrostriatal pathway in Parkinson’s disease and CDNF expression in the substantia nigra of 6-OHDA lesioned rats attenuated expression of inflammatory markers (nitrosative stress and gliosis), but did not affect cytokine expression (e.g. TNFα and IL1β) in the striatum (Nadella et al., 2014). Using a rodent model of stroke, Teppo et al. found that MANF diminished the expression S100A8 and S100A9, markers of inflammation that are upregulated by stroke in the peri-infarct area (Teppo et al., 2020). Lastly, in both Drosophila and mice the retina, MANF was shown modulate the activation of innate immune cells and cause neuroprotection (Neves et al., 2016). All of these data support an anti-inflammatory role of MANF and CDNF in cells of the nervous system.
Liu et al (2015) showed that MANF is expressed in human spleen, a central organ for immune system, in patients that had undergone spleen trauma (Liu et al., 2015). The MANF expression was evident in the plasma cells and macrophages, but not in T-cells or B-cells. Expression was accompanied by other UPR proteins further reinforcing MANF as a UPR gene. Plasma cells are responsible for secreting antibodies in vast amounts, which may put a higher burden on the ER and secretory pathway leading to ER stress and thus accompanied increase in UPR proteins (Liu et al., 2015). Knocking down MANF from mouse mono-macrophages lead to increased levels of splenic M1 macrophages under basal conditions, whereas hepatic fibrosis induced by CCl4 treatment lead to increased number of M2 macrophages, the cell type responsible for TGF-β1 secretion, in the spleens of these mice (Hou et al., 2019). Furthermore, under hepatic fibrosis conditions knocking out MANF from macrophages lead to more severe splenomegaly and higher amount of splenic plasma cells compared to WT mice. The authors conclude that the absence of MANF amplifies the inflammatory response in the immune system cells (Hou et al., 2019). Although additional investigation into the mechanism of MANFs action in the spleen are needed, these studies support that MANF acts as a negative regulator of inflammation.
Further evidence of the anti-inflammatory role of MANF and CDNF in the periphery is shown in human pancreatic β-cells. These β-cells can undergo cell death in response to proinflammatory cytokines and MANF expression is increased in human β-cells treated with proinflammatory cytokines. Knockdown of MANF increases ER stress and cell death caused by a cytokine challenge whereas augmenting MANF reduces cytokine-induced cell death in pancreatic β-cells. The ability of MANF to improve cell viability correlated with its ability to repress the NFκB signaling pathway (Hakonen et al., 2018). These data suggest that MANF serves as an anti-inflammatory molecule to protect β-cells from cytokine-induced stress. In a separate study, MANF was shown to interact with the NFκB via the SAP-like domain located in the C-terminus of MANF (Chen et al., 2015). MANF overexpression in HEK293T cells inhibited TNFα-induced NFκB activation of transcription whereas a MANF mutant lacking the C-terminus had no effect. Recently, MANF was shown to negatively regulate the p65 subunit of NFκB in a hepatoma cell line where knockdown of MANF upregulated TNFα, IL6 and IL-1α (Liu et al., 2020). In mice, MANF overexpression reduced liver inflammation associated with a high fat diet and aging (Sousa-Victor et al., 2019). Together these data support a role for MANF in negatively regulating a key transcriptional activator of inflammation in non-neuronal tissues.
Further evidence for MANF’s role in regulating inflammation comes from mice that are heterozygous null for Manf (MANFHet). Mice with a single Manf allele express 50% less MANF than wild-type littermates and exhibit signs of chronic inflammation. For example, MANFHet mice had increased infiltration and activation of macrophages in several tissues including adipose tissue, liver and pancreas. Systemic delivery of MANF-expressing plasmids alleviated age-related increase of activated macrophages and cytokine expression in the liver (Sousa-Victor et al., 2019). These data further support a role of MANF in regulating systemic inflammation in the mouse.
In summary, both MANF and CDNF have the ability to decreased cytokine production. The current data suggests that CDNF works through the JNK pathway and MANF works through p38 pathway. There are numerous studies that support a coordinated activation of UPR and inflammation (Reverendo et al., 2019). Perhaps the ability of MANF and CDNF to reduce production of pro-inflammatory proteins is part of a negative-feedback loop to shut down the coordinated activation of UPR and inflammation. Additional studies in other cell and tissue types are needed to further understand how MANF and CDNF work to negatively regulate the immune response such as the expression of pro-inflammatory cytokines.
Lipid binding and metabolism
As described above, MANF and CDNF have a SAPLIP domain within the N-terminal portion of each protein (Parkash et al., 2009). The SAPLIP domains resemble those of granulysin and NK-lysin both of which are proteins secreted by immune cells in order to lyse invading bacteria by forming a pore in their lipid membrane (Linde et al., 2005). The structural similarity of the MANF and CDNF SAPLIP domain to granulysin led to speculation that MANF and CDNF may also bind to lipids (Parkash et al., 2009). Nearly 10 years after MANF and CDNF were proposed to interact with lipids, Bai et al (2018) showed that MANF interacts with sulfatide, a lipid present in the extracellular leaflet of cell membranes and in extracellular fluid such as serum (Bai et al., 2018). Although CDNF is structurally similar to MANF and contains a SAPLIP domain, it was not shown to bind sulfatide. The inability of CDNF to bind to sulfatide may be attributed to a conserved lysine residue K122 that is present in human MANF, but is a leucine in CDNF. A K112L substitution in human MANF significantly reduced sulfatide binding (Bai et al., 2018). Unlike other SAPLIP proteins, MANF does not degrade the bound lipid nor does it change detectable sulfatide levels (Bai et al., 2018). The binding of sulfatide to MANF is sensitive to pH such that binding is highest at pH 6 to 6.75 (the same pH found in Golgi). Supplementing cell culture medium with sulfatide enhances the internalization of MANF. Together, these data support a model in which MANF is combined with sulfatide in the Golgi then secreted as a MANF-sulfatide complex that can be taken up by neighboring cells (Bai et al., 2018).
In addition to interacting directly with lipids, MANF and CDNF were identified as potential regulators of membrane lipids based on studies using mutants of the Drosophila homolog, DmManf (Palgi et al., 2012). Loss of DmManf in either the larval or embryonic stage caused severe disruption in vesicular transport and accumulation of debris-filled vesicles proximal to the plasma membrane. Both exocytosis and endocytosis were altered in the DmManf mutants, with a downregulation of genes involved in multivesicular body formation and a decreased expression of several transmembrane receptors for growth factors. Conversely, MANF overexpression had the opposite effect and it increased expression of these receptors (Palgi et al., 2012). These data were the first to implicate MANF and CDNF in lipid regulation and in 2019, MANF was shown to regulate lipid metabolism in the liver. In Manf heterozygous knockout (MANFHet) mice, the reduced level of MANF correlated with increased expression of genes involved in lipid metabolism in the liver (Sousa-Victor et al., 2019). Subsequently, MANFHet mice exhibited increased accumulation of lipids in hepatocytes and developed hepatosteatosis at a greater rate than control mice. Furthermore, MANF protein levels were lower in the plasma of mice fed a high fat diet (Sousa-Victor et al., 2019) and in the liver of ob/ob mice (He et al., 2020b). Fat accumulation in the liver caused by high fat diet was reduced in mice overexpressing MANF. Similarly, overexpressing MANF in aged mice attenuated the fat accumulation and markers of aging in their livers (Sousa-Victor et al., 2019). Systemic MANF levels were shown to decrease with aging in humans, mice and flies. In line with these observations, MANF knock down was shown to increase the accumulation of lipid droplets in HepG2 human hepatocyte cell line treated with free fatty acids, whereas overexpression of MANF had the opposite effect and suppressed lipogenesis (He et al., 2020b). Surprisingly, whereas in the pancreatic β-cells MANF knock down induces UPR activation and consequently cell death (Lindahl et al., 2014), lower levels of MANF in the liver cells of mice or knock down of MANF from hepatocytes did not induce UPR activation (Sousa-Victor et al., 2019). Instead, the deleterious effects from decreased MANF expression in the liver appeared to be related to the upregulation of lipid metabolism-related genes, especially G0/G1 Switch gene 2 (G0S2), G0S2 protein is known as a critical regulator of energy homeostasis and lipid flux. Increased levels of G0S2 have been shown to cause hepatic steatosis but also lead macrophages to a pro-inflammatory state (Zhang et al., 2017b; Sousa-Victor et al., 2019).
MANF also interacts with the lipid kinase PIP4K2b, the enzyme responsible for phosphorylating PI-5-P to PI-4,5-P2 which is an important lipid species for cellular signaling (Yang et al., 2017). In the brain of mice that overexpress MANF, the overall levels of PIP4K2b were comparable to wild-type mice, but the levels in the ER were significantly increased compared to wild-type. Rat PC12 cells that overexpress MANF have elevated levels of PIP4K2b in the ER. Using truncated mutants of PIP4K2b and recombinant MANF, co-immunoprecipitation and in vitro binding experiments indicate that the N-terminus of PIP4K2b interacts with MANF. PIP4K2b mutants lacking the N-terminus do not become enriched in the ER even when overexpressed. The authors put forth that MANF regulates the activity of PIP4K2b by localizing it to the ER where its substrate PI-5-P is abundant. In this manner, MANF may regulate the overall levels of PIP2 in the cells and affect not only the membrane composition of ER but also other membrane compartments as well (Yang et al., 2017). The role of CDNF in regulating PIP4k2b was not reported and remains to be determined.
The lipid interaction of the saposin-like domain of MANF, but not CDNF raises an interesting question about the fundamental differences in MANF and CDNF. Does this interaction explain interaction why the phenotype of MANF knockout mice is much more severe than CDNF knockout mice? Does CDNF bind a different set of lipids than MANF, or bind them under different conditions (redox state, and pH for example)? The possible roles of MANF and CDNF in the regulation of lipids and membranes may reflect the diverse functions they have on maintaining ER homeostasis.
Trophic activity of MANF and CDNF in diseases associated with ER stress
The expression of MANF and CDNF are both upregulated in diseases associated with ER stress including brain and heart ischemia (Apostolou et al., 2008; Tadimalla et al., 2008; Glembotski et al., 2012; Shen et al., 2012; Zhang et al., 2018a), Parkinson’s disease (Galli et al., 2019a; Virachit et al., 2019), Alzheimer’s disease (Xu et al., 2019), diabetes (Lindahl et al., 2014), chondrodysplasia (Cameron et al., 2011; Hartley et al., 2013), spinocerebellar ataxia 17 (Guo et al., 2018) and systemic lupus erythematosus (Wang et al., 2014) and others. A summary of conditions where MANF and CDNF expression are changed is summarized in Table 1. Given this broad range of diseases associated with diverse etiologies, it is not surprising that MANF and CDNF are capable of conferring protective effects in many different cells and tissues. Publications reporting the restorative and protective effects of recombinant MANF and CDNF in different disease models are summarized in Table 2. Although most of the research following the discovery of MANF and CDNF was done in the CNS, these proteins are now known to be crucial for the development and survival of cells in other parts of the body. Furthermore, they are shown to function both intracellularly and extracellularly. A more in-depth look at the trophic actions of MANF and CDNF in a variety of diseases is found below. CDNF has mainly been studied in diseases of the central nervous system and there is limited information on its role in most of the diseases discussed in this chapter which therefore focuses mainly on MANF. The associated diseases are listed in the chronological order of appearance in the literature.
TABLE 1.
Human conditions with reported changes in MANF and CDNF expression
| Disease | Change | Reference |
|---|---|---|
| Atrial Fibrillation | Decreased MANF mRNA and protein in serum | Wang et al 2019 |
| Diabetes Type 1 | Increased MANF protein in plasma | Galli et al 2016 |
| Diabetes Type 2 | Increased MANF protein in plasma | Wu et al 2017, Fu et al 2020 |
| Fasting | Increased MANF protein in plasma | Galli et al 2019a |
| Hepatocellular carcinoma | Increased MANF mRNA and protein in tissue | He et al 2020a |
| Hyperlipidemia | Increased MANF protein in serum | Fu et al 2020 |
| Nonalcoholic Steatohepatitis | Decreased MANF protein in serum | Sousa-Victor et al 2019 |
| Parkinson’s disease | Increased MANF protein in plasma | Galli et al 2019b |
| Parkinson’s disease | Increased CDNF protein in hippocampus | Virachit et al 2019 |
| Physiological ageing | Decreased MANF protein in plasma | Sousa-Victor et al 2019 |
| Polycystic ovary syndrome | Decreased MANF protein in plasma | Wei et al 2020 |
| Proliferative diabetic retinopathy | Increased MANF protein in vitreous | Gao et al 2017 |
| Retinal detachment | Increased MANF protein in vitreous | Gao et al 2017 |
| Rheumatoid arthritis | Increased MANF mRNA in white blood cells | Chen et al 2015 |
| Stroke | Decreased CDNF mRNA levels in blood platelets | Joshi et al 2020 |
| Systemic lupus erythematosus | Increased MANF mRNA in white blood cells | Chen et al 2015 |
TABLE 2.
Disease models in which MANF and CDNF were effective trophic factors
| MANF | Disease | Model System | Species | Reference |
|---|---|---|---|---|
| Alzheimer’s disease | In vitro – Challenging with amyloid β | Human (Neuro 2a and SH-SY5Y cell lines) | Xu et al 2019 | |
| Brain ischemia | In vivo - distal middle cerebral artery occlusion (dMCAO) | Rat | Airavaara et al 2009, Airavaara et al 2010, Yang et al 2014, Wang et al 2016, Tseng et al 2018, Mätlik et al 2018, Gao et al 2020 | |
| In vitro – Tunicamycin treatment, Oxygen-glucose deprivation | Rat (Primary neurons and primary astrocytes) | Yu et al 2010, Zhao et al 2013 | ||
| Cardiac ischemia/reperfusion | In vitro – simulated ischemia or tunicamycin treatment | Rat Mouse (primary cardiac myocytes) | Tadimalla et al 2008, Arrieta et al 2020 | |
| In vivo – coronary vessel occlusion | Mouse | Glembotski et al 2012 | ||
| Diabetes | In vitro - Challenge with cytokines | Human (primary pancreatic islets) | Hakonen et al 2018, Cunha et al 2017 | |
| In vitro - Challenge with hyperglycemia | Mouse (primary pancreatic islets) | Danilova et al 2019 | ||
| Diabetic keratopathy | In vivo – Corneal epithelium wound healing model, diabetic and normoglycemic | Mouse | Wang et al 2020 | |
| Glaucoma | In vivo - chronic ocular hypertension (COHT) model | Rat | Gao et al 2017 | |
| Intracerebral hemorrhage | In vivo - Perforation model | Rat | Xu et al 2018 | |
| Liver damage | In vivo – aged mice | Mouse | Sousa-Victor et al 2019 | |
| Liver steatosis | In vitro – Challenging with free fatty acids | Human (HepG2 cell line) | He et al 2020b | |
| Myocardial infarction | In vivo – coronary occlusion/reperfusion | Mouse | Glembotski et al 2012 | |
| Nephrotic syndrome (Tg-C321R) | In vitro | Mouse (primary podocytes) | Park et al 2019 | |
| Parkinson’s disease | In vivo - 6-hydroxy dopamine (6-OHDA) lesioning | Rat, Mouse | Voutilainen et al 2009, Cordero-Llana et al 2015 | |
| In vivo - 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) lesioning | Mouse | Liu et al 2018 | ||
| In vitro - 6-OHDA treatment | Human (SH-SY5Y cell line) | Zhang et al 2017a | ||
| In vivo – 6-OHDA treatment and α-synuclein overexpression | Roundworm | Zhang et al 2018 | ||
| Retinal degeneration | In vivo – 5 klux light exposure Crxtvrm65 and Pde6bRd1 mutants, aged mice | Mouse | Neves et al 2016, Neves et al 2020 | |
| In vivo - rhodopsin S334ter mutation | Rat | Lu et al 2018 | ||
| Traumatic spinal cord injury | In vivo - clip compressive crush injury | Rat | Gao et al 2018 | |
| Traumatic brain injury | In vivo - Subarachnoid hemorrhage induction | Rat | Li et al 2019 | |
| CDNF | Alzheimer’s disease | In vivo - APP/PS1 mutant | Mouse | Kemppainen et al 2015 |
| In vitro – Challenging with β-amyloid | Rat (primary hippocampal cells) | Zhou et al 2016 | ||
| Brain ischemia | In vivo - MCAO | Rat | Zhang et al 2018a | |
| Parkinson’s disease | In vivo - 6-OHDA lesioning | Rat | Lindholm et al 2007, Voutilainen et al 2011, Bäck et al 2013, Ren et al 2013, Wang et al 2017, Huotarinen et al 2018, Cordero-Llana et al 2015 | |
| In vivo - MPTP lesioning | Mouse | Airavaara et al 2012 | ||
| In vivo - 6-OHDA lesioning | Marmoset monkeys | Garea-Rodriquez et al 2016 | ||
| Peripheral nerve injury | In vivo – Nerve transection | Rat | Cheng et al 2013, Liu et al 2014 |
Parkinson’s disease (PD)
As both MANF and CDNF were first shown to protect dopaminergic neurons (Petrova et al., 2003; Lindholm et al., 2007) the subsequent studies concentrated on models of PD especially with CDNF which has entered clinical trials for the treatment of PD (Huttunen and Saarma, 2019). Parkinson’s disease has been a primary focus in the field of MANF and CDNF research and many thorough reviews cover the history of MANF and CDNF as putative therapeutics for Parkinson’s disease so those studies will not be reviewed here (Voutilainen et al., 2015; Albert and Airavaara, 2019; Huttunen and Saarma, 2019). A summary of studies evaluating MANF and CDNF in PD models can be found in Table 2. Several recent results not captured in the prior reviews warrant mention. For example, Galli et al (2019) showed that MANF protein levels were elevated in the serum of Parkinson’s patients compared to control group. No differences in MANF mRNA was detected in the whole blood samples. CDNF protein and mRNA levels were same between PD patients and controls (Galli et al., 2019a). A separate study showed that MANF levels were not altered, but the expression of CDNF protein, examined by immunohistochemical staining and ELISA, was significantly increased in hippocampi of post-mortem PD patients when compared to control (Virachit et al., 2019). To study the role of endogenous MANF in the brain, Pakarinen et al 2020 used Cre-conditional Manf knock-out mice crossed with Nestin-Cre driver mice (Pakarinen et al., 2020). UPR genes were upregulated in the midbrain but despite the UPR activation, they did not show increased loss of dopaminergic neurons during aging, compared to control mice. No differences were observed in the levels of dopamine or its metabolites and no behavioural deficits were detected. These data suggest that MANF F is not necessary for the survival and function of the dopaminergic neurons (Pakarinen et al., 2020), even though exogenous MANF has been shown to protect them (Petrova et al., 2003, Lindholm et al., 2007). Mice that lack MANF expression completely (Manf−/−) are diabetic and show growth deficiency (Lindahl et al., 2014). Knocking out Cdnf from mice does not result in any obvious defects and the mice are viable and fertile (Lindahl et al., 2020). The midbrain dopaminergic neurons of Cdnf knockout mice are similar to those of WT mice in both number and morphology. Unlike the conditional MANF knockout mice, the lack of CDNF did not upregulate UPR genes in the brain. However, Cdnf−/− mice show a loss of enteric neurons in the submucosal plexus with ageing due to neurodegeneration. This leads to slowing of GI movements, which is a typical early symptom in PD patients (Lindahl et al., 2020). Subsequent studies into the role CDNF and MANF on peripheral neurons may further illuminate the mechanistic differences between MANF and CDNF.
Ischemia
Soon after discovery of its relationship with dopaminergic neurons and PD, it became clear that MANF is upregulated following ischemia in both brain and heart (Apostolou et al., 2008; Lindholm et al., 2008; Tadimalla et al., 2008; Airavaara et al., 2009; Yu et al., 2010). Cerebral ischemia/reperfusion has been shown to induce ER stress via multiple mechanisms (DeGracia and Montie, 2004). The ischemia-related upregulation of MANF and CDNF are consistent with a function as UPR genes. Although fewer studies describe CDNF and brain ischemia, CDNF levels are elevated 2 hours after stroke in the rat brain and CDNF has cytoprotective effects following cerebral ischemia in rats (Zhang et al., 2018a). Treating rats with CDNF after cortical ischemia-reperfusion improved their functional recovery as well (Anttila et al., 2019).
The connection between MANF and brain ischemia has been extensively studied. In a rat model of brain ischemia, pre-treatment with recombinant MANF or AAV induced-mediated MANF overexpression in rat cerebral cortex reduced the infarction area. This demonstrated that MANF protected the neurons from ischemia-induced cell death (Airavaara et al., 2009; Airavaara et al., 2010). In these studies MANF levels were increased prior to the ischemia/reperfusion, however, treatment with MANF following the stroke was also capable of improving recovery (Yang et al., 2014; Mätlik et al., 2018). The timing of recombinant MANF delivery relative to the middle cerebral artery occlusion altered the effectiveness of MANF on behavioral or histopathologic outcomes. For example, MANF administered two hours post-occlusion reduced neuronal death as measured by TUNEL and propidium iodide staining (Yang et al., 2014). MANF had no effect on the infarction volume when delivered as recombinant protein or AAV vector at 2 to 3 days post-stroke, but the rats showed improved functional recovery compared to vehicle or control AAV groups (Mätlik et al., 2018). How does MANF exert these functions so long after the stroke given that these effects cannot be attributed to its role in protecting the neurons from the ischemia itself? According to a rodent study by Tseng et al (2018) MANF increased the migration of neural progenitor cells to the infarction site and promotes their differentiation to replace neuronal function lost due to the stroke (Tseng et al., 2018). MANF also transiently increased the phagocytic immune cells around the infarct area in rat brain which may have contributed to more effective removal of the cellular debris and dead cells (Mätlik et al., 2018). Recently, Gao et al (2020) reported increased VEGF expression and functional microvessels in the infarction area when MANF was administered 24 hours after the stroke suggesting that MANF promotes angiogenesis (Gao et al., 2020). An earlier study, however, found no MANF-related changes to angiogenesis in the peri-infarct area (Mätlik et al., 2018). Using metabolomics, RNA sequencing and proteomics Teppo et al (2020) studied the effects of MANF overexpression in the peri-infarct area of rat brain 4 days post-stroke. The changes between animals treated with AAV-MANF and AAV-GFP were subtle, but support an immunomodulatory role of MANF (Teppo et al., 2020). Additional studies are needed to understand the function of MANF and CDNF in recovery from brain ischemia and optimize their therapeutic potential for stroke.
The protective effects of MANF have also been evaluated for cardiac ischemia both in vivo and in vitro. In transgenic mice harboring a tamoxifen-inducible ATF6, the level of MANF mRNA was increased more than 10-fold in response to ATF6 activation supporting that MANF is a target of the UPR (Tadimalla et al., 2008). At 4–7 days following an infarction, MANF protein levels were increased in the cardiomyocytes as well as other cells surrounding the infarct area in mouse heart. Interestingly, using miRNAs to lower the levels of MANF expression (by up to 90 %) in rat neonatal ventricular myocytes, did not affect their viability under normal conditions, but after simulated infarction/reperfusion (sI/R), the cell death was significantly higher in these cells compared to the control. In addition, recombinant MANF protected these miRNA treated cells from sI/R induced death (Tadimalla et al., 2008). Although the intracellular expression of MANF in cultured myocytes is increased by a range of ER stress inducers, its secretion was only increased in response to ischemia or ER stress induced by thapsigargin, indicating that disturbances in calcium homeostasis of ER are needed to trigger the secretion (Glembotski et al., 2012). GRP78 and MANF interact as part of a macromolecular complex inside the ER and this complex is dependent on calcium. When ER calcium is depleted the complex is disrupted and MANF gets secreted (Glembotski et al., 2012). Mice that received MANF protein by systemic infusion from subdermal osmotic minipumps prior to ischemia/reperfusion had a decreased infarct area in the hearts which reinforces that MANF is a cardioprotective factor (Glembotski et al., 2012). A recent study from the same laboratory demonstrated that selective knockout of MANF in cardiomyoctes increased the tissue damage and decreased the viability of myocytes following I/R (Arrieta et al., 2020). Re-expressing MANF in the hearts of these mice, by AAV9 encoding a MANF not targeted by the used miRNA, reversed the effects of cardiac-specific MANF knockout. According to the study, MANF acts as chaperone and binds the misfolded ER proteins to counteract the negative effects of cardiac ischemia. They demonstrate that the conserved cysteine residues, including the CXXC domains, are necessary for the chaperone function of MANF (Arrieta et al., 2020). While the protective effects of CDNF on cardiac ischemia have not been reported, CDNF has been shown to protect cardiomyocytes from tunicamycin-induced ER stress (Liu et al., 2018a).
Diabetes
The development of diabetes has been shown to be one of the primary features in MANF knockout mice (Lindahl et al., 2014). Specifically, the pancreatic β-cells showed increased apoptotic cell death, reduced proliferation and the expression of ER stress markers ATF6, Grp78 and CHOP in pancreatic β-cells were increased in MANF knockout mice compared to control mice (Lindahl et al., 2014). Consistent with this finding, pancreatic β-cell specific knockout of MANF lead to diabetes in embryonic stage but also in adult mice (Danilova et al., 2019). Mouse embryos lacking MANF exhibited reduced expansion of β-cells whereas adult mice lacking MANF had reduced β-cells mass due to apoptotic cells death (Danilova et al., 2019). Furthermore, treating pancreatic β-cells in vitro with recombinant MANF lead to their increased proliferation but also protected cells from thapsigargin- or hyperglycemia-induced cell death (Lindahl et al., 2014; Danilova et al., 2019). MANF has also been shown to protect the pancreas from pro-inflammatory cytokines (Cunha et al., 2017; Hakonen et al., 2018), which are thought to be the main driver of pancreatic β-cell death in type 1 diabetes (Gurzov and Eizirik, 2011). MANF has been also studied in human pancreatic β-cells derived from organ donors (Hakonen et al., 2018). Treating human pancreatic islets with cytokines lead to increased MANF secretion and recombinant MANF reduced cell death induced by inflammatory cytokines suggesting MANF might be a potential therapeutic to treat diabetes (Hakonen et al., 2018). As described above, MANF’s positive effects correlate with repressed inflammation and UPR (Hakonen et al., 2018; Danilova et al., 2019). In accordance with the studies described above, MANF was shown to increase the proliferation of pancreatic β-cells in mouse model of Wolfram syndrome (Mahadevan et al., 2020). Wolfram syndrome is a prototype of ER stress related diseases, and it causes juvenile onset diabetes, optic nerve atrophy, hearing loss and neurodegeneration. The condition is usually caused by a recessive mutation in WFS1 gene, that codes a protein involved in regulation of ER calcium homeostasis. Mahadevan et al (2020) utilized βWfs1−/− mice and both in vivo AAV mediated overexpression of MANF and treating pancreatic islands from these mice in vitro with recombinant MANF increased the proliferation of the pancreatic β-cells. However, the insulin secretion was not affected by either recombinant MANF treatment or MANF overexpression (Mahadevan et al., 2020). Clinical studies have shown a correlation between serum MANF levels and diabetes (Galli et al., 2016; Wu et al., 2017). Serum MANF levels were increased both in adults with either recently diagnosed type 2 diabetes or prediabetes and in children 1–9 years of age recently diagnosed with type 1 diabetes but not in older diabetic children (Galli et al., 2016; Wu et al., 2017). Given that the recombinant MANF shows cytoprotective effects in preclinical studies, it may seem contradictory that diabetic patients have increased serum levels of MANF. However, this could be explained by increased ER stress in pancreatic β-cells due to the increased need to produce insulin as this population of cells is decreasing. As described above, the C-terminal tail of MANF is also sensitive to ER calcium conditions and perhaps the elevated MANF is a biomarker for the inability of the cells to retain ERS-containing proteins, a phenomenon termed “exodosis” (Trychta et al., 2018). Furthermore, as mentioned above the inflammatory cytokines thought to play a key part in the development of type 1 diabetes also increase the secretion of MANF (Hakonen et al., 2018), highlighting the complex interplay between MANF expression and secretion that may exist in different tissues in vivo.
MANF overexpression in the hypothalamus has been linked with insulin resistance (Yang et al., 2017), while MANF expression in the hypothalamus was shown to increase following fasting and transgenic mice overexpressing MANF in their brain showed increased feeding and obesity, which was a consequence of overeating and not changes in the metabolic rate or locomotor activity of the animals. Reduced MANF levels in the hypothalamus, by knock-down with AAV expressing MANF shRNA, led to hypophagia. The transgenic mice with reduced hypothalamic MANF levels were glucose intolerant but there was no change in inflammatory molecule levels, indicating that the insulin insensitivity was not due to increased inflammation (Yang et al., 2017). In humans, fasting led to an increase of MANF protein levels in serum; however, the BMI of the study subjects did not correlate with the plasma MANF levels or the fasting-induced change in MANF levels did not correlate with plasma lipids, glucose or insulin levels nor insulin resistance as evaluated by homeostatic model assessment (Galli et al., 2019b). Collectively, these data indicate that MANF acts at several locations to modulate glucose homeostasis and deficiencies in MANF can lead to diabetic phenotype while elevated levels of serum MANF may reflect disruption of ER proteostasis in the pancreas. The role of CDNF in diabetes has not been reported, however, CDNF knockout mice do not display altered glucose levels or other gross differences compared to wild-type mice (Lindahl et al., 2020). Perhaps this relates to the differences in MANF and CDNF to interact with lipids described above.
Alzheimer’s disease
Although most studies on MANF and CDNF in neurodegenerative diseases have been focused on PD there is evidence that MANF and CDNF have beneficial effects in Alzheimer’s disease (AD) models as well. The amyloid precursor protein/presenilin1 (APP/PS1) double transgenic mouse is a widely used animal model for AD and these mice show β-amyloid deposits accumulating in the brain over time, with strong deposits visible as early as at six months of age (Kurt et al., 2001). Additionally, these mice display memory deficits, with short-term memory affected at 3 months of age and long-term memory at 6 months of age (Trinchese et al., 2004). In the APP/PS1 mouse model, recombinant CDNF protein or AAV2 overexpressing CDNF in the hippocampus improved the long-term memory of the transgenic mice at the age of one year, but did not ameliorate short-term memory or decrease the amount of amyloid deposits in the brain (Kemppainen et al., 2015). Interestingly, CDNF also improved long-term memory in wild type mice. A separate study using the APP/PS1 mice found that MANF expression was increased in the brain compared to age matched wildtype mice (Xu et al., 2019). Furthermore, treating neuronal cell lines (Neuro-2a and SH-SY5Y) with β-amyloid-induced UPR pathways and treatment with recombinant MANF or MANF overexpression alleviated the β-amyloid-induced UPR activation, indicating that MANF reduces the ER stress triggered by β-amyloid (Xu et al., 2019). Zhou et al (2016) used rat primary hippocampal cells to show that treatment with recombinant CDNF alleviated the ER stress caused by β-amyloid treatment and protected cells from β-amyloid synaptotoxicity which is thought to precede neuronal loss and coincide with the cognitive decline observed in AD (Zhou et al., 2016).
Retinal disorders
MANF is expressed in different parts of the vision system, including the optic nerve, retina and vitreous (Gao et al., 2016; Gao et al., 2017). MANF was proposed as a biomarker for certain types of retinal disorders because the amount of MANF in vitreous was increased in patients suffering from proliferative diabetic retinopathy or retinal detachment compared to patients undergoing vitreoretinal surgery unrelated to diabetes or detachment (Gao et al., 2017). Conversely, in a recent study Wang et al (2020) showed that MANF expression was significantly reduced in corneal epithelial cells of the streptozotocin-induced type I diabetic mice (Wang et al., 2020). They also demonstrated that recombinant human MANF improved the healing of a wounded corneal epithelium in both normoglycemic and diabetic mice (Wang et al., 2020b). In addition, recombinant MANF has been shown to be protective for both retinal ganglion cells undergoing hypoxia and photoreceptor cells in a transgenic (S334ter rhodopsin mutant) rat model of retinal degeneration (Gao et al., 2017; Lu et al., 2018). MANF protects different cell types in the eye and promotes the repair of damaged retina by modulating the immune response, leading to more successful transplantation and integration of photoreceptor cells in the damaged retina (Neves et al., 2016). In two different genetic mouse models of retinal degeneration and in a light induced retinal damage model, injection of recombinant human MANF into the vitreous had protective effects in all of three models. Furthermore, Neves et al (2016) injected AAV9-MANF transduced fibroblasts that expressed and secreted MANF, into the vitreous of a transgenic mouse model of retinal degeneration showing that persistent MANF delivery delayed the retinal degeneration and protected the optic nerve cells (Neves et al., 2016). Aging leads to decreased MANF levels in the choroid of both mice and humans, which corresponds with an increase in the amount of activated macrophages (Neves et al., 2020). Additionally, light induced damage of photoreceptor cells was more severe in the eyes of old animals than in those of young. MANF delivery to the retina of the old mice modulated the immune response, leading to reduction of apoptotic cells in the mouse retina following light induced damage (Neves et al., 2020). These data further support the role of MANF as an immunomodulator and encourages further studies into the use of MANF as a regenerative therapy for retinal degeneration.
Nephrotic syndrome
In nephrotic syndrome the kidneys secrete proteins into the urine due to a kidney malfunction. ER stress caused by accumulation of misfolded proteins and activation of UPR has been associated with many tubular and glomerular diseases (Inagi et al., 2014). In glomeruli isolated from a mouse model of ER stress induced nephrotic syndrome, MANF mRNA and protein expression were upregulated and MANF secretion was detected in the growth medium of primary podocytes. Most importantly, Kim et al (2016) showed that MANF was detectable in the urine of the mouse model of nephrotic syndrome in its initial state. MANF was secreted in the urine following induction of a renal ischemic insult or ER stress by tunicamycin injections suggesting that the appearance of MANF in the urine could be used as a biomarker for several ER stress related kidney malfunctions (Kim et al., 2016). A study conducted in rats produced similar findings (Tousson-Abouelazm et al., 2020). Although ER stress is associated with nephrotic syndrome, there are no treatments that specifically target ER stress (Park et al., 2019). Using the aforementioned mouse model of nephrotic syndrome, Park et al (2019) identified an increase in the RYR2 phosphorylation in podocytes which caused the RYR to leak calcium from the ER and therefore cause ER stress and cell death. Treatment with MANF reduced the phosphorylation of RYR2 and stabilized ER calcium leading to increased podocyte survival (Park et al., 2019). These findings support further investigation of MANF’s role in nephrotic syndrome.
Chondrodysplasia
In addition to being necessary for the development of pancreatic β-cells, MANF also plays a role in cartilage development (Bell et al., 2019). Two types of skeletal disorders, metaphyseal chondrodysplasia (Schmid type, MCDS) and multiple epiphyseal dysplasia (MED) are results of mutations that cause normally secreted proteins to be misfolded and retained inside of cells (Bell et al., 2019). The retention of these misfolded proteins (type X collagen in MCDS and matrilin-3 in MED) causes ER stress. In mouse models of both conditions, Manf is one of the most upregulated genes (Nundlall et al., 2010; Cameron et al., 2011). Manf deletion from cartilage induced upregulation of ER stress proteins, decreased chondrocyte proliferation and resulted in impaired long bone growth (Bell et al., 2019). Exogenous MANF increased the proliferation of tunicamycin treated chondrocytes and attenuated tunicamycin induced GRP78 expression in them. In matrilin-3 mutant chondrocytes exogenous MANF decreased GRP78 expression but levels and localization of intracellular matrilin-3 were unaffected (Bell et al., 2019).
Hearing loss
In addition to the visual system, MANF has been recently shown to contribute to hearing loss. A loss of outer hair cells of the inner ear is commonly associated with hearing loss and MANF knockout mice have a reduced number of outer hair cell. MANF knockout mice also have increased expression of UPR-related genes GRP78, PDI and CHOP in their cochlear sensory cells suggesting that, once again, the loss of MANF leads to increased UPR (Herranen et al., 2020). Additional work into the role of MANF and CDNF in the auditory system are warranted.
Summary
MANF and CDNF represent a unique class of trophic factors that reside in the lumen of the ER. In response to environmental stressors that cause disruption of ER functions (e.g. protein misfolding, calcium storage, lipid metabolism, etc.) the expression of MANF and CDNF is increased. Their function as UPR genes appears to serve a primary purpose of negatively regulating the UPR but can also attenuate an inflammatory response. Their trophic actions convey support across a wide range of tissues including brain, liver, heart, pancreas, bone and kidney. While a receptor has not been identified, MANF has been shown to interact with a lipid and with BiP/GRP78, a primary chaperone central to the ER functions. The use of CDNF in a clinical trial for Parkinson’s disease is evidence for the therapeutic potential of this family of molecules. Given the diverse pathologies that MANF and CDNF can alleviate, additional clinical applications are anticipated especially for diseases where the UPR is activated.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, NIDA. The authors thank Dr. Mark Henderson, Dr. Kathleen Trychta, Dr. Deon Harvey, Ms. Lacey Kennedy, Ms. Helena Baffoe-Bonnie for their comments on the manuscript. We also thank Ms. Katie Nolan for her comments and assistance preparing Figure 1.
Funding:
This work was supported by the Intramural Research Program of the NIH, NIDA.
Footnotes
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest: The authors have no conflicts to declare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Shen H, Kuo CC, Peränen J, Saarma M, Hoffer B, Wang Y (2009) Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J Comp Neurol 515:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, Chiocco MJ, Howard DB, Zuchowski KL, Peränen J, Liu C, Fang S, Hoffer BJ, Wang Y, Harvey BK (2010) Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol 225:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, Harvey BK, Voutilainen MH, Shen H, Chou J, Lindholm P, Lindahl M, Tuominen RK, Saarma M, Hoffer B, Wang Y (2012) CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplant 21:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Airavaara M (2019) Neuroprotective and reparative effects of endoplasmic reticulum luminal proteins - mesencephalic astrocyte-derived neurotrophic factor and cerebral dopamine neurotrophic factor. Croat Med J 60:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti RH, Bradshaw RA (1971) Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci U S A 68:2417–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila JE, Pöyhönen S, Airavaara M (2019) Secondary Pathology of the Thalamus after Focal Cortical Stroke in Rats is not Associated with Thermal or Mechanical Hypersensitivity and is Not Alleviated by Intra-Thalamic Post-Stroke Delivery of Recombinant CDNF or MANF. Cell Transplant 28:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou A, Shen YX, Liang Y, Luo J, Fang S (2008) Armet, a UPR-upregulated protelin, inhibits cell proliferation and ER stress-induced cell death. Experimental Cell Research 314:2454–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Ellgaard L (2008) The human PDI family: versatility packed into a single fold.Biochim Biophys Acta 1783:535–548. [DOI] [PubMed] [Google Scholar]
- Arancibia D, Zamorano P, Andrés ME (2018) CDNF induces the adaptive unfolded protein response and attenuates endoplasmic reticulum stress-induced cell death. Biochim Biophys Acta Mol Cell Res 1865:1579–1589. [DOI] [PubMed] [Google Scholar]
- Arrieta A, Blackwood EA, Stauffer WT, Domingo MS, Bilal AS, Thuerauf DJ, Pentoney AN, Aivati C, Sarakki AV, Doroudgar S, Glembotski CC (2020) Mesencephalic astrocyte-derived neurotrophic factor is an ER-resident chaperone that protects against reductive stress in the heart. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Vozdek R, Hnízda A, Jiang C, Wang B, Kuchar L, Li T, Zhang Y, Wood C, Feng L, Dang Y, Ma DK (2018) Conserved roles of C. elegans and human MANFs in sulfatide binding and cytoprotection. Nat Commun 9:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell PA, Dennis EP, Hartley CL, Jackson RM, Porter A, Boot-Handford RP, Pirog KA, Briggs MD (2019) Mesencephalic astrocyte-derived neurotropic factor is an important factor in chondrocyte ER homeostasis. Cell Stress Chaperones 24:159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TJ, Fregno I, Fumagalli F, Rinaldi A, Bertoni F, Boersema PJ, Picotti P, Molinari M (2018) Chemical stresses fail to mimic the unfolded protein response resulting from luminal load with unfolded polypeptides. J Biol Chem 293:5600–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Božok V, Yu LY, Palgi J, Arumäe U (2018) Antioxidative CXXC Peptide Motif From Mesencephalic Astrocyte-Derived Neurotrophic Factor Antagonizes Programmed Cell Death. Front Cell Dev Biol 6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T, Voutilainen MH, Raasmaja A, Saarma M, Männistö PT, Tuominen RK (2013) Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease. Brain Behav 3:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron TL, Bell KM, Tatarczuch L, Mackie EJ, Rajpar MH, McDermott BT, Boot-Handford RP, Bateman JF (2011) Transcriptional profiling of chondrodysplasia growth plate cartilage reveals adaptive ER-stress networks that allow survival but disrupt hypertrophy. PLoS One 6:e24600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara M, Prischi F, Ali MM (2013) UPR Signal Activation by Luminal Sensor Domains. Int J Mol Sci 14:6454–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Feng L, Wang X, Du J, Chen Y, Yang W, Zhou C, Cheng L, Shen Y, Fang S, Li J (2015) Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-κB pathway. Sci Rep 5:8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhao H, Zhang W, Liu B, Liu Y, Guo Y, Nie L (2013) Overexpression of conserved dopamine neurotrophic factor (CDNF) in astrocytes alleviates endoplasmic reticulum stress-induced cell damage and inflammatory cytokine secretion. Biochem Biophys Res Commun 435:34–39. [DOI] [PubMed] [Google Scholar]
- Cordero-Llana Ó, Houghton BC, Rinaldi F, Taylor H, Yáñez-Muñoz RJ, Uney JB, Wong LF, Caldwell MA (2015) Enhanced efficacy of the CDNF/MANF family by combined intranigral overexpression in the 6-OHDA rat model of Parkinson’s disease. Mol Ther 23:244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha DA, Cito M, Grieco FA, Cosentino C, Danilova T, Ladrière L, Lindahl M, Domanskyi A, Bugliani M, Marchetti P, Eizirik DL, Cnop M (2017) Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J Biol Chem 292:14977–14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova T, Galli E, Pakarinen E, Palm E, Lindholm P, Saarma M, Lindahl M (2019) Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Is Highly Expressed in Mouse Tissues With Metabolic Function. Front Endocrinol (Lausanne) 10:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, Montie HL (2004) Cerebral ischemia and the unfolded protein response. J Neurochem 91:1–8. [DOI] [PubMed] [Google Scholar]
- Fu J, Nchambi KM, Wu H, Luo X, An X, Liu D (2020) Liraglutide protects pancreatic β cells from endoplasmic reticulum stress by upregulating MANF to promote autophagy turnover. Life Sci 252:117648. [DOI] [PubMed] [Google Scholar]
- Galli E, Planken A, Kadastik-Eerme L, Saarma M, Taba P, Lindholm P (2019a) Increased Serum Levels of Mesencephalic Astrocyte-Derived Neurotrophic Factor in Subjects With Parkinson’s Disease. Front Neurosci 13:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli E, Rossi J, Neumann T, Andressoo JO, Drinda S, Lindholm P (2019b) Mesencephalic Astrocyte-Derived Neurotrophic Factor Is Upregulated with Therapeutic Fasting in Humans and Diet Fat Withdrawal in Obese Mice. Sci Rep 9:14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli E, Härkönen T, Sainio MT, Ustav M, Toots U, Urtti A, Yliperttula M, Lindahl M, Knip M, Saarma M, Lindholm P (2016) Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci Rep 6:29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Deng J, Zhang X, Sun H, Jia G, Li J, Zhang K, Wan C, Wang L, Yan LJ, Cai Z, Ma J (2020) Effects of mesencephalic astrocyte-derived neurotrophic factor on cerebral angiogenesis in a rat model of cerebral ischemia. Neurosci Lett 715:134657. [DOI] [PubMed] [Google Scholar]
- Gao FJ, Zhang SH, Li TT, Wu JH, Wu Q (2016) Expression and Distribution of Mesencephalic Astrocyte-Derived Neurotrophic Factor in the Retina and Optic Nerve. Front Hum Neurosci 10:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FJ, Wu JH, Li TT, Du SS, Wu Q (2017) Identification of Mesencephalic Astrocyte-Derived Neurotrophic Factor as a Novel Neuroprotective Factor for Retinal Ganglion Cells. Front Mol Neurosci 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garea-Rodríguez E, Eesmaa A, Lindholm P, Schlumbohm C, König J, Meller B, Krieglstein K, Helms G, Saarma M, Fuchs E (2016) Comparative Analysis of the Effects of Neurotrophic Factors CDNF and GDNF in a Nonhuman Primate Model of Parkinson’s Disease. PLoS One 11:e0149776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glembotski CC, Thuerauf DJ, Huang C, Vekich JA, Gottlieb RA, Doroudgar S (2012) Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem 287:25893–25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Cui Y, Liu Q, Yang Y, Li Y, Weng L, Tang B, Jin P, Li XJ, Yang S, Li S (2018) Piperine ameliorates SCA17 neuropathology by reducing ER stress. Mol Neurodegener 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurzov EN, Eizirik DL (2011) Bcl-2 proteins in diabetes: mitochondrial pathways of β-cell death and dysfunction. Trends Cell Biol 21:424–431. [DOI] [PubMed] [Google Scholar]
- Hakonen E, Chandra V, Fogarty CL, Yu NY, Ustinov J, Katayama S, Galli E, Danilova T, Lindholm P, Vartiainen A, Einarsdottir E, Krjutškov K, Kere J, Saarma M, Lindahl M, Otonkoski T (2018) MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia 61:2202–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CL, Edwards S, Mullan L, Bell PA, Fresquet M, Boot-Handford RP, Briggs MD (2013) Armet/Manf and Creld2 are components of a specialized ER stress response provoked by inappropriate formation of disulphide bonds: implications for genetic skeletal diseases. Hum Mol Genet 22:5262–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman JH, Richie CT, Gordon KL, Mello DF, Castillo P, Zhu A, Wang Y, Hoffer BJ, Sherwood DR, Meyer JN, Harvey BK (2019) MANF deletion abrogates early larval Caenorhabditis elegans stress response to tunicamycin and Pseudomonas aeruginosa. Eur J Cell Biol 98:151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Li G, Liu X, Ma L, Zhang P, Zhang J, Zheng S, Wang J, Liu J (2020a) Diagnostic and Prognostic Values of MANF Expression in Hepatocellular Carcinoma. Biomed Res Int 2020:1936385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Wang C, Long XH, Peng JJ, Liu DF, Yang GY, Jensen MD, Zhang LL (2020b) Mesencephalic astrocyte-derived neurotrophic factor ameliorates steatosis in HepG2 cells by regulating hepatic lipid metabolism. World J Gastroenterol 26:1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman M, Peränen J, Saarma M, Permi P (2010) 1H, 13C and 15N resonance assignments of the human mesencephalic astrocyte-derived neurotrophic factor. Biomol NMR Assign 4:215–217. [DOI] [PubMed] [Google Scholar]
- Henderson MJ, Richie CT, Airavaara M, Wang Y, Harvey BK (2013) Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J Biol Chem 288:4209–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MJ, Wires ES, Trychta KA, Richie CT, Harvey BK (2014) SERCaMP: a carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol Biol Cell 25:2828–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranen A, Ikäheimo K, Lankinen T, Pakarinen E, Fritzsch B, Saarma M, Lindahl M, Pirvola U (2020) Deficiency of the ER-stress-regulator MANF triggers progressive outer hair cell death and hearing loss. Cell Death Dis 11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Wang D, Li X, He Y, Wei C, Jiang R, Liu J, Feng L, Shen Y (2019) MANF regulates splenic macrophage differentiation in mice. Immunol Lett 212:37–45. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotarinen A, Penttinen AM, Bäck S, Voutilainen MH, Julku U, Piepponen TP, Männistö PT, Saarma M, Tuominen R, Laakso A, Airavaara M (2018) Combination of CDNF and Deep Brain Stimulation Decreases Neurological Deficits in Late-stage Model Parkinson’s Disease. Neuroscience 374:250–263. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Saarma M (2019) CDNF Protein Therapy in Parkinson’s Disease. Cell Transplant 28:349–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagi R, Ishimoto Y, Nangaku M (2014) Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease. Nat Rev Nephrol 10:369–378. [DOI] [PubMed] [Google Scholar]
- Joshi H, McIntyre WB, Kooner S, Rathbone M, Gabriele S, Gabriele J, Baranowski D, Frey BN, Mishra RK (2020) Decreased Expression of Cerebral Dopamine Neurotrophic Factor in Platelets of Stroke Patients. J Stroke Cerebrovasc Dis 29:104502. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Lindholm P, Galli E, Lahtinen HM, Koivisto H, Hämäläinen E, Saarma M, Tanila H (2015) Cerebral dopamine neurotrophic factor improves long-term memory in APP/PS1 transgenic mice modeling Alzheimer’s disease as well as in wild-type mice. Behav Brain Res 291:1–11. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee H, Manson SR, Lindahl M, Evans B, Miner JH, Urano F, Chen YM (2016) Mesencephalic Astrocyte-Derived Neurotrophic Factor as a Urine Biomarker for Endoplasmic Reticulum Stress-Related Kidney Diseases. J Am Soc Nephrol 27:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt MA, Davies DC, Kidd M, Duff K, Rolph SC, Jennings KH, Howlett DR (2001) Neurodegenerative changes associated with beta-amyloid deposition in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Exp Neurol 171:59–71. [DOI] [PubMed] [Google Scholar]
- Latgé C, Cabral KM, Almeida MS, Foguel D (2013) (1)H-, (13)C- and (15)N-NMR assignment of the N-terminal domain of human cerebral dopamine neurotrophic factor (CDNF). Biomol NMR Assign 7:101–103. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R (1987) The nerve growth factor 35 years later. Science 237:1154–1162. [DOI] [PubMed] [Google Scholar]
- Li T, Xu W, Gao L, Guan G, Zhang Z, He P, Xu H, Fan L, Yan F, Chen G (2019) Mesencephalic astrocyte-derived neurotrophic factor affords neuroprotection to early brain injury induced by subarachnoid hemorrhage via activating Akt-dependent prosurvival pathway and defending blood-brain barrier integrity. FASEB J 33:1727–1741. [DOI] [PubMed] [Google Scholar]
- Lindahl M, Danilova T, Palm E, Lindholm P, Võikar V, Hakonen E, Ustinov J, Andressoo JO, Harvey BK, Otonkoski T, Rossi J, Saarma M (2014) MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep 7:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Chalazonitis A, Palm E, Pakarinen E, Danilova T, Pham TD, Setlik W, Rao M, Võikar V, Huotari J, Kopra J, Andressoo JO, Piepponen PT, Airavaara M, Panhelainen A, Gershon MD, Saarma M (2020) Cerebral dopamine neurotrophic factor-deficiency leads to degeneration of enteric neurons and altered brain dopamine neuronal function in mice. Neurobiol Dis 134:104696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde CM, Grundström S, Nordling E, Refai E, Brennan PJ, Andersson M (2005) Conserved structure and function in the granulysin and NK-lysin peptide family. Infect Immun 73:6332–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm P, Peränen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O, Timmusk T, Saarma M (2008) MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci 39:356–371. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M (2007) Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448:73–77. [DOI] [PubMed] [Google Scholar]
- Lindström R, Lindholm P, Kallijärvi J, Yu LY, Piepponen TP, Arumäe U, Saarma M, Heino TI (2013) Characterization of the structural and functional determinants of MANF/CDNF in Drosophila in vivo model. PLoS One 8:e73928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yu C, Yu H, Zhong L, Wang Y, Liu J, Zhang S, Sun J, Duan L, Gong L, Yang J (2018a) Cerebral dopamine neurotrophic factor protects H9c2 cardiomyocytes from apoptosis. Herz 43:346–351. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou C, Tao X, Feng L, Wang X, Chen L, Li C, Huang D, Fang S, Shen Y (2015) ER stress-inducible protein MANF selectively expresses in human spleen. Hum Immunol 76:823–830. [DOI] [PubMed] [Google Scholar]
- Liu J, Wu Z, Han D, Wei C, Liang Y, Jiang T, Chen L, Sha M, Cao Y, Huang F, Geng X, Yu J, Shen Y, Wang H, Feng L, Wang D, Fang S, Wang S (2020) Mesencephalic Astrocyte-Derived Neurotrophic Factor Inhibits Liver Cancer Through Small Ubiquitin-Related Modifier (SUMO)ylation-Related Suppression of NF-κB/Snail Signaling Pathway and Epithelial-Mesenchymal Transition. Hepatology 71:1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang J, Jiang M, Cai Q, Fang J, Jin L (2018b) MANF improves the MPP. Am J Transl Res 10:1284–1294. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nie L, Zhao H, Zhang W, Zhang YQ, Wang SS, Cheng L (2014) Conserved dopamine neurotrophic factor-transduced mesenchymal stem cells promote axon regeneration and functional recovery of injured sciatic nerve. PLoS One 9:e110993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Luo L, Huang D, Liu X, Xia X, Wang Z, Lam BL, Yi J, Wen R, Li Y (2018) Photoreceptor Protection by Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF). eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan J, Morikawa S, Yagi T, Abreu D, Lu S, Kanekura K, Brown CM, Urano F (2020) A soluble endoplasmic reticulum factor as regenerative therapy for Wolfram syndrome. Lab Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei M, Zhai C, Li X, Zhou Y, Peng W, Ma L, Wang Q, Iverson BL, Zhang G, Yi L (2017) Characterization of aromatic residue-controlled protein retention in the endoplasmic reticulum of. J Biol Chem 292:20707–20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki JI, Naitoh M, Koizumi A, Nagata K (2007) ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Structure and Function 32:41–50. [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham HR (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48:899–907. [DOI] [PubMed] [Google Scholar]
- Mätlik K, Yu LY, Eesmaa A, Hellman M, Lindholm P, Peränen J, Galli E, Anttila J, Saarma M, Permi P, Airavaara M, Arumäe U (2015) Role of two sequence motifs of mesencephalic astrocyte-derived neurotrophic factor in its survival-promoting activity. Cell Death Dis 6:e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mätlik K, Anttila JE, Kuan-Yin T, Smolander OP, Pakarinen E, Lehtonen L, Abo-Ramadan U, Lindholm P, Zheng C, Harvey B, Arumäe U, Lindahl M, Airavaara M (2018) Poststroke delivery of MANF promotes functional recovery in rats. Sci Adv 4:eaap8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadella R, Voutilainen MH, Saarma M, Gonzalez-Barrios JA, Leon-Chavez BA, Jiménez JM, Jiménez SH, Escobedo L, Martinez-Fong D (2014) Transient transfection of human CDNF gene reduces the 6-hydroxydopamine-induced neuroinflammation in the rat substantia nigra. J Neuroinflammation 11:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Chirco KR, Cedron-Craft W, Chew S, Zhu J, Jasper H, Lamba DA (2020) MANF delivery improves retinal homeostasis and cell replacement therapies in ageing mice. Exp Gerontol 134:110893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Zhu J, Sousa-Victor P, Konjikusic M, Riley R, Chew S, Qi Y, Jasper H, Lamba DA (2016) Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 353:aaf3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nundlall S, Rajpar MH, Bell PA, Clowes C, Zeeff LA, Gardner B, Thornton DJ, Boot-Handford RP, Briggs MD (2010) An unfolded protein response is the initial cellular response to the expression of mutant matrilin-3 in a mouse model of multiple epiphyseal dysplasia. Cell Stress Chaperones 15:835–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Hashi K, Tanaka K, Koga H, Hirata Y, Kiuchi K (2012) Intracellular trafficking and secretion of mouse mesencephalic astrocyte-derived neurotrophic factor. Mol Cell Biochem 363:35–41. [DOI] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakarinen E, Danilova T, Võikar V, Chmielarz P, Piepponen P, Airavaara M, Saarma M, Lindahl M (2020) MANF Ablation Causes Prolonged Activation of the UPR without Neurodegeneration in the Mouse Midbrain Dopamine System. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palgi M, Greco D, Lindström R, Auvinen P, Heino TI (2012) Gene expression analysis of Drosophilaa Manf mutants reveals perturbations in membrane traffic and major metabolic changes. BMC Genomics 13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Kim Y, Yang SM, Henderson MJ, Yang W, Lindahl M, Urano F, Chen YM (2019) Discovery of endoplasmic reticulum calcium stabilizers to rescue ER-stressed podocytes in nephrotic syndrome. Proc Natl Acad Sci U S A 116:14154–14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash V, Lindholm P, Peränen J, Kalkkinen N, Oksanen E, Saarma M, Leppänen VM, Goldman A (2009) The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel 22:233–241. [DOI] [PubMed] [Google Scholar]
- Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20:173–188. [DOI] [PubMed] [Google Scholar]
- Ren X, Zhang T, Gong X, Hu G, Ding W, Wang X (2013) AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp Neurol 248:148–156. [DOI] [PubMed] [Google Scholar]
- Reverendo M, Mendes A, Argüello RJ, Gatti E, Pierre P (2019) At the crossway of ER-stress and proinflammatory responses. FEBS J 286:297–310. [DOI] [PubMed] [Google Scholar]
- Richman C, Rashid S, Prashar S, Mishra R, Selvaganapathy PR, Gupta BP (2018) MANF Homolog Is Necessary for the Protection of Dopaminergic Neurons and ER Unfolded Protein Response. Front Neurosci 12:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno D, Müller WEG, Bausen M, Elkhooly TA, Markl JS, Wiens M (2017) An evolutionary perspective on the role of mesencephalic astrocyte-derived neurotrophic factor (MANF): At the crossroads of poriferan innate immune and apoptotic pathways. Biochem Biophys Rep 11:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Sun A, Wang Y, Cha D, Wang H, Wang F, Feng L, Fang S (2012) Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J Neuroinflammation 9:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR, Su AI, Kelly JW, Wiseman RL (2013) Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep 3:1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, Kolumam GA, Villeda SA, Lamba DA, Jasper H (2019) MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab 1:276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Jiang M, Fu X, Cai Q, Zhang J, Yin Y, Guo J, Yu L, Jiang Y, Liu Y, Feng L, Nie Z, Fang J, Jin L (2017) reduces cell apoptosis via upregulating HSP70 in SHSY-5Y cells. Transl Neurodegener 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC (2008) Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res 103:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppo J, Vaikkinen A, Stratoulias V, Mätlik K, Anttila JE, Smolander OP, Pöhö P, Harvey BK, Kostiainen R, Airavaara M (2020) Molecular profile of the rat peri-infarct region four days after stroke: Study with MANF. Exp Neurol 329:113288. [DOI] [PubMed] [Google Scholar]
- Tousson-Abouelazm N, Papillon J, Guillemette J, Cybulsky AV (2020) Urinary ERdj3 and mesencephalic astrocyte-derived neutrophic factor identify endoplasmic reticulum stress in glomerular disease. Lab Invest. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O (2004) Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol 55:801–814. [DOI] [PubMed] [Google Scholar]
- Trychta KA, Bäck S, Henderson MJ, Harvey BK (2018) KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency. Cell Rep 25:1829–1840.e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Danilova T, Domanskyi A, Saarma M, Lindahl M, Airavaara M (2017) MANF Is Essential for Neurite Extension and Neuronal Migration in the Developing Cortex. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Anttila JE, Khodosevich K, Tuominen RK, Lindahl M, Domanskyi A, Airavaara M (2018) MANF Promotes Differentiation and Migration of Neural Progenitor Cells with Potential Neural Regenerative Effects in Stroke. Mol Ther 26:238–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virachit S, Mathews KJ, Cottam V, Werry E, Galli E, Rappou E, Lindholm P, Saarma M, Halliday GM, Shannon Weickert C, Double KL (2019) Levels of glial cell line-derived neurotrophic factor are decreased, but fibroblast growth factor 2 and cerebral dopamine neurotrophic factor are increased in the hippocampus in Parkinson’s disease. Brain Pathol 29:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen MH, Arumäe U, Airavaara M, Saarma M (2015) Therapeutic potential of the endoplasmic reticulum located and secreted CDNF/MANF family of neurotrophic factors in Parkinson’s disease. FEBS Lett 589:3739–3748. [DOI] [PubMed] [Google Scholar]
- Voutilainen MH, Bäck S, Peränen J, Lindholm P, Raasmaja A, Männistö PT, Saarma M, Tuominen RK (2011) Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson’s disease. Exp Neurol 228:99–108. [DOI] [PubMed] [Google Scholar]
- Voutilainen MH, Bäck S, Pörsti E, Toppinen L, Lindgren L, Lindholm P, Peränen J, Saarma M, Tuominen RK (2009) Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci 29:9651–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen MH, De Lorenzo F, Stepanova P, Bäck S, Yu LY, Lindholm P, Pörsti E, Saarma M, Männistö PT, Tuominen RK (2017) Evidence for an Additive Neurorestorative Effect of Simultaneously Administered CDNF and GDNF in Hemiparkinsonian Rats: Implications for Different Mechanism of Action. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. [DOI] [PubMed] [Google Scholar]
- Wang C, Yu S, Bao Q, Qiang W, Wu C, Zhang C, Jiang Y, Cai Y, Huang D, Chen Y, Hou C, Wang D (2020a) Circulating Mesencephalic Astrocyte-Derived Neurotrophic Factor Negatively Correlates With Atrial Apoptosis in Human Chronic Atrial Fibrillation. J Cardiovasc Pharmacol 75:141–147. [DOI] [PubMed] [Google Scholar]
- Wang D, Hou C, Cao Y, Cheng Q, Zhang L, Li H, Feng L, Shen Y (2018) XBP1 activation enhances MANF expression via binding to endoplasmic reticulum stress response elements within MANF promoter region in hepatitis B. Int J Biochem Cell Biol 99:140–146. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang X, Ke ZJ, Comer AL, Xu M, Frank JA, Zhang Z, Shi X, Luo J (2015) Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol 283:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Q, Wang X, Zu B, Xu J, Xu Y, Zuo X, Shen Y (2014) Deficiency of IRE1 and PERK signal pathways in systemic lupus erythematosus. Am J Med Sci 348:465–473. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Z, Zhu R, Bi J, Feng X, Liu W, Wu J, Zhang H, Wu H, Kong W, Yu B, Yu X (2017) Therapeutic efficacy of AAV8-mediated intrastriatal delivery of human cerebral dopamine neurotrophic factor in 6-OHDA-induced parkinsonian rat models with different disease progression. PLoS One 12:e0179476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Zhou Q, Li J, Zhang J, Li D, Qi X, Liu T, Zhao X, Li S, Yang L, Xie L (2020b) MANF Promotes Diabetic Corneal Epithelial Wound Healing and Nerve Regeneration by Attenuating Hyperglycemia-Induced Endoplasmic Reticulum Stress. Diabetes 69:1264–1278. [DOI] [PubMed] [Google Scholar]
- Wang XY, Song MM, Bi SX, Shen YJ, Shen YX, Yu YQ (2016) MRI Dynamically Evaluates the Therapeutic Effect of Recombinant Human MANF on Ischemia/Reperfusion Injury in Rats. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wang C, Yang G, Jia Y, Li Y, Deng W, Long M, Liu D (2020) Decreased Circulating MANF in Women with PCOS is Elevated by Metformin Therapy and is Inversely Correlated with Insulin Resistance and Hyperandrogenism. Horm Metab Res 52:109–116. [DOI] [PubMed] [Google Scholar]
- Wu T, Zhang F, Yang Q, Zhang Y, Liu Q, Jiang W, Cao H, Li D, Xie S, Tong N, He J (2017) Circulating mesencephalic astrocyte-derived neurotrophic factor is increased in newly diagnosed prediabetic and diabetic patients, and is associated with insulin resistance. Endocr J 64:403–410. [DOI] [PubMed] [Google Scholar]
- Xu S, Di Z, He Y, Wang R, Ma Y, Sun R, Li J, Wang T, Shen Y, Fang S, Feng L (2019) Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress. J Neuroinflammation 16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Gao L, Li T, Zheng J, Shao A, Zhang J (2018) Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Protects Against Neuronal Apoptosis via Activation of Akt/MDM2/p53 Signaling Pathway in a Rat Model of Intracerebral Hemorrhage. Front Mol Neurosci 11:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Rato C, Rohland L, Preissler S, Ron D (2019) MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat Commun 10:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yang H, Chang R, Yin P, Yang Y, Yang W, Huang S, Gaertig MA, Li S, Li XJ (2017) MANF regulates hypothalamic control of food intake and body weight. Nat Commun 8:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Shen Y, Chen Y, Chen L, Wang L, Wang H, Xu S, Fang S, Fu Y, Yu Y (2014) Mesencephalic astrocyte-derived neurotrophic factor prevents neuron loss via inhibiting ischemia-induced apoptosis. J Neurol Sci 344:129–138. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Liu LC, Wang FC, Liang Y, Cha DQ, Zhang JJ, Shen YJ, Wang HP, Fang S, Shen YX (2010) Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J Cereb Blood Flow Metab 30:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GL, Wang LH, Liu XY, Zhang YX, Hu MY, Liu L, Fang YY, Mu Y, Zhao Y, Huang SH, Liu T, Wang XJ (2018a) Cerebral Dopamine Neurotrophic Factor (CDNF) Has Neuroprotective Effects against Cerebral Ischemia That May Occur through the Endoplasmic Reticulum Stress Pathway. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cai Q, Jiang M, Liu Y, Gu H, Guo J, Sun H, Fang J, Jin L (2017a) Mesencephalic astrocyte-derived neurotrophic factor alleviated 6-OHDA-induced cell damage via ROS-AMPK/mTOR mediated autophagic inhibition. Exp Gerontol 89:45–56. [DOI] [PubMed] [Google Scholar]
- Zhang X, Heckmann BL, Campbell LE, Liu J (2017b) G0S2: A small giant controller of lipolysis and adipose-liver fatty acid flux. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiang Y, Wang X, Zhu L, Li H, Wang S, Pan X, Zhao H (2019) Cerebral dopamine neurotrophic factor protects microglia by combining with AKT and by regulating FoxO1/mTOR signaling during neuroinflammation. Biomed Pharmacother 109:2278–2284. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shen Y, Luo H, Zhang F, Peng D, Jing L, Wu Y, Xia X, Song Y, Li W, Jin L (2018b) MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol 308:59–71. [DOI] [PubMed] [Google Scholar]
- Zhao H, Liu Y, Cheng L, Liu B, Zhang W, Guo YJ, Nie L (2013) Mesencephalic astrocyte-derived neurotrophic factor inhibits oxygen-glucose deprivation-induced cell damage and inflammation by suppressing endoplasmic reticulum stress in rat primary astrocytes. J Mol Neurosci 51:671–678. [DOI] [PubMed] [Google Scholar]
- Zhao H, Cheng L, Liu Y, Zhang W, Maharjan S, Cui Z, Wang X, Tang D, Nie L (2014) Mechanisms of anti-inflammatory property of conserved dopamine neurotrophic factor: inhibition of JNK signaling in lipopolysaccharide-induced microglia. J Mol Neurosci 52:186–192. [DOI] [PubMed] [Google Scholar]
- Zhou W, Chang L, Fang Y, Du Z, Li Y, Song Y, Hao F, Lv L, Wu Y (2016) Cerebral dopamine neurotrophic factor alleviates Aβ. Neurosci Lett 633:40–46. [DOI] [PubMed] [Google Scholar]
- Zhu W, Li J, Liu Y, Xie K, Wang L, Fang J (2016) Mesencephalic astrocyte-derived neurotrophic factor attenuates inflammatory responses in lipopolysaccharide-induced neural stem cells by regulating NF-κB and phosphorylation of p38-MAPKs pathways. Immunopharmacol Immunotoxicol 38:205–213. [DOI] [PubMed] [Google Scholar]