Abstract
SFTSV, a tick-borne bunyavirus causing a severe hemorrhagic fever termed as severe fever with thrombocytopenia syndrome (SFTS). To evaluate the potential role of rodents and its ectoparasitic chiggers in the transmission of SFTSV, we collected wild rodents and chiggers on their bodies from a rural area in Qingdao City, Shandong Province, China in September 2020. PCR amplification of the M and L segments of SFTSV showed that 32.3% (10/31) of rodents and 0.2% (1/564) of chiggers (Leptotrombidium deliense) from the rodents were positive to SFTSV. Our results suggested that rodents and chiggers may play an important role in the transmission of SFTSV, although the efficiency of chiggers to transmit SFTSV needs to be further investigated experimentally.
Author summary
Severe fever with thrombocytopenia syndrome (SFTS), an emerging hemorrhagic fever in Asia, is caused by SFTSV, a tick-borne bunyavirus. In this study, we analyzed the prevalence of SFTSV in rodents and their ectoparasite chigger mites (Leptotrombidium delicense) with RT-PCR. RT-PCR results showed that one third (32.3%, 10/31) rodents and one chigger (0.2%, 1/564) was positive to SFTSV. Our study suggested that rodents and chiggers may play an important role in the ecology of SFTSV.
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging hemorrhagic fever disease, caused by a tick-borne bunyavirus SFTSV (Dabie bandavirus) [1–3]. SFTS cases had non-specific clinical manifestations, such as fever, fatigue, myalgia, and gastrointestinal symptoms [1], which can be easily misdiagnosed as other febrile disease such as scrub typhus and hemorrhagic fever with renal syndrome (caused by hantaviruses) with similar clinical manifestation and increased the risk of death [4,5]. The case fatality rate of SFTS ranges from 3% to 30% among patients in different countries in East Asia [1,6–8], and lack of specific anti-SFTSV medicine or vaccines to combat the SFTSV. Since the disease was reported in China in 2011, SFTS cases has been confirmed by isolation of SFTSV or detection of SFTSV RNA in Japan, South Korea, Myanmar, Vietnam, and Thailand [1,6,9–12]. As it causes a severe disease and its endemic area continues to expand, SFTSV had been included in a list of priority pathogens with the potential to generate an international public health emergency by the World Health Organization in 2015 [13]. SFTS is typically considered as a tick-borne disease, and the virus has been detected in many tick species and laboratory transmitted in several tick species [2,14], but SFTSV can also be transmitted through mouth mucosa or conjunctiva to cause person-to-person or animal-to-human infection [15–17]. SFTSV genome had been detected from ticks in China, South Korea, and Japan [2,18–21].
Mites of the family Trombiculidae (known as chigger mites, sand mites, and harvest mites) are a large group of medical-relevant arthropods lived in moist soil covered with vegetation [22,23]. The word “chigger” refers to the larval stage of trombiculid mites among their life cycle (egg, larva, nymph, adult), as it is the only parasitic stage and feeds on decomposed tissues and lymph fluid of the hosts [24]. Chiggers have a worldwide distribution and infest a wide range of animals, including vertebrate and non-vertebrate, particularly rodents [25]. The most important medical significance of chigger is as the vector of Orientia tsutsugamushi, which causes scrub typhus [26]. Only a minority of chiggers can spread scrub typhus effectively, and they belong to the genus Leptotrombidium [27]. Leptotrombidium scutellare is ranked only second to L. deliense as the important vector of scrub typhus in China [28].
In recent years, with more and more reports of co-infection of SFTSV and O. tsutsugamushi [12,29–31], as well as, SFTS and scrub typhus had overlapped epidemic seasons [30,32,33], it is suspected that chiggers might play a role in the transmission of SFTSV. In addition, a Chinese literature reported that SFTSV was detected by PCR in mites (Laelaps echidninus, family Laelapidae and L. scutellare, family Trombiculidae) collected from rodents and goats in Jiangsu Province [34], which further deepened the suspicion of chigger’s role in transmission of SFTSV. However, only a single study reporting PCR detection of SFTSV in chiggers without isolation of the virus, the role of chiggers in transmission of SFTSV is still not clear because we do not know whether the PCR-detected SFTSV was degraded or inactivated viral genome in mites in the previous study. To understand the infection of SFTSV in chiggers, we used molecular method to analysis SFTSV infection in rodents and chiggers collected on the rodents from Qingdao City of Shandong Province, China.
Material and methods
Ethics statement
Collection of rodents was approved by the ethics committee of Medical School, Wuhan University (WHU2020-YF0023) and carried out in accordance with Wuhan University Guidelines on the Care and Use of Laboratory Animals.
Sampling
In September 2020, rodents and insectivore were captured from Qingdao City of Shandong Province in Eastern China. Rodents and insectivore were captured with snap traps baited with peanuts in wine, which were set bordering crop field each night, and checked the next morning. The captured animals were placed individually in transparent sealing bags labeled with the sampling number, date, and site of collection, and were taken back to the laboratory of a local center for disease control and prevention to collect rodent tissue and ectoparasites. Under a digital microscope, mites from animals and sealing bags were carefully collected using the sharp end of an acne needle. Mites of same species from a host were pooled into microcentrifuge tubes according to morphology [35]. Tick was not found on any rodents. Samples were placed into a bucket of dry ice for transportation, and once arrived at the laboratory, samples were immediately stored at -80°C.
Nucleic acid extraction and molecular detection
Mites in each pool were rinsed with sterile phosphate buffer saline (PBS) and homogenized with steel beads before nucleic acid extraction. DNA and RNA were extracted from each pool of mites and each spleen sample of rodents using the AllPrep DNA/RNA Mini Kit (QIAGEN, Hilden, Germany) and were stored at -80°C.
The RNA template was reverse transcribed into cDNA with RT First Strand cDNA Synthesis Kit (Servicebio, Wuhan, China). Nested polymerase chain reaction (PCR) targeting L and M segments of the SFTSV was performed. Both primary and nested PCRs were performed at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 30–60 s, and a final extension at 72°C for 10 min. The species of mites and small animals were identified by PCR amplification of the barcording region of the mitochondrial cytochrome c oxidase subunit I gene (COI) as described previously [36,37]. Primers used in this study were shown in Table 1. Each PCR reaction included a negative control with diethyl pyrocarbonate (DEPC)-treated ddH2O instead of cDNA as the template. Nucleic acid extraction, PCR preparation, and agarose gel electrophoresis were performed in separate rooms to avoid cross-contamination.
Table 1. Polymerase chain reaction primers used in this study.
| Target genes | Primers | Primer Sequence (5’-3’) | Size (bp) | Reference |
|---|---|---|---|---|
| SFTSV L segment | SFTSV-L-F1 | AATGATGCCAAGAAGTGGAAT | 855 | This study |
| SFTSV-L-R1 | ATGTAAGCATAGTCCTAGAAGC | |||
| SFTSV-L-F2 | CCACAGATTCATTTGGGCT | 367 | ||
| SFTSV-L-R2 | ATCATGATCGCTGAGTCGTC | |||
| SFTSV M segment | SFTSV-M-F1 | TCTGCAGTTCAGACTCAGGGA | 761 | [38] |
| SFTSV-M-R1 | GACGTGTATTGCTGTTTTCCC | |||
| SFTSV-M-F2 | TGTTGCTTGTCAGCCTATGAC | 674 | ||
| SFTSV-M-R2 | CAACCAATGATCCTGAGTGGA | |||
| Mites COI | COI-F | GGTCAACAAATCATAAAGATATTGG | 710 | [36] |
| COI-R | TAAACTTCAGGGTGACCAAAAAATCA | |||
| Small mammals COI | COI-F | ACTTCTGGGTGTCCAAAGAATCA | 750 | [37] |
| COI-R | CCTACTCRGCCATTTTACCTATG |
COI: mitochondrial cytochrome c oxidase subunit I gene
PCR products were analyzed in a 1.5% agarose gel containing 4S GelRed (Sangon, Shanghai, China) and checked under an ultraviolet light. PCR products with expected size were excised and purified using Gel Extraction Kit (Tsingke, Beijing, China). Purified DNA was inserted into pMD 19-T vector (TaKaRa, Dalian, China) and transformed into Escherichia coli DH5α competent cells for cloning and sequencing (Sangon, Shanghai, China).
Phylogenetic analysis
Chromatograms were checked with Chromas (Technelysium, Tewantin, QLD, Australia) to ensure the accuracy of sequencing. The obtained sequences were identified by comparing with sequences in GenBank with BLAST programs (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence alignment and editing were performed with the MEGA 7.0 software (http://www.megasoftware.net). Phylogenetic trees were constructed based on Maximum Likelihood (ML) method with the Kimura 2-parameter model using the MEGA 7.0 software, bootstrap values were calculated with 1,000 replicates.
Results
Small mammal and mite collection
Thirty rodents (12 Apodemus agrarius, 11 Tscherskia triton, 6 Mus musculus and 1 Cricetulus griseus) and one shrew (Crocidura lasiura) were captured from rural area in Huangdao District, Qingdao City of Shandong Province, China. A total of 564 mites were collected from small mammals (Fig 1). Mites from each animal were pooled together (average 23 mites/pool, ranging from 9 to 50 mites/pool) to analyze SFTSV. All mites belonged to the family Laelapidae and family Trombiculidae based on COI gene sequence (Table 2).
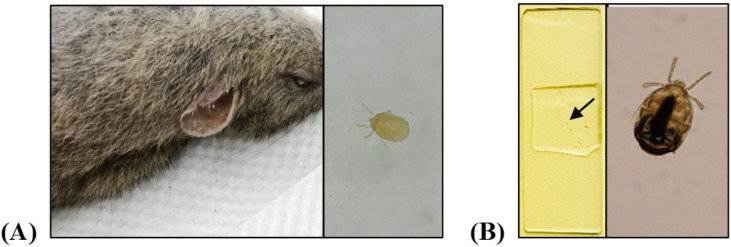
Fig 1. Photograph of chiggers collected from small mammals.
(A) A Tscherskia triton infested by chiggers in the external ear canal (left) and a chigger photographed with digital microscope (right). (B) A chigger was fixed on the microscope slide (left, as indicated by the arrow) and photographed with an Olympus microscope (right).
Table 2. Prevalence of SFTSV in rodents, insectivore and mites.
| Host species | No. of host animals (%) | SFTSV positive rate (%) | No. of mites (%) | No. of mite pools | No. of mite pool positive to SFTSV |
|---|---|---|---|---|---|
| Apodemus agrarius | 12 (38.7) | 4 (33.3) | 107 (19.0) | 8 (2 Laelapidae; 6 Trombiculidae) | 0 |
| Tscherskia triton | 11 (35.5) | 4 (36.4) | 429 (76.1) | 15 (Trombiculidae) | 1 |
| Mus musculus | 6 (19.4) | 2 (33.3) | 9 (1.6) | 0 | 0 |
| Cricetulus griseus | 1 (3.2) | 0 | 10 (1.8) | 1 (Trombiculidae) | 0 |
| Crocidura lasiura | 1 (3.2) | 0 | 9 (1.6) | 1 (Laelapidae) | 0 |
| Total | 31 | 10 (32.3) | 564 | 25 (3 Laelapidae; 22 Trombiculidae) | 1 |
Identification of SFTSV in rodents and mites
The SFTSV M segments were amplified from 10 (32.3%, 10/31) rodents, including 4 Apodemus agrarius, 4 Tscherskia triton, and 2 Mus musculus, but none in shrew. One pool of chiggers (L. deliense, n = 23) from a greater long-tailed hamster (Tscherskia triton) was also positive to SFTSV by the M and L segments.
Phylogenetic analysis
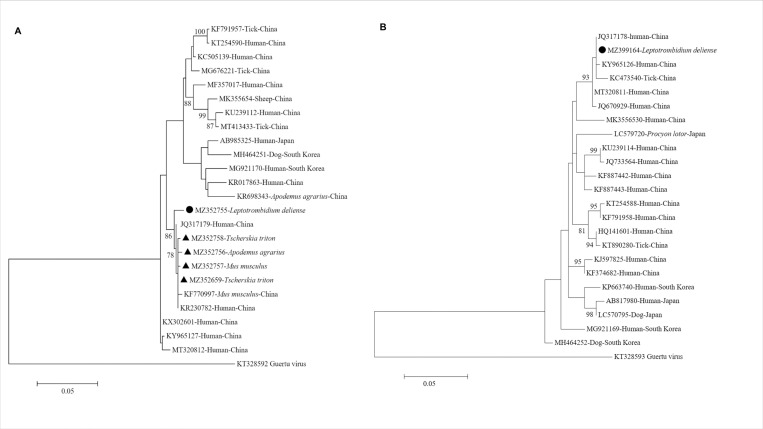
Phylogenetic analysis showed that the partial SFTSV M segment obtained from rodents in this study shared 99.4%-100.0% nucleotide homology with each other, and were closely related to those obtained from a Mus musculus (KF770997) in Shandong Province and a SFTSV patient (KR230782) in Jiangsu Province, China. The partial SFTSV M segment sequence obtained from L. deliense in this study exhibited 98.6%-98.9% nucleotide identity with the SFTSV sequences from rodents, and shared 99.1% nucleotide identity with the sequence from a SFTS patient (JQ317179) in Jiangsu Province, China. Coincidentally, the SFTSV positive chiggers were collected from a long-tailed hamster that was also positive to SFTSV, but the sequences of SFTSV M segment from the chiggers and the hamster were different (98.9% homology), indicating the SFTSV in the chigger was not from the hamster. The M segment obtained from hamster had 99.8% nucleotide identity with SFTS patient (JQ317179) from Jiangsu Province, China (Fig 2A). The partial SFTSV L segment detected in L. deliense shared 100% nucleotide identity to the sequence from a SFTS patient (JQ317178) in Jiangsu Province, China (Fig 2B). Sequences of this study were deposited in GenBank with accession numbers: MZ352755-MZ352765, MZ399164, MZ389783-MZ389787, MZ389209-MZ389211.
Fig 2. Phylogenetic analysis based on the partial nucleotide sequences of SFTSV M and L segments.
(A) M segment (638bp). (B) L segment (327bp). The SFTSV sequences obtained in this study were marked by triangles (from rodents) and a circle (from chiggers). The phylogenetic trees were generated with Maximum Likelihood method by the MEGA 7.0 software (1,000 bootstrap replicates). Bootstrap value >75% was shown at nodes. The Guertu virus, belonging to the genus Phlebovirus of the family Phenuiviridae, was used as outgroup. Scale bars indicated substitutions per site. The reference sequences were named as: GenBank accession number-host-country.
Discussion
In this study, we found that a high prevalence of SFTSV in rodents (32.3%) from rural area in Qingdao City, Shandong Province, China by PCR amplification. The positive rodents included Apodemus agrarius, Tscherskia triton, and Mus musculus. PCR preparation and agarose gel electrophoresis were performed in separate rooms, which could eliminate cross-contamination. Furthermore, the SFTSV sequences from different animals varied, confirmed that the STSV sequences obtained in this study were genuine and were not from contamination. To our knowledge, the positive rate of SFTSV in the rodents was higher than any other animal species investigated previously [38–41], suggesting rodents may be the major animal hosts of SFTSV. In a previous study we demonstrated that rodents were positive to SFTSV at a low positive rate (0.7%) [38]. The sequences of M segment for SFTSV obtained from rodents and chigger in this study also closely related to corresponding sequences from Mus musculus in previous study (KF770997, Fig 2A). Both our previous study and this study have captured rodents in rural areas of Huangdao District of Qingdao City, but at different seasons and years. The rodents in the previous study were captured from January to August in 2013 and the rodents in this study were captured in September, 2020. It was reported that density of field rodents had a peak in the third quarter (July to September) in Qingdao City [42]. The optimum temperature for the development and reproduction of L. deliense was 18–28°C [43], and the average temperature in Qingdao was 22.3°C in September, 2020, according to the Meteorological Bureau of Shandong Province [44]. As the only parasitic stage among its life cycle, chiggers parasitize mainly on rodents and other small mammals [45]. Collectively, high density of rodents and chiggers might contribute to the high prevalence of SFTSV in rodents in this study. The seasonal variation of SFTSV positive rate in rodents need to be further studied.
We also demonstrated that a pool of chiggers (L. deliense) fed on an infected hamster was positive to SFTSV with low infection rate (0.2%). The SFTSV sequence from the chiggers was different from the SFTSV sequence from the hamster host, indicating that the chigger might obtain SFTSV from another animal or vertically. We used PCR to detect the host COI gene to demonstrate whether host DNA existed in the chigger’s digestion system when we extracted chiggers’ DNA. We could not amplify the host COI gene in the extracted chiggers’ nucleic acid, indicating that the host nucleic acid had been degraded in the chigger’s midgut. By analogy, any SFTSV in the chiggers’ meal (liquefied host tissues) should also been degraded in the midgut. Thus, the SFTSV RNA detected in the chiggers must be from SFTSV existing in the chigger body, but not degraded SFTSV RNA in the liquefied host tissue in the meal. This result indicated that SFTSV survived in the body of chiggers. These results suggested that chigger may play a role in transmission of SFTSV among rodents and to humans. In total we analyzed 564 chiggers, with 179 from 10 infected rodents, only one chigger was positive to SFTSV. However, the extreme small body size (0.4 mm) of chigger may limit the amount of SFTSV RNA in a chigger and reduce the sensitivity of PCR for detection SFTSV, which may make us underestimate the positive rate of SFTSV in chigger. In order to detect SFTSV, we pooled chiggers together, which could further reduce the sensitivity of PCR detection of SFTSV in chiggers. Therefore, the role of chigger in transmission of SFTSV needs to be further experimentally verified.
It is worth noting that the prevalence of SFTSV and viral loads in the major vector, Haemaphysalis longicornis, are very limited. The prevalence of SFTSV in H. longicornis in China and South Korea were 0.2% (8/3,300) and 0.5% (55/11,856), respectively [2,46]. It was reported that SFTSV RNA was detected in 6.0% (233/3,898) individual ticks collected from the Deogyusan National Park, including 5.9% (218/3,671) H. longicornis and 7.7% (15/194) H. flava, but none (0/32) in Ixodes nipponensis in the Republic of Korea [47]. Another study in the Republic of Korea reported SFTSV was detected in 4.77% (48/1,006) H. longicornis, 1.15% (5/436) H. flava, and 20% (1/5) in A. testudinarium, also none (0/23) in I. nipponensis, collected from vegetation in five national parks [48]. These studies indicated H. longicornis and H. flava were main vectors for SFTSV. Ticks were not identified on the rodents in this study. A possible explanation is that ticks had abandoned dead hosts and another possibility is that the active season of ticks has passed when the rodents were collected. The peak season of SFTS in Shandong Province is May to August [49].
In this study, we only used M segment for phylogenetic analysis of chiggers and hosts. We also tried to amplified S segment and L segment of SFTSV, but only got 2 positive results for SFTSV L segment of rodents. We got positive result from chiggers with primers from a conserved region of SFTSV L segment. However, the L segment of SFTSV obtained from rodents and chigger were not overlapped, we could not compare the homology between them.
In conclusion, SFTSV RNA was identified in a high proportion of rodents and in a low proportion of chiggers (L. deliense). Our results indicated that rodents may play an important role in transmission of SFTSV and the role of chiggers in transmission of SFTSV need to be further investigated.
Acknowledgments
We are grateful to Qingdao West Coast New District Center for Disease Control and Prevention for organizing the fieldwork to trap small mammals.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was supported by National Natural Science Funds of China [grant number 81971939] (Xue-Jie Yu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32. doi: 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis. 2015;21(10):1770–6. doi: 10.3201/eid2110.150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn JH, Adkins S, Alioto D, Alkhovsky SV, Amarasinghe GK, Anthony SJ, et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch Virol. 2020;165(12):3023–72. doi: 10.1007/s00705-020-04731-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu XL, Qi R, Li WQ, Jiao YJ, Yu H, Yu XJ. Misdiagnosis of scrub typhus as hemorrhagic fever with renal syndrome and potential co-infection of both diseases in patients in Shandong Province, China, 2013–2014. PLoS Negl Trop Dis. 2021;15(3):e0009270. doi: 10.1371/journal.pntd.0009270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi R, Qin XR, Wang L, Han HJ, Cui F, Yu H, et al. Severe fever with thrombocytopenia syndrome can masquerade as hemorrhagic fever with renal syndrome. PLoS Negl Trop Dis. 2019;13(3):e0007308. doi: 10.1371/journal.pntd.0007308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi Y, Kato H, Yamagishi T, Shimada T, Matsui T, Yoshikawa T, et al. Severe Fever with Thrombocytopenia Syndrome, Japan, 2013–2017. Emerg Infect Dis. 2020;26(4):692–9. doi: 10.3201/eid2604.191011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu XJ. Risk factors for death in severe fever with thrombocytopenia syndrome. Lancet Infect Dis. 2018;18(10):1056–7. doi: 10.1016/S1473-3099(18)30312-8 [DOI] [PubMed] [Google Scholar]
- 8.Choi SJ, Park SW, Bae IG, Kim SH, Ryu SY, Kim HA, et al. Severe Fever with Thrombocytopenia Syndrome in South Korea, 2013–2015. PLoS Negl Trop Dis. 2016;10(12):e0005264. doi: 10.1371/journal.pntd.0005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran XC, Yun Y, Van An L, Kim SH, Thao NTP, Man PKC, et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis. 2019;25(5):1029–31. doi: 10.3201/eid2505.181463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun SM, Park SJ, Kim YI, Park SW, Yu MA, Kwon HI, et al. Genetic and pathogenic diversity of severe fever with thrombocytopenia syndrome virus (SFTSV) in South Korea. JCI insight. 2020;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supitcha O, Ruedeerat W, Photchana R. Severe fever with thrombocytopenia syndrome virus: the first case report in Thailand. Bangk Med J. 2020;16(2):204–6. [Google Scholar]
- 12.Win AM, Nguyen YTH, Kim Y, Ha NY, Kang JG, Kim H, et al. Genotypic heterogeneity of Orientia tsutsugamushi in scrub typhus patients and thrombocytopenia syndrome co-infection, Myanmar. Emerg Infect Dis. 2020;26(8):1878–81. doi: 10.3201/eid2608.200135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehand MS, Al-Shorbaji F, Millett P, Murgue B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res. 2018;159:63–7. doi: 10.1016/j.antiviral.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu YY, Zhuang L, Liu K, Sun Y, Dai K, Zhang XA, et al. Role of three tick species in the maintenance and transmission of severe fever with thrombocytopenia syndrome virus. PLoS Negl Trop Dis. 2020;14(6):e0008368. doi: 10.1371/journal.pntd.0008368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12(2):156–60. doi: 10.1089/vbz.2011.0758 [DOI] [PubMed] [Google Scholar]
- 16.Tsuru M, Suzuki T, Murakami T, Matsui K, Maeda Y, Yoshikawa T, et al. Pathological characteristics of a patient with severe fever with thrombocytopenia syndrome (SFTS) infected with SFTS virus through a sick cat’s bite. Viruses. 2021;13(2). doi: 10.3390/v13020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou C-M, Qi R, Qin X-R, Fang L-Z, Han H-J, Lei X-Y, et al. Oral and ocular transmission of severe fever with thrombocytopenia syndrome virus. Infectious Medicine. 2022. 10.1016/j.imj.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, et al. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014;20(8):1358–61. doi: 10.3201/eid2008.131857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh SS, Chae JB, Kang JG, Kim HC, Chong ST, Shin JH, et al. Detection of severe fever with thrombocytopenia syndrome virus from wild animals and ixodidae ticks in the Republic of Korea. Vector Borne Zoonotic Dis. 2016;16(6):408–14. doi: 10.1089/vbz.2015.1848 [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Mekata H, Sudaryatma PE, Kirino Y, Yamamoto S, Ando S, et al. Isolation of severe fever with thrombocytopenia syndrome virus from various tick species in area with human severe fever with thrombocytopenia syndrome cases. Vector Borne Zoonotic Dis. 2021;21(5):378–84. doi: 10.1089/vbz.2020.2720 [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Bao C, Hu J, Liu W, Wang X, Zhang L, et al. Ecology of the tick-borne phlebovirus causing severe fever with thrombocytopenia syndrome in an endemic area of China. PLoS Negl Trop Dis. 2016;10(4):e0004574. doi: 10.1371/journal.pntd.0004574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panzer R, Krebs S. Mites, caterpillars and moths. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology: JDDG. 2020;18(8):867–80. [DOI] [PubMed] [Google Scholar]
- 23.Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, Silva-de La Fuente MC, Jiang J, Richards AL, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species. PLoS Negl Trop Dis. 2020;14(1):e0007619. doi: 10.1371/journal.pntd.0007619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohenberger ME, Elston DM. What’s eating you? chiggers. Cutis. 2017;99(6):386–8. [PubMed] [Google Scholar]
- 25.Huang XD, Cheng P, Zhao YQ, Li WJ, Zhao JX, Liu HM, et al. Chigger mite (Acari: Trombiculidae) survey of rodents in Shandong Province, northern China. Korean J Parasitol. 2017;55(5):555–9. doi: 10.3347/kjp.2017.55.5.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitzel T, Dittrich S, López J, Phuklia W, Martinez-Valdebenito C, Velásquez K, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375(10):954–61. doi: 10.1056/NEJMoa1603657 [DOI] [PubMed] [Google Scholar]
- 27.Sungvornyothin S, Kumlert R, Paris DH, Prasartvit A, Sonthayanon P, Apiwathnasorn C, et al. Geometric morphometrics of the scutum for differentiation of trombiculid mites within the genus Walchia (Acariformes: Prostigmata: Trombiculidae), a probable vector of scrub typhus. Ticks Tick Borne Dis. 2019;10(2):495–503. doi: 10.1016/j.ttbdis.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 28.Xiang R, Guo XG. Research advances of Leptotrombidium scutellare in China. Korean J Parasitol. 2021;59(1):1–8. doi: 10.3347/kjp.2021.59.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo JR, Heo ST, Kang JH, Park D, Kim JS, Bae JH, et al. Mixed infection with severe fever with thrombocytopenia syndrome virus and two genotypes of scrub typhus in a patient, South Korea, 2017. Am J Trop Med Hyg. 2018;99(2):287–90. doi: 10.4269/ajtmh.18-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wi YM, Woo HI, Park D, Lee KH, Kang CI, Chung DR, et al. Severe fever with thrombocytopenia syndrome in patients suspected of having scrub typhus. Emerg Infect Dis. 2016;22(11):1992–5. doi: 10.3201/eid2211.160597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thi Hai Yen N, Kim C, Jeong S, Jeon K, Choi H, Ro HJ, et al. Severe fever with thrombocytopenia syndrome virus infection or mixed infection with scrub typhus in South Korea in 2000–2003. Am J Trop Med Hyg. 2019;101(5):1096–9. doi: 10.4269/ajtmh.19-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YC, Qian Q, Soares Magalhaes RJ, Han ZH, Hu WB, Haque U, et al. Spatiotemporal dynamics of scrub typhus transmission in mainland China, 2006–2014. PLoS Negl Trop Dis. 2016;10(8):e0004875. doi: 10.1371/journal.pntd.0004875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Lu L, Wu H, Yang J, Ren J, Liu Q. The changing epidemiological characteristics of severe fever with thrombocytopenia syndrome in China, 2011–2016. Sci Rep. 2017;7(1):9236. doi: 10.1038/s41598-017-08042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang QK, Ge HM, Li ZF, Shan YF, Cui L, Wang YP. Vector research of severe fever with thrombocytopenia syndrome virus in gamasid mites and chigger mites [in Chinese]. Chinese Journal of Vector Biology and Control. 2012;23(005):452–4. [Google Scholar]
- 35.Xiangguang Y. Atlas of common medical ticks and mites. 2020. [Google Scholar]
- 36.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–9. [PubMed] [Google Scholar]
- 37.Liu WJ, Xu GY, Xiao FZ, Lin DH, Liu J, Han TW. Application of DNA barcoding in identifying rodent species in Fujian province, China [in Chinese]. Chinese Journal of Vector Biology and Control. 2020;31(2):175–9. [Google Scholar]
- 38.Liu JW, Wen HL, Fang LZ, Zhang ZT, He ST, Xue ZF, et al. Prevalence of SFTSV among Asian house shrews and rodents, China, January-August 2013. Emerg Infect Dis. 2014;20(12):2126–8. doi: 10.3201/eid2012.141013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang JG, Cho YK, Han SW, Jeon K, Choi H, Kim JH, et al. Molecular and serological investigation of severe fever with thrombocytopenia syndrome virus in cats. Vector Borne Zoonotic Dis. 2020;20(12):916–20. doi: 10.1089/vbz.2020.2649 [DOI] [PubMed] [Google Scholar]
- 40.Kang JG, Oh SS, Jo YS, Chae JB, Cho YK, Chae JS. Molecular detection of severe fever with thrombocytopenia syndrome virus in Korean domesticated pigs. Vector Borne Zoonotic Dis. 2018;18(8):450–2. doi: 10.1089/vbz.2018.2310 [DOI] [PubMed] [Google Scholar]
- 41.Rim JM, Han SW, Cho YK, Kang JG, Choi KS, Jeong H, et al. Survey of severe fever with thrombocytopenia syndrome virus in wild boar in the Republic of Korea. Ticks Tick Borne Dis. 2021;12(6):101813. doi: 10.1016/j.ttbdis.2021.101813 [DOI] [PubMed] [Google Scholar]
- 42.Jiang F, Wang L, Wang S, Zhu L, Dong L, Zhang Z, et al. Meteorological factors affect the epidemiology of hemorrhagic fever with renal syndrome via altering the breeding and hantavirus-carrying states of rodents and mites: a 9 years’ longitudinal study. Emerg Microbes Infect. 2017;6(11):e104. doi: 10.1038/emi.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv Y, Guo XG, Jin DC. Research Progress on Leptotrombidium deliense. Korean J Parasitol. 2018;56(4):313–24. doi: 10.3347/kjp.2018.56.4.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meteorological Bureau of Shandong Province. Climate impact assessment of Shandong Province in September, 2020 [Internet]. Available from: http://sd.cma.gov.cn/xxgk_3518/sjtj/202010/t20201009_2243918.html.
- 45.Peng PY, Guo XG, Ren TG, Dong WG, Song WY. An updated distribution and hosts: trombiculid mites (Acari: Trombidiformes) associated with small mammals in Yunnan Province, southwest China. Parasitol Res. 2016;115(5):1923–38. doi: 10.1007/s00436-016-4934-4 [DOI] [PubMed] [Google Scholar]
- 46.Park SW, Song BG, Shin EH, Yun SM, Han MG, Park MY, et al. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick Borne Dis. 2014. Oct;5(6):975–7. doi: 10.1016/j.ttbdis.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 47.Kang JG, Cho YK, Jo YS, Han SW, Chae JB, Park JE, et al. Severe fever with thrombocytopenia syndrome virus in ticks in the Republic of Korea. Korean J Parasitol. 2022. Feb;60(1):65–71. doi: 10.3347/kjp.2022.60.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jo YS, Kang JG, Chae JB, Cho YK, Shin JH, Jheong WH, et al. Prevalence of severe fever with thrombocytopenia syndrome virus in ticks collected from national parks in Korea. Vector Borne Zoonotic Dis. 2019. Apr;19(4):284–9. doi: 10.1089/vbz.2018.2338 [DOI] [PubMed] [Google Scholar]
- 49.Pang B, Ding SJ, Zhang XM, Jiang XL, Wang XJ, Bi ZQ. Analysis on epidemiological features of severe fever with thrombocytopenia syndrome, Shandong province, 2013–2015 [in Chinese]. Preventive Medicine Tribune. 2017, 23(2):4 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.