Summary
In a recent Cell study, Leonardi et al (2022) show that commensal mucosa-associated gut fungi profoundly impact host immunity, epithelial barrier function and, unexpectedly, neuroimmune modulation of social behavior. All of these events are controlled by fungal-induced activation of Type 17 cytokines that act on both epithelial cells and neurons.
The human digestive tract harbors trillions of microorganisms, most famously bacteria but also viruses, parasites and fungi. Development of high-throughput sequencing techniques enabled significant advances in our understanding of the composition of commensal microbial communities and insights into the resulting impact on physiology has revealed extraordinary interplay between host and microbiome. To date, the overwhelming majority of studies has focused on elucidating bacterial components of the gut. Fungi constitute less than 1% of the enteric microbiome, but nonetheless exhibit profound biologic impacts that are still not well defined. To date, our understanding of the diverse array of gut fungal communities (the “mycobiota”) is a relatively new and rapidly emerging field. Several studies have shown that not only does the composition of the mycobiota change during disease, but commensal fungi play vital roles in maintaining intestinal homeostasis and regulating the systemic immune response (Hallen-Adams and Suhr, 2017).
A nuanced look at the microbiome has indicated that not only the composition but also the specific ‘geography’ of the bacterial microbiota are functionally important. Detailed analyses of gut bacterial composition, density and localization along the gastrointestinal (GI) tract revealed heterogenous bacterial communities residing along the longitudinal and transverse axes (Tropini et al., 2017). However, such investigations have been completely lacking for gut fungal species. This new report by the Iliev group characterized the biogeography of the mycobiota along the GI tract in mice and humans (Leonardi et al., 2022). In this study, ITS sequencing of fungal ribosomal DNA was performed on both luminal and mucosal samples isolated from distinct areas of the GI tract. In contrast to gut bacterial populations, the mycobiota showed a strong clustering based on luminal vs. mucosal sites, rather than by longitudinal location as observed for bacteria (Hooper et al., 2012). These analyses further revealed a high diversity of luminal fungal communities independent of the longitudinal site of GI tract, whereas mucosa-associated fungi (MAF) were surprisingly less diverse. Specifically, consortia of luminal versus mucosal fungi were identified in the mouse gut, which importantly was found to closely resemble that in the human GI tract (Leonardi et al., 2022).
The MAF described in this work include genera such as Candida, Saccharomyces and Saccharomycopsis, many of which have been well described to modulate host immunity (Bacher et al., 2019). In contrast, the luminal fungi included genera that primarily represent transient-environmental organisms such as Aspergillus and Cladosporium, which not surprisingly lack the ability to adhere, colonize or interact with the intestinal epithelium. These findings suggest that, by allowing colonization of a restricted and defined subset of specialized fungi, the host is able to limit unwanted and excessive stimulation of inflammation by mycobiota at the intestinal mucosa.
To test the possibility that luminal and MAF differentially regulate local immunity within the intestinal milieu, Leonardi et al. colonized mice with defined consortia of either luminal fungi or MAF and assessed the outcome on intestinal injury induced by the chemical irritant DSS. Strikingly, colonization with MAF but not luminal fungi was sufficient to protect against intestinal injury and associated mortality (Leonardi et al., 2022). The authors further demonstrated that the proximity of MAF with the intestinal lining led to increased epithelial barrier function and repair. In keeping with this observation, colonization with the MAF consortium protected against colitis triggered by an attaching-effacing bacterium, Citrobacter rodentium. Together, these data underscore the importance of spatial organization of intestinal fungal communities as a determinant of intestinal health. Precisely how epithelial cell-derived components serve as nutrient and chemical cues for adaptation of MAF within the GI tract warrants further investigation.
The past decade has seen exciting progress in defining the bidirectional communication between enteric bacteria and the nervous system, known as the gut-brain axis (GBA). It is clear now that dysbiosis of gut bacteria contribute to driving anxiety, depressive-like behavior and autistic traits (Desbonnet et al., 2015; Strati et al., 2017). However, remarkably little is known about the mycobiome’s impact on neuroimmune and neurodegenerative conditions. This report identified an unexpected role for MAF in promoting social behavior (Leonardi et al., 2022) (Figure 1). In contrast to gut bacterial communities, MAF did not affect repetitive, anxiety and compulsion-related behaviors. Rather, MAF colonization promoted social behavior in mice even in the absence of bacterial dysbiosis. Accordingly, tonic stimulation of the neuroimmune axis by MAF influences the social behavior in mice with unperturbed intestinal microbiota. This study reinforces the intimate relationship between MAF and neuroimmune system and may have ramifications for development of therapeutic interventions in neurodegenerative disorders, where impairment of social behavior is a clinical feature.
Fig 1: Spatial organization of gut microbiota influence immunity, epithelial barrier function and social behavior in mice.
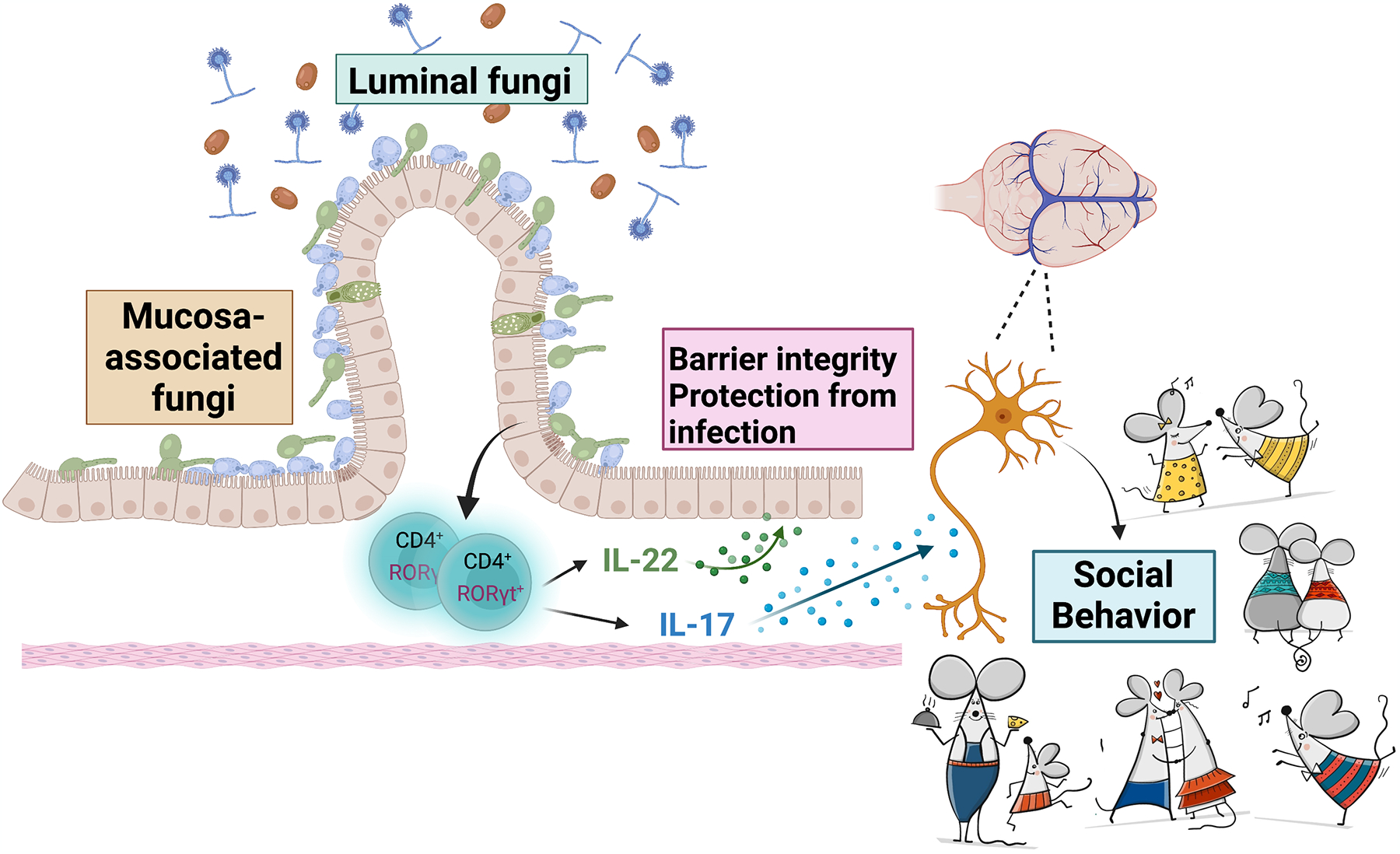
Sequencing of ribosomal DNA on luminal and mucosal fungi identified diverse luminal fungal communities but comparatively less diverse mucosa-associated fungal (MAF) communities in the gut. MAF but not luminal fungi could promote barrier function through induction of Type 17 cytokines (IL-22 and IL-17) by CD4+ T cells, which protect mice against DSS-induced intestinal epithelial injury and also Citrobacter rodentium bacterial infection. MAF also influences neuroimmune modulation of mouse social behavior by virtue of IL-17 receptor signaling in neurons. Image created with BioRender.com. Images of mice was purchased from ©www.gograph.com / Kudryashka.
Fungal infections of the central nervous system (CNS) are not uncommon in humans, and can be accompanied by severe neurological symptoms (Goralska et al., 2018). However, it was unclear from a mechanistic standpoint how changes in fungi in the GI tract could influence neural activity and consequently social behavior in the CNS. Unexpectedly, this report found that MAF-driven IL-17 production (and not IL-22, another Type 17 cell-derived cytokine) was the basis for this observation. Recently, IL-17 was described to promote social behavior in mouse models of neurodevelopmental disorders through actions on the somatosensory cortex (Reed et al., 2020; Ribeiro et al., 2019). This current study created mice with a neuron-specific deletion of the IL-17 receptor, which were resistant to the beneficial impacts of MAF on social behavior. Hence, this report is among just a handful of reports showing that IL-17 can act directly on neurons to modulate behavioral responses. Of course, much more work will be required to determine to what extent this concept can be translated to human behavior, although links of anti-IL-17RA therapy and suicide risk have been reported (Lebwohl et al., 2021). Together, these results reveal a surprising neuroimmune mechanism by which production of IL-17 augmented by gut-resident fungi promotes social behavior by directly affecting neural activity.
In summary, this intriguing study by Iliev and colleagues reveals an important relationship between the spatial “geography” of mycobiota within the GI tract and its far-reaching impact not only on health versus disease but on the behavioral attributes of mammals. A fascinating open question to emerge from this work is exactly what the neuron-intrinsic signaling events downstream of IL-17 are, and whether such events can in any way be extrapolated to humans. In the long run, the findings described in this report could set the stage for understanding – perhaps even exploiting - fungal contributions to inflammatory as well as neurodegenerative disorders. To paraphrase the Greek philosopher Epicurus, “let’s eat [fungi], drink and be merry!”
Acknowledgments
This work is supported in part by NIH grants DE022550 and AI147383 to S.L.G.; AI142354, DK104680 and AI145242 to P.S.B. We thank H. Shaw for helpful input.
Footnotes
Declaration of interests
The authors declare no competing interests
References:
- Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S, Rohmel J, Eschenhagen P, Grehn C, Seidel K, et al. (2019). Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 176, 1340–1355 e1315. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, and Cryan JF (2015). Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun 48, 165–173. [DOI] [PubMed] [Google Scholar]
- Goralska K, Blaszkowska J, and Dzikowiec M (2018). Neuroinfections caused by fungi. Infection 46, 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen-Adams HE, and Suhr MJ (2017). Fungi in the healthy human gastrointestinal tract. Virulence 8, 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, and Macpherson AJ (2012). Interactions between the microbiota and the immune system. Science 336, 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M, Leonardi C, Armstrong A, Rawnsley N, Alexander B, Goehring E Jr., Kerdel F, and Jacobson A (2021). Three-year U.S. pharmacovigilance report of brodalumab. Dermatol Ther 34, e15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, Andina M, King HO, Waisman A, Halassa MM, et al. (2020). IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C, Coelho JE, Marques-Morgado I, Valente CA, Omenetti S, et al. (2019). Meningeal gammadelta T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabro A, and De Filippo C (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropini C, Earle KA, Huang KC, and Sonnenburg JL (2017). The Gut Microbiome: Connecting Spatial Organization to Function. Cell Host Microbe 21, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]


