Keywords: fluid homeostasis, fluid intake, loop diuretic, sodium-glucose cotransporter 2 inhibition, vasopressin
Abstract
Loop diuretics are commonly used diuretics in the treatment of fluid retention but induce hypovolemia-related renal dysfunction. Na+-glucose cotransporter 2 (SGLT2) inhibitors induce osmotic diuresis, but body fluid volume is maintained by stimulating vasopressin-induced fluid intake and collecting duct water reabsorption as previously reported in diabetic rats. We aimed to test the hypothesis that unlike SGLT2 inhibitors, loop diuretics lack activation of similar fluid homeostatic mechanisms. Nondiabetic male Sprague-Dawley rats were treated daily by oral gavage with vehicle, the SGLT2 inhibitor ipragliflozin (5 mg/kg), or the loop diuretic furosemide (50 mg/kg) and monitored in metabolic cages for 2 or 7 days. Ipragliflozin and furosemide similarly increased urine volume on day 2. This was associated with increased serum Na+ concentration, urine vasopressin excretion, fluid intake, and solute-free water reabsorption in response to ipragliflozin but not to furosemide. Ipragliflozin maintained fluid balance (fluid intake – urine volume) on day 2 and total body water measured by bioimpedance spectroscopy and serum creatinine on day 7. In comparison, furosemide decreased fluid balance on day 2 and decreased total body water and increased serum creatinine on day 7. Furosemide, but not ipragliflozin, increased plasma renin activity, and systolic blood pressure was similar among the groups. In conclusion, the osmotic diuresis of the SGLT2 inhibitor increased serum Na+ concentration and the vasopressin-related stimulation of fluid intake and renal water retention maintained fluid balance, whereas the loop diuretic did not engage the compensatory vasopressin system. The data suggest differences in vasopressin and fluid homeostatic responses between SGLT2 inhibitors and loop diuretics.
NEW & NOTEWORTHY In nondiabetic rats, the Na+-glucose cotransporter 2 (SGLT2) inhibitor ipragliflozin increased vasopressin-related stimulation of fluid intake and free water reabsorption and maintained fluid balance and serum creatinine, whereas the loop diuretic furosemide reduced vasopressin and induced a negative fluid balance followed by a subsequent increase in serum creatinine. This study suggests that differences in vasopressin secretion in response to a SGLT2 inhibitor or loop diuretic may contribute to differences in body fluid status and subsequent renal function.
INTRODUCTION
Diuretics act on the tubules of kidneys to increase urine output. Loop diuretics such as furosemide (Furo), which inhibit Na+-K+-2Cl− cotransporter in the loop of Henle, are the most commonly used diuretics in the treatment of fluid retention such as congestive heart failure, renal dysfunction, and ascites (1). Loop diuretics exhibit fast and strong diuretic action, but they do not improve long-term cardiorenal outcomes and are associated with increased mortality, hospitalization of heart failure and renal dysfunction in a dose-dependent manner (2–5). Intravascular hypovolemia and the activation of the renin-angiotensin system and sympathetic nervous system have been implicated in the unfavorable outcomes of loop diuretics (1, 2, 6).
Na+-glucose cotransporter 2 (SGLT2) inhibitors are antihyperglycemic agents that suppress glucose reabsorption by inhibiting SGLT2 in the early proximal tubule (7). SGLT2 inhibitors induce mild natriuresis and glucose-induced osmotic diuresis (7–9). In accordance, treatment of patients with diabetic kidney disease for 1 wk with the SGLT2 inhibitor dapagliflozin increased urinary Na+, glucose, and fluid excretion and ameliorated extracellular fluid retention (10, 11). Moreover, compared with the loop diuretic Furo, SGLT2 inhibitors have a low tendency for hypovolemia (8, 9, 12). In this regard, treatment of patients with diabetic kidney disease with dapagliflozin for 1 wk did not decrease extracellular volume in patients without fluid retention (12). This is consistent with animal studies in which long-term administration (8 wk) of the SGLT2 inhibitor ipragliflozin (Ipra) in nondiabetic Sprague-Dawley (SD) rats (8) and type 2 diabetic Goto–Kakizaki rats (9) did not decrease body fluid volume despite an increase in urine Na+, glucose, and fluid excretion. This unique response to the SGLT2 inhibitor was due to 1) a compensatory increase in fluid and food intake (8, 9) and 2) suppression of excessive urine volume loss by vasopressin-induced solute-free water reabsorption in the collecting duct (9). Thus, chronic SGLT2 inhibition can ameliorate extracellular fluid retention while activating mechanisms to prevent hypovolemia.
Several unresolved critical issues remain. First, does short-term SGLT2 inhibition likewise maintain body fluid volume by increasing vasopressin-induced water reabsorption and fluid intake? Second, how is the short-term response on fluid homeostasis different to a loop diuretic? The clinical use of SGLT2 inhibitors has recently been expanded to nondiabetic chronic kidney disease and heart failure (13–15), and comparative studies on effects of SGLT2 inhibitors and loop diuretics on fluid homeostasis in nondiabetic states are needed. We therefore compared the short-term effects of a loop diuretic with a SGLT2 inhibitor on vasopressin secretion and body fluid status in nondiabetic rats.
MATERIALS AND METHODS
Experimental Animals
The protocol of this study was approved by the Jichi Medical University Animal Ethics Committee (Approval No. 17187-01). Nondiabetic male SD rats from 5 to 9 wk of age were purchased from the CLEA Japan (Tokyo, Japan). Because the relative expression of tubular transporters including SGLT2 is different between male and female rats (16, 17), only male rats were used to reduce potential data variation. SD rats were housed at a 12:12-h light-dark cycle in normal cages with free access to fluid and food (0.33% Na+, 1.12% K+, 4.60% fat, CLEA Rodent Diet CE2, CLEA Japan). SD rats older than 10 wk of age (average: 19.7 ± 0.7 wk of age) were acclimatized to metabolic cages for 5 days with free access to fluid and food (CLEA Rodent Diet CE2). Thereafter, rats in the metabolic cages were randomly divided to vehicle (Veh), SGLT2 inhibitor Ipra (Astellas Pharma, Tokyo, Japan), or loop diuretic Furo (LKT Laboratories, St. Paul, MN) treatment with free access to fluid and food (CLEA Rodent Diet CE2). Ipra (5 mg/kg) (18, 19) and Furo (50 mg/kg) (20, 21) were suspended in 0.5% methylcellulose and given by gavage. Methylcellulose (0.5%) was given by gavage in the Veh-treated group at 10 AM. Body weight and food and fluid intake were measured, and 24-h urine volume was collected at 10 AM for 1, 2, or 7 consecutive days. Blood pressure was measured by a tail-cuff method (Softron BP-98A, Softron, Tokyo, Japan) at 2 days of treatment, as previously described (8, 9). After completion of the metabolic cage experiments, some of the rats were used for blood pressure measurements. Bioimpedance spectroscopy (BIS) using the ImpediVet BIS1 system (ImpediMed, San Diego, CA) was performed to measure body fluid volume at 7 days of treatment, as previously described (8, 9, 22). The number of rats for each category was as follows: n = 7–20 per group for serum creatinine and total body water, n = 23–30 per group for body weight, food and fluid intake, urine volume, and fluid balance, n = 4–16 per group for urine and serum parameters, and n = 5–11 per group for the change in plasma volume and systolic blood pressure. Because body weight, food and fluid intake, urine volume, and fluid balance are fundamental parameters of diuretic agents, a larger number of rats was used.
Blood and Urine Analysis
Blood was collected under short-term isoflurane anesthesia by penetrating the retroorbital plexus with a sterile hematocrit capillary tube. Blood samples were taken each day (days 0, 2, and 7) from the same animals for the measurement of serum creatinine and blood urea nitrogen (BUN). The measurement of serum and plasma and urine parameters, including urinary vasopressin concentrations and plasma renin activity, was entrusted to the SRL laboratory (Hachioji, Tokyo, Japan), as previously described (8, 9). Osmolar clearance and solute-free water reabsorption were calculated by the following formulas: osmolar clearance = urine osmolality × urine volume/serum osmolality and solute-free water reabsorption = osmolar clearance – urine volume (9). The change in serum Na+ + Cl− was calculated with the following formula: [(serum Na+ + Cl−)d2 – (serum Na+ + Cl−)d0]/(serum Na+ + Cl−)d0 × 100, where d0 is day 0 and d2 is day 2. Hemoglobin and hematocrit were measured with a hematology analyzer (MEK-6558 Celltac α, Nihon Kohden, Tokyo, Japan). Changes in plasma volume were calculated by the Strauss formula (23) = Hbd0/Hbd2 × [(100 − Htd2)/(100 − Htd0) − 1] × 100, where Hb is hemoglobin and Ht is hematocrit. The values of the Strauss formula are used as a proxy to assess traditional human and rodent plasma volume measurements with 125I-labeled human serum albumin (24, 25).
Statistical Analysis
Data are expressed as means ± SE. One-way ANOVA was performed to identify statistically significant differences between three groups, whereas a post hoc Tukey honestly significant difference test or Student’s t test was used to examine differences between pairs of groups. Changes in body weight and data for serum creatinine and BUN, which were determined on multiple days using the same rats, were analyzed by repeated-measure ANOVA followed by a Tukey honestly significant difference test. A matched-pairs t test was performed to compare the values of serum creatinine and BUN between day 7 and day 0 in the Furo-treated group. Correlations among some parameters were analyzed using Spearman’s correlation. P values of <0.05 were considered to be statistically significant.
RESULTS
The Loop Diuretic Furo, but Not the SGLT2 Inhibitor Ipra, Decreased Body Fluid Volume, and Increased Serum Creatinine over 7 Days
In nondiabetic SD rats, body weight was largely maintained in Veh- and Ipra-treated groups but fell gradually in the Furo-treated group over the 7 days of treatment (Fig. 1A). On day 2, serum creatinine and BUN were similar among the groups (Fig. 1, B and C). On day 7, serum creatinine and BUN were significantly higher and total body water (measured by BIS) was significantly lower in response to Furo, but these were unaffected by Ipra versus Veh (Fig. 1, B and C). P values of serum creatinine and BUN between day 7 and day 0 in the Furo-treated group were 0.041 and 0.006, respectively. There were no significant differences in urinary protein among the groups on day 2 (Table 1) and day 7 (Veh: 2.1 ± 0.5, Ipra: 3.0 ± 0.5, and Furo: 1.7 ± 0.6 mg/day, ANOVA P = 0.265).
Figure 1.
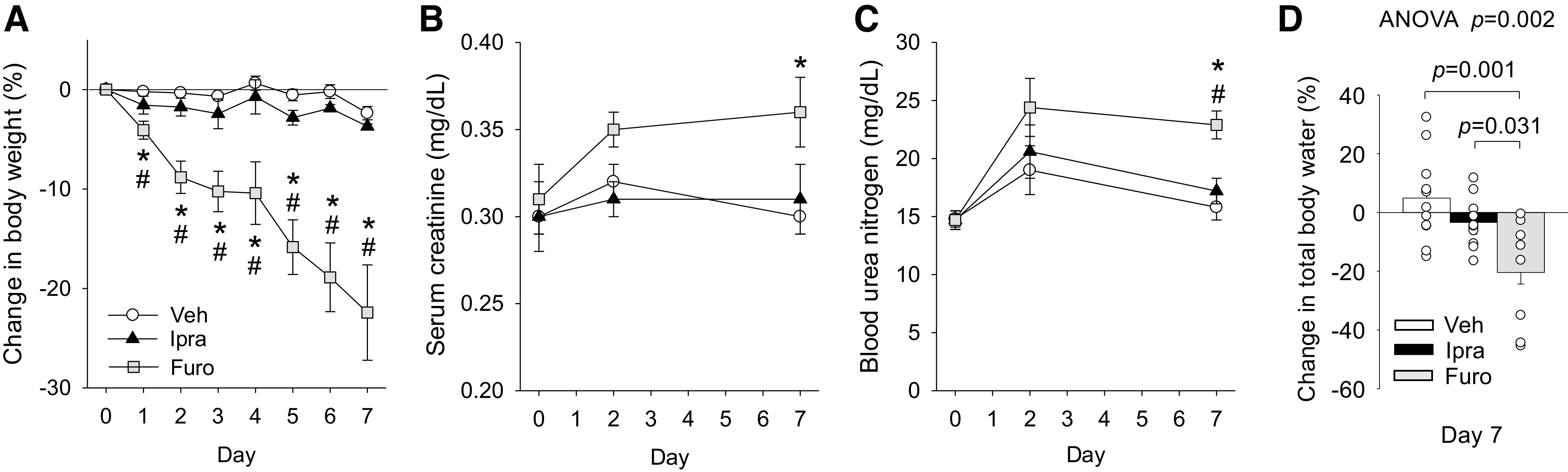
Effect of ipragliflozin (Ipra), furosemide (Furo), or vehicle (Veh) administered for 7 days in nondiabetic Sprague–Dawley rats on change in body weight (A), serum creatinine (B), blood urea nitrogen (C), and change in total body water (D). Veh: 0.5% methylcellulose solution, Ipra: 5 mg/kg body wt in 0.5% mehylcellulose, Furo: 50 mg/kg body wt in 0.5% methylcellulose. Repeated-measures or one-way ANOVA followed by a post hoc Tukey honestly significant difference test were used for statistical analysis. *P < 0.05 vs. Veh; #P < 0.05 vs. Ipra. Values are expressed as means ± SE; n = 5–12/group.
Table 1.
Urine and blood parameters after ipragliflozin and furosemide treatment on day 2
| Vehicle | Ipragliflozin | Furosemide | ANOVA P Value |
Post Hoc Analysis P Value |
|||
|---|---|---|---|---|---|---|---|
| Vehicle vs. Ipragliflozin | Vehicle vs. Furosemide | Ipragliflozin vs. Furosemide | |||||
| Urinary protein, mg/day | 2.1 ± 0.3 | 3.2 ± 0.4 | 2.9 ± 0.4 | 0.125 | 0.122 | 0.378 | 0.839 |
| Food intake, g/day/100 g body wt | 4.3 ± 0.3 | 4.5 ± 0.3 | 2.3 ± 0.3 | <0.001 | 0.881 | <0.001 | <0.001 |
| Fluid intake, mL/day/100 g body wt | 7.6 ± 0.7 | 10.3 ± 0.7 | 8.5 ± 0.8 | 0.020 | 0.017 | 0.655 | 0.209 |
| Urine volume, mL/day/100 g body wt | 2.7 ± 0.4 | 5.0 ± 0.4 | 4.7 ± 0.4 | <0.001 | <0.001 | 0.002 | 0.897 |
| Fluid balance, mL/day/100 g body wt* | 4.6 ± 0.5 | 5.1 ± 0.5 | 3.5 ± 0.6 | 0.072 | 0.712 | 0.313 | 0.057 |
| Serum osmolality, mosmol/kgH2O | 297.9 ± 2.7 | 297.0 ± 3.4 | 296.5 ± 2.7 | 0.930 | 0.972 | 0.925 | 0.988 |
| Urinary osmolality, mosmol/kgH2O | 2712 ± 287 | 2077 ± 275 | 1387 ± 287 | 0.010 | 0.262 | 0.007 | 0.208 |
| Osmolar clearance, mL/day/100 g body wt | 13.2 ± 2.1 | 24.0 ± 2.0 | 11.5 ± 2.3 | <0.001 | <0.001 | 0.723 | <0.001 |
| Serum Na+, mEq/L | 141 ± 1 | 143 ± 1 | 140 ± 1 | 0.036 | 0.133 | 0.767 | 0.039 |
| Urinary Na+, mEq/L | 170 ± 21 | 62 ± 20 | 51 ± 21 | <0.001 | 0.002 | <0.001 | 0.917 |
| Urinary Na+, mEq/day/100 g body wt | 0.22 ± 0.03 | 0.19 ± 0.03 | 0.13 ± 0.03 | 0.097 | 0.775 | 0.088 | 0.299 |
| Fractional excretion of Na+, % | 0.35 ± 0.11 | 0.38 ± 0.11 | 0.23 ± 0.11 | 0.597 | 0.982 | 0.717 | 0.605 |
| Serum Cl−, mEq/L | 101 ± 1 | 103 ± 1 | 96 ± 1 | <0.001 | 0.636 | 0.005 | <0.001 |
| Urinary Cl−, mEq/L | 253 ± 24 | 110 ± 23 | 96 ± 24 | <0.001 | <0.001 | <0.001 | 0.913 |
| Urinary Cl−, mEq/day/100 g body wt | 0.37 ± 0.06 | 0.33 ± 0.06 | 0.26 ± 0.06 | 0.413 | 0.860 | 0.386 | 0.681 |
| Fractional excretion of Cl−, % | 0.69 ± 0.15 | 0.45 ± 0.17 | 0.70 ± 0.16 | 0.458 | 0.511 | 0.999 | 0.519 |
| Serum Na+ + Cl−, mEq/L | 241 ± 2 | 246 ± 2 | 236 ± 2 | 0.009 | 0.219 | 0.221 | 0.006 |
| Serum glucose, mg/dL | 140 ± 9 | 111 ± 9 | 145 ± 9 | 0.022 | 0.075 | 0.910 | 0.026 |
| Urinary glucose, mg/dL | 58 ± 543 | 9705 ± 543 | 10 ± 543 | <0.001 | <0.001 | 0.998 | <0.001 |
| Urinary glucose, g/day/100 g body wt | 0.003 ± 0.148 | 1.727 ± 0.186 | 0.001 ± 0.186 | <0.001 | <0.001 | 1.000 | <0.001 |
| Fractional excretion of glucose, % | 0.04 ± 1.79 | 38.0 ± 2.7 | 0.02 ± 1.79 | <0.001 | <0.001 | 1.000 | <0.001 |
| Urinary urea nitrogen, mg/dL | 3548 ± 570 | 1685 ± 546 | 1326 ± 570 | 0.021 | 0.062 | 0.026 | 0.893 |
| Urinary urea nitrogen, mg/day/100 g body wt | 33.8 ± 6.2 | 42.6 ± 4.7 | 27.1 ± 4.9 | 0.094 | 0.507 | 0.677 | 0.079 |
| Fractional excretion of urea, % | 42.9 ± 4.4 | 36.8 ± 4.7 | 24.8 ± 4.4 | 0.024 | 0.615 | 0.020 | 0.168 |
| Urinary creatinine, mg/dL | 250 ± 51 | 111 ± 49 | 102 ± 51 | 0.083 | 0.133 | 0.115 | 0.992 |
| Urinary creatinine, mg/day/100 g body wt | 1.9 ± 0.2 | 2.4 ± 0.2 | 1.8 ± 0.2 | 0.200 | 0.334 | 0.951 | 0.209 |
Values are means ± SE; n = 4–30/group. *Fluid balance = fluid intake – urine volume. One‐way ANOVA followed by post hoc Tukey’s honestly significant difference test was used for statistical analysis. Bold font indicates statistical significance.
Because reduced renal function affects tubular transport and function (26), we examined in more detail an early time point (2-day treatment) when serum creatinine was still similar among the groups (Fig. 1B).
The Diuretic Action of Furo, but Not of Ipra, Was Associated with Negative Fluid Balance
Baseline food intake (Veh: 3.8 ± 0.3 g/day/100 g body wt, Ipra: 3.9 ± 0.4 g/day/100 g body wt, and Furo: 3.7 ± 0.3 g/day/100 g body wt, ANOVA P = 0.925), fluid intake (Veh: 7.6 ± 0.7 mL/day/100 g body wt, Ipra: 8.4 ± 0.6 mL/day/100 g body wt, and Furo: 7.8 ± 0.6 mL/day/100 g body wt, ANOVA P = 0.586), and urine volume (Veh: 3.0 ± 0.5 mL/day/100 g body wt, Ipra: 3.1 ± 0.4 mL/day/100 g body wt, and Fura: 2.9 ± 0.4 mL/day/100 g body, ANOVA P = 0.929) were similar among the groups. On day 2 and as expected, Ipra, but not Furo, increased absolute and fractional urinary glucose excretion (Table 1). Ipra and Furo similarly increased urinary fluid excretion versus Veh (Fig. 2C and Table 1). At the same time, Ipra, but not Furo, increased fluid intake (Fig. 2B and Table 1). As a consequence, fluid balance (fluid intake – urine volume) became negative in response to Furo but was not affected by Ipra (Fig. 2D). Furo, but not Ipra, reduced food intake (Fig. 2A and Table 1), and neither increased absolute or fractional urinary Na+ or Cl− excretion versus Veh (Table 1).
Figure 2.
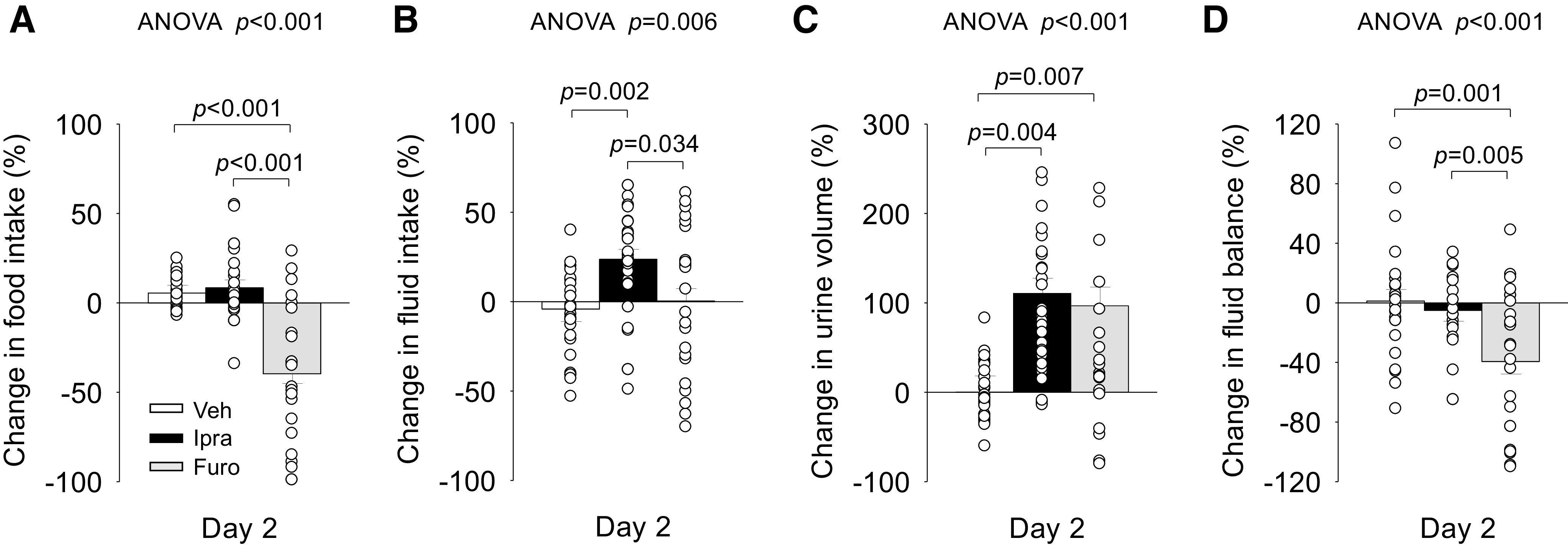
Effect of ipragliflozin (Ipra), furosemide (Furo), or vehicle (Veh) on changes in food intake (A), fluid intake (B), urine volume (C), and fluid balance (D) on day 2 in nondiabetic Sprague-Dawley rats. Drug doses are as in Fig. 1. One-way ANOVA followed by a post hoc Tukey honestly significant difference test or Student’s t test were used for statistical analysis. Values are expressed as means ± SE; n = 23–30/group.
Ipra, but Not Furo, Increased Urine Vasopressin and Renal Solute-Free Water Reabsorption
Ipra significantly increased urine vasopressin excretion and solute-free water reabsorption, whereas Furo did not (Fig. 3, A and B). Notably, Furo even significantly reduced urine vasopressin excretion (Fig. 3A). Lesser vasopressin stimulation in response to Furo may have limited the upregulation of solute-free water reabsorption in the kidney (Fig. 3B).
Figure 3.
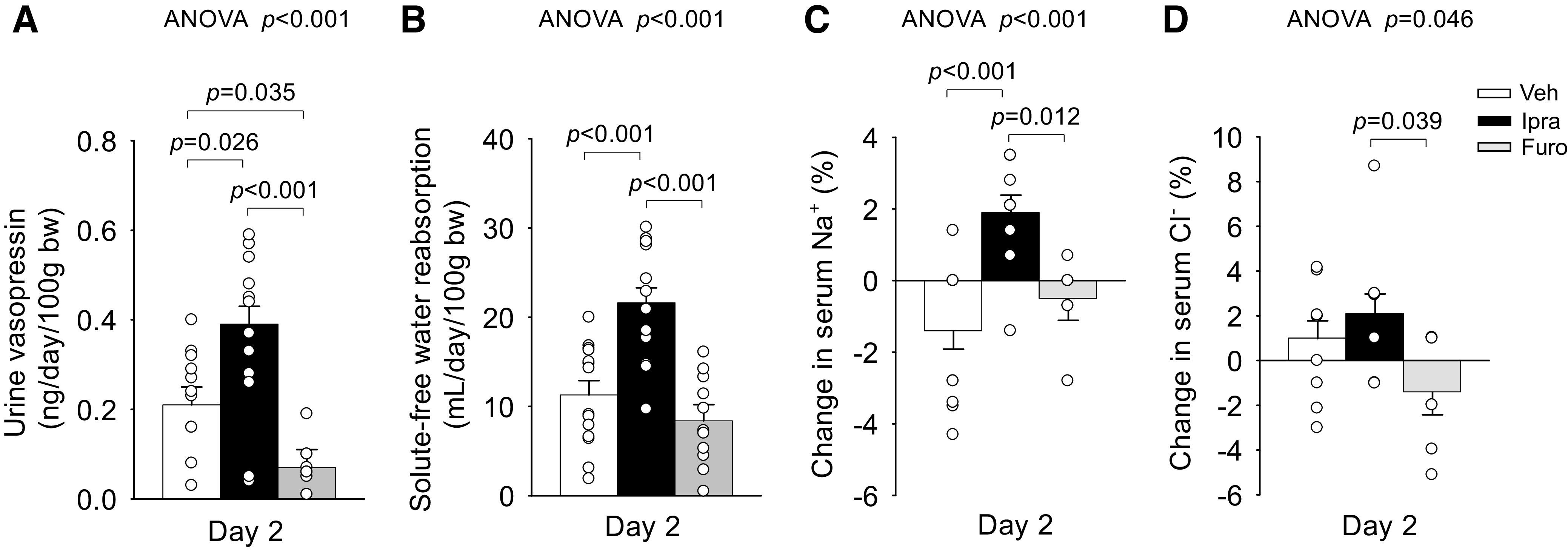
Effect of ipragliflozin (Ipra), furosemide (Furo), or vehicle (Veh) on urine vasopressin excretion (A), solute-free water reabsorption (B), change in serum Na+ (C), and change in serum Cl− (D) on day 2 in nondiabetic Sprague-Dawley rats. Drug doses are as in Fig. 1. One-way ANOVA followed by a post hoc Tukey honestly significant difference test were used for statistical analysis. Values are expressed as means ± SE; n = 7–15/group. bw, body weight.
Ipra, but Not Furo, Induced Positive Changes of Serum Na+ and Cl− Concentrations, Which Correlated With Urine Vasopressin Excretion
On day 2, serum osmolality was similar among the groups (Table 1). Serum glucose and BUN, both of which are minor determinants of serum osmolality (27, 28), were different among the groups (Table 1). Consistent with the glucosuric effect, serum glucose levels in the Ipra-treated group were numerically lower versus the Veh-treated group and significantly lower versus the Furo-treated group (Table 1). BUN levels, an indicator of volume depletion, were increased in response to Furo but not Ipra (Table 1).
Serum concentrations of Na+ and Cl−, which are the major determinants of serum osmolality and vasopressin release (29, 30), were different among the groups (Fig. 3, C and D, and Table 1). Ipra, but not Furo, induced positive changes in serum Na+ concentration versus Veh (Fig. 3C). This was associated with lesser serum Cl− concentration (Table 1) and more negative changes in serum Cl− concentration in response to Furo versus Ipra and Veh (Fig. 3D). The concentration of serum Na+ + Cl− tended to be higher with Ipra versus Veh and was significantly lower in response to Furo versus Ipra (Table 1).
Changes in serum Na+ (r = 0.306, P = 0.031), serum Cl− (r = 0.435, P = 0.002), and serum Na+ + Cl− (r = 0.497, P < 0.001) were positively correlated with changes in urine vasopressin when all rats were pooled on days 1 and 2 (Fig. 4, A−C). On the other hand, the change in serum osmolality was not significantly correlated with the change in urine vasopressin (r = 0.048, P = 0.809; Fig. 4D).
Figure 4.
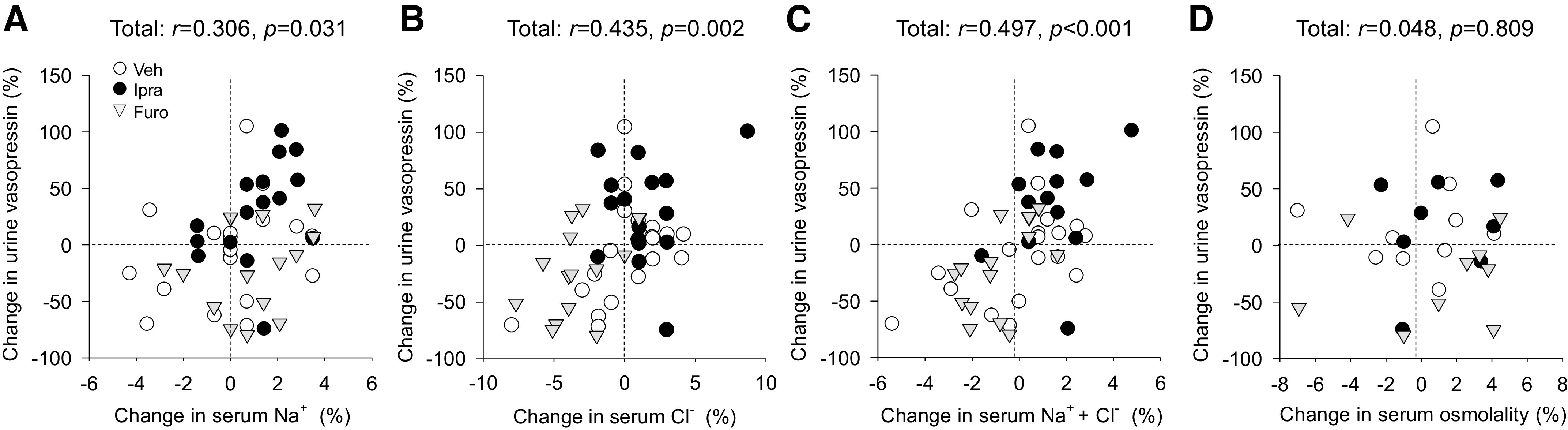
Relationship between changes in serum concentrations of Na+ (A), Cl− (B), Na+ + Cl− (C), and serum osmolality (D) and changes in urine vasopressin excretion on day 2 when the three groups of rats were pooled. Abbreviations and drug doses are as in Fig. 1. Correlations were analyzed using Spearman’s correlation. n = 8–19/group for days 1 or 2.
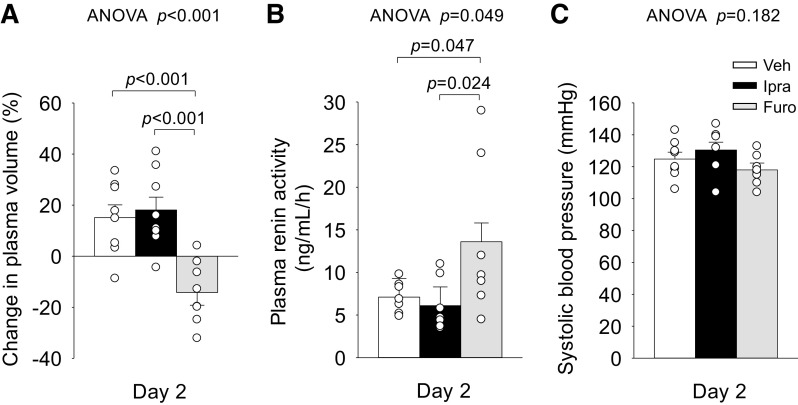
Furo, but Not Ipra, Reduced Plasma Volume
The change in plasma volume on day 2 was more negative in response to Furo versus Veh and not affected by Ipra (Fig. 5A). This was associated with higher plasma renin activity in response to Furo versus Veh and Ipra (Fig. 5B), probably to compensate for the reduction in plasma volume and to preserve systolic blood pressure (Fig. 5C).
Figure 5.
Effect of ipragliflozin (Ipra), furosemide (Furo), or vehicle (Veh) on change in plasma volume (A), plasma renin activity (B), and systolic blood pressure (C) on day 2 in nondiabetic Sprague–Dawley rats. Drug doses are as in Fig. 1. One-way ANOVA followed by a post hoc Tukey honestly significant difference test were used for statistical analysis. Values are expressed as means ± SE; n = 6–11/group.
DISCUSSION
The present study in nondiabetic rats shows that fluid balance and serum creatinine were maintained in response to the SGLT2 inhibitor Ipra, whereas the loop diuretic Furo induced a negative fluid balance evident after 2 days and increased serum creatinine after 7 days. Ipra and Furo induced a similar increase in urine volume after 2 days, associated with preserved renal function. However, by this time, Furo already had induced a negative fluid balance and reduced plasma volume associated with a lack of compensatory increases in vasopressin secretion, solute-free water reabsorption, and fluid intake, which likely contributed to reduced body fluid volume and potentially reduced renal function.
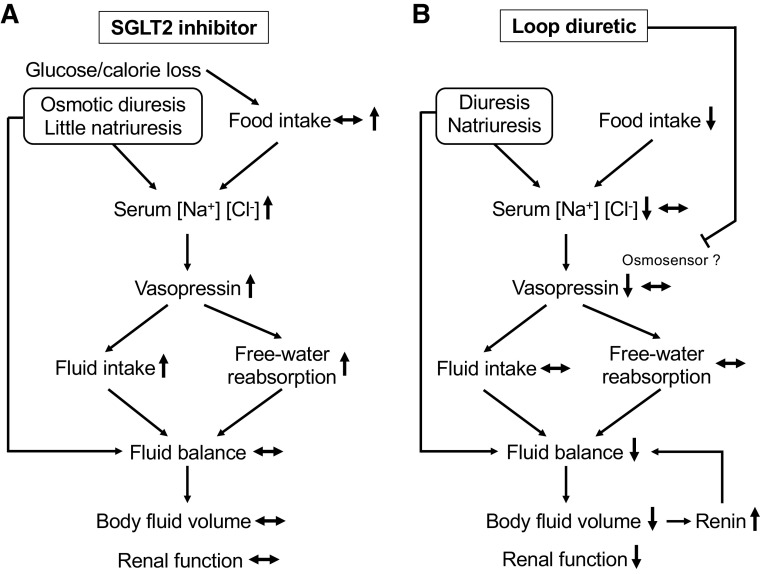
In comparison, the diuretic action of short-term SGLT2 inhibition with Ipra enhanced urine vasopressin excretion and renal water reabsorption and fluid intake, i.e., effects that can maintain body fluid volume and thereby potentially renal function (Fig. 6). These results are consistent with our previous study of long-term SGLT2 inhibition in type 2 diabetic rats (9). Consequently, SGLT2 inhibitor may stimulate vasopressin-induced water reabsorption to maintain body fluid volume, regardless of the duration of administration and the presence or absence of diabetes. The fluid homeostatic mechanism of SGLT2 inhibition shown in our previous study (9) and the present study may reduce their risk for hypovolemia in both patients with and without diabetes.
Figure 6.
Proposed effects of Na+-glucose cotransporter 2 (SGLT2) inhibitor and loop diuretic on vasopressin and fluid homeostatic responses. A: the increase in serum Na+ and Cl− concentrations mainly due to osmotic diuresis by SGLT2 inhibitor induces vasopressin release, which stimulates fluid intake and free water reabsorption. These compensatory mechanisms in response to a diuretic action of SGLT2 inhibitor maintains fluid balance (fluid intake – urine volume), body fluid volume, and renal function. B: the decrease in serum Na+ and Cl− concentrations due to natriuresis and the reduction of food intake by furosemide attenuated vasopressin release, which fails to stimulate fluid intake and free water reabsorption. As a result, loop diuretic induces a negative fluid balance and the reduction in body fluid volume and potentially reduces renal function.
In the present study, the loop diuretic Furo even decreased urine vasopressin excretion, which is expected to lower renal free water retention, and in the face of its diuretic action facilitated a negative fluid balance and reduce plasma volume, body fluid volume, and, subsequently, renal function (Fig. 6). Similar to the present study, several rodent and human studies have reported that vasopressin secretion was not increased by Furo despite substantial fluid loss. In nondiabetic SD rats, repeated subcutaneous injections of Furo over 32 h reduced body weight and increased hematocrit, but plasma vasopressin was similar to the Veh control (31). A study in healthy adults concluded that the loop diuretics Furo and piretanide in doses that induced robust diuresis have little effect on plasma vasopressin and osmolality but reduced blood volume (7%) when monitored over a period of 6 h (32). Similarly, in healthy subjects, intravenous administration of Furo reduced free water reabsorption (negative value of free water clearance) and extracellular water at 2 h compared with placebo and did not change plasma vasopressin (33). Taken together, the previous and present studies show that the natriuretic and diuretic effect of furosemide is not a robust stimulus to increase vasopressin secretion, which is expected to blunt a compensatory increase in fluid intake and free water reabsorption (also due to reduced medullary tonicity) and attenuate the ability to maintain euvolemia.
Why is the effect on vasopressin secretion different in response to SGLT2 inhibition and a loop diuretic? Ipra slightly increased serum Na+ + Cl− concentrations (more pronounced for the increase in serum Na+), whereas Furo caused a small reduction (more pronounced for the decrease in serum Cl−). Serum osmolality, a major stimulus of vasopressin release, was not significantly changed by either Ipra or Furo. In Ipra-treated rats, the mild increase in serum concentrations of Na+ and Cl− appeared to be offset by the mild decrease in serum glucose levels, similar to our previous long-term study in type 2 diabetic rats (9). In Furo-treated rats, serum osmolality was maintained by small decreases in serum concentrations of Na+ and Cl− being balanced by small increases in serum glucose and BUN levels. These nuances are functionally important because among the determinants of serum osmolality, Na+ and Cl− are effective osmolytes with regard to vasopressin release, whereas glucose (in the presence of insulin) and urea are noneffective osmolytes (27, 28). In accordance and as shown in Fig. 4, A–C, the changes in serum Na+, Cl−, and Na+ + Cl− concentrations significantly and positively correlated with changes in urine vasopressin excretion across the pooled groups whereas total osmolality did not. These results are consistent with the notion that the increase in serum Na+ and Cl− concentrations by Ipra increased vasopressin release, whereas the decrease in serum Na+ and Cl− concentrations by Furo attenuated vasopressin release (Fig. 6). Similar to the present study, a previous study has shown that serum Na+ and Cl− concentrations were mildly decreased by oral administration of Furo in nondiabetic Wistar rats (34). In nondiabetic SD rats, repeated subcutaneous injections of Furo over 32 h was associated with lower body weight and plasma Na+ concentration, and plasma vasopressin levels were similar to the Veh control (31). Likewise, in healthy subjects, intravenous administration of Furo mildly decreased serum Na+ concentration and did not increase plasma vasopressin (33). In contrast, when healthy subjects were intravenously given Furo and hypertonic saline (3% saline), plasma concentrations of Na+, Cl−, and vasopressin were significantly increased compared with their baseline values (35). Therefore, changes in serum Na+ and Cl− concentrations may contribute to the different vasopressin responses to SGLT2 inhibition and a loop diuretic.
Glucosuria-induced osmotic diuresis can concentrate serum levels of Na+ and Cl− in response to Ipra. In comparison, a decrease in food intake might have contributed to lower serum Na+ and Cl− concentrations in response to Furo. A dose-dependent decrease in food intake has been previously reported with Furo in rodent studies (36, 37). General food avoidance, an increased preference for salty food, and some aversive effects to Furo have been considered as mechanisms for the decrease in food intake (36–38). By inhibiting renal Na+-K+-2Cl− cotransporters, a single dose of Furo application induces an increase in urinary Na+ and Cl− excretion (which can also lower serum concentrations), but this short-term saluretic effect is in part offset by a compensatory antisaluretic effect after the drug wears off such that daily excretion of NaCl was similar between Veh and Furo treatment. Similar Na+ and Cl− excretion despite lesser food intake/NaCl indicated a natriuretic tone of Furo.
Decreases in blood pressure and plasma volume are important and potent nonosmotic stimuli for vasopressin release (21, 39, 40). In the present study, blood pressure was similar among the groups, suggesting little impact of blood pressure on vasopressin release in response to Furo versus Ipra treatments. Furo reduced plasma volume by >10% (average: 14.2%) and increased renin activity but did not increase vasopressin levels. Further studies are needed to test the hypothesis that vasopressin release can be attenuated by direct inhibitory effects of Furo on osmosensors in the brain (41).
An intact homeostatic mechanism to prevent excessive changes in body fluid volume may play an important role in the favorable cardiorenal outcomes of SGLT2 inhibitors, which were shown in large-scale clinical trials (13–15, 42, 43). Limiting the extent of hypovolemia may reduce the incidence of acute kidney injury and suppresses the activation of the renin-angiotensin system and sympathetic nervous system, which can worsen cardiorenal dysfunction (7, 44). In contrast, a stronger acute natriuresis and poor responses in vasopressin secretion may contribute to hypovolemia and the observed unfavorable cardiorenal outcomes of loop diuretics (2–5, 45).
This study includes several limitations and remaining issues. First, a causative relationship between the fluid homeostatic response to SGLT2 inhibitors or loop diuretics and vasopressin secretion was not shown. Second, the results of this study may not be directly applied to humans, because there are several differences between current and human studies, including the administration time of drugs (rest period in this study vs. active period in human studies) and weight changes induced by SGLT2 inhibitors (no change in this study versus reduction in most human studies, due to initial fluid loss and reduction in fat mass with more long-term application) (7, 12). Third, the expression of tubular transporters including the water channel aquaporin 2, which was increased by longer-term treatment of ipragliflozin in type 2 diabetic model (9), could not be assessed in this study. Finally, confirmation of the findings in female rats is needed, because this study used only male rats.
Perspectives and Significance
In nondiabetic rats, the SGLT2 inhibitor Ipra increased vasopressin and maintained fluid balance and serum creatinine, whereas the loop diuretic Furo reduced vasopressin and induced a negative fluid balance followed by a subsequent increase in serum creatinine. Differences in vasopressin secretion in response to a SGLT2 inhibitor or a loop diuretic may contribute to differences in body fluid status and subsequent renal function. Further experiments with central administration of a SGLT2 inhibitor and loop diuretic are required to evaluate potential central effects and mechanisms affecting the thirst and satiety centers and vasopressin secretion.
GRANTS
This work was supported in part by a Jichi Medical University Young Investigator Award (to T.M.), the Salt Science Research Foundation (to T.M.), Grant-in-Aid for Young Scientists 15K21321 (to T.M.), and National Institutes of Health Grants R01DK112042, R01HL142814, R01AG061296, and P30DK079337 (University of Alabama at Birmingham/University of California-San Diego O’Brien Center of Acute Kidney Injury; to V.V.).
DISCLOSURES
Astellas Pharma (Tokyo, Japan) provided the SGLT2 inhibitor ipragliflozin for this study. Over the past 12 mo, V.V. has served as a consultant and received honoraria from Fibrocor and Lexicon and received grant support for investigator-initiated research from Astra-Zeneca, Gilead, Kyowa-Kirin, Novo-Nordisk, and Janssen. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.M. and V.V. conceived and designed research; T.M. performed experiments; T.M. analyzed data; T.M. and V.V. interpreted results of experiments; T.M. prepared figures; T.M. and V.V. drafted manuscript; T.M., K.O., V.V., and D.N. edited and revised manuscript; T.M., K.O., V.V., and D.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge critical comments by Masahide Yoshida (Division of Brain and Neurophysiology, Department of Physiology, Jichi Medical University) and technical support provided by Tomoko Tateno, Minami Watanabe, and Keiko Fukuda (Division of Nephrology, Department of Internal Medicine, Jichi Medical University).
REFERENCES
- 1. Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol 310: F958–F971, 2016. doi: 10.1152/ajprenal.00476.2015. [DOI] [PubMed] [Google Scholar]
- 2. Miura M, Sugimura K, Sakata Y, Miyata S, Tadaki S, Yamauchi T, Onose T, Tsuji K, Abe R, Oikawa T, Kasahara S, Nochioka K, Takahashi J, Shimokawa H; CHART-2 Investigators. Prognostic impact of loop diuretics in patients with chronic heart failure–effects of addition of renin-angiotensin-aldosterone system inhibitors and β-blockers. Circ J 80: 1396–1403, 2016. doi: 10.1253/circj.CJ-16-0216. [DOI] [PubMed] [Google Scholar]
- 3. Kapelios CJ, Bonou M, Malliaras K, Athanasiadi E, Vakrou S, Skouloudi M, Masoura C, Barbetseas J. Association of loop diuretics use and dose with outcomes in outpatients with heart failure: a systematic review and meta-analysis of observational studies involving 96,959 patients. Heart Fail Rev 27: 147–161, 2022. doi: 10.1007/s10741-020-09995-z. [DOI] [PubMed] [Google Scholar]
- 4. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 97: 1759–1764, 2006. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 5. Inomata T, Ikeda Y, Kida K, Shibagaki Y, Sato N, Kumagai Y, Shinagawa H, Ako J, Izumi T; Kanagawa Aquaresis Investigators. Effects of additive tolvaptan vs. increased furosemide on heart failure with diuretic resistance and renal impairment—results from the K-STAR study. Circ J 82: 159–167, 2018. doi: 10.1253/circj.CJ-17-0179. [DOI] [PubMed] [Google Scholar]
- 6. Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 82: 1724–1729, 1990. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 7. Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 83: 503–528, 2021. doi: 10.1146/annurev-physiol-031620-095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masuda T, Watanabe Y, Fukuda K, Watanabe M, Onishi A, Ohara K, Imai T, Koepsell H, Muto S, Vallon V, Nagata D. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Renal Physiol 315: F653–F664, 2018. doi: 10.1152/ajprenal.00143.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masuda T, Muto S, Fukuda K, Watanabe M, Ohara K, Koepsell H, Vallon V, Nagata D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep 8: e14360, 2020. doi: 10.14814/phy2.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohara K, Masuda T, Murakami T, Imai T, Yoshizawa H, Nakagawa S, Okada M, Miki A, Myoga A, Sugase T, Sekiguchi C, Miyazawa Y, Maeshima A, Akimoto T, Saito O, Muto S, Nagata D. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: a comparison study with furosemide and tolvaptan. Nephrology (Carlton) 24: 904–911, 2019. doi: 10.1111/nep.13552. [DOI] [PubMed] [Google Scholar]
- 11. Masuda T, Ohara K, Murakami T, Imai T, Nakagawa S, Okada M, Miki A, Myoga A, Onishi A, Sekiguchi C, Miyazawa Y, Akimoto T, Saito O, Muto S, Nagata D. Sodium-glucose cotransporter 2 inhibition with dapagliflozin ameliorates extracellular volume expansion in diabetic kidney disease patients. POJ Diabetes Obes 1: 1–8, 2017. [Google Scholar]
- 12. Ohara K, Masuda T, Morinari M, Okada M, Miki A, Nakagawa S, Murakami T, Oka K, Asakura M, Miyazawa Y, Maeshima A, Akimoto T, Saito O, Nagata D. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr 12: 37, 2020. doi: 10.1186/s13098-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjostrom CD, Toto RD, Langkilde AM, Wheeler DC. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 15. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 16. Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Q, McDonough AA, Layton HE, Layton AT. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol 315: F692–F700, 2018. doi: 10.1152/ajprenal.00171.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol 385: 423–436, 2012. doi: 10.1007/s00210-011-0713-z. [DOI] [PubMed] [Google Scholar]
- 19. Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Antidiabetic effects of SGLT2-selective inhibitor ipragliflozin in streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci 120: 36–44, 2012. doi: 10.1254/jphs.12089fp. [DOI] [PubMed] [Google Scholar]
- 20. Hu L, Maslanik T, Zerebeckyj M, Plato CF. Evaluation of bioimpedance spectroscopy for the measurement of body fluid compartment volumes in rats. J Pharmacol Toxicol Methods 65: 75–82, 2012. doi: 10.1016/j.vascn.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 21. Pool AH, Wang T, Stafford DA, Chance RK, Lee S, Ngai J, Oka Y. The cellular basis of distinct thirst modalities. Nature 588: 112–117, 2020. doi: 10.1038/s41586-020-2821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masuda T, Fu Y, Eguchi A, Czogalla J, Rose MA, Kuczkowski A, Gerasimova M, Feldstein AE, Scadeng M, Vallon V. Dipeptidyl peptidase IV inhibitor lowers PPARγ agonist-induced body weight gain by affecting food intake, fat mass, and beige/brown fat but not fluid retention. Am J Physiol Endocrinol Physiol 306: E388–E398, 2014. doi: 10.1152/ajpendo.00124.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest 30: 862–868, 1951. doi: 10.1172/JCI102501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 26: 72–76, 1985. [PubMed] [Google Scholar]
- 25. Dekkers CCJ, Sjöström CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab 21: 2667–2673, 2019. doi: 10.1111/dom.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vallon V. Tubular transport in acute kidney injury: relevance for diagnosis, prognosis and intervention. Nephron 134: 160–166, 2016. doi: 10.1159/000446448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zerbe RL, Robertson GL. Osmoregulation of thirst and vasopressin secretion in human subjects: effect of various solutes. Am J Physiol Endocrinol Physiol 244: E607–E614, 1983. doi: 10.1152/ajpendo.1983.244.6.E607. [DOI] [PubMed] [Google Scholar]
- 28. Sladek CD, Knigge KM. Osmotic control of vasopressin release by rat hypothalamo-neurohypophyseal explants in organ culture. Endocrinology 101: 1834–1838, 1977. doi: 10.1210/endo-101-6-1834. [DOI] [PubMed] [Google Scholar]
- 29. Mahon WA, Holland J, Urowitz MB. Hyperosmolar, non-ketotic diabetic coma. Can Med Assoc J 99: 1090–1092, 1968. [PMC free article] [PubMed] [Google Scholar]
- 30. Hayama N, Wang W, Schneider EG. Osmolality-induced changes in aldosterone secretion involve a chloride-dependent process. Am J Physiol Regul Integr Comp Physiol 268: R8–R13, 1995. doi: 10.1152/ajpregu.1995.268.1.R8. [DOI] [PubMed] [Google Scholar]
- 31. Iwasaki Y, Gaskill MB, Robertson GL. Adaptive resetting of the volume control of vasopressin secretion during sustained hypovolemia. Am J Physiol Regul Integr Comp Physiol 268: R349–R357, 1995. doi: 10.1152/ajpregu.1995.268.2.R349. [DOI] [PubMed] [Google Scholar]
- 32. Baylis PH, De Beer FC. Human plasma vasopressin response to potent loop-diuretic drugs. Eur J Clin Pharmacol 20: 343–346, 1981. doi: 10.1007/BF00615403. [DOI] [PubMed] [Google Scholar]
- 33. Mose FH, Oczachowska-Kulik AE, Fenton RA, Bech JN. Effect of furosemide on body composition and urinary proteins that mediate tubular sodium and sodium transport—a randomized controlled trial. Physiol Rep 8: e14653, 2021. doi: 10.14814/phy2.14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujimura A, Ohashi K, Ebihara A. Chronopharmacological study of furosemide; (VII). Influence of repeated administration on biochemical parameters in blood. Life Sci 47: 2277–2281, 1990. doi: 10.1016/0024-3205(90)90159-o. [DOI] [PubMed] [Google Scholar]
- 35. Mose FH, Jörgensen AN, Vrist MH, Ekelof NP, Pedersen EB, Bech JN. Effect of 3% saline and furosemide on biomarkers of kidney injury and renal tubular function and GFR in healthy subjects—a randomized controlled trial. BMC Nephrol 20: 200, 2019. doi: 10.1186/s12882-019-1342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lundy RF Jr, Blair M, Horvath N, Norgren R. Sodium appetite, and ingestive behavior. Physiol Behav 78: 449–458, 2003. doi: 10.1016/S0031-9384(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 37. Dutra SGV, Paterson A, Monteiro LRN, Greenwood MP, Greenwood MP, Amaral LS, Melo MR, Colombari DSA, Colombari E, Reis LC, Hindmarch CCT, Elias LLK, Antunes-Rodrigues J, Murphy D, Mecawi AS. Physiological and transcriptomic changes in the hypothalamic-neurohypophysial system after 24 h of furosemide-induced sodium depletion. Neuroendocrinology 111: 70–86, 2021. doi: 10.1159/000505997. [DOI] [PubMed] [Google Scholar]
- 38. Bertino M, Tordoff MG. Sodium depletion increases rats' preferences for salted food. Behav Neurosci 102: 565–573, 1988. doi: 10.1037//0735-7044.102.4.565. [DOI] [PubMed] [Google Scholar]
- 39. Kanbay M, Yilmaz S, Dincer N, Ortiz A, Sag AA, Covic A, Sánchez-Lozada LG, Lanaspa MA, Cherney DZI, Johnson RJ, Afsar B. Antidiuretic hormone and serum osmolarity physiology and related outcomes: what is old, what is new, and what is unknown? J Clin Endocrinol Metab 104: 5406–5420, 2019. doi: 10.1210/jc.2019-01049. [DOI] [PubMed] [Google Scholar]
- 40. Mutlu GM, Factor P. Role of vasopressin in the management of septic shock. Intensive Care Med 30: 1276–1291, 2004. doi: 10.1007/s00134-004-2283-8. [DOI] [PubMed] [Google Scholar]
- 41. Kim JS, Kim WB, Kim YB, Lee Y, Kim YS, Shen FY, Lee SW, Park D, Choi HJ, Hur J, Park JJ, Han HC, Colwell CS, Cho YW, Kim YI. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci 31: 13312–13322, 2011. doi: 10.1523/JNEUROSCI.1440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 43. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 1800, 2016. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 44. Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, Häring HU, Stefan N, Fritsche A, Artunc F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol 18: 46, 2019. doi: 10.1186/s12933-019-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 21: 137–155, 2019. doi: 10.1002/ejhf.1369. [DOI] [PubMed] [Google Scholar]