Abstract
Objective
To review the evidence on the effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality.
Design
Systematic review and meta-analysis.
Data sources
Medline, Embase, CINAHL, Biosis, Joanna Briggs, Global Health, and World Health Organization COVID-19 database (preprints).
Eligibility criteria for study selection
Observational and interventional studies that assessed the effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality.
Main outcome measures
The main outcome measure was incidence of covid-19. Secondary outcomes included SARS-CoV-2 transmission and covid-19 mortality.
Data synthesis
DerSimonian Laird random effects meta-analysis was performed to investigate the effect of mask wearing, handwashing, and physical distancing measures on incidence of covid-19. Pooled effect estimates with corresponding 95% confidence intervals were computed, and heterogeneity among studies was assessed using Cochran’s Q test and the I2 metrics, with two tailed P values.
Results
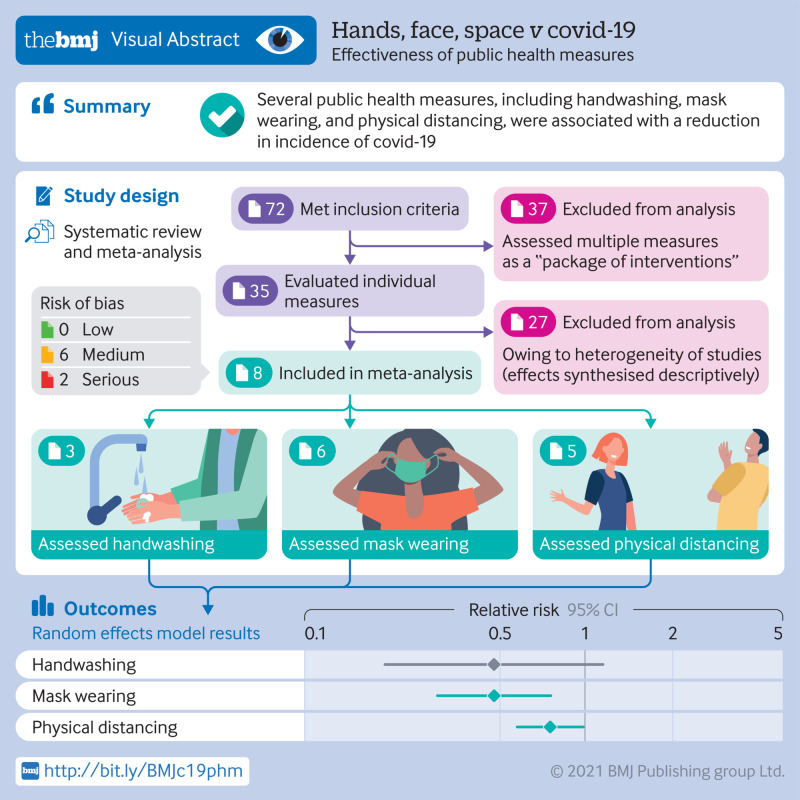
72 studies met the inclusion criteria, of which 35 evaluated individual public health measures and 37 assessed multiple public health measures as a “package of interventions.” Eight of 35 studies were included in the meta-analysis, which indicated a reduction in incidence of covid-19 associated with handwashing (relative risk 0.47, 95% confidence interval 0.19 to 1.12, I2=12%), mask wearing (0.47, 0.29 to 0.75, I2=84%), and physical distancing (0.75, 0.59 to 0.95, I2=87%). Owing to heterogeneity of the studies, meta-analysis was not possible for the outcomes of quarantine and isolation, universal lockdowns, and closures of borders, schools, and workplaces. The effects of these interventions were synthesised descriptively.
Conclusions
This systematic review and meta-analysis suggests that several personal protective and social measures, including handwashing, mask wearing, and physical distancing are associated with reductions in the incidence covid-19. Public health efforts to implement public health measures should consider community health and sociocultural needs, and future research is needed to better understand the effectiveness of public health measures in the context of covid-19 vaccination.
Systematic review registration
PROSPERO CRD42020178692.
Introduction
The impact of SARS-CoV-2 on global public health and economies has been profound.1 As of 14 October 2021, there were 239 007 759 million cases of confirmed covid-19 and 4 871 841 million deaths with covid-19 worldwide.2
A variety of containment and mitigation strategies have been adopted to adequately respond to covid-19, with the intention of deferring major surges of patients in hospitals and protecting the most vulnerable people from infection, including elderly people and those with comorbidities.3 Strategies to achieve these goals are diverse, commonly based on national risk assessments that include estimation of numbers of patients requiring hospital admission and availability of hospital beds and ventilation support.
Globally, vaccination programmes have proved to be safe and effective and save lives.4 5 Yet most vaccines do not confer 100% protection, and it is not known how vaccines will prevent future transmission of SARS-CoV-2,6 given emerging variants.7 8 9 The proportion of the population that must be vaccinated against covid-19 to reach herd immunity depends greatly on current and future variants.10 This vaccination threshold varies according to the country and population’s response, types of vaccines, groups prioritised for vaccination, and viral mutations, among other factors.6 Until herd immunity to covid-19 is reached, regardless of the already proven high vaccination rates,11 public health preventive strategies are likely to remain as first choice measures in disease prevention,12 particularly in places with a low uptake of covid-19 vaccination. Measures such as lockdown (local and national variant), physical distancing, mandatory use of face masks, and hand hygiene have been implemented as primary preventive strategies to curb the covid-19 pandemic.13
Public health (or non-pharmaceutical) interventions have been shown to be beneficial in fighting respiratory infections transmitted through contact, droplets, and aerosols.14 15 Given that SARS-CoV-2 is highly transmissible, it is a challenge to determine which measures might be more effective and sustainable for further prevention.
Substantial benefits in reducing mortality were observed in countries with universal lockdowns in place, such as Australia, New Zealand, Singapore, and China. Universal lockdowns are not, however, sustainable, and more tailored interventions need to be considered; the ones that maintain social lives and keep economies functional while protecting high risk individuals.16 17 Substantial variation exists in how different countries and governments have applied public health measures,18 and it has proved a challenge for assessing the effectiveness of individual public health measures, particularly in policy decision making.19
Previous systematic reviews on the effectiveness of public health measures to treat covid-19 lacked the inclusion of analytical studies,20 a comprehensive approach to data synthesis (focusing only on one measure),21 a rigorous assessment of effectiveness of public health measures,22 an assessment of the certainty of the evidence,23 and robust methods for comparative analysis.24 To tackle these gaps, we performed a systematic review of the evidence on the effectiveness of both individual and multiple public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality. When feasible we also did a critical appraisal of the evidence and meta-analysis.
Methods
This systematic review and meta-analysis were conducted in accordance with PRISMA25 (supplementary material 1, table 1) and with PROSPERO (supplementary material 1, table 2).
Eligibility criteria
Articles that met the population, intervention, comparison, outcome, and study design criteria were eligible for inclusion in this systematic review (supplementary material 1, table 3). Specifically, preventive public health measures that were tested independently were included in the main analysis. Multiple measures, which generally contain a “package of interventions”, were included as supplementary material owing to the inability to report on the individual effectiveness of measures and comparisons on which package led to enhanced outcomes. The public health measures were identified from published World Health Organization sources that reported on the effectiveness of such measures on a range of communicable diseases, mostly respiratory infections, such as influenza.
Given that the scientific community is concerned about the ability of the numerous mathematical models, which are based on assumptions, to predict the course of virus transmission or effectiveness of interventions,26 this review focused only on empirical studies. We excluded case reports and case studies, modelling and simulation studies, studies that provided a graphical summary of measures without clear statistical assessments or outputs, ecological studies that provided a descriptive summary of the measures without assessing linearity or having comparators, non‐empirical studies (eg, commentaries, editorials, government reports), other reviews, articles involving only individuals exposed to other pathogens that can cause respiratory infections, such as severe acute respiratory syndrome or Middle East respiratory syndrome, and articles in a language other than English.
Information sources
We carried out electronic searches of Medline, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature, Ebsco), Global Health, Biosis, Joanna Briggs, and the WHO COVID-19 database (for preprints). A clinical epidemiologist (ST) developed the initial search strategy, which was validated by two senior medical librarians (LR and MD) (supplementary material 1, table 4). The updated search strategy was last performed on 7 June 2021. All citations identified from the database searches were uploaded to Covidence, an online software designed for managing systematic reviews,27 for study selection.
Study selection
Authors ST, DG, SS, AM, ET, JR, XL, WX, IME, and XZ independently screened the titles and abstracts and excluded studies that did not match the inclusion criteria. Discrepancies were resolved in discussion with the main author (ST). The same authors retrieved full text articles and determined whether to include or exclude studies on the basis of predetermined selection criteria. Using a pilot tested data extraction form, authors ST, SS, AM, JR, XL, WX, AM, IME, and XZ independently extracted data on study design, intervention, effect measures, outcomes, results, and limitations. ST, SS, AM, and HW verified the extracted data. Table 5 in supplementary material 1 provides the specific criteria used to assess study designs. Given the heterogeneity and diversity in how studies defined public health measures, we took a common approach to summarise evidence of these interventions (supplementary material 1, table 6).
Risk of bias within individual studies
SS, JR, XL, WX, IME, and XZ independently assessed risk of bias for each study, which was cross checked by ST and HW. For non-interventional observational studies, a ROBINS-I (risk of bias in non-randomised studies of interventions) risk of bias tool was used.28 For interventional studies, a revised tool for assessing risk of bias in randomised trials (RoB 2) tool was used.29 Reviewers rated each domain for overall risk of bias as low, moderate, high, or serious/critical.
Data synthesis
The DerSimonian and Laird method was used for random effects meta-analysis, in which the standard error of the study specific estimates was adjusted to incorporate a measure of the extent of variation, or heterogeneity, among the effects observed for public health measures across different studies. It was assumed that the differences between studies are a result of different, yet related, intervention effects being estimated. If fewer than five studies were included in meta-analysis, we applied a recommended modified Hartung-Knapp-Sidik-Jonkman method.30
Statistical analysis
Because of the differences in the effect metrics reported by the included studies, we could only perform quantitative data synthesis for three interventions: handwashing, face mask wearing, and physical distancing. Odds ratios or relative risks with corresponding 95% confidence intervals were reported for the associations between the public health measures and incidence of covid-19. When necessary, we transformed effect metrics derived from different studies to allow pooled analysis. We used the Dersimonian Laird random effects model to estimate pooled effect estimates along with corresponding 95% confidence intervals for each measure. Heterogeneity among individual studies was assessed using the Cochran Q test and the I2 test.31 All statistical analyses were conducted in R (version 4.0.3) and all P values were two tailed, with P=0.05 considered to be significant. For the remaining studies, when meta-analysis was not feasible, we reported the results in a narrative synthesis.
Public and patient involvement
No patients or members of the public were directly involved in this study as no primary data were collected. A member of the public was, however, asked to read the manuscript after submission.
Results
A total of 36 729 studies were initially screened, of which 36 079 were considered irrelevent. After exclusions, 650 studies were eligible for full text review and 72 met the inclusion criteria. Of these studies, 35 assessed individual interventions and were included in the final synthesis of results (fig 1) and 37 assessed multiple interventions as a package and are included in supplementary material 3, tables 2 and 3. The included studies comprised 34 observational studies and one interventional study, eight of which were included in the meta-analysis.
Fig 1.
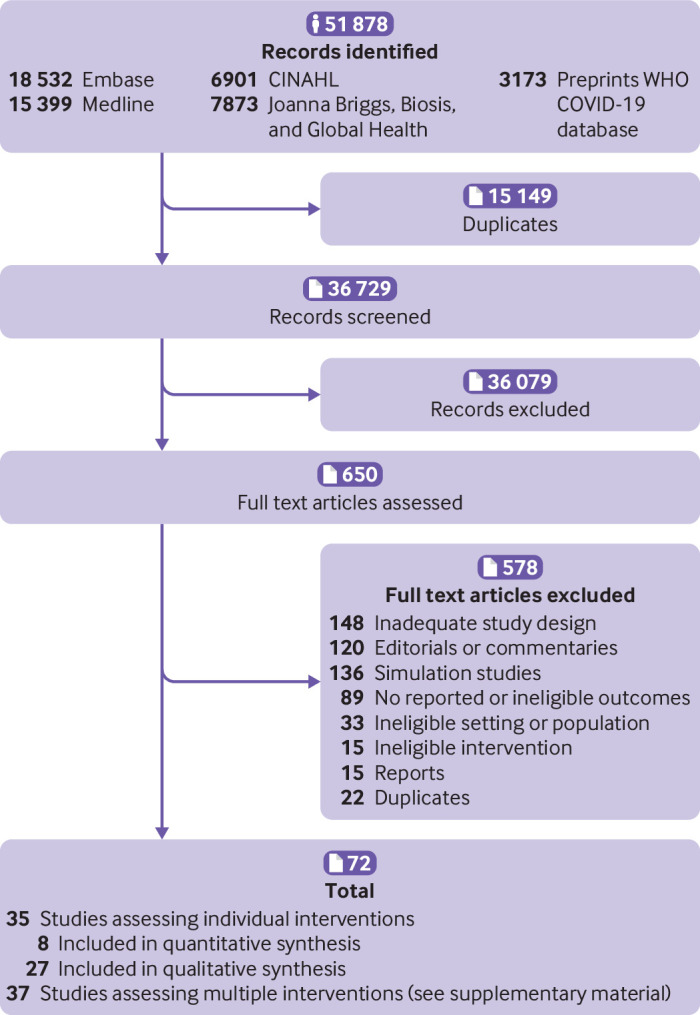
Flow of articles through the review. WHO=World Health Organization
Risk of bias
According to the ROBINS-I tool,28 the risk of bias was rated as low in three studies,32 33 34 moderate in 24 studies,35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 and high to serious in seven studies.59 60 61 62 63 64 65 One important source of serious or critical risk of bias in most of the included studies was major confounding, which was difficult to control for because of the novel nature of the pandemic (ie, natural settings in which multiple interventions might have been enforced at once, different levels of enforcement across regions, and uncaptured individual level interventions such as increased personal hygiene). Variations in testing capacity and coverage, changes to diagnostic criteria, and access to accurate and reliable outcome data on covid-19 incidence and covid-19 mortality, was a source of measurement bias for numerous studies (fig 2). These limitations were particularly prominent early in the pandemic, and in low income environments.47 52 62 63 65 The randomised controlled trial66 was rated as moderate risk of bias according to the ROB-2 tool. Missing data, losses to follow-up, lack of blinding, and low adherence to intervention all contributed to the reported moderate risk. Tables 1 and 2 in supplementary material 2 summarise the risk of bias assessment for each study assessing individual measures.
Fig 2.
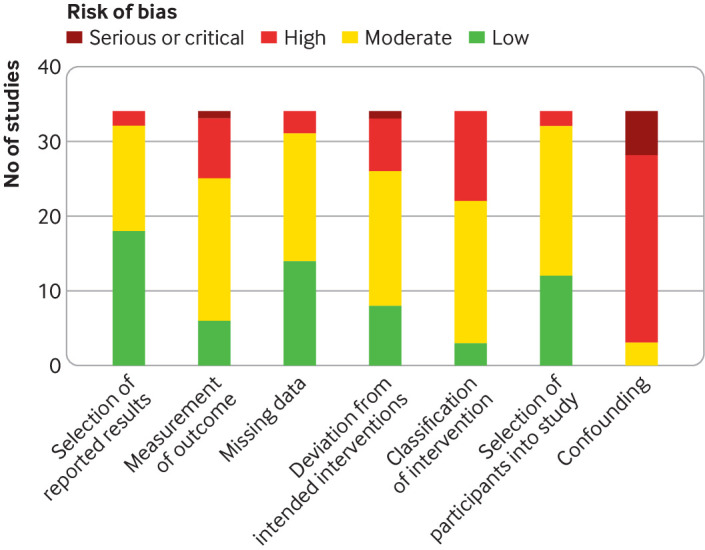
Summary of risk of bias across studies assessing individual measures using risk of bias in non-randomised studies of interventions (ROBINS-I) tool
Study characteristics
Studies assessing individual measures
Thirty five studies provided estimates on the effectiveness of an individual public health measures. The studies were conducted in Asia (n=11), the United States (n=9), Europe (n=7), the Middle East (n=3), Africa (n=3), South America (n=1), and Australia (n=1). Thirty four of the studies were observational and one was a randomised controlled trial. The study designs of the observational studies comprised natural experiments (n=11), quasi-experiments (n=3), a prospective cohort (n=1), retrospective cohorts (n=8), case-control (n=2), and cross sectional (n=9). Twenty six studies assessed social measures,32 34 35 37 38 39 40 41 42 44 46 47 48 52 53 55 56 57 58 59 60 61 63 64 65 67 12 studies assessed personal protective measures,36 43 45 49 50 57 58 60 63 66 68 three studies assessed travel related measures,54 58 62 and one study assessed environmental measures57 (some interventions overlapped across studies). The most commonly measured outcome was incidence of covid-19 (n=18), followed by SARS-CoV-2 transmission, measured as reproductive number, growth number, or epidemic doubling time (n=13), and covid-19 mortality (n=8). Table 1 in supplementary material 3 provides detailed information on each study.
Effects of interventions
Personal protective measures
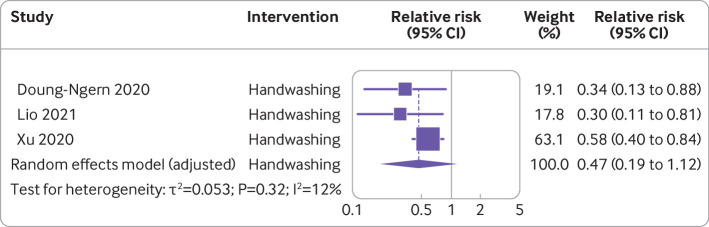
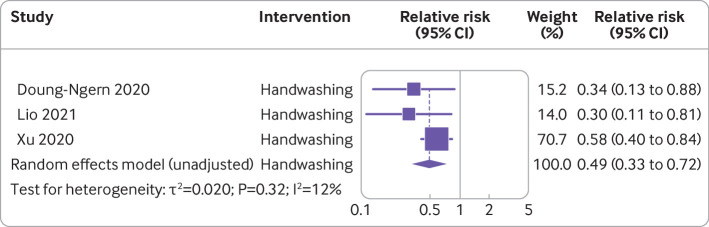
Handwashing and covid-19 incidence—Three studies with a total of 292 people infected with SARS-CoV-2 and 10 345 participants were included in the analysis of the effect of handwashing on incidence of covid-19.36 60 63 Overall pooled analysis suggested an estimated 53% non-statistically significant reduction in covid-19 incidence (relative risk 0.47, 95% confidence interval 0.19 to 1.12, I2=12%) (fig 3). A sensitivity analysis without adjustment showed a significant reduction in covid-19 incidence (0.49, 0.33 to 0.72, I2=12%) (fig 4). Risk of bias across the three studies ranged from moderate36 60 to serious or critical63 (fig 2).
Fig 3.
Meta-analysis of evidence on association between handwashing and incidence of covid-19 using modified Hartung-Knapp-Sidik-Jonkman adjusted random effect model
Fig 4.
Meta-analysis of evidence on association between handwashing and incidence of covid-19 using unadjusted random effect model
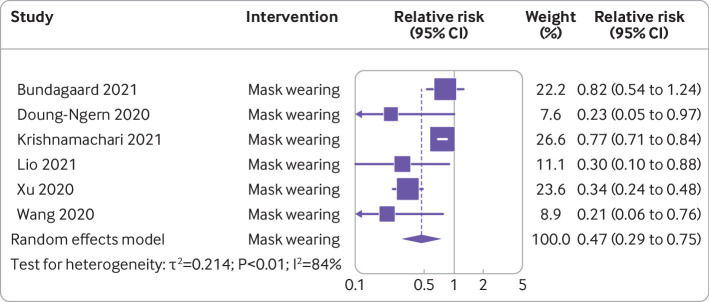
Mask wearing and covid-19 incidence—Six studies with a total of 2627 people with covid-19 and 389 228 participants were included in the analysis examining the effect of mask wearing on incidence of covid-19 (table 1).36 43 57 60 63 66 Overall pooled analysis showed a 53% reduction in covid-19 incidence (0.47, 0.29 to 0.75), although heterogeneity between studies was substantial (I2=84%) (fig 5). Risk of bias across the six studies ranged from moderate36 57 60 66 to serious or critical43 63 (fig 2).
Table 1.
Study characteristics and main results from studies that assessed individual personal protective and environmental measures
| Reference, country | Study design | Public health measure | Sample size | Outcome measure | Study duration | Effect estimates: conclusions | Risk of bias |
|---|---|---|---|---|---|---|---|
| Doung-Ngern et al,63 Thailand | Case-control | Handwashing | 211 cases, 839 controls | Incidence | 1-31 Mar 2020 | Regular handwashing: adjusted odds ratio 0.34 (95% confidence interval 0.13 to 0.87): associated with lower risk of SARS-CoV-2* | Serious or critical |
| Lio et al,36 China | Case-control | Handwashing | 24 cases, 1113 controls | Incidence | 17 Mar-15 Apr 2020 | Adjusted odds ratio 0.30 (95% confidence interval 0.11 to 0.80): reduction in odds of becoming infectious* | Moderate |
| Xu et al,60 China | Cross sectional comparative | Handwashing | n=8158 | Incidence | 22 Feb-5 Mar 2020 | Relative risk 3.53 (95% confidence interval 1.53 to 8.15): significantly increased risk of infection with no handwashing* | Moderate |
| Bundgaard et al,66 Denmark | Randomised controlled | Mask wearing | 2392 cases, 2470 controls | Incidence | Apr and May 2020 | Odds ratio 0.82 (95% confidence interval 0.54 to 1.23): 46% reduction to 23% increase in infection* | Moderate |
| Doung-Ngern et al,63 Thailand | Case-control | Mask wearing | 211 cases, 839 controls | Incidence | 1-31 Mar 2020 | Adjusted odds ratio 0.23 (95% confidence interval 0.09 to 1.60): associated with lower risk of SARS-CoV-2 infection* | Serious or critical |
| Lio et al,36 China | Case-control | Mask wearing | 24 cases, 1113 controls | Incidence | 17 Mar-15 Apr 2020 | Odds ratio 0.30 (95% confidence interval 0.10 to 0.86): 70% risk reduction* | Moderate |
| Xu et al,60 China | Cross sectional comparative | Mask wearing | 8158 people | Incidence | 22 Feb-5 Mar 2020 | Relative risk 12.38 (95% confidence interval 5.81 to 26.36): significantly increased risk of infection* | Moderate |
| Krishnamachari et al,43 US | Natural experiment | Mask wearing | 50 states | Incidence (cumulative rate) | Apr 2020 | 3-6 months, adjusted odds ratio 1.61 (95% confidence interval 1.23 to 2.10): >6 months, 2.16 (1.64 to 2.88): higher incidence rate with later mask mandate than with mask mandate in first month* | Serious or critical |
| Wang et al,57 China | Retrospective cohort | Mask wearing | 335 people | Incidence (assessed as attack rate†) | 28 Feb-27 Mar 2020 | Odds ratio 0.21 (95% confidence interval 0.06 to 0.79): 79% reduction in transmission of SARS-CoV-2* | Moderate |
| Cheng et al,68 China | Longitudinal comparative | Mask wearing (South Korea v HKSAR) | 961 cases (HKSAR), average control not available | Incidence | 31 Dec 2019-8 Apr 2020 | Incidence rate 49.6% (South Korea) v 11.8% (HKSAR) P <0.001: 37.8% less SARS-CoV-2 cases* | Moderate |
| Leffler et al,49 US | Natural experiment | Mask wearing | 200 countries | Mortality (per capita) | Jan-9 May 2020 | No masks: mortality rate 61.9% (95% confidence interval 37.0% to 91.0%); masks: 16.2% (−14.4% to 57.4%): 45.7% fewer mortality* | Moderate |
| Lyu et al,50 US | Natural experiment | Mask wearing | 15 states | Case growth rate | 31 Mar-22 May 2020 | Mandatory mask wearing: case growth rate 2%: 2% decrease in daily covid-19 growth rate at ≥21 days (P<0.05)* | Moderate |
| Rader et al,45 US | Cross sectional | Mask wearing | 378 207 people | R0 | 3 Jun-27 Jul | Adjusted odds ratio 3.53 (95% confidence interval 2.03 to 6.43): 10% increase in self-reported mask wearing was associated with an increased odds of transmission control* | Moderate |
| Liu et al,58 US | Natural experiment | Mask wearing | 50 states | Rt | 21 Jan-31 May 2020 | Risk ratio 0.71 (95% confidence interval 0.58 to 0.75): 29% reduction in Rt* | Moderate |
| Wang et al,57 China | Retrospective cohort | Chlorine or ethanol based disinfectant | 335 people | Incidence (attack rate†) | 28 Feb-27 Mar 2020 | Odds ratio 0.23 (95% confidence interval 0.07 to 0.84): 77% reduction in transmission of SARS-CoV-2* | Moderate |
HKSAR=Hong Kong Special Administrative Region of China; R0=reproductive number; Rt=time varying reproductive number.
Interpretation of findings as reported in the original manuscript.
Percentage of individuals who tested positive over a specified period.
Fig 5.
Meta-analysis of evidence on association between mask wearing and incidence of covid-19 using unadjusted random effect model
Mask wearing and transmission of SARS-CoV-2, covid-19 incidence, and covid-19 mortality—The results of additional studies that assessed mask wearing (not included in the meta-analysis because of substantial differences in the assessed outcomes) indicate a reduction in covid-19 incidence, SARS-CoV-2 transmission, and covid-19 mortality. Specifically, a natural experiment across 200 countries showed 45.7% fewer covid-19 related mortality in countries where mask wearing was mandatory (table 1).49 Another natural experiment study in the US reported a 29% reduction in SARS-CoV-2 transmission (measured as the time varying reproductive number Rt) (risk ratio 0.71, 95% confidence interval 0.58 to 0.75) in states where mask wearing was mandatory.58
A comparative study in the Hong Kong Special Administrative Region reported a statistically significant lower cumulative incidence of covid-19 associated with mask wearing than in selected countries where mask wearing was not mandatory (table 1).68 Similarly, another natural experiment involving 15 US states reported a 2% statistically significant daily decrease in covid-19 transmission (measured as case growth rate) at ≥21 days after mask wearing became mandatory,50 whereas a cross sectional study reported that a 10% increase in self-reported mask wearing was associated with greater odds for control of SARS-CoV-2 transmission (adjusted odds ratio 3.53, 95% confidence interval 2.03 to 6.43).45 The five studies were rated at moderate risk of bias (fig 2).
Environmental measures
Disinfection in household and covid-19 incidence
Only one study, from China, reported the association between disinfection of surfaces and risk of secondary transmission of SARS-CoV-2 within households (table 1).57 The study assessed disinfection retrospectively by asking participants about their “daily use of chlorine or ethanol-based disinfectant in households,” and observed that use of disinfectant was 77% effective at reducing SARS-CoV-2 transmission (odds ratio 0.23, 95% confidence interval 0.07 to 0.84). The study did not collect data on the concentration of the disinfectant used by participants and was rated at moderate risk of bias (fig 2).
Social measures
Physical distancing and covid-19 incidence
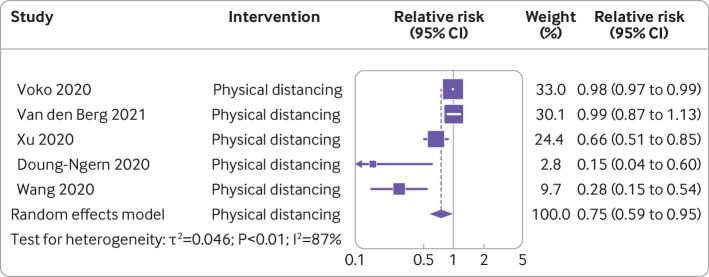
Five studies with a total of 2727 people with SARS-CoV-2 and 108 933 participants were included in the analysis that examined the effect of physical distancing on the incidence of covid-19.37 53 57 60 63 Overall pooled analysis indicated a 25% reduction in incidence of covid-19 (relative risk 0.75, 95% confidence interval 0.59 to 0.95, I2=87%) (fig 6). Heterogeneity among studies was substantial, and risk of bias ranged from moderate37 53 57 60 to serious or critical63(fig 2).
Fig 6.
Meta-analysis of evidence on association between physical distancing and incidence of covid-19 using unadjusted random effect model
Physical distancing and transmission of SARS-CoV-2 and covid-19 mortality
Studies that assessed physical distancing but were not included in the meta-analysis because of substantial differences in outcomes assessed, generally reported a positive effect of physical distancing (table 2). A natural experiment from the US reported a 12% decrease in SARS-CoV-2 transmission (relative risk 0.88, 95% confidence interval 0.86 to 0.89),40 and a quasi-experimental study from Iran reported a reduction in covid-19 related mortality (β −0.07, 95% confidence interval −0.05 to −0.10; P<0.001).47 Another comparative study in Kenya also reported a reduction in transmission of SARS-CoV-2 after physical distancing was implemented, reporting 62% reduction in overall physical contacts (reproductive number pre-intervention was 2.64 and post-intervention was 0.60 (interquartile range 0.50 to 0.68)).61 These three studies were rated at moderate risk of bias40 61 to serious or critical risk of bias47 (fig 2).
Table 2.
Study characteristics and main results from studies assessing individual social measures
| Reference, country | Study design | Public health measure | Sample size | Outcome | Study duration | Effect estimates: conclusions | Risk of bias |
|---|---|---|---|---|---|---|---|
| Jarvis et al,65 UK | Cross sectional | Stay at home or isolation | 1356 cases | R0 | Feb-24 Mar 2020 | R0: pre-intervention 3.6, post-intervention 0.60 (95% confidence interval 0.37 to 0.89): 3.0 R0 decrease | Serious or critical |
| Khosravi et al,55 Iran | Cross sectional | Stay at home or isolation | 993 cases | R0 | 20 Feb-01 Apr 2020 | R0: pre-intervention 2.70 (95% confidence interval 2.10 to 3.40), post-intervention 1.13 (1.03 to 1.25): 1.5 R0 decrease | Moderate |
| Dreher et al,41 US | Retrospective cohort | Stay at home or isolation | 49 states and territories | R0 | NS | Odds ratio 0.07 (95% confidence interval 0.01 to 0.37): decrease in odds of having a positive R0 result* | Low |
| Liu et al,58 US | Natural experiment | Stay at home or isolation | 50 states | Rt | 21 Jan-31 May 2020 | Risk ratio 0.49 (95% confidence interval 0.43 to 0.54): contributed about 51% to reduction in Rt* | Moderate |
| Alfano et al,52 Italy | Natural experiment | Lockdown | 202 countries, 22 018 people | Incidence | 22 Jan-10 May 2020 | β coefficient −235.8 (standard error −11.04), P<0.01 | Serious or critical |
| Thayer et al,56 India | Quasi-experimental | Lockdown | NS | Incidence (% median) | 2 Mar-1 Sept 2020 | Incidence rate: pre-lockdown 15.8% (95% confidence interval 7.0% to 20.2%), post-lockdown 5.0% (4.7% to 5.4%): 10.8% reduction in average incidence rate* | Moderate |
| Pillai et al,46 South Africa | Retrospective cohort | Lockdown | 162 528 | Attack rate† | 5 Mar-30 June | Attack rate: pre-lockdown 18.5%, full lockdown 4.1%: 14.1% reduction in risk* | Moderate |
| Siedner et al,35 US | Natural experiment | Lockdown | 45 states | Case growth rate, mortality growth rate | 10-25 Mar 2020 | Case growth rate 0.9% decrease (95% confidence interval 1.40% to 0.4%)/day (after 4 days)*; mortality growth rate 2.0% mortality decrease (−3.0% to 0.9%)/day* | Moderate |
| Silva et al,42 Brazil | Quasi-experimental | Lockdown | Nationwide | Mortality | 5-30 Mar 2020 | Post-intervention changes in mortality, São Luís (β coefficient −0.13, P<0.001), Recife (β coefficient −0.06, P<0.001), Belém (β coefficient −0.10, P<0.001), Fortaleza (β coefficient −0.09, P<0.001): 27.4% average difference in mortality | Moderate |
| Tobias et al,38 Spain | Natural experiment | Lockdown | Spain and Italy | Mortality | 24 Feb-5 Apr 2020 | Mortality rates: Italy pre-intervention −32.8 (95% confidence interval 21.0 to 44.6), Italy post-intervention −0.2 (−1.5 to 1.0), Spain pre-intervention 59.3 (23.0 to 95.2), Spain post-intervention −1.8 (−5.0 to 3.1): beneficial effect in both countries* | Moderate |
| Wang et al,69 China | Retrospective cohort | Lockdown | Nationwide | R0 | 10 Jan-16 Feb 2020 | R0: pre-intervention 4.95 (95% confidence interval 4.26 to 5.67), post-intervention 0.98 (0.96 to 1.03): 3.97 decrease | Low |
| Guzzetta et al,39 Italy | Longitudinal comparative | Lockdown | Nationwide | R0 | 10-25 Mar 2020 | R0: pre-intervention 2.03, 3 weeks 0.76 (95% confidence interval 0.67 to 0.85): 1.27 decrease | Low |
| Basu et al,64 India | Retrospective cohort | Lockdown | Nationwide | R0 | 24 Mar-31 May 2020 | R0: pre-intervention 3.36 (95% confidence interval 3.03 to 3.71), post-intervention 1.27 (1.26 to 1.28): 2.09 decrease | Moderate |
| Guo et al,40 US | Natural experiment | Lockdown | 50 states and one territory (Virgin Islands) | Rt | 29 Jan-31 Jul 2020 | Relative risk 0.89 (95% confidence interval 0.88 to 0.91): associated with a 11% decrease in risk of Rt* | Moderate |
| Al-Tawfiq et al,34 Saudi Arabia | Prospective cohort | Quarantine | 1928 cases | Incidence | 14 Mar-6 Jun | Incidence rate: 4 weeks 5.9%, 8 weeks 1.0%, 13 weeks 0%: 4.9% decrease at 8 weeks | Low |
| Vaman et al,59 India | Retrospective cohort | Quarantine | 179 cases | Risk of transmission | 24 Mar-30 Apr 2020 | Odds ratio 14.44 (95% confidence interval 2.42 to 86.17), relative risk 11.85 (95% confidence interval 2.91 to 48.23): >14 times higher risk without quarantine compared with strict quarantine.* Significant risk of transmission* | Moderate |
| Auger et al,48 US | Longitudinal comparative | School closure | Nationwide | Incidence, mortality (adjusted relative change) | 9 Mar-7 May 2020 | Incidence −62% (95% confidence interval −49% to −71%), mortality rate −58% (95% confidence interval −46% to −68%): decreased covid-19 incidence and mortality* | Moderate |
| Vlachos et al,32 Sweden | Cross sectional comparative | School closure | Teachers and parents, number not specified | Incidence | 25 Mar-1 Apr 2020 | Odds ratio 2.01 (95% confidence interval 1.52 to 2.67): teachers in lower secondary schools twice as likely to become infected with SARS-CoV-2 than teachers in upper secondary school* | Moderate |
| Iwata et al,44 Japan | Natural experiment | School closure | Not specified | Incidence | 27-Feb 31 Mar 2020 | α coefficient 0.08 (95% confidence interval −0.36 to 0.65): no decrease in incidence of SARS-CoV-2‡ | Moderate |
| Liu et al,58 US | Natural experiment | School closure | 50 states | Rt | 21 Jan-31 May 2020 | Risk ratio 0.90 (95% confidence interval 0.86 to 0.93): contributed about 10% to reduction in Rt* | Moderate |
| Guo et al,40 US | Natural experiment | School closure | 50 states and one territory (Virgin Islands) | Rt | 29 Jan-31 July 2020 | Relative risk 0.87 (95% confidence interval 0.86 to 0.89): associated with 13% decrease in risk of Rt* | Moderate |
| Liu et al,58 US | Natural experiment | Business closure | 50 states | Rt | 21 Jan-31 May 2020 | Risk ratio 0.84 (95% confidence interval 0.79 to 0.90): contributed about 26% reduction in Rt* | Moderate |
| Guo et al,40 US | Natural experiment | Business closure | 50 states and one territory (Virgin Islands) | Rt | 29 Jan-31 July 2020 | Relative risk 0.88 (95% confidence interval 0.86 to 0.89): associated with 12% decrease in risk of Rt* | Moderate |
| Voko et al,53 Europe | Natural experiment | Physical distancing | 28 countries | Incidence | 1 Feb-18 Apr 2020 | Incidence rate ratio 1.23 (95% confidence interval 1.19 to 1.28), 0.98 (0.97 to 0.99): 26% decrease in incidence* | Moderate |
| Van den Berg et al,37 US | Retrospective cohort | Physical distancing | 99 390 staff | Incidence (adjusted) | 24 Sep 2020-27 Jan 2021 | ≥3 v ≥6 feet adjusted incidence rate ratio 1.01 (95% confidence interval 0.75 to 1.36), larger physical distancing not associated with lower rates of SARS-CoV-2*‡ | Moderate |
| Xu et al,60China | Cross sectional comparative | Physical distancing | 8158 people | Incidence | 22 Feb-5 Mar 2020 | Relative risk 2.63 (95% confidence interval 1.48 to 4.67): significantly increased risk of infection* | Moderate |
| Doung-Ngern et al,63 Thailand | Case-control | Physical distancing | 211 cases, 839 controls | Incidence | 1-31 Mar 2020 | >1m physical distance adjusted odds ratio 0.15; 95% confidence interval 0.04 to 0.63)): associated with lower risk of SARS-CoV-2 infection* | Serious or critical |
| Wang et al,57 China | Retrospective cohort | Physical distancing | 335 people | Incidence (proportions assessed as attack rate†) | 28 Feb-27 Mar 2020 | Odds ratio 18.26 (95% confidence interval 3.93 to 84.79): risk of household transmission was 18 times higher with frequent daily close contact with the primary case* | Moderate |
| Alimohamadi et al,47 Iran | Quasi-experimental | Physical distancing | NS | Incidence, mortality | 20 Feb-13 May 2020 | Incidence β coefficient −1.70 (95% confidence interval −2.3 to 1.1), mortality β coefficient −0.07 (−0.05 to −0.10): reduced incidence and mortality* | Serious or critical |
| Quaife et al,61 Africa | Cross-sectional comparative | Physical distancing | 237 cases | R0 | 1 -31 May 2020 | R0: pre-intervention 2.64, post-intervention 0.60 (interquartile range 0.50-0.68): 2.04 decrease in R0 | Moderate |
| Guo et al,40 US | Natural experiment | Physical distancing | 50 states and one territory (Virgin Islands) | Rt | 29 Jan-31 Jul 2020 | Relative risk 0.88 (95% confidence interval 0.86 to 0.89): associated with a 12% decrease in risk of Rt* | Moderate |
R0=reproductive number; Rt=time varying reproductive number.
Interpretation of findings as reported in the original manuscript.
Percentage of individuals who tested positive over a specified period.
Not an effective intervention.
Stay at home or isolation and transmission of SARS-CoV-2
All the studies that assessed stay at home or isolation measures reported reductions in transmission of SARS-CoV-2 (table 2). A retrospective cohort study from the US reported a significant reduction in the odds of having a positive reproductive number (R0) result (odds ratio 0.07, 95% confidence interval 0.01 to 0.37),41 and a natural experiment reported a 51% reduction in time varying reproductive number (Rt) (risk ratio 0.49, 95% confidence interval 0.43 to 0.54).58
A study from the UK reported a 74% reduction in the average daily number of contacts observed for each participant and estimated a decrease in reproductive number: the reproductive number pre-intervention was 3.6 and post-intervention was 0.60 (95% confidence interval 0.37 to 0.89).65 Similarly, an Iranian study projected the reproductive number using serial interval distribution and the number of incidence cases and found a significant decrease: the reproductive number pre-intervention was 2.70 and post-intervention was 1.13 (95% confidence interval 1.03 to 1.25).55 Three of the studies were rated at moderate to serious or critical risk of bias,55 58 65 and one study was rated at low risk of bias41 (fig 2).
Quarantine and incidence and transmission of SARS-CoV-2
Quarantine was assessed in two studies (table 2).34 59 A prospective cohort study from Saudi Arabia reported a 4.9% decrease in the incidence of covid-19 at eight weeks after the implementation of quarantine.34 This study was rated at low risk of bias (fig 2). A retrospective cohort study from India reported a 14 times higher risk of SARS-CoV-2 transmission associated with no quarantine compared with strict quarantine (odds ratio 14.44, 95% confidence interval 2.42 to 86.17).59 This study was rated at moderate risk of bias (fig 2).
School closures and covid-19 incidence and covid-19 mortality
Two studies assessed the effectiveness of school closures on transmission of SARS-CoV-2, incidence of covid-19, or covid-19 mortality (table 2).44 48 A US population based longitudinal study reported on the effectiveness of state-wide closure of primary and secondary schools and observed a 62% decrease (95% confidence interval −49% to −71%) in incidence of covid-19 and a 58% decrease (−46% to−68%) in covid-19 mortality.48 Conversely, a natural experiment from Japan reported no effect of school closures on incidence of covid-19 (α coefficient 0.08, 95% confidence interval −0.36 to 0.65).44 Both studies were rated at moderate risk of bias (fig 2).
School closures and transmission of SARS-CoV-2
Two natural experiments from the US reported a reduction in transmission (ie, reproductive number); with one study reporting a reduction of 13% (relative risk 0.87, 95% confidence interval 0.86 to 0.89)40 and another reporting a 10% (0.90, 0.86 to 0.93) reduction (table 2).58 A Swedish study reported an association between school closures and a small increase in confirmed SARS-CoV-2 infections in parents (odds ratio 1.17, 95% confidence interval 1.03 to 1.32), but observed that teachers in lower secondary schools were twice as likely to become infected than teachers in upper secondary schools (2.01, 1.52 to 2.67).32 All three studies were rated at moderate risk of bias (fig 2).
Business closures and transmission of SARS-CoV-2
Two natural experiment studies assessed business closures across 50 US states and reported reductions in transmission of SARS-CoV-2 (table 2).40 58 One of the studies observed a significant reduction in transmission of 12% (relative risk 0.88, 95% confidence interval 0.86 to 0.89)40 and the other reported a significant 16% (risk ratio 0.84, 0.79 to 0.90) reduction.58 Both studies were rated at moderate risk of bias (fig 2).
Lockdown and incidence of covid-19
A natural experiment involving 202 countries suggested that countries that implemented universal lockdown had fewer new cases of covid-19 than countries that did not (β coefficient −235.8 (standard error −11.04), P<0.01) (table 2).52 An Indian quasi-experimental study reported a 10.8% reduction in incidence of covid-19 post-lockdown,56 whereas a South African retrospective cohort study observed a 14.1% reduction in risk after implementation of universal lockdown (table 2).46 These studies were rated at high risk of bias52 and moderate risk of bias46 56 (fig 2).
Lockdown and covid-19 mortality
The three studies that assessed universal lockdown and covid-19 mortality generally reported a decrease in mortality (table 2).35 38 42 A natural experiment study involving 45 US states reported a decrease in covid-19 related mortality of 2.0% (95% confidence interval −3.0% to 0.9%) daily after lockdown had been made mandatory.35 A Brazilian quasi-experimental study reported a 27.4% average difference in covid-19 related mortality rates in the first 25 days of lockdown.42 In addition, a natural experiment study reported about 30% and 60% reductions in covid-19 related mortality post-lockdown in Italy and Spain over four weeks post-intervention, respectively.38 All three studies were rated at moderate risk of bias (fig 2).
Lockdown and transmission of SARS-CoV-2
Four studies assessed universal lockdown and transmission of SARS-CoV-2 during the first few months of the pandemic (table 2). The decrease in reproductive number (R0) ranged from 1.27 in Italy (pre-intervention 2.03, post-intervention 0.76)39 to 2.09 in India (pre-intervention 3.36, post-intervention 1.27),64 and 3.97 in China (pre-intervention 4.95, post-intervention 0.98).33 A natural experiment from the US reported that lockdown was associated with an 11% reduction in transmission of SARS-CoV-2 (relative risk 0.89, 95% confidence interval 0.88 to 0.91).40 All the studies were rated at low risk of bias33 39 to moderate risk40 64 (fig 2).
Travel related measures
Restricted travel and border closures
Border closure was assessed in one natural experiment study involving nine African countries (table 3).62 Overall, the countries recorded an increase in the incidence of covid-19 after border closure. These studies concluded that the implementation of border closures within African countries had minimal effect on the incidence of covid-19. The study had important limitations and was rated at serious or critical risk of bias. In the US, a natural experiment study reported that restrictions on travel between states contributed about 11% to a reduction in SARS-CoV-2 transmission (table 3).36 The study was rated at moderate risk of bias (fig 2).
Table 3.
Study characteristics and main results from studies that assessed individual travel measures
| Reference, country | Study design | Public health measure | Sample size | Outcome measure | Study duration | Effect estimates: conclusions | Risk of bias |
|---|---|---|---|---|---|---|---|
| Emeto et al,62 Africa | Natural experiment | Border closure | 9 countries | Rt | 14 Feb-19 Jul 2020 | See supplementary table for data on all countries: minimal effect on reducing transmission (Rt)*† | Serious or critical |
| Liu et al,58 USA | Natural experiment | Interstate travel restrictions | 50 states | Rt | 21 Jan-31 May 2020 | Risk ratio 0.89 (95% confidence interval 0.84 to 0.95): contributed about 11% to reduction in Rt* | Moderate |
| Mitra et al,54 Australia | Retrospective cohort | Screening for fever | 65 000 people | Daily growth rate | 9 Mar-13 May 2020 | Sensitivity 24%: 86% of cases not detected—poor sensitivity of identifying people with SARS-CoV-2* | Moderate |
R0=reproductive number; Rt=time varying reproductive number.
Interpretation of findings as reported in the original manuscript.
Not an effective intervention
Entry and exit screening (virus or symptom screening)
One retrospective cohort study assessed screening of symptoms, which involved testing 65 000 people for fever (table 3).54 The study found that screening for fever lacked sensitivity (ranging from 18% to 24%) in detecting people with SARS-CoV-2 infection. This translated to 86% of the population with SARS-CoV-2 remaining undetected when screening for fever. The study was rated at moderate risk of bias (fig 2).
Multiple public health measures
Study characteristics
Overall, 37 studies provided estimates on the effectiveness of multiple public health measures, assessed as a collective group. Studies were mostly conducted in Asia (n=15), the US (n=11), Europe (n=6), Africa (n=4), and South America (n=1). All the studies were observational. The most commonly measured outcome was transmission of disease (ie, measured as reproductive number, growth number, or epidemic doubling time) (n=23), followed by covid-19 incidence (n=19) and covid-19 mortality (n=8). This review attempted to assess the overall effectiveness of the public health intervention packages by reporting the percentage difference in outcome before and after implementation of measures or between regions or countries studied. Eleven of the 37 included studies noted a difference of between 26% and 50% in transmission of SARS-CoV-2 and incidence of covid-19,70 71 72 73 74 75 76 77 78 79 80 nine noted a difference of between 51% and 75% in SARS-CoV-2 transmission, covid-19 incidence, and covid-19 mortality,81 82 83 84 85 86 87 88 89 and 14 noted a difference of more than 75% in transmission of SARS-CoV-2, covid-19 incidence and covid-19 mortality.79 80 89 90 91 92 93 94 95 96 97 98 99 100 For the remaining studies, the overall effectiveness was not assessed owing to a lack of comparators (see supplementary material 3, table 3). Two studies that assessed universal lockdown and physical distancing reported a decrease of between 0% and 25% in SARS-CoV-2 transmission and covid-19 incidence.79 101 Studies that included school and workplace closures,91 95 96 isolation or stay at home measures,80 94 or a combination of both79 89 93 97 98 99 reported decreases of more than 75% in SARS-CoV-2 transmission. Supplementary material 3, table 2 provides detailed information on each study.
Discussion
Worldwide, government and public health organisations are mitigating the spread of SARS-CoV-2 by implementing various public health measures. This systematic review identified a statistically significant reduction in the incidence of covid-19 through the implementation of mask wearing and physical distancing. Handwashing interventions also indicated a substantial reduction in covid-19 incidence, albeit not statistically significant in the adjusted model. As the random effects model tends to underestimate confidence intervals when a meta-analysis includes a small number of individual studies (<5), the adjusted model for handwashing showed a statistically non-significant association in reducing the incidence of covid-19 compared with the unadjusted model.
Overall effectiveness of these interventions was affected by clinical heterogeneity and methodological limitations, such as confounding and measurement bias. It was not possible to evaluate the impact of type of face maks (eg, surgical, fabric, N95 respirators) and compliance and frequency of wearing masks owing to a lack of data. Similarly, it was not feasible to assess the differences in effect that different recommendations for physical distancing (ie, 1.5 m, 2m, or 3 m) have as preventive strategies.
The effectiveness of measures such as universal lockdowns and closures of businesses and schools for the containment of covid-19 have largely been effective, but depended on early implementation when incidence rates of covid-19 were still low.42 52 58 Only Japan reported no decrease in covid-19 incidence after school closures,44 and other studies found that different public health measures were sometimes implemented simultaneously or soon after one another, thus the results should be interpreted with caution.32 46 56
Isolation or stay at home was an effective measure in reducing the transmission of SARS-CoV-2, but the included studies used results for mobility to assess stay at home or isolation and therefore could have been limited by potential flaws in publicly available phone data,41 58 102 and variations in the enforcement of public health measures in different states or regions were not assessed.55 58 102 Quarantine was found to be as effective in reducing the incidence of covid-19 and transmission of SARS-CoV-2, yet variation in testing and case detection in low income environments was substantial.59 96 98 Another study reported that quarantine was effective in reducing the transmission of SARS-CoV-2 in a cohort with a low prevalence of the virus, yet it is unknown if the same effect would be observed with higher prevalence.34
It was not possible to draw conclusions about the effectiveness of restricted travel and full border closures because the number of empirical studies was insufficient. Single studies identified that border closure in Africa had a minimal effect in reducing SARS-CoV-2 transmission, but the study was assessed as being at high risk of bias.62 Screening for fever was also identified to be ineffective, with only 24% of positive cases being captured by screening.54
Comparison with other studies
Previous literature reviews have identified mask wearing as an effective measure for the containment of SARS-CoV-2103; the caveat being that more high level evidence is required to provide unequivocal support for the effectiveness of the universal use of face masks.104 105 Additional empirical evidence from a recent randomised controlled trial (originally published as a preprint) indicates that mask wearing achieved a 9.3% reduction in seroprevalence of symptomatic SARS-CoV-2 infection and an 11.9% reduction in the prevalence of covid-19-like symptoms.106 Another systematic review showed stronger effectiveness with the use of N95, or similar, respirators than disposable surgical masks,107 and a study evaluating the protection offered by 18 different types of fabric masks found substantial heterogeneity in protection, with the most effective mask being multilayered and tight fitting.108 However, transmission of SARS-CoV-2 largely arises in hospital settings in which full personal protective measures are in place, which suggests that when viral load is at its highest, even the best performing face masks might not provide adequate protection.51 Additionally, most studies that assessed mask wearing were prone to important confounding bias, which might have altered the conclusions drawn from this review (ie, effect estimates might have been underestimated or overestimated or can be related to other measures that were in place at the time the studies were conducted). Thus, the extent of such limitations on the conclusions drawn remain unknown.
A 2020 rapid review concluded that quarantine is largely effective in reducing the incidence of covid-19 and covid-19 mortality. However, uncertainty over the magnitude of such an effect still remains,109 with enhanced management of quality quarantine facilities for improved effective control of the epidemics urgently needed.110 In addition, findings on the application of school and workplace closures are still inconclusive. Policy makers should be aware of the ambiguous evidence when considering school closures, as other potentially less disruptive physical distancing interventions might be more appropriate.21 Numerous findings from studies on the efficacy of school closures showed that the risk of transmission within the educational environment often strongly depends on the incidence of covid-19 in the community, and that school closures are most successfully associated with control of SARS-CoV-2 transmission when other mitigation strategies are in place in the community.111 112 113 114 115 116 117 School closures have been reported to be disruptive to students globally and are likely to impair children’s social, psychological, and educational development118 119 and to result in loss of income and productivity in adults who cannot work because of childcare responsibilities.120
Speculation remains as how best to implement physical distancing measures.121 Studies that assess physical distancing measures might interchangeably study physical distancing with lockdown35 52 56 64 and other measures and thus direct associations are difficult to assess.
Empirical evidence from restricted travel and full border closures is also limited, as it is almost impossible to study these strategies as single measures. Current evidence from a recent narrative literature review suggested that control of movement, along with mandated quarantine, travel restrictions, and restricting nationals from entering areas of high infection, are effective measures, but only with good compliance.122 A narrative literature review of travel bans, partial lockdowns, and quarantine also suggested effectiveness of these measures,123 and another rapid review further supported travel restrictions and cross border restrictions to stop the spread of SARS-CoV-2.124 It was impossible to make such observations in the current review because of limited evidence. A German review, however, suggested that entry, exit, and symptom screening measures to prevent transmission of SARS-CoV-2 are not effective at detecting a meaningful proportion of cases,125 and another review using real world data from multiple countries found that border closures had minimal impact on the control of covid-19.126
Although universal lockdowns have shown a protective effect in lowering the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality, these measures are also disruptive to the psychosocial and mental health of children and adolescents,127 global economies,128 and societies.129 Partial lockdowns could be an alternative, as the associated effectiveness can be high,125 especially when implemented early in an outbreak,85 and such measures would be less disruptive to the general population.
It is important to also consider numerous sociopolitical and socioeconomic factors that have been shown to increase SARS-CoV-2 infection130 131 and covid-19 mortality.132 Immigration status,82 economic status,81 101 and poverty and rurality98 can influence individual and community compliance with public health measures. Poverty can impact the ability of communities to physically distance,133 especially in crowded living environments,134 135 as well as reduce access to personal protective measures.134 135 A recent study highlights that “a one size fits all” approach to public health measures might not be effective at reducing the spread of SARS-CoV-2 in vulnerable communities136 and could exacerbate social and economic inequalities.135 137 As such, a more nuanced and community specific approach might be required. Even though screening is highly recommended by WHO138 because a proportion of patients with covid-19 can be asymptomatic,138 screening for symptoms might miss a larger proportion of the population with covid-19. Hence, temperature screening technologies might need to be reconsidered and evaluated for cost effectiveness, given such measures are largely depended on symptomatic fever cases.
Strengths and limitations of this review
The main strength of this systematic review was the use of a comprehensive search strategy to identify and select studies for review and thereby minimise selection bias. A clinical epidemiologist developed the search strategy, which was validated by two senior medical librarians. This review followed a comprehensive appraisal process that is recommended by the Cochrane Collaboration31 to assess the effectiveness of public health measures, with specifically validated tools used to independently and individually assess the risk of bias in each study by study design.
This review has some limitations. Firstly, high quality evidence on SARS CoV-2 and the effectiveness of public health measures is still limited, with most studies having different underlying target variables. Secondly, information provided in this review is based on current evidence, so will be modified as additional data become available, especially from more prospective and randomised studies. Also, we excluded studies that did not provide certainty over the effect measure, which might have introduced selection bias and limited the interpretation of effectiveness. Thirdly, numerous studies measured interventions only once and others multiple times over short time frames (days v month, or no timeframe). Additionally, the meta-analytical portion of this study was limited by significant heterogeneity observed across studies, which could neither be explored nor explained by subgroup analyses or meta-regression. Finally, we quantitatively assessed only publications that reported individual measures; studies that assessed multiple measures simultaneously were narratively analysed with a broader level of effectiveness (see supplementary material 3, table 3). Also, we excluded studies in languages other than English.
Methodological limitations of studies included in the review
Several studies failed to define and assess for potential confounders, which made it difficult for our review to draw a one directional or causal conclusion. This problem was mainly because we were unable to study only one intervention, given that many countries implemented several public health measures simultaneously; thus it is a challenge to disentangle the impact of individual interventions (ie, physical distancing when other interventions could be contributing to the effect). Additionally, studies measured different primary outcomes and in varied ways, which limited the ability to statistically analyse other measures and compare effectiveness.
Further pragmatic randomised controlled trials and natural experiment studies are needed to better inform the evidence and guide the future implementation of public health measures. Given that most measures depend on a population’s adherence and compliance, it is important to understand and consider how these might be affected by factors. A lack of data in the assessed studies meant it was not possible to understand or determine the level of compliance and adherence to any of the measures.
Conclusions and policy implications
Current evidence from quantitative analyses indicates a benefit associated with handwashing, mask wearing, and physical distancing in reducing the incidence of covid-19. The narrative results of this review indicate an effectiveness of both individual or packages of public health measures on the transmission of SARS-CoV-2 and incidence of covid-19. Some of the public health measures seem to be more stringent than others and have a greater impact on economies and the health of populations. When implementing public health measures, it is important to consider specific health and sociocultural needs of the communities and to weigh the potential negative effects of the public health measures against the positive effects for general populations. Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved. It is likely that further control of the covid-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures.
What is already known on this topic
Public health measures have been identified as a preventive strategy for influenza pandemics
The effectiveness of such interventions in reducing the transmission of SARS-CoV-2 is unknown
What this study adds
The findings of this review suggest that personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of covid-19
More stringent measures, such as lockdowns and closures of borders, schools, and workplaces need to be carefully assessed by weighing the potential negative effects of these measures on general populations
Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage
Acknowledgments
We thank medical subject librarians Lorena Romero (LR) and Marshall Dozier (MD) for their expert advice and assistance with the study search strategy.
Web extra.
Extra material supplied by authors
Supplementary information: additional material
Contributors: ST, DG, DI, DL, and ZA conceived and designed the study. ST, DG, SS, AM, HW, WX, JR, ET, AM, XL, XZ, and IME collected and screened the data. ST, DG, and DI acquired, analysed, or interpreted the data. ST, HW, and SS drafted the manuscript. All authors critically revised the manuscript for important intellectual content.. XL and ST did the statistical analysis. NA obtained funding. LR and MD provided administrative, technical, or material support. ST and DI supervised the study. ST and DI had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ST is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: No funding was available for this research. ET is supported by a Cancer Research UK Career Development Fellowship (grant No C31250/A22804). XZ is supported by The Darwin Trust of Edinburgh.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/and declare: ET is supported by a Cancer Research UK Career Development Fellowship and XZ is supported by The Darwin Trust of Edinburgh; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
The lead author (ST) affirms that the manuscript is an honest, accurate, and transparent account of the study reported; no important aspects of the study have been omitted. Dissemination to participants and related patient and public communities: It is anticipated to disseminate the results of this research to wider community via press release and social media platforms.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1. McKee M, Stuckler D. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat Med 2020;26:640-2. 10.1038/s41591-020-0863-y. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/
- 3. Parodi SM, Liu VX. From Containment to Mitigation of COVID-19 in the US. JAMA 2020;323:1441-2. 10.1001/jama.2020.3882. [DOI] [PubMed] [Google Scholar]
- 4. Bernal JL, Andrews N, Gower C, et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv 2021:2021.03.01.21252652.
- 5. Chodick G, Tene L, Patalon T, et al. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw Open 2021;4:e2115985. 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson RM, Vegvari C, Truscott J, Collyer BS. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020;396:1614-6. 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khateeb J, Li Y, Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit Care 2021;25:244. 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh J, Pandit P, McArthur AG, Banerjee A, Mossman K. Evolutionary trajectory of SARS-CoV-2 and emerging variants. Virol J 2021;18:166. 10.1186/s12985-021-01633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanyaolu A, Okorie C, Marinkovic A, et al. The emerging SARS-CoV-2 variants of concern. Ther Adv Infect Dis 2021;8:20499361211024372. 10.1177/20499361211024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19. 2020. www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19
- 11. Henry DA, Jones MA, Stehlik P, Glasziou PP. Effectiveness of COVID-19 vaccines: findings from real world studies. Med J Aust 2021;215:149-151.e1. 10.5694/mja2.51182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. COVID-19 strategy update. 2020. www.who.int/docs/default-source/coronaviruse/covid-strategy-update-14april2020.pdf?sfvrsn=29da3ba0_19
- 13. Hollingsworth TD, Klinkenberg D, Heesterbeek H, Anderson RM. Mitigation strategies for pandemic influenza A: balancing conflicting policy objectives. PLoS Comput Biol 2011;7:e1001076-76. 10.1371/journal.pcbi.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aledort JE, Lurie N, Wasserman J, Bozzette SA. Non-pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base. BMC Public Health 2007;7:208-08. 10.1186/1471-2458-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Non-pharmaceutical public health measures for mitigating the risk and impact of epidemic and pandemic influenza 2019. 2019. https://apps.who.int/iris/bitstream/handle/10665/329438/9789241516839-eng.pdf?ua=1.
- 16. Yang Chan EY, Shahzada TS, Sham TST, et al. Narrative review of non-pharmaceutical behavioural measures for the prevention of COVID-19 (SARS-CoV-2) based on the Health-EDRM framework. Br Med Bull 2020;136:46-87. 10.1093/bmb/ldaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han E, Tan MMJ, Turk E, et al. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet 2020;396:1525-34. 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong MC, Huang J, Teoh J, Wong SH. Evaluation on different non-pharmaceutical interventions during COVID-19 pandemic: An analysis of 139 countries. J Infect 2020;81:e70-1. 10.1016/j.jinf.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellewell J, Abbott S, Gimma A, et al. Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group . Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health 2020;8:e488-96. 10.1016/S2214-109X(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mendez-Brito A, El Bcheraoui C, Pozo-Martin F. Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19. J Infect 2021;83:281-93. 10.1016/j.jinf.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health 2020;4:397-404. 10.1016/S2352-4642(20)30095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Regmi K, Lwin CM. Factors Associated with the Implementation of Non-Pharmaceutical Interventions for Reducing Coronavirus Disease 2019 (COVID-19): A Systematic Review. Int J Environ Res Public Health 2021;18:4274. 10.3390/ijerph18084274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizvi RF, Craig KJT, Hekmat R, et al. Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis. SAGE Open Med 2021;9:20503121211022973. 10.1177/20503121211022973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayouni I, Maatoug J, Dhouib W, et al. Effective public health measures to mitigate the spread of COVID-19: a systematic review. BMC Public Health 2021;21:1015. 10.1186/s12889-021-11111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26. Holmdahl I, Buckee C. Wrong but Useful - What Covid-19 Epidemiologic Models Can and Cannot Tell Us. N Engl J Med 2020;383:303-5. 10.1056/NEJMp2016822. [DOI] [PubMed] [Google Scholar]
- 27.Covidence Systematic Review Software. Veritas Health Innovation, Melbourne Australia. www.covidence.org
- 28. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 30. Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol 2015;15:99. 10.1186/s12874-015-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ. Welch VA, ed. Cochrane Handbook for Systematic Reviews of Interventions.: Chichester, UK: Wiley; 2019. 2nd edn. https://training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 32. Vlachos J, Hertegård E, B Svaleryd H. The effects of school closures on SARS-CoV-2 among parents and teachers. Proc Natl Acad Sci U S A 2021;118:e2020834118. 10.1073/pnas.2020834118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J, Liao Y, Wang X, et al. Incidence of novel coronavirus (2019-nCoV) infection among people under home quarantine in Shenzhen, China. Travel Med Infect Dis 2020;37:101660. 10.1016/j.tmaid.2020.101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al-Tawfiq JA, Sattar A, Al-Khadra H, et al. Incidence of COVID-19 among returning travelers in quarantine facilities: A longitudinal study and lessons learned. Travel Med Infect Dis 2020;38:101901-01. 10.1016/j.tmaid.2020.101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siedner MJ, Harling G, Reynolds Z, et al. Correction: Social distancing to slow the US COVID-19 epidemic: Longitudinal pretest-posttest comparison group study. PLoS Med 2020;17:e1003376. 10.1371/journal.pmed.1003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lio CF, Cheong HH, Lei CI, et al. Effectiveness of personal protective health behaviour against COVID-19. BMC Public Health 2021;21:827. 10.1186/s12889-021-10680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van den Berg P, Schechter-Perkins EM, Jack RS, et al. Effectiveness of 3 Versus 6 ft of Physical Distancing for Controlling Spread of Coronavirus Disease 2019 Among Primary and Secondary Students and Staff: A Retrospective, Statewide Cohort Study. Clin Infect Dis 2021. 10.1093/cid/ciab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tobías A. Evaluation of the lockdowns for the SARS-CoV-2 epidemic in Italy and Spain after one month follow up. Sci Total Environ 2020;725:138539. 10.1016/j.scitotenv.2020.138539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guzzetta G, Riccardo F, Marziano V, et al. COVID-19 Working Group,2 . Impact of a Nationwide Lockdown on SARS-CoV-2 Transmissibility, Italy. Emerg Infect Dis 2021;27:267. 10.3201/eid2701.202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo C, Chan SHT, Lin C, et al. Physical distancing implementation, ambient temperature and Covid-19 containment: An observational study in the United States. Sci Total Environ 2021;789:147876. 10.1016/j.scitotenv.2021.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dreher N, Spiera Z, McAuley FM, et al. Policy Interventions, Social Distancing, and SARS-CoV-2 Transmission in the United States: A Retrospective State-level Analysis. Am J Med Sci 2021;361:575-84. 10.1016/j.amjms.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silva L, Figueiredo Filho D, Fernandes A. The effect of lockdown on the COVID-19 epidemic in Brazil: evidence from an interrupted time series design. Cad Saude Publica 2020;36:e00213920. 10.1590/0102-311x00213920. [DOI] [PubMed] [Google Scholar]
- 43. Krishnamachari B, Morris A, Zastrow D, Dsida A, Harper B, Santella AJ. The role of mask mandates, stay at home orders and school closure in curbing the COVID-19 pandemic prior to vaccination. Am J Infect Control 2021;49:1036-42. 10.1016/j.ajic.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwata K, Doi A, Miyakoshi C. Was school closure effective in mitigating coronavirus disease 2019 (COVID-19)? Time series analysis using Bayesian inference. Int J Infect Dis 2020;99:57-61. 10.1016/j.ijid.2020.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rader B, White LF, Burns MR, et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: a cross-sectional study. Lancet Digit Health 2021;3:e148-57. 10.1016/S2589-7500(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pillai J, Motloba P, Motaung KSC, et al. The effect of lockdown regulations on SARS-CoV-2 infectivity in Gauteng Province, South Africa. S Afr Med J 2020;110:1119-23. 10.7196/SAMJ.2020.v110i11.15222. [DOI] [PubMed] [Google Scholar]
- 47. Alimohamadi Y, Holakouie-Naieni K, Sepandi M, Taghdir M. Effect of Social Distancing on COVID-19 Incidence and Mortality in Iran Since February 20 to May 13, 2020: An Interrupted Time Series Analysis. Risk Manag Healthc Policy 2020;13:1695-700. 10.2147/RMHP.S265079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Auger KA, Shah SS, Richardson T, et al. Association Between Statewide School Closure and COVID-19 Incidence and Mortality in the US. JAMA 2020;324:859-70. 10.1001/jama.2020.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leffler CT, Ing E, Lykins JD, Hogan MC, McKeown CA, Grzybowski A. Association of Country-wide Coronavirus Mortality with Demographics, Testing, Lockdowns, and Public Wearing of Masks. Am J Trop Med Hyg 2020;103:2400-11. 10.4269/ajtmh.20-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lyu W, Wehby GL. Community Use Of Face Masks And COVID-19: Evidence From A Natural Experiment Of State Mandates In The US. Health Aff (Millwood) 2020;39:1419-25. 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 51. Cheng Y, Ma N, Witt C, et al. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021;372:1439-43. 10.1126/science.abg6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alfano V, Ercolano S. The Efficacy of Lockdown Against COVID-19: A Cross-Country Panel Analysis. Appl Health Econ Health Policy 2020;18:509-17. 10.1007/s40258-020-00596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vokó Z, Pitter JG. The effect of social distance measures on COVID-19 epidemics in Europe: an interrupted time series analysis. Geroscience 2020;42:1075-82. 10.1007/s11357-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mitra B, Luckhoff C, Mitchell RD, O’Reilly GM, Smit V, Cameron PA. Temperature screening has negligible value for control of COVID-19. Emerg Med Australas 2020;32:867-9. 10.1111/1742-6723.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khosravi A, Chaman R, Rohani-Rasaf M, Zare F, Mehravaran S, Emamian MH. The basic reproduction number and prediction of the epidemic size of the novel coronavirus (COVID-19) in Shahroud, Iran. Epidemiol Infect 2020;148:e115. 10.1017/S0950268820001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thayer WM, Hasan MZ, Sankhla P, Gupta S. An interrupted time series analysis of the lockdown policies in India: a national-level analysis of COVID-19 incidence. Health Policy Plan 2021;36:620-9. 10.1093/heapol/czab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health 2020;5:e002794. 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu X, Xu X, Li G, et al. Differential impact of non-pharmaceutical public health interventions on COVID-19 epidemics in the United States. BMC Public Health 2021;21:965. 10.1186/s12889-021-10950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaman RS, Valamparampil MJ, Varghese B, et al. Quarantine practices and COVID-19 transmission in a low-resource setting: Experience of Kerala, India. J Family Med Prim Care 2021;10:1003-8. 10.4103/jfmpc.jfmpc_2034_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu H, Gan Y, Zheng D, et al. Relationship Between COVID-19 Infection and Risk Perception, Knowledge, Attitude, and Four Nonpharmaceutical Interventions During the Late Period of the COVID-19 Epidemic in China: Online Cross-Sectional Survey of 8158 Adults. J Med Internet Res 2020;22:e21372. 10.2196/21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Quaife M, van Zandvoort K, Gimma A, et al. CMMID COVID-19 Working Group . The impact of COVID-19 control measures on social contacts and transmission in Kenyan informal settlements. BMC Med 2020;18:316. 10.1186/s12916-020-01779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Emeto TI, Alele FO, Ilesanmi OS. Evaluation of the effect of border closure on COVID-19 incidence rates across nine African countries: an interrupted time series study. Trans R Soc Trop Med Hyg 2021;115:1174-83. 10.1093/trstmh/trab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Doung-Ngern P, Suphanchaimat R, Panjangampatthana A, et al. Case-Control Study of Use of Personal Protective Measures and Risk for SARS-CoV 2 Infection, Thailand. Emerg Infect Dis 2020;26:2607-16. 10.3201/eid2611.203003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Basu D, Salvatore M, Ray D, et al. A Comprehensive Public Health Evaluation of Lockdown as a Non-pharmaceutical Intervention on COVID-19 Spread in India: National Trends Masking State Level Variations. medRxiv 2020:2020.05.25.20113043. [DOI] [PMC free article] [PubMed]
- 65. Jarvis CI, Van Zandvoort K, Gimma A, et al. CMMID COVID-19 working group . Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med 2020;18:124. 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers: A Randomized Controlled Trial. Ann Intern Med 2020;174:335-43. 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang L, Li J, Guo S, et al. Real-time estimation and prediction of mortality caused by COVID-19 with patient information based algorithm. Sci Total Environ 2020;727:138394. 10.1016/j.scitotenv.2020.138394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cheng VC-C, Wong S-C, Chuang VW-M, et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect 2020;81:107-14. 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang K, Zhao S, Li H, et al. Real-time estimation of the reproduction number of the novel disease (COVID-19) in China in 2020 based on incidence data. Ann Transl Med 2020;8:689. 10.21037/atm-20-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ryu S, Ali ST, Jang C, Kim B, Cowling BJ. Effect of Nonpharmaceutical Interventions on Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, South Korea, 2020. Emerg Infect Dis 2020;26:2406-10. 10.3201/eid2610.201886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Malheiro R, Figueiredo AL, Magalhães JP, et al. Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: a retrospective cohort study. Public Health 2020;189:54-9. 10.1016/j.puhe.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dasgupta S, Kassem AM, Sunshine G, et al. Differences in rapid increases in county-level COVID-19 incidence by implementation of statewide closures and mask mandates - United States, June 1-September 30, 2020. Ann Epidemiol 2021;57:46-53. 10.1016/j.annepidem.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yeoh EK, Chong KC, Chiew CJ, et al. Assessing the impact of non-pharmaceutical interventions on the transmissibility and severity of COVID-19 during the first five months in the Western Pacific Region. One Health 2021;12:100213-13. 10.1016/j.onehlt.2021.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bendavid E, Oh C, Bhattacharya J, Ioannidis JPA. Assessing mandatory stay-at-home and business closure effects on the spread of COVID-19. Eur J Clin Invest 2021;51:e13484. 10.1111/eci.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jüni P, Rothenbühler M, Bobos P, et al. Impact of climate and public health interventions on the COVID-19 pandemic: a prospective cohort study. CMAJ 2020;192:E566-73. 10.1503/cmaj.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rubin D, Huang J, Fisher BT, et al. Association of Social Distancing, Population Density, and Temperature With the Instantaneous Reproduction Number of SARS-CoV-2 in Counties Across the United States. JAMA Netw Open 2020;3:e2016099-99. 10.1001/jamanetworkopen.2020.16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thu TPB, Ngoc PNH, Hai NM, Tuan LA. Effect of the social distancing measures on the spread of COVID-19 in 10 highly infected countries. Sci Total Environ 2020;742:140430-30. 10.1016/j.scitotenv.2020.140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang C, Chen C, Shen W, et al. Impact of population movement on the spread of 2019-nCoV in China. Emerg Microbes Infect 2020;9:988-90. 10.1080/22221751.2020.1760143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lau H, Khosrawipour V, Kocbach P, et al. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med 2020;27:taaa037. 10.1093/jtm/taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patel P, Athotra A, Vaisakh TP, Dikid T, Jain SK, NCDC COVID Incident Management Team . Impact of nonpharmacological interventions on COVID-19 transmission dynamics in India. Indian J Public Health 2020;64(Supplement):S142-6. 10.4103/ijph.IJPH_510_20. [DOI] [PubMed] [Google Scholar]
- 81. Courtemanche C, Garuccio J, Le A, Pinkston J, Yelowitz A. Strong social distancing measures in the united states reduced the covid-19 growth rate. Health Aff (Millwood) 2020;39:1237-46. 10.1377/hlthaff.2020.00608. [DOI] [PubMed] [Google Scholar]
- 82. Al Wahaibi A, Al Manji A, Al Maani A, et al. COVID-19 epidemic monitoring after non-pharmaceutical interventions: The use of time-varying reproduction number in a country with a large migrant population. Int J Infect Dis 2020;99:466-72. 10.1016/j.ijid.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Timelli L, Girardi E. Effect of timing of implementation of containment measures on Covid-19 epidemic. The case of the first wave in Italy. PLoS One 2021;16:e0245656-56. 10.1371/journal.pone.0245656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bo Y, Guo C, Lin C, et al. Effectiveness of non-pharmaceutical interventions on COVID-19 transmission in 190 countries from 23 January to 13 April 2020. Int J Infect Dis 2021;102:247-53. 10.1016/j.ijid.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Koh WC, Naing L, Wong J. Estimating the impact of physical distancing measures in containing COVID-19: an empirical analysis. Int J Infect Dis 2020;100:42-9. 10.1016/j.ijid.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tariq A, Undurraga EA, Laborde CC, et al. Transmission dynamics and control of COVID-19 in Chile, March-October, 2020. PLoS Negl Trop Dis 2021;15:e0009070. 10.1371/journal.pntd.0009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ghosal S, Bhattacharyya R, Majumder M. Impact of complete lockdown on total infection and death rates: A hierarchical cluster analysis. Diabetes Metab Syndr 2020;14:707-11. 10.1016/j.dsx.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Castillo RC, Staguhn ED, Weston-Farber E. The effect of state-level stay-at-home orders on COVID-19 infection rates. Am J Infect Control 2020;48:958-60. 10.1016/j.ajic.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu H, Chen C, Cruz-Cano R, Guida JL, Lee M. Public Compliance With Social Distancing Measures and SARS-CoV-2 Spread : A Quantitative Analysis of 5 States. Public Health Rep 2021;136:475-82. 10.1177/00333549211011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Son H, Lee H, Lee M, et al. Epidemiological characteristics of and containment measures for COVID-19 in Busan, Korea. Epidemiol Health 2020;42:e2020035-35. 10.4178/epih.e2020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yehya N, Venkataramani A, Harhay MO. Statewide Interventions and Covid-19 Mortality in the United States: An Observational Study. Clin Infect Dis 2020;73:e1863-9. 10.1093/cid/ciaa923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pan A, Liu L, Wang C, et al. Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA 2020;323:1915-23. 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang KW, Gao J, Wang H, et al. Epidemiology of 2019 novel coronavirus in Jiangsu Province, China after wartime control measures: A population-level retrospective study. Travel Med Infect Dis 2020;35:101654. 10.1016/j.tmaid.2020.101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ruan L, Wen M, Zeng Q, et al. New measures for COVID-19 response: a lesson from the Wenzhou experience. Clin Infect Dis 2020;71:866-9. 10.1093/cid/ciaa386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tsai AC, Harling G, Reynolds Z, Gilbert RF, Siedner MJ. Coronavirus Disease 2019 (COVID-19) Transmission in the United States Before Versus After Relaxation of Statewide Social Distancing Measures. Clin Infect Dis 2021;73(Suppl 2):S120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tchole AIM, Li ZW, Wei JT, et al. Cheeloo EcoHealth Consortium (CLEC) . Epidemic and control of COVID-19 in Niger: quantitative analyses in a least developed country. J Glob Health 2020;10:020513. 10.7189/jogh.10.020513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Singh S, Shaikh M, Hauck K, Miraldo M. Impacts of introducing and lifting nonpharmaceutical interventions on COVID-19 daily growth rate and compliance in the United States. Proc Natl Acad Sci U S A 2021;118:1. 10.1073/pnas.2021359118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. McCreesh N, Dlamini V, Edwards A, et al. Impact of social distancing regulations and epidemic risk perception on social contact and SARS-CoV-2 transmission potential in rural South Africa: analysis of repeated cross-sectional surveys. medRxiv 2020:2020.12.01.20241877. [DOI] [PMC free article] [PubMed]
- 99. Haapanen M, Renko M, Artama M, Kuitunen I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children - A nationwide register study in Finland. EClinicalMedicine 2021;34:100807-07. 10.1016/j.eclinm.2021.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McGrail DJ, Dai J, McAndrews KM, Kalluri R. Enacting national social distancing policies corresponds with dramatic reduction in COVID19 infection rates. PLoS One 2020;15:e0236619-19. 10.1371/journal.pone.0236619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Clipman SJ, Wesolowski AP, Gibson DG, et al. Rapid real-time tracking of non-pharmaceutical interventions and their association with SARS-CoV-2 positivity: The COVID-19 Pandemic Pulse Study. medRxiv 2020:2020.07.29.20164665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Erim DO, Oke GA, Adisa AO, et al. Associations of Government-Mandated Closures and Restrictions With Aggregate Mobility Trends and SARS-CoV-2 Infections in Nigeria. JAMA Netw Open 2021;4:e2032101-01. 10.1001/jamanetworkopen.2020.32101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Howard J, Huang A, Li Z, et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci U S A 2021;118:e2014564118. 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brainard J, Jones NR, Lake IR, Hooper L, Hunter PR. Community use of face masks and similar barriers to prevent respiratory illness such as COVID-19: a rapid scoping review. Euro Surveill 2020;25:2000725. 10.2807/1560-7917.ES.2020.25.49.2000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Camargo MC, Martinez-Silveira MS, Lima AA, et al. Effectiveness of the use of non-woven face mask to prevent coronavirus infections in the general population: a rapid systematic review. Cien Saude Colet 2020;25:3365-76. 10.1590/1413-81232020259.13622020. [DOI] [PubMed] [Google Scholar]
- 106.Abaluck J, Kwong LH, Styczynskyi A, et al. The Impact of Community Masking on COVID-19: A Cluster-Randomized Trial in Bangladesh. 2021. www.poverty-action.org/sites/default/files/publications/Mask_RCT____Symptomatic_Seropositivity_083121.pdf [DOI] [PMC free article] [PubMed]
- 107. Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ 2016;188:567-74. 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. O’Kelly E, Arora A, Pirog S, et al. Improving Fabric Face Masks: Impact of Design Features on the Protection Offered by Fabric Face Masks. medRxiv 2021:2021.01.21.20228569.
- 109. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev 2020;4:CD013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nafees M, Khan F. Pakistan’s response to COVID-19 pandemic and efficacy of quarantine and partial lockdown: A review. Electronic Journal of General Medicine 2020;17:em240.
- 111. Macartney K, Quinn HE, Pillsbury AJ, et al. NSW COVID-19 Schools Study Team . Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health 2020;4:807-16. 10.1016/S2352-4642(20)30251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Link-Gelles R, DellaGrotta AL, Molina C, et al. Limited Secondary Transmission of SARS-CoV-2 in Child Care Programs - Rhode Island, June 1-July 31, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1170-2. 10.15585/mmwr.mm6934e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Heavey L, Casey G, Kelly C, Kelly D, McDarby G. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill 2020;25:2000903. 10.2807/1560-7917.ES.2020.25.21.2000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fontanet A, Tondeur L, Madec Y, et al. Cluster of COVID-19 in northern France: A retrospective closed cohort study. medRxiv 2020:2020.04.18.20071134.
- 115.National Centre for Immunisation Research and Surveliance. COVID-19 in schools and early childhood education and care services – the Term 3 experience in NSW. 2020, NSW government. www.ncirsorgau/sites/default/files/2020-10/COVID-19%20Transmission%20in%20educational%20settings%20in%20NSW%20Term%203%20report_0pdf
- 116.National Centre for Immunisation Research and Surveliance. COVID-19 in schools and early childhood education and care services – the Term 1 experience in NSW. 2020, NSW government. www.ncirsorgau/sites/default/files/2020-08/COVID-19%20Transmission%20in%20educational%20settings%20in%20NSW%20Term%201%20report_0pdf
- 117.National Centre for Immunisation Research and Surveliance. COVID-19 in schools and early childhood education and care services – the Term 2 experience in NSW. 2020, NSW government. www.ncirsorgau/sites/default/files/2020-08/COVID-19%20Transmission%20in%20educational%20settings%20in%20NSW%20Term%202%20report_0pdf
- 118. Dubey S, Biswas P, Ghosh R, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr 2020;14:779-88. 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ghosh R, Dubey MJ, Chatterjee S, Dubey S. Impact of COVID -19 on children: special focus on the psychosocial aspect. Minerva Pediatr 2020;72:226-35. 10.23736/S0026-4946.20.05887-9. [DOI] [PubMed] [Google Scholar]
- 120. Bayham J, Fenichel EP. Impact of school closures for COVID-19 on the US health-care workforce and net mortality: a modelling study. Lancet Public Health 2020;5:e271-8. 10.1016/S2468-2667(20)30082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lahiri A, Jha SS, Bhattacharya S, Ray S, Chakraborty A. Effectiveness of preventive measures against COVID-19: A systematic review of In Silico modeling studies in indian context. Indian J Public Health 2020;64(Supplement):S156-67. 10.4103/ijph.IJPH_464_20. [DOI] [PubMed] [Google Scholar]
- 122. Tang KHD. Movement control as an effective measure against Covid-19 spread in Malaysia: an overview. Z Gesundh Wiss 2020. 10.1007/s10389-020-01316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Issac A, Stephen S, Jacob J, et al. The pandemic league of COVID-19: Korea versus the United States, with lessons for the entire world. J Prev Med Public Health 2020;53:228-32. 10.3961/jpmph.20.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Burns J, Movsisyan A, Stratil JM, et al. Travel-related control measures to contain the COVID-19 pandemic: a rapid review. Cochrane Database Syst Rev 2020;10:CD013717. 10.1002/14651858.CD013717. [DOI] [PubMed] [Google Scholar]
- 125. Haug N, Geyrhofer L, Londei A, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav 2020;4:1303-12. 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 126. Patiño-Lugo DF, Vélez M, Velásquez Salazar P, et al. Non-pharmaceutical interventions for containment, mitigation and suppression of COVID-19 infection. Colomb Med (Cali) 2020;51:e4266. 10.25100/cm.v51i2.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Singh S, Roy D, Sinha K, Parveen S, Sharma G, Joshi G. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res 2020;293:113429. 10.1016/j.psychres.2020.113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Felsenthal M. COVID-19 to plunge global economy into worst recession since World War II 2020. www.worldbank.org/en/news/press-release/2020/06/08/covid-19-to-plunge-global-economy-into-worst-recession-since-world-war-ii.
- 129. Brodeur A, Clark AE, Fleche S, Powdthavee N. COVID-19, lockdowns and well-being: Evidence from Google Trends. J Public Econ 2021;193:104346. 10.1016/j.jpubeco.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Niedzwiedz CL, O’Donnell CA, Jani BD, et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med 2020;18:160. 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chadeau-Hyam M, Bodinier B, Elliott J, et al. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK biobank data. Int J Epidemiol 2020;49:1454-67. 10.1093/ije/dyaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Williamson E, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020:2020.05.06.20092999.
- 133. Garnier R, Benetka JR, Kraemer J, Bansal S. Socioeconomic Disparities in Social Distancing During the COVID-19 Pandemic in the United States: Observational Study. J Med Internet Res 2021;23:e24591. 10.2196/24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bong C-L, Brasher C, Chikumba E, McDougall R, Mellin-Olsen J, Enright A. The COVID-19 Pandemic: Effects on Low- and Middle-Income Countries. Anesth Analg 2020;131:86-92. 10.1213/ANE.0000000000004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Corburn J, Vlahov D, Mberu B, et al. Slum Health: Arresting COVID-19 and Improving Well-Being in Urban Informal Settlements. J Urban Health 2020;97:348-57. 10.1007/s11524-020-00438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet 2020;395:1243-4. 10.016/S0140-6736(20)30893-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lou J, Shen X, Niemeier D. Are stay-at-home orders more difficult to follow for low-income groups? J Transp Geogr 2020;89:102894. 10.1016/j.jtrangeo.2020.102894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.World Health Organization. Transmission of COVID-19 by asymptomatic cases. 2020. www.emro.who.int/health-topics/corona-virus/transmission-of-covid-19-by-asymptomatic-cases.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional material
Data Availability Statement
No additional data available.